Abstract
Pancreatic cancer is a malignant neoplasm with a high mortality rate. Therapeutic agents that activate TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis have shown promising efficacy, but many pancreatic cancers are resistant to TRAIL therapy. Epigenetic regulation plays important roles in tumor pathogenesis and resistance, and a recent study indicated that the long non-coding RNA HOX transcript antisense RNA (HOTAIR) is overexpressed in pancreatic cancer. However, the role of HOTAIR in pancreatic cancer resistance to anticancer agents is unknown. The present study determined the role of HOTAIR in pancreatic cancer TRAIL resistance and investigated the underlying molecular mechanisms. We observed that TRAIL-resistant pancreatic cancer cells had higher levels of HOTAIR expression, whereas TRAIL-sensitive pancreatic cancer cells had lower HOTAIR levels. Overexpressing HOTAIR in TRAIL-sensitive cells attenuated TRAIL-induced apoptosis, and shRNA-mediated HOTAIR knockdown in TRAIL-resistant PANC-1 cells sensitized them to TRAIL-induced apoptosis. These results support a causative effect of HOTAIR on TRAIL sensitivity. Mechanistically, we found that increased HOTAIR expression inhibited the expression of the TRAIL receptor death receptor 5 (DR5), whereas HOTAIR knockdown increased DR5 expression. We further demonstrated that HOTAIR regulates DR5 expression via the epigenetic regulator enhancer of zeste homolog 2 (EZH2) and that EZH2 controls histone H3 lysine 27 trimethylation on the DR5 gene. Taken together, these results demonstrate that high HOTAIR levels increase the resistance of pancreatic cancer cells to TRAIL-induced apoptosis via epigenetic regulation of DR5 expression. Our study therefore supports the notion that targeting HOTAIR function may represent a strategy to overcome TRAIL resistance in pancreatic cancer.
Keywords: apoptosis; histone methylation; long non-coding RNA (long ncRNA, lncRNA); pancreatic cancer; TRAIL; death receptor 5; DR5
Introduction
Pancreatic cancer is a malignant neoplasm with a high mortality rate. The 5-year survival rate remains very low despite the use of comprehensive therapies, including surgical resection, radiation therapy, and chemotherapy (1). Given the concealed location, pancreatic cancer is seldom diagnosed until serious clinical symptoms and signs are present. Most patients with pancreatic cancer are not candidates for surgical resection. Chemotherapy, radiotherapy, and immunotherapy have been employed to treat patients with pancreatic cancer. However, resistance of pancreatic cancer to current treatment protocols is a major obstacle to obtaining a better prognosis for patients with pancreatic cancer (2, 3).
Dysregulation of cell apoptosis not only is involved in pathogenesis and progression of various cancers, including pancreatic cancer, but also contributes to resistance of cancer cells to chemotherapy-, radiotherapy-, and immunotherapy-induced cytotoxicity (4). Apoptosis pathways may be initiated through different entry sites, including mitochondria and death receptors, leading to activation of effector caspases (5, 6). Activating death receptor-induced apoptosis via the TRAIL2 receptors death receptor 4 (DR4) and death receptor 5 (DR5) has been demonstrated in a wide variety of tumor cells in vitro and in vivo, and has been consistently highly selective for tumor cells over normal cells (7). Many cancer cells are resistant to TRAIL-induced cell death, however, especially some highly malignant tumors such as pancreatic cancer (8). Some TRAIL and DR4/DR5 agonist antibodies, including conatumumab (AMG655, antibody for DR5) and tigatuzumab (CS-1008/TRA-8, antibody for DR5), have also been used for treating several types of cancers. Unfortunately, overall they have shown limited anti-tumor efficacy (9, 10). Therefore, further understanding of the molecular and cellular mechanisms of TRAIL resistance is critical for the successful application of TRAIL and DR4 or DR5 agonist antibodies in cancer therapy.
Multifaceted mechanisms including cross-talk of signaling pathways, genetic and epigenetic mutations, inactivation of tumor suppressors, and activation of oncogenes in cancer can lead to dysregulation of cell apoptosis. Epigenetic regulation by non-coding RNA (ncRNA) has been shown to play a major biological role in cellular development and metabolism (11–13). Non-coding RNAs are transcripts without an open reading frame. Aberrant regulation of ncRNAs can lead to developmental abnormalities and a variety of diseases, including cancer (14–16). MicroRNAs are small ncRNAs with ∼22 nucleotides and have been extensively investigated in many cellular systems, including various cancers (16). On the other hand, long non-coding RNAs (lncRNAs) are mRNA-like transcripts ranging in length from 200 nucleotides to ∼100 kb. A relatively small number of human lncRNAs have been characterized in a spectrum of biological processes (17). Some lncRNAs have been associated with the pathogenesis of cancer (17–19), including the HOX transcript antisense RNA (HOTAIR). Recent studies have shown that HOTAIR is overexpressed in many tumors, including pancreatic cancer, which may regulate cancer pathogenesis via epigenetic regulation (20–24). The lncRNA HOTAIR is a 2158-bp link RNA localized to a boundary in the HOXC gene cluster (20). Inhibition of HOTAIR was associated with decreased pancreatic cell invasion and proliferation (24). However, the function of HOTAIR in regulating pancreatic cancer TRAIL resistance has never been explored previously.
In this report, we determine the contribution of HOTAIR to resistance of pancreatic cancer cells to TRAIL-induced apoptosis. We identified a higher level of HOTAIR expression in TRAIL-resistant pancreatic cancer cells, whereas TRAIL-sensitive pancreatic cancer cells expressed a lower level of HOTAIR, suggesting a negative correlation between HOTAIR expression and the sensitivity of pancreatic cancer cells to TRAIL-induced apoptosis. Using gain and loss of function approaches, we further demonstrated a causative effect of HOTAIR on TRAIL-induced apoptosis via modulating DR5 expression. Mechanistically, HOTAIR was found to regulate DR5 expression via enhancer of zeste homolog 2 (EZH2), which modulated histone H3K27 trimethylation on DR5 gene. These studies have revealed a new causative link between lncRNA HOTAIR and pancreatic cancer TRAIL resistance, via epigenetic regulation of DR5 expression. Our results support the notion that targeting HOTAIR function may represent a novel strategy to improve efficacy of TRAIL therapy for resistant pancreatic cancer.
Results
HOTAIR expression in TRAIL-sensitive and -resistant pancreatic cancer cells
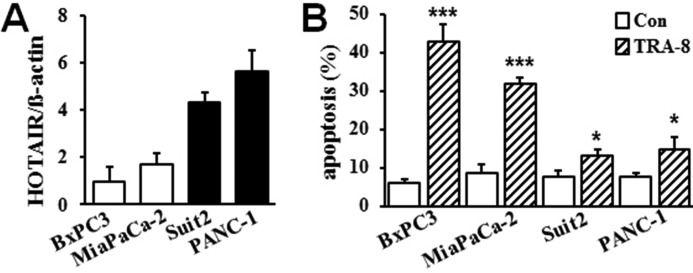
To determine the role of HOTAIR in pancreatic cancer cell TRAIL resistance, we analyzed the expression of HOTAIR in pancreatic cancer cells with different sensitivity to TRA-8. As shown in Fig. 1, TRA-8-sensitive MiaPaCa-2 and BxPC3 cells expressed relatively lower levels of HOTAIR, whereas higher levels of HOTAIR expression were demonstrated in the TRA-8-resistant Suit2 and PANC-1 cells. The results suggest that higher levels of HOTAIR expression may contribute to the resistance of pancreatic cancer cells to TRAIL-induced apoptosis.
Figure 1.
HOTAIR expression in pancreatic cancer cells with different sensitivity to TRAIL-induced apoptosis. A, HOTAIR expression in pancreatic cell lines, BxPC3, MiaPaCa-2, Suit2, and PANC-1, as determined by qRT-PCR and normalized by the expression of β-actin. Results shown are means ± S.D. of three independent experiments performed in duplicate. B, TRA-8-induced apoptosis. Pancreatic cancer cells were exposed to TRA-8 (1 μg/ml) for 24 h; and apoptotic cells were detected by flow cytometry (n = 3, *, p < 0.05, ***, p < 0.001).
Increased expression of HOTAIR inhibits TRAIL-induced apoptosis in sensitive pancreatic cancer cells
To determine a causative effect of HOTAIR expression on pancreatic cancer TRAIL resistance, we first increased the expression of HOTAIR in TRAIL-sensitive BxPC3 and MiaPaCa-2 cells (Fig. 2). BxPC3 and MiaPaCa-2 cells stably expressing 2- to 3-fold increase of HOTAIR over basal levels were generated (Fig. 2, Aa and Ba). Increased HOTAIR expression in these TRAIL-sensitive pancreatic cancer cells markedly inhibited TRA-8-induced apoptosis (Fig. 2, Ab and Bb). Furthermore, TRA-8 induced activation (cleavage) of caspase-8 in the control cells, which was blocked in the HOTAIR-overexpressing BxPC3 (Fig. 2Ac) and MiaPaCa-2 cells (Fig. 2Bc), further supporting that increased HOTAIR expression inhibits death receptor-activated apoptotic signaling. Therefore, increased HOTAIR expression in sensitive pancreatic cancer cells reduces their sensitivity to TRAIL-induced apoptosis.
Figure 2.
Overexpression of HOTAIR attenuates TRA-8-induced apoptosis in sensitive pancreatic cancer cells. A and B, BxPC3 (A) and MiaPaCa-2 (B) cells were infected with lentiviruses carrying control vector or HOTAIR cDNA (HOTAIR), and stable clones were selected by puromycin. Panels Aa and Ba, HOTAIR expression, as determined by qRT-PCR and normalized by β-actin expression (n = 3, ***, p < 0.001). Panels Ab and Bb, TRA-8-induced apoptosis. BxPC3 and MiaPaCa-2 cells with HOTAIR overexpression and their control vector cells were seeded into 6-well plates at 2 × 105 per well. After culturing for 24 h, cells were exposed to TRA-8 (1 μg/ml) for 24 h, and apoptosis was determined by flow cytometry using Annexin PE and 7 AAD staining kit. TRA-8-induced apoptosis is shown in the hatched bars (n = 3, ***, p < 0.001). Panels Ac and Bc, Western blot analysis of the expression of caspase-8 (Casp8). The expression of β-actin was used as a loading control. Representative blots from three independent experiments are shown.
HOTAIR down-regulation promotes TRAIL-induced apoptosis in TRAIL-resistant pancreatic cancer cells
A definitive role of HOTAIR expression in mediating TRAIL resistance was further demonstrated by knocking down HOTAIR in the TRAIL-resistant PANC-1 and Suit 2 cells (Fig. 3). PANC-1 and Suit2 cells with HOTAIR knockdown were generated with shRNA specific for HOTAIR (Fig. 3, Aa and Ba). Knockdown of HOTAIR in the TRAIL-resistant PANC-1 and Suit2 sensitized them to TRA-8-induced apoptosis (Fig. 3, Ab and Bb). Therefore, together with results in Fig. 2, these studies demonstrate that the expression of HOTAIR in pancreatic cancer cells is correlated with their sensitivity to TRAIL-induced apoptosis.
Figure 3.
Down-regulation of HOTAIR promotes TRA-8-induced apoptosis in resistant pancreatic cells. A and B, PANC-1 (A) and Suit2 (B) cells were infected with lentiviruses carrying scrambled shRNA (shScr) or shRNA for HOTAIR (shHOTAIR) and selected by puromycin. Panels Aa and Ba, HOTAIR expression, as determined by qRT-PCR and normalized by β-actin expression (n = 3, **, p < 0.01, ***, p < 0.001). Panels Ab and Bb, TRA-8-induced apoptosis. Cells were exposed to TRA-8 (1 μg/ml) for 24 h, and apoptosis was determined by flow cytometry. TRA-8-induced apoptosis is shown in the hatched bars (n = 3, **, p < 0.01, ***, p < 0.001).
HOTAIR regulates DR5 expression in pancreatic cancer cells
To determine HOTAIR-regulated signals that contribute to its effects on TRAIL-induced apoptosis, we analyzed the expression of the mediators in the TRAIL-activated apoptotic signaling pathways. Among the TRAIL death receptors, we found that the expression of DR5 was modified when HOTAIR expression was altered in pancreatic cancer cells (Fig. 4). Overexpression of HOTAIR in the sensitive BxPC3 and MiaPaCa-2 cells decreased the expression of DR5 mRNA, as determined by real-time PCR (Fig. 4, Aa and Ba). Western blot analysis further demonstrated inhibition of DR5 protein expression in HOTAIR-overexpressed cells (Fig. 4, Ab and Bb). On the other hand, knockdown of HOTAIR in the resistant PANC-1 and Suit2 cells increased the expression of DR5 at both mRNA and protein levels (Fig. 4, C and D). Of note, the expression of other TRAIL death receptor DR4 was not affected by HOTAIR expression (data not shown). Therefore, HOTAIR regulates DR5 expression, thus contributing to the sensitivity/resistance of pancreatic cancer cells to TRAIL-induced apoptosis.
Figure 4.
HOTAIR regulates DR5 expression in pancreatic cancer cells. A and B, overexpression of HOTAIR inhibited DR5 expression BxPC3 and MiaPaCa-2 cells stably expressing HOTAIR cDNA (HOTAIR) or control vector were selected by puromycin. Panels Aa and Ba, DR5 mRNA expression, as determined by qRT-PCR and normalized by β-actin expression (n = 3, **, p < 0.01). Panels Ab and Bb, DR5 protein expression, as determined by Western blot analysis. The expression of β-actin expression was used as a loading control. Representative blots from three independent experiments are shown. C and D, HOTAIR knockdown increased DR5 expression. PANC-1 and Suit2 cells stably expressing scrambled shRNA (shScr) or shRNA for HOTAIR (shHOTAIR) were selected by puromycin. Panels Ca and Da, DR5 mRNA expression, as determined by qRT-PCR and normalized by β-actin expression (n = 3, *, p < 0.05, **, p < 0.01). Panels Cb and Db, DR5 protein expression, as determined by Western blotting. The expression of β-actin was used as a loading control. Representative blots from three independent experiments are shown.
HOTAIR/EZH2 signaling regulates DR5 expression and TRAIL-induced apoptosis
HOTAIR has been shown to regulate the expression of tumor suppressor genes through interacting with polycomb repressive complex 2 (PRC2) containing EZH2 (enhancer of zeste homolog 2), which binds to the promoter regions of target genes that regulate chromatin structure and silence gene transcription (20). Using DZNeP (5 μm), a specific pharmacological inhibitor for the important PRC2 component EZH2 as reported (25), we found that inhibiting HOTAIR/EZH2 function sensitized TRA-8-induced apoptosis in the resistant PANC-1 and Suit2 cells (Fig. 5A, a and b), similar to the effects of HOTAIR knockdown (Fig. 3). More importantly, DZNeP treatment resulted in recovery of TRAIL sensitivity in HOTAIR-overexpressed BxPC3 and MiaPaCa-2 cells, further supporting a direct role of EZH2 in mediating the function of HOTAIR-regulated TRAIL sensitivity (Fig. 5B, a and b).
Figure 5.
Inhibition of EZH2 increases TRA-8-induced apoptosis. A, DZNeP increased TRA-8-induced apoptosis in resistant cells. PANC-1 (panel a) and Suit2 (panel b) cells were exposed to DZNeP (5 μm), an EZH2 inhibitor, and subsequently treated with TRA-8 (1 μg/ml) for 24 h. Apoptosis was determined by flow cytometry (n = 3, ***, p < 0.001). B, DZNeP attenuated HOTAIR-overexpression-induced TRA-8 resistance. BxPC3 (panel a) and MiaPaCa-2 (panel b) cells with stably HOTAIR overexpression were exposed to DZNeP (5 μm) and subsequently treated with TRA-8 (1 μg/ml) for 24 h. Apoptosis was determined by flow cytometry (n = 3, *, p < 0.05, **, p < 0.01). C and D, EZH2 knockdown enhanced DR5 expression and TRA-8-induced apoptosis. PANC-1 and Suit2 cells stably expressing scrambled shRNA (shScr) or shRNA for EZH2 (shEZH2) were selected by puromycin. Panels Ca and Da, cells were exposed to TRA-8 (1 μg/ml) for 24 h; apoptosis was determined by flow cytometry (n = 3, *, p < 0.05, ***, p < 0.001). Panels Cb and Db, Western blot analyses of the expression of EZH2 and DR5. The expression of β-actin was used as a loading control. Representative blots from three independent experiments are shown.
In addition, the specificity of the EZH2 in regulating DR5 and TRAIL-induced apoptosis was determined using shRNA for EZH2. Consistent with the observations using DZNeP, EZH2 knockdown in PANC-1 and Suit2 cells sensitized these resistant cells to TRA-8-induced apoptosis (Fig. 5, Ca and Da). The knockdown of EZH2 in these cells was confirmed by Western blot analysis (Fig. 5, Cb and Db). Importantly, EZH2 knockdown markedly increased DR5 expression in these resistant pancreatic cancer cells (Fig. 5, Cb and Db). Taken together, these results have demonstrated that inhibition of HOTAIR/EZH2 signals increases DR5 expression and sensitizes TRAIL-induced apoptosis in pancreatic cancer cells.
HOTAIR inhibits DR5 transcription via increased histone H3 trimethylation (H3K27me3) on DR5 gene
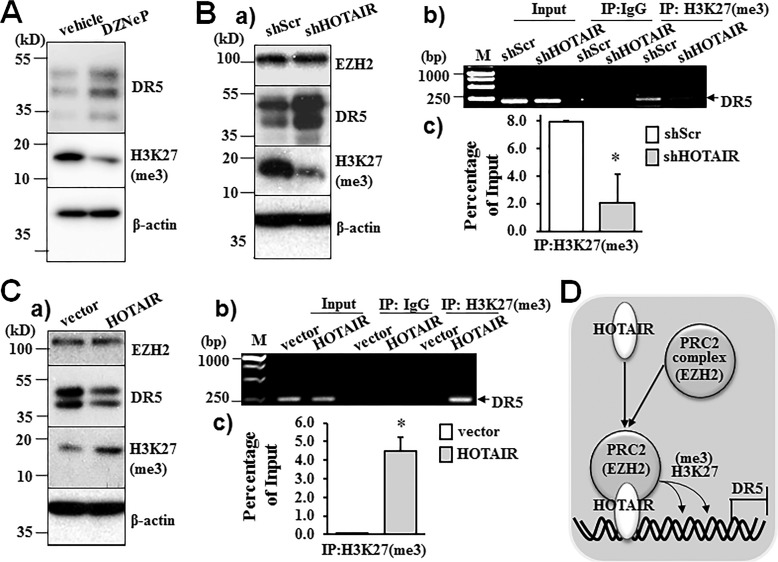
EZH2 enzyme catalyzes histone H3 lysine 27 trimethylation (H3K27me3), an important epigenetic modulation on histone that controls chromatin structure and inhibits gene transcription (26). Accordingly, we first determined the effects of HOTAIR/EZH2 signal on H3K27me3. Inhibition of EZH2 by DZNeP decreased H3K27me3, confirming the function of EZH2 in regulating H3K27me3 in pancreatic cancer cells (Fig. 6A). Knockdown of HOTAIR expression by shRNA did not affect the expression of EZH2, whereas H3K27me3 was decreased (Fig. 6Ba). Furthermore, HOTAIR knockdown markedly decreased H3K27me3 binding to the DR5 promoter, as demonstrated by chromatin immunoprecipitation (ChIP) analysis using an antibody for H3K27me3 (Fig. 6B, b and c). Consistently, overexpression of HOTAIR did not affect EZH2 expression, but greatly enhanced H3K27me3 binding to the DR5 promoter (Fig. 6C, a–c). Altogether, these studies with gain or loss of function of HOTAIR have demonstrated that HOTAIR/EZH2 increases H3K27me3 on the DR5 gene, thus inhibiting DR5 transcription and decreasing DR5 expression as depicted in Fig. 6D.
Figure 6.
HOTAIR regulates DR5 expression via EZH2-mediated modulation of histone H3 lysine 27 trimethylation (H3K27me3) on DR5 gene. A, DZNeP inhibited H3K27me3 in pancreatic cancer cells. PANC-1 cells were exposed to DZNeP (5 μm) for 24 h. H3K27me3 and DR5 expression was determined by Western blot analysis. The expression of β-actin was used as a loading control. Representative blots from three independent experiments are shown. B, HOTAIR knockdown inhibited H3K27me3 on DR5 gene. PANC-1 cells stably expressing scrambled shRNA (shScr) or shRNA for HOTAIR (shHOTAIR) were selected by puromycin. Panel a, Western blot analyses of EZH, DR5, and H3K27me3. Panels b and c, ChIP was performed using mouse anti-trimethyl-histone 3 (lysine 27) antibody or control mouse IgG. H3K27me3-bound DR5 gene was determined by PCR (panel b) and quantitative PCR (panel c). Representative results of three independent experiments are shown. *, p < 0.05. C, HOTAIR overexpression increased H3K27me3 on DR5 gene. MiaPaCa-2 cells stably expressing HOTAIR cDNA or control vector were selected by puromycin. Panel a, Western blot analysis of the expression of EZH2, DR5, and H3K27me3. The expression of β-actin was used as a loading control. Panels b and c, ChIP analysis of H3K27me3 on DR5 gene, determined by PCR (panel b) and quantitative PCR (panel c). Representative results of three independent experiments are shown. *, p < 0.05. D, schematic model of HOTAIR/EZH2-mediated H3K27me3 and DR5 expression in pancreatic cancer cells.
Discussion
Many recombinant TRAIL or anti-human DR4 or DR5 monoclonal antibodies have been tested in phase I–III clinical trials for their anti-tumor efficacy, including antibodies for DR5, conatumumab, and tigatuzumab that have been tested for treating pancreatic tumors (Clinicaltrials.gov) (9, 10, 27, 28). These reagents show low toxicity and are well tolerated in patients in clinical trials (7, 29, 30). However, TRAIL and DR4/DR5 agonist antibodies have shown only limited anti-tumor efficacy in clinical trials (9, 31). The resistance of tumor cells to TRAIL-induced apoptosis and a lack of ability for the other anticancer agents to sensitize TRAIL-induced apoptosis likely contribute to the limited efficacy of the current TRAIL therapies. The present studies provide the first evidence that epigenetic modulation of the DR5 gene by long non-coding RNA HOTAIR regulates the resistance of pancreatic cancer cells to TRA-8-induced apoptosis, which may contribute to TRAIL resistance.
Although a positive correlation of TRAIL resistance and the expression levels of TRAIL receptors, including DR4 and DR5, are not consistently demonstrated, previous studies from our group and others have suggested that strategies to up-regulate the death receptors DR4 and DR5 enhance TRAIL-induced apoptosis in cancer cells (32–36). The discovery of higher levels of lncRNA HOTAIR in the TRAIL-resistant cells led us to investigate whether there was a direct role of HOTAIR in TRAIL resistance. HOTAIR expression was previously linked to pancreatic cell invasion and proliferation (24); however, its function in regulating TRAIL-induced apoptosis has not been studied. Using gain and loss of function approaches, we demonstrated a causative effect of HOTAIR on modulating the expression of DR5 and thus regulating TRAIL sensitivity/resistance. Furthermore, the direct effect of HOTAIR on the expression of death receptors was specific for DR5, as we found that altered HOTAIR expression did not affect the expression of DR4 in either resistant or sensitive pancreatic cancer cells (data not shown).
Epigenetic modulation of regulators in the TRAIL-induced apoptotic pathway by non-coding RNAs has been studied previously, but largely by small non-coding RNAs or microRNAs. For instance, miR-148a is down-regulated in non-small cell lung cancer cells with acquired TRAIL resistance, and enforced expression of miR-148a sensitizes cells to TRAIL (37). In addition, other microRNAs, such as miR-145, miR-216, miR-182, and miR-96, were predicted to regulate DR4/DR5 and Fas-associated death domain (38), thus contributing to the regulation of TRAIL-induced apoptosis. To the best of our knowledge, the present studies represent the first effort to explore the regulatory effects of long non-coding RNA on TRAIL-induced apoptosis.
We demonstrated that inhibition of EZH2 by a pharmacological inhibitor or shRNA for EZH2 duplicated the effects of HOTAIR inhibition on DR5 expression and TRA-8-induced apoptosis. This observation is consistent with the known function of HOTAIR, which interacts with PRC2 containing the histone H3 methylation enzyme, EZH2, and silences gene transcription via EZH2-catalyzed H3K27me3 (20, 26). Importantly, EZH2 inhibition attenuated HOTAIR-overexpression-induced TRAIL resistance, supporting a direct role of EZH2 in mediating the function of HOTAIR in regulating TRAIL-induced apoptosis. A previous study has shown that EZH2 expression in pancreatic cancer cells was significantly higher than in normal ductal pancreatic cells and fibroblasts. Thus, inhibition of EZH2 function by DZNeP inhibits pancreatic cancer cell proliferation and promotes anti-cancer drug-induced apoptosis (25). Of note, the expression of EZH2 was similar in TRAIL-sensitive and -resistant pancreatic cancer cells in the present studies. Moreover, altered HOTAIR expression did not affect the expression of EZH2, indicating the effect of HOTAIR on DR5 expression was not through modulating EZH2 expression, but through regulating H3K27me3 of the chromatin on the promoter region of DR5 gene. It is likely that regulating H3K27me3 by altered HOTAIR/EZH2 may affect the expression of other molecules that may contribute to HOTAIR/EZH2-regulated TRAIL sensitivity. Nonetheless, our studies utilizing the gain of function and loss of function of HOTAIR and ChIP analysis of H3K27me3 binding to DR5 promoter clearly support that HOTAIR/EZH2 regulated DR5 expression, which contributed to TRA-8-induced apoptosis. Therefore, a high level of HOTAIR may lead to increased recruitment of HOTAIR/EZH2 complex to the promoter region of DR5 that leads to H3 trimethylation, thus silencing DR5 expression.
In summary, we have demonstrated a causative effect of HOTAIR expression on resistance of pancreatic cancer cells to TRAIL-induced apoptosis and identified a novel mechanism underlying HOTAIR-regulated DR5 expression via EZH2-mediated H3K27me3 and its direct binding on the DR5 gene. These studies support the notion that targeting HOTAIR/EZH2 to increase nascent DR5 expression in resistant pancreatic cancer cells may represent a new strategy to sensitizing TRAIL-induced apoptosis and thus improving TRAIL therapy.
Experimental procedures
Cell lines
Human pancreatic cancer cell lines MiaPaCa-2, BxPC3, Suit2, and PANC-1 were purchased from the American Type Culture Collection (ATCC) and maintained in DMEM or RPMI 1640 medium with 10% FBS.
Reagents and antibodies
DR5 agonist antibody, TRA-8, was generated as described previously (39). Antibodies were purchased as follows: rabbit anti-DR5 antibody (ProSci, no. 2019, lot number 5355–1502), mouse anti-caspase 8 (BIOSOURCE no. AHZ0502, lot number 22363–01S), mouse anti-β-actin (Sigma, no. A5541–2MI, lot number 014M4759), rabbit anti-EZH2 (Cell Signaling Technology, no. D2C9, lot number 7), and mouse anti-trimethyl-histone H3 (lysine 27) (Active Motif, no. 61017, lot number 23115012). The EZH2 inhibitor DZNeP was purchased from ApexBio Technology.
Western blot analysis
Whole cell lysates were prepared using extraction buffer (50 mm Tris-HCL-buffered saline, pH 7.4, 1% Triton X-100, 1% Nonidet P-40, and protease inhibitor mixture). Protein concentrations were measured with a BCA protein assay kit (Thermo Scientific). Proteins were separated by SDS-PAGE and transferred to Immobilon-P membranes. Membranes were blocked in 5% nonfat milk, incubated with primary antibodies and horseradish peroxidase-conjugated secondary antibodies. Signals were detected using Amersham ECL Western Blotting Detection Reagents (GE Healthcare).
Lentiviral constructs
Human HOTAIR cDNA was amplified by PCR and then inserted into lentivirus cloning vector (40) through Bamh 1 and Xho l restriction enzyme sites as we described previously (40). For shRNA specific targeting HOTAIR or EZH2, shRNA oligos were generated according to Addgene instruction for construction of PLKO.1 shRNA lentivirus vector and inserted into pLKO.1-TRC cloning vector (Addgene).
Sequences for shRNA HOTAIR and shRNA EZH2 were as follows: shRNA-HOTAIR1, 5′-GAACGGGAGTACAGAGAGATT-3′ (GenBank®, NR_047528.1); shRNA-EZH2, 5′-GCTCCTCTAACCATGTTTACA-3′ (GenBank®, NM_001203247.1).
The lentiviral vectors were subjected for sequencing analysis and packaged into HEK-293T cells to generate lentiviruses as we described previously (32, 41).
Stable clone with HOTAIR overexpression or HOTAIR/EZH2 knockdown was generated as we described previously (32). For HOTAIR overexpression, lentiviruses carrying control vector or HOTAIR cDNA were infected in TRAIL-sensitive cells, MiaPaCa-2 and BxPC3. For HOTAIR/EZH2 knockdown, lentiviruses carrying control-scrambled shRNA or shRNA specific for HOTAIR or EZH2 were infected in TRAIL-resistant cells, PANC-1 and Suit2. Stably infected clones were selected by puromycin (2 μg/ml).
Quantitative real-time PCR analysis
The RNA expression of HOTAIR, DR5, and β-actin were determined by real-time PCR using SYBR Green Master Mix kit (Bio-Rad) as we reported (42). Primer sequences were as follows: human HOTAIR, 5′-GACACCACTGGAGGGTGACT-3′ (forward) and 5′-CAGGTCCACATGGTCTTCCT-3′(reverse); human DR5, 5′-GAACAGCTGTGTCTGCCAAA-3′ (forward) and 5′-TGGACTGTGACATCCCAGAA-3′ (reverse); and human β-actin, 5′-GACATCCTGGAACTGCCCTA-3′ (forward) and 5′-GGTCATGTTGCCTTTCCAGT-3 (reverse).
Analysis of apoptosis
Apoptosis of cells was determined by flow cytometry using Annexin V-fluorescein isothiocyanate (FITC) propidium iodide (PI) staining kit (BD Biosciences) or Annexin PE and 7 AAD staining kit (BD Biosciences) as we reported previously (32, 33).
Chromatin immunoprecipitation (ChIP) assay
ChIP was performed according to previously published method (43–45). In brief, cells were cross-linked by addition of formaldehyde (1% final concentration); and nuclei were collected by a serial of centrifugation, resuspended in buffer containing protease inhibitors, and sonicated on ice. After centrifugation, supernatant was adjusted to RIPA buffer. After being precleared with protein-agarose beads, samples were incubated with 2 μg/ml of mouse anti-trimethyl-histone H3 (lysine 27) (Active Motif, no. 61017) or control IgG and subsequently incubated with protein A-agarose beads. The immune complexes were recovered, washed, treated with proteinase K followed by overnight reversal of cross-links. The resulting DNA was purified and analyzed by PCR and quantitative PCR (qPCR) using primers that recognize promoter sequences that flank DR5 gene promoter. The primers were as follows: 5′-GGGAAGGGGAGAAGATCAAG-3′ (forward), 5′-AGTCTCGTCAACGCAATCCT-3′ (reverse).
Statistical analysis
Results were statistically analyzed using an unpaired Student's t test or one-way analysis of variance (ANOVA). Data presented are means ± S.D. p values less than 0.05 were considered significant.
Author contributions
S. Y. and Y. C. designed the experiments. S. Y., F. X., and T. Z. performed the experiments. S. Y., T. Z., J. M. M., and Y. C. analyzed data. S. Y., J. M. M., X. Z., and Y. C. wrote the manuscript.
This work was supported by Veterans Affairs Research Department Grants BX002296 and BX003617 (Y.C.). The authors declare that they have no conflicts of interest with the contents of this article.
- TRAIL
- TNF-related apoptosis-inducing ligand
- DR4
- death receptor 4
- DR5
- death receptor 5
- EZH2
- enhancer of zeste homolog 2
- ncRNA
- non-coding RNA
- lncRNA
- long non-coding RNA
- PRC2
- polycomb repressive complex 2.
References
- 1. Mocan T., Matea C. T., Cojocaru I., Ilie I., Tabaran F. A., Zaharie F., Iancu C., Bartos D., and Mocan L. (2014) Photothermal treatment of human pancreatic cancer using pegylated multi-walled carbon nanotubes induces apoptosis by triggering mitochondrial membrane depolarization mechanism. J. Cancer 5, 679–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mayor S. (2015) Immunotherapy improves overall survival in pancreatic cancer. Lancet Oncol. 16, e58. [DOI] [PubMed] [Google Scholar]
- 3. Bergmann L., Maute L., Heil G., Rüssel J., Weidmann E., Köberle D., Fuxius S., Weigang-Köhler K., Aulitzky W. E., Wörmann B., Hartung G., Moritz B., Edler L., Burkholder I., Scheulen M. E., and Richly H. (2015) A prospective randomised phase-II trial with gemcitabine versus gemcitabine plus sunitinib in advanced pancreatic cancer: a study of the CESAR Central European Society for Anticancer Drug Research-EWIV. Eur. J. Cancer 51, 27–36 [DOI] [PubMed] [Google Scholar]
- 4. Hanahan D., and Weinberg R. A. (2000) The hallmarks of cancer. Cell 100, 57–70 [DOI] [PubMed] [Google Scholar]
- 5. Schütze S., Tchikov V., and Schneider-Brachert W. (2008) Regulation of TNFR1 and CD95 signalling by receptor compartmentalization. Nat. Rev. Mol. Cell Biol. 9, 655–662 [DOI] [PubMed] [Google Scholar]
- 6. Fulda S., and Debatin K. M. (2006) Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 25, 4798–4811 [DOI] [PubMed] [Google Scholar]
- 7. Holoch P. A., and Griffith T. S. (2009) TNF-related apoptosis-inducing ligand (TRAIL): a new path to anti-cancer therapies. Eur. J. Pharmacol. 625, 63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Matsuzaki H., Schmied B. M., Ulrich A., Standop J., Schneider M. B., Batra S. K., Picha K. S., and Pour P. M. (2001) Combination of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and actinomycin D induces apoptosis even in TRAIL-resistant human pancreatic cancer cells. Clin. Cancer Res. 7, 407–414 [PubMed] [Google Scholar]
- 9. Herbst R. S., Kurzrock R., Hong D. S., Valdivieso M., Hsu C. P., Goyal L., Juan G., Hwang Y. C., Wong S., Hill J. S., Friberg G., and LoRusso P. M. (2010) A first-in-human study of conatumumab in adult patients with advanced solid tumors. Clin. Cancer Res. 16, 5883–5891 [DOI] [PubMed] [Google Scholar]
- 10. Forero-Torres A., Shah J., Wood T., Posey J., Carlisle R., Copigneaux C., Luo F. R., Wojtowicz-Praga S., Percent I., and Saleh M. (2010) Phase I trial of weekly tigatuzumab, an agonistic humanized monoclonal antibody targeting death receptor 5 (DR5). Cancer Biother. Radiopharm. 25, 13–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kornfeld J. W., and Brüning J. C. (2014) Regulation of metabolism by long, non-coding RNAs. Front. Genet. 5, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stefani G., and Slack F. J. (2008) Small non-coding RNAs in animal development. Nat. Rev. Mol. Cell Biol. 9, 219–230 [DOI] [PubMed] [Google Scholar]
- 13. Schmitt A. M., and Chang H. Y. (2016) Long noncoding RNAs in cancer pathways. Cancer Cell 29, 452–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ambros V. (2004) The functions of animal microRNAs. Nature 431, 350–355 [DOI] [PubMed] [Google Scholar]
- 15. Esteller M. (2011) Non-coding RNAs in human disease. Nat. Rev. Genet. 12, 861–874 [DOI] [PubMed] [Google Scholar]
- 16. Montano M. (2011) MicroRNAs: miRRORS of health and disease. Transl. Res. 157, 157–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gibb E. A., Brown C. J., and Lam W. L. (2011) The functional role of long non-coding RNA in human carcinomas. Mol. Cancer 10, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huarte M., Guttman M., Feldser D., Garber M., Koziol M. J., Kenzelmann-Broz D., Khalil A. M., Zuk O., Amit I., Rabani M., Attardi L. D., Regev A., Lander E. S., Jacks T., and Rinn J. L. (2010) A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell 142, 409–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huarte M., and Rinn J. L. (2010) Large non-coding RNAs: missing links in cancer? Hum. Mol. Genet. 19, R152–R161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gupta R. A., Shah N., Wang K. C., Kim J., Horlings H. M., Wong D. J., Tsai M. C., Hung T., Argani P., Rinn J. L., Wang Y., Brzoska P., Kong B., Li R., West R. B., van de Vijver M. J., Sukumar S., and Chang H. Y. (2010) Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 464, 1071–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tsai M. C., Manor O., Wan Y., Mosammaparast N., Wang J. K., Lan F., Shi Y., Segal E., and Chang H. Y. (2010) Long noncoding RNA as modular scaffold of histone modification complexes. Science 329, 689–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ishibashi M., Kogo R., Shibata K., Sawada G., Takahashi Y., Kurashige J., Akiyoshi S., Sasaki S., Iwaya T., Sudo T., Sugimachi K., Mimori K., Wakabayashi G., and Mori M. (2013) Clinical significance of the expression of long non-coding RNA HOTAIR in primary hepatocellular carcinoma. Oncol. Rep. 29, 946–950 [DOI] [PubMed] [Google Scholar]
- 23. Kogo R., Shimamura T., Mimori K., Kawahara K., Imoto S., Sudo T., Tanaka F., Shibata K., Suzuki A., Komune S., Miyano S., and Mori M. (2011) Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 71, 6320–6326 [DOI] [PubMed] [Google Scholar]
- 24. Kim K., Jutooru I., Chadalapaka G., Johnson G., Frank J., Burghardt R., Kim S., and Safe S. (2013) HOTAIR is a negative prognostic factor and exhibits pro-oncogenic activity in pancreatic cancer. Oncogene 32, 1616–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Avan A., Crea F., Paolicchi E., Funel N., Galvani E., Marquez V. E., Honeywell R. J., Danesi R., Peters G. J., and Giovannetti E. (2012) Molecular mechanisms involved in the synergistic interaction of the EZH2 inhibitor 3-deazaneplanocin A with gemcitabine in pancreatic cancer cells. Mol. Cancer Ther. 11, 1735–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chase A., and Cross N. C. (2011) Aberrations of EZH2 in cancer. Clin. Cancer Res. 17, 2613–2618 [DOI] [PubMed] [Google Scholar]
- 27. Kindler H. L., Richards D. A., Garbo L. E., Garon E. B., Stephenson J. J. Jr., Rocha-Lima C. M., Safran H., Chan D., Kocs D. M., Galimi F., McGreivy J., Bray S. L., Hei Y., Feigal E. G., Loh E., and Fuchs C. S. (2012) A randomized, placebo-controlled phase 2 study of ganitumab (AMG 479) or conatumumab (AMG 655) in combination with gemcitabine in patients with metastatic pancreatic cancer. Ann. Oncol. 23, 2834–2842 [DOI] [PubMed] [Google Scholar]
- 28. Rajeshkumar N. V., Rasheed Z. A., García-García E., López-Ríos F., Fujiwara K., Matsui W. H., and Hidalgo M. (2010) A combination of DR5 agonistic monoclonal antibody with gemcitabine targets pancreatic cancer stem cells and results in long-term disease control in human pancreatic cancer model. Mol. Cancer Ther. 9, 2582–2592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang S. (2010) TRAIL: a sword for killing tumors. Curr. Med. Chem. 17, 3309–3317 [DOI] [PubMed] [Google Scholar]
- 30. Wiezorek J., Holland P., and Graves J. (2010) Death receptor agonists as a targeted therapy for cancer. Clin. Cancer Res. 16, 1701–1708 [DOI] [PubMed] [Google Scholar]
- 31. Younes A., Vose J. M., Zelenetz A. D., Smith M. R., Burris H. A., Ansell S. M., Klein J., Halpern W., Miceli R., Kumm E., Fox N. L., and Czuczman M. S. (2010) A phase 1b/2 trial of mapatumumab in patients with relapsed/refractory non-Hodgkin's lymphoma. Br. J. Cancer 103, 1783–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yuan K., Sun Y., Zhou T., McDonald J., and Chen Y. (2013) PARP-1 regulates resistance of pancreatic cancer to TRAIL therapy. Clin. Cancer Res. 19, 4750–4759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yuan K., Yong S., Xu F., Zhou T., McDonald J. M., and Chen Y. (2015) Calmodulin antagonists promote TRA-8 therapy of resistant pancreatic cancer. Oncotarget 6, 25308–25319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dimberg L. Y., Anderson C. K., Camidge R., Behbakht K., Thorburn A., and Ford H. L. (2013) On the TRAIL to successful cancer therapy? Predicting and counteracting resistance against TRAIL-based therapeutics. Oncogene 32, 1341–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ozawa F., Friess H., Kleeff J., Xu Z. W., Zimmermann A., Sheikh M. S., and Büchler M. W. (2001) Effects and expression of TRAIL and its apoptosis-promoting receptors in human pancreatic cancer. Cancer Lett. 163, 71–81 [DOI] [PubMed] [Google Scholar]
- 36. Keane M. M., Ettenberg S. A., Nau M. M., Russell E. K., and Lipkowitz S. (1999) Chemotherapy augments TRAIL-induced apoptosis in breast cell lines. Cancer Res. 59, 734–741 [PubMed] [Google Scholar]
- 37. Joshi P., Jeon Y. J., Laganà A., Middleton J., Secchiero P., Garofalo M., and Croce C. M. (2015) MicroRNA-148a reduces tumorigenesis and increases TRAIL-induced apoptosis in NSCLC. Proc. Natl. Acad. Sci. U.S.A. 112, 8650–8655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ovcharenko D., Kelnar K., Johnson C., Leng N., and Brown D. (2007) Genome-scale microRNA and small interfering RNA screens identify small RNA modulators of TRAIL-induced apoptosis pathway. Cancer Res. 67, 10782–10788 [DOI] [PubMed] [Google Scholar]
- 39. Ichikawa K., Liu W., Zhao L., Wang Z., Liu D., Ohtsuka T., Zhang H., Mountz J. D., Koopman W. J., Kimberly R. P., and Zhou T. (2001) Tumoricidal activity of a novel anti-human DR5 monoclonal antibody without hepatocyte cytotoxicity. Nat. Med. 7, 954–960 [DOI] [PubMed] [Google Scholar]
- 40. Chen J., Yuan K., Mao X., Miano J. M., Wu H., and Chen Y. (2012) Serum response factor regulates bone formation via IGF-1 and Runx2 signals. J. Bone Miner. Res. 27, 1659–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Heath J. M., Sun Y., Yuan K., Bradley W. E., Litovsky S., Dell'Italia L. J., Chatham J. C., Wu H., and Chen Y. (2014) Activation of AKT by O-linked N-acetylglucosamine induces vascular calcification in diabetes mellitus. Circ. Res. 114, 1094–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sun Y., Byon C. H., Yuan K., Chen J., Mao X., Heath J. M., Javed A., Zhang K., Anderson P. G., and Chen Y. (2012) Smooth muscle cell-specific runx2 deficiency inhibits vascular calcification. Circ. Res. 111, 543–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Takahashi Y., Rayman J. B., and Dynlacht B. D. (2000) Analysis of promoter binding by the E2F and pRB families in vivo: distinct E2F proteins mediate activation and repression. Genes Dev. 14, 804–816 [PMC free article] [PubMed] [Google Scholar]
- 44. Byon C. H., Sun Y., Chen J., Yuan K., Mao X., Heath J. M., Anderson P. G., Tintut Y., Demer L. L., Wang D., and Chen Y. (2011) Runx2-upregulated receptor activator of nuclear factor κB ligand in calcifying smooth muscle cells promotes migration and osteoclastic differentiation of macrophages. Arterioscler. Thromb. Vasc. Biol. 31, 1387–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tran N. T., Su H., Khodadadi-Jamayran A., Lin S., Zhang L., Zhou D., Pawlik K. M., Townes T. M., Chen Y., Mulloy J. C., and Zhao X. (2016) The AS-RBM15 lncRNA enhances RBM15 protein translation during megakaryocyte differentiation. EMBO Rep. 17, 887–900 [DOI] [PMC free article] [PubMed] [Google Scholar]