Abstract
Maintenance of whole-body glucose homeostasis is critical to glycemic function. Genetic variants mapping to chromosome 8p23.1 in genome-wide association studies have been linked to glycemic traits in humans. The gene of known function closest to the mapped region, PPP1R3B (protein phosphatase 1 regulatory subunit 3B), encodes a protein (GL) that regulates glycogen metabolism in the liver. We therefore sought to test the hypothesis that hepatic PPP1R3B is associated with glycemic traits. We generated mice with either liver-specific deletion (Ppp1r3bΔhep) or liver-specific overexpression of Ppp1r3b. The Ppp1r3b deletion significantly reduced glycogen synthase protein abundance, and the remaining protein was predominantly phosphorylated and inactive. As a consequence, glucose incorporation into hepatic glycogen was significantly impaired, total hepatic glycogen content was substantially decreased, and mice lacking hepatic Ppp1r3b had lower fasting plasma glucose than controls. The concomitant loss of liver glycogen impaired whole-body glucose homeostasis and increased hepatic expression of glycolytic enzymes in Ppp1r3bΔhep mice relative to controls in the postprandial state. Eight hours of fasting significantly increased the expression of two critical gluconeogenic enzymes, phosphoenolpyruvate carboxykinase and glucose-6-phosphatase, above the levels in control livers. Conversely, the liver-specific overexpression of Ppp1r3b enhanced hepatic glycogen storage above that of controls and, as a result, delayed the onset of fasting-induced hypoglycemia. Moreover, mice overexpressing hepatic Ppp1r3b upon long-term fasting (12–36 h) were protected from blood ketone-body accumulation, unlike control and Ppp1r3bΔhep mice. These findings indicate a major role for Ppp1r3b in regulating hepatic glycogen stores and whole-body glucose/energy homeostasis.
Keywords: gluconeogenesis, glycogen, glycogen storage disease, glycogen synthase, glycolysis, liver, Alb-Cre, Ppp1r3b
Introduction
The Meta-Analysis of Glucose and Insulin related traits Consortium (MAGIC) study analyzed data sets from multiple cohorts that contained both genome-wide genotype data and quantitative clinical measurements of human plasma glycemic traits. Among the 12 loci identified in this study, one signal mapping to chromosome 8p23.1 was associated both with fasting plasma glucose and fasting plasma insulin levels. The lead variant, rs4841132, most strongly associated with fasting plasma glucose mapped to an intergenic region 1 kb from the 5′ end of LOC157273 gene, a long non-coding RNA transcript of unknown function, and 174 kb from the protein-coding PPP1R3B gene. Despite the 174-kb separation from the PPP1R3B gene, rs9987289 has been identified as an expression quantitative trait locus for PPP1R3B: the minor allele is associated with higher levels of PPP1R3B mRNA in the liver (and is also associated with higher fasting glucose and insulin levels). This observation prompted us to investigate the possibility that the genetic association with glycemic traits identified by these non-coding variants was due to their effects on the expression of PPP1R3B.
PPP1R3B (protein phosphatase 1 regulatory subunit 3B; also historically known as GL) is a regulator of liver glycogen metabolism. In the liver, sequestration of dietary glucose into glycogen is an important and tightly regulated component of whole-body glucose homeostasis, promoting maintenance of systemic homeostasis in blood glucose levels through glycogen storage during the postprandial period and its breakdown during fasting (1–4). Dysregulation of this pathway is associated with metabolic disorders including glycogen storage diseases and diabetes. Glycogen synthase (GS)3 and glycogen phosphorylase (GP) promote glycogen synthesis and glycogen catabolism, respectively, and their activities are tightly controlled by endocrine signaling pathways that are in turn coupled to nutritional status. Glycogen-targeting subunits recruit the phosphatases and kinases that post-translationally regulate the activation of GS and inactivation of GP. Several proteins have been characterized that target protein phosphatase 1 (PP1) to glycogen (5, 6). In liver, two major subunits, PPP1R3B (GL) and PPP1R3C (PTG) are expressed at approximately equivalent levels and together facilitate the storage of hepatic glycogen.
Previous studies of GL and PTG in cell culture have revealed that overexpression increases glycogen content (7–9), likely because of a redistribution of PP1 and GS to glycogen particles and a corresponding increase in GS activity through PP1-mediated dephosphorylation of GS and GP, resulting in augmentation of glycogen synthesis and inhibition of glycogenolysis, respectively. Ppp1r3c knock-out mice had reduced hepatic glycogen and were glucose-intolerant and insulin-resistant (10); conversely, Ppp1r3c transgenic mice had increased hepatic glycogen and improved glucose and insulin sensitivity (11). Surprisingly, although the glycogen phenotypes of the Ppp1r3c knock-out and transgenic overexpression mice were opposite in direction, both mouse models had reduced hepatic TG content compared with controls (11, 12). In the case of the transgenic Ppp1r3c mouse, hepatic fat remained lower than controls despite 12–24 weeks of high fat diet (45% kcal fat) feeding. However, mice genetically modified to lack or overexpress Ppp1r3b have not been reported to date.
GL is unique among the glycogen-targeting subunits, in that in addition to promoting the activation of glycogen synthase by PP1 dephosphorylation, GL also targets PP1 to GP, where the dephosphorylation inactivates GP. Thus, under conditions of abundant cellular glucose (as glucose-6-phosphate), the PP1-GL complex simultaneously activates the glycogen synthetic pathway and inactivates glycogen catabolism. Mice in which this GP regulatory binding site has been mutated improved hepatic glycogen storage capacity and systemic glucose disposal (13).
Inspired by the human genetics suggesting that variation in hepatic expression of PPP1R3B is an important determinant of glycemic traits, we generated mice with liver-specific deletion of Ppp1r3b (Ppp1r3bΔhep) or with hepatic overexpression of Ppp1r3b using adeno-associated virus (AAV). Floxed Ppp1r3b was inactivated in liver either by a transgenic albumin promoter-driven Cre recombinase, or by injecting adeno-associated virus expressing Cre (AAV-Cre). Despite an almost complete lack of hepatic glycogen in the fed state as well as upon fasting, Ppp1r3bΔhep mice are viable but are prone to hypoglycemia upon fasting and utilize non-carbohydrate precursors to maintain fasted blood glucose levels. Conversely, liver-specific overexpression of Ppp1r3b enhanced hepatic glycogen storage, and as a consequence, AAV-Ppp1r3b mice had a delayed decrease in plasma glucose and exhibited significantly delayed accumulation of blood ketone bodies in response to long-term fasting. These observations are consistent with the fact that natural human genetic variants associated with higher liver expression of PPP1R3B have higher fasting plasma glucose.
Results
Hepatic Ppp1r3b deficiency leads to depletion of hepatic glycogen stores with significant reduction in incorporation of glucose into hepatic glycogen
To investigate the role of Ppp1r3b in the liver, we generated mice lacking this gene exclusively in the liver (see “Experimental procedures” and supplemental Fig. S1A). Liver-specific Ppp1r3b knock-out mice (Ppp1r3bΔhep) were viable, born at the expected frequency for Mendelian inheritance, and showed normal fertility. In all subsequent experiments, mice without the Cre transgene (Ppp1r3bflox/flox) were used as controls. In Ppp1r3bΔhep mice, transcript levels were negligible in the liver, with 97% deletion efficiency observed, and there was no compensatory increase in gene expression of other glycogen-targeting subunits: GM (Ppp1r3a) and PTG (Ppp1r3c) (Fig. 1A). Ppp1r3bΔhep mice showed normal growth rates on chow diet, with no differences observed in body weights between genotypes up to 50 weeks of age (supplemental Fig. S1B).
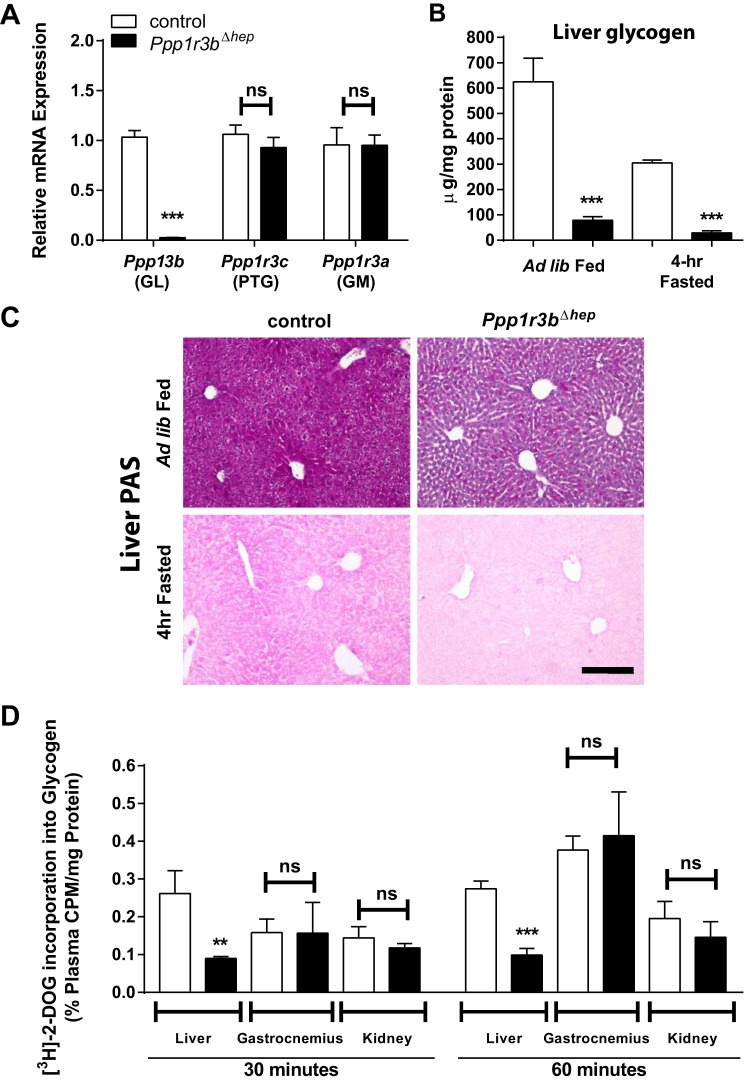
Figure 1.
Liver-specific deletion of Ppp1r3b results in depletion of hepatic glycogen content. A, hepatic mRNA levels of Ppp1r3a, Ppp1r3b, and Ppp1r3c measured by SYBR-Green RT quantitative PCR in control and Ppp1r3bΔhep [Alb-Cre] mice (n = 10–14/genotype). B, hepatic glycogen content in ad libitum chow-fed and 4-h fasted control and Ppp1r3bΔhep mice (n = 6/genotype). C, PAS staining of paraffin-embedded liver sections from ad lib fed and 4-h fasted Ppp1r3bΔhep and control mice shows depletion of glycogen (purple stain). Note that the 4-h fasted sections were not counterstained with hemotoxylin. Scale bar, 1 mm. D, 2-deoxy-d-[3H] glucose (2-DOG) incorporation into glycogen was measured after 6 h of fasting in control and Ppp1r3bΔhep mice (n = 3–4/genotype). Label incorporation was measured as cpm from precipitated glycogen from tissue lysates and normalized to plasma cpm/mg protein. The results were replicated in at least two independent experiments. All mice were adults (2–8 months) and were age-matched within experiments. The data are expressed as the means ± S.E. Significance was determined in all panels by unpaired Student's t test. *, p < 0.05; **, p < 0.005; ***, p < 0.0001. ns, not significant.
Hepatic glycogen content was significantly reduced in ad libitum fed state in Ppp1r3bΔhep mice, which was rapidly depleted upon short-term, 4-h fasting as measured biochemically (Fig. 1B). Periodic acid-Schiff (PAS) staining of liver sections showed positive staining for glycogen in control hepatocytes as indicated by the purple color under these two conditions (Fig. 1C). The weight of the liver of Ppp1r3bΔhep mice was also modestly decreased compared with controls (supplemental Fig. S1C), likely because of a lack of glycogen stores, which with their associated water content account for 5% of liver mass (3). We used AAV-Cre in Ppp1r3bflox/flox mice as a second approach to generate liver-specific knock-out of Ppp1r3b and found effects on hepatic glycogen and plasma glucose (supplemental Fig. S2) comparable to those in the Alb-Cre mice. Alb-Cre mice were used for the studies presented here, unless otherwise specified. Glycogen content in skeletal muscle presented no substantial differences among genotypes under ad libitum fed and 4-h fasted conditions (supplemental Fig. S1D). To investigate the glycogen synthesis rate in these mice, we performed 2-deoxy-d-[3H]glucose incorporation into glycogen in glucose-responsive tissues: liver, skeletal muscle (gastrocnemius), and kidney, as a surrogate measurement of GS activity. Radioactivity in the glycogen pellet from tissue lysates was measured at 30 and 60 min and quantified as a percentage of glucose specific activity of total radioactivity in plasma and of total protein content. In this experiment, Ppp1r3bΔhep mice exhibited significantly reduced glycogen synthase activity in the liver, whereas its activity was not different in skeletal muscle or kidney (Fig. 1D). Thus, the loss of hepatic Ppp1r3b results in a smaller liver with reduced GS activity and glycogen content in ad libitum fed and 4-h fasted mice.
Deletion of Ppp1r3b in the liver causes significant reduction in total glycogen synthase protein and activity state
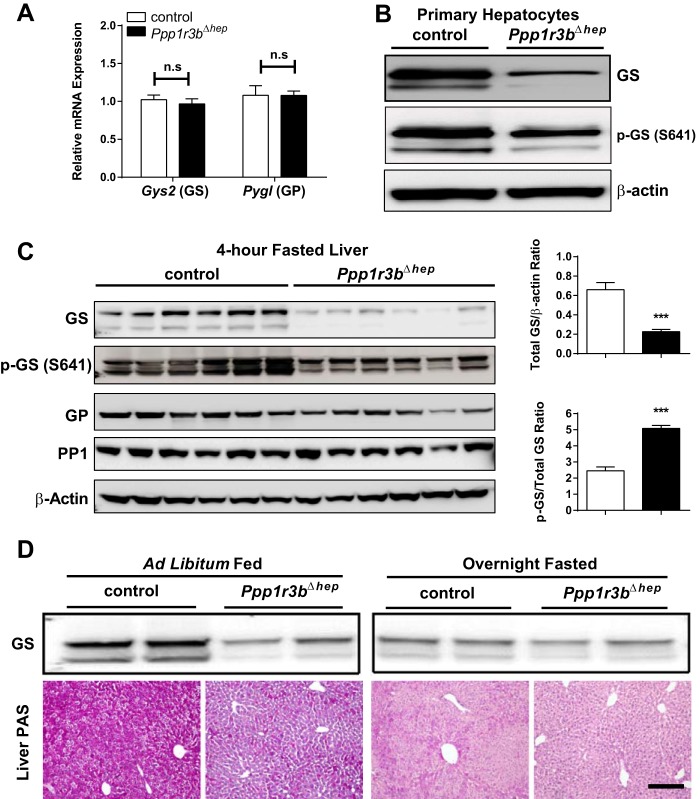
Ppp1r3bΔhep mice have significant reduction in GS but not GP at the protein level, but no differences at the mRNA levels in both the liver and primary hepatocytes (Fig. 2). The ratio of GS phosphorylated at serine position 641 to total GS, which determines the activity status of GS, is increased, suggesting that GS is phosphorylated and largely inactivated in Ppp1r3bΔhep mice (Fig. 2C). The nearly complete loss of dephosphorylated [activated] GS protein likely parallels the depletion of glycogen granules because overnight fasted control mice also reached a similar depletion of glycogen, and protein levels of active/dephosphorylated GS were similar in control and Ppp1r3bΔhep livers after an overnight fast (Fig. 2D). The molecular mechanism by which Ppp1r3bΔhep deletion leads to hyperphosphorylation of GS and marked reduction in total GS protein content even during the ad libitum fed state remains to be further explored. Thus, a loss of hepatic Ppp1r3b results in a reduced glycogen synthase protein and activity state in the liver, consistent with the observation of reduced glucose incorporation of into liver glycogen and overall glycogen depletion.
Figure 2.
Ppp1r3b-deficient livers have reduced total GS protein but higher relative phospho-GS. A, 4-h fasted liver lysates were used for measurement of transcript levels of Gys2 and Pygl (n = 6–9/genotype). B, protein expression of total and p-GS GS in primary hepatocytes isolated from ad libitum chow-fed control and Ppp1r3bΔhep mice. C, protein expression of total GS, P-GS (Ser-641), total GP, and PP1 in liver lysates from 4-h fasted control and Ppp1r3bΔhep mice. The ratio of total GS to β-actin is decreased, and P-GS to total GS is increased Ppp1r3bΔhep compared with control mice (n = 6/genotype). D, liver GS protein expression and PAS staining in ad libitum chow-fed and overnight fasted Ppp1r3bΔhep mice compared with control mice (n = 4–6/genotype). Scale bar, 1 mm. All mice were adults (2–8 months) and were age-matched within experiments. The results were replicated in at least two independent experiments. The data are expressed as the means ± S.E. Significance was determined in all panels by unpaired Student's t test. *, p < 0.05; **, p < 0.005; ***, p < 0.0001. n.s, not significant.
Hepatic loss of Ppp1r3b promotes dysregulated glycogen metabolism and is associated with impaired glucose homeostasis and glucose mobilization
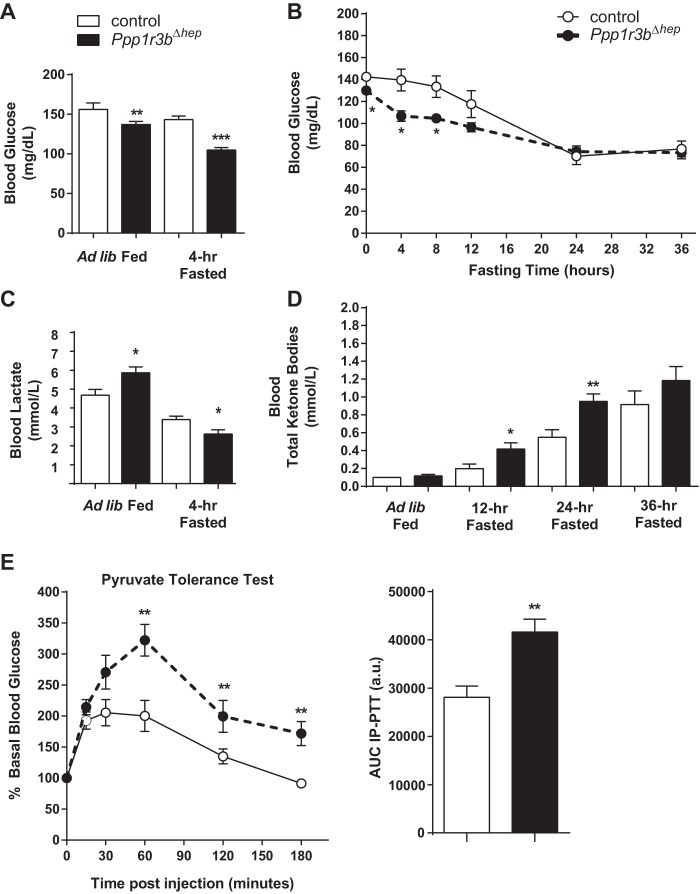
Postprandial glucose levels in Ppp1r3bΔhep mice are modestly lower, and Ppp1r3bΔhep mice become mildly hypoglycemic after a 4-h fast (Fig. 3A). Within 4–8 h of fasting, blood glucose in Ppp1r3bΔhep mice dropped to a significantly lower value than control mice, which reached similarly low blood glucose only after longer-term fasting of up to 24 h. (Fig. 3B). We hypothesized that alternative metabolites compensate for the lack of hepatic glycogen stores to substitute for the maintenance of blood glucose as an energy source to peripheral tissues and the central nervous system. In the postprandial state, plasma lactate levels were increased in Ppp1r3bΔhep mice compared with controls (Fig. 3C). Upon prolonged fasting (12 h), total plasma ketone bodies were significantly increased and were progressively elevated by 24- and 36-h fasting periods in Ppp1r3bΔhep mice, compared with controls (Fig. 3D). Moreover, after an overnight fast, gluconeogenesis was increased in Ppp1r3bΔhep mice, as evidenced by a challenge with intraperitoneal injection of sodium pyruvate in which these mice were more efficiently able to synthesize glucose from an exogenous pyruvate load, compared with controls (Fig. 3E). Taken together, these data suggest that although the Ppp1r3bΔhep mice are more prone to enter into a quasifasted state following short-term food deprivation, they are nonetheless able to maintain stable long-term fasting plasma glucose levels by compensatory up-regulation of the gluconeogenic pathway from alternative precursors.
Figure 3.
Plasma glucose homeostasis is impaired in Ppp1r3bΔhep mice. A, blood glucose levels of ad libitum chow-fed and 4-h fasted mice (n = 10–14/genotype). B, blood glucose levels during a 36-h fasting period in Ppp1r3bΔhep mice compared with control mice (n = 6/genotype). C, blood lactate levels in ad libitum chow-fed and in 4-h fasted control and Ppp1r3bΔhep mice (n = 10–14/genotype). D, total ketone bodies in blood measured during ad libitum ad fed and 12-, 24-, and 36-h fasting conditions in Ppp1r3bΔhep mice compared with control mice (n = 6–8/genotype). E, PTT in control and Ppp1r3bΔhep mice (n = 6/genotype). PTT was performed in mice by administering 2 g/kg body weight sodium pyruvate by intraperitoneal injection after overnight (14–16 h) fasting. The values are reported as percentages of basal glucose levels; the area under the curve (AUC) is expressed as arbitrary units. All mice were adults (2–8 months) and were age-matched within experiments). The results were replicated in at least two independent experiments. The data are expressed as the means ± S.E. Significance was determined in all panels by unpaired Student's t test. *, p < 0.05; **, p < 0.005; ***, p < 0.0001. n.s, not significant.
Hepatic deletion of Ppp1r3b causes up-regulation of glycolytic genes in the postprandial state and up-regulation of gluconeogenic genes within 8 h of fasting
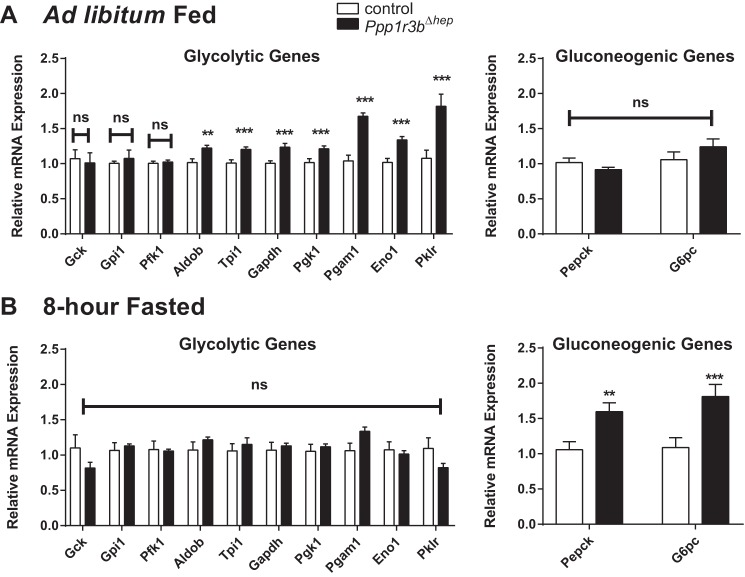
In the Ppp1r3b-deficient livers, under ad lib fed conditions, genes that encode enzymes are critical in the glycolytic pathway up-regulated compared with control livers (Fig. 4A); these included aldolase (Aldob), triose phosphate isomerase (Tpi), glyceraldehyde phosphate dehydrogenase (Gapdh), phosphoglycerate kinase (Pgk1), phosphoglycerate mutase (Pgam1), enolase (Eno1), and l-pyruvate kinase (Pklr). Upon an 8-h fasting period, there was an up-regulation of gluconeogenic genes (Pepck and G6pc) in Ppp1r3bΔhep mice (Fig. 4B), suggesting that de novo synthesis of glucose is initiated much earlier on in these mice compared with controls. Collectively, these data suggest that with Ppp1r3b deficiency, there is a significant perturbation in nutrient balance that favors shunting of glucose into the glycolysis pathway in the fed state and an early up-regulation of the gluconeogenic pathway in response to fasting.
Figure 4.
Ppp1r3b-deficient livers undergo rapid switching from glycolysis to gluconeogenesis. A, ad libitum chow-fed liver lysates were used for measurement of transcript levels of glycolytic and gluconeogenic genes (Gck, Gpi1, Pfk1, Aldob, Tpi1, Gapdh, Pgk1, Pgam1, Eno1, Pklr, Pepck, and G6pc (n = 6–9/genotype). B, 8-h fasted liver lysates were used for measurement of transcript levels of the above glycolytic and gluconeogenic genes (n = 5/genotype). The results were replicated in at least two independent experiments. The data are expressed as the means ± S.E. Significance was determined in all panels by unpaired Student's t test. *, p < 0.05; **, p < 0.005; ***, p < 0.0001. n.s, not significant.
Overexpression of Ppp1r3b in the liver enhances hepatic glycogen storage
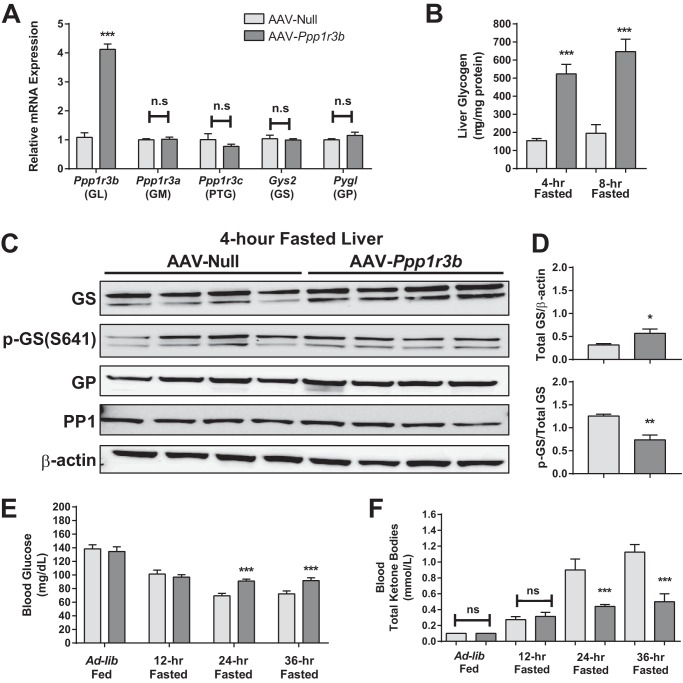
We generated a reciprocal mouse model to express Ppp1r3b at higher than wild-type levels, combining the tropism of AAV serotype 8 and the liver specificity of the TBG/Serpina7 promoter, which has been previously shown to promote efficient, specific, and persistent gene expression in the liver (14), to overexpress Ppp1r3b in hepatocytes in vivo. Ppp1r3b was ∼4-fold higher compared with control mice receiving a null AAV vector (Fig. 5A). There were no compensatory changes in the mRNA expression of other glycogen-targeting subunits (Ppp1r3a and Ppp1r3c), as well as rate-limiting enzymes in glycogen metabolism: glycogen synthase (Gys2) or glycogen phosphorylase (Pygl), in mice overexpressing Ppp1r3b. Following a short-term 4-h fasting period, mice overexpressing Ppp1r3b had ∼4-fold higher levels of hepatic glycogen content than controls, and this was maintained at 8 h of fasting (Fig. 5, B and C). Levels of total GS protein were also higher in livers from mice overexpressing Ppp1r3b, and the ratio of phospho-GS to total GS protein was decreased, consistent with increased activation status after 4- and 8-h fasting periods (Fig. 5D). Additionally, total GP or PP1 protein content was not affected (Fig. 5D). Blood glucose levels were not different between the two groups with ad libitum and 12-h fasting conditions, indicating that despite the larger reservoir of available glycogen, glucose levels are maintained, without exceeding the normal physiological range (Fig. 5E). Between 24 and 48 h of fasting, AAV-Ppp1r3b mice maintained persistently higher (by ∼15 mg/dl) plasma glucose levels than the control group (Fig. 5E). Upon long-term fasting, AAV-Ppp1r3b were protected from the onset of ketogenesis (Fig. 5F), suggesting that the enhanced glycogen storage buffered the onset of the fasting-induced metabolic shift to β-oxidation. Taken together, these data suggest that enhancement of hepatic glycogen reservoir in mice overexpressing Ppp1r3b improves the capacity for maintenance of plasma glucose homeostasis and minimizes the dependence on other energy substrates under longer-term fasting conditions.
Figure 5.
Overexpression of Ppp1r3b in liver enhances hepatic glycogen storage and delayed responses to fasting. A, 4-h fasted liver lysates were used for measurement of transcript levels of Ppp1r3b, Ppp1r3c (PTG), Ppp1r3a (GM), Gys2, and Pygl from C57BL/6J mice injected with AAV-null and AAV-Ppp1r3b (n = 4/group). B, hepatic glycogen content in 4- and 8-h fasted liver lysates from C57BL/6J mice injected with AAV-null and AAV-Ppp1r3b (n = 3–4/group). C, PAS staining of paraffin-embedded liver sections from 4- and 8-h fasted C57BL/6J mice injected with AAV-null and AAV-Ppp1r3b, showing increased amount of glycogen (purple stain) in AAV-Ppp1r3b-overexpressing mice. D, protein expression of total GS, P-GS (Ser-641), total GP, and PP1 in liver lysates from 4-h fasted from C57BL/6J mice injected with AAV-null and AAV-Ppp1r3b (n = 4/group). The ratio of total GS to β-actin is increased and P-GS to total GS is decreased in AAV-Ppp1r3b-overexpressing mice compared with the Null group (n = 4/genotype). E, blood glucose levels during a 36-h fasting period in C57BL/6J mice injected with AAV-null and AAV-Ppp1r3b (n = 7–8/group). F, total ketone bodies in blood measured during ad libitum fed and 12-, 24-, and 36-h fasting conditions in C57BL/6J mice injected with AAV-null and AAV-Ppp1r3b (n = 7–8/group). The mice were injected at 9 weeks of age, and liver lysates were analyzed at 11–12 weeks post injection. The results were replicated in at least two independent experiments. The data are expressed as the means ± S.E. Significance was determined in all panels by unpaired Student's t test. *, p < 0.05; **, p < 0.005; ***, p < 0.0001. n.s, not significant.
Discussion
Variants at chromosomal locus 8p23.1, in an intergenic region 174 kb from the PPP1R3B gene, have recently been associated with human glycemic traits including fasting plasma insulin and glucose in a large-scale meta-analysis of genome wide association studies (15). Rs4841132 has two alleles (G or A) in the 1000 Genomes database (phase 3), of which the minor A allele has an overall frequency of 0.093. The rs4841132 A allele is associated with increases in both fasting plasma glucose and insulin (15). Rs4841132 is in high linkage disequilibrium with another SNP, rs9987289 (linkage disequilibrium R2 = 1; physical distance = 238 base pairs). The minor allele at rs9987289 was associated with increased plasma lactate levels in individuals of European ancestry, and this effect was found to be strongest in diabetics taking metformin, a drug known to limit excessive hepatic glucose production (16). The locus was also identified with other plasma lipid traits (total cholesterol, and both high- and low-density lipoprotein cholesterol) (17). Lastly, several subsequent independent GWAS studies have associated the locus with plasma lactate levels (16) and with hepatic steatosis (18, 19).
The centrality of the liver in the maintenance of whole-body glucose homeostasis in both fed and fasted states has been well established, and glycogen metabolism is in turn a critical mediator of this function, serving as an immediate source of glucose that can be rapidly mobilized to buffer falling plasma glucose levels, and conversely, as a reservoir to capture excess glucose. GS is a rate-limiting glycogen synthetic enzyme that is allosterically regulated by glucose-6-phosphate. Binding of glucose-6-phosphate induces a conformational shift that promotes recruitment of PP1 that dephosphorylates and activates GS (4, 20). Phosphorylation of Ser-641 inactivates GS. PP1 promotes dephosphorylation of GS at Ser-641 by juxtaposing PP1 to GS through glycogen-targeting subunits (21). Moorhead et al. (22) demonstrated that heterodimeric complexes consisting of PP1-catalytic subunit (α and β isoforms) and Ppp1r3b/GL (then termed PP1G) purified from rat liver bound both glycogen and glycogen phosphorylase-a (GP α) with high affinity. The GL-GPα interaction is thought to antagonize PP1G-mediated GS activation in an allosteric fashion. Later, Printen et al. (9) showed that FLAG-Ppp1r3c/PTG fusion protein simultaneously bound PP1-catalytic subunit and glycogen. Independent of insulin stimulation, PTG could form stable complexes with the PP1-catalytic subunit, GS, and phosphorylase kinase, an activator of glycogen phosphorylase, but not with other phosphatases or kinases. Ppp1r3a/GM/RGL is the most abundant glycogen-targeting subunit in rodent skeletal muscle, which upon phosphorylation dissociates from PP1 and regulates GS activation in a similar fashion to that of PTG (23). Lastly, Gasa et al. (8) characterized different glycogenic capacities of three major subunits (Ppp1r3b (GL), Ppp1r3a (GM), and Ppp1r3c (PTG) in rat primary hepatocytes with respect to their regulatory role in activation and deactivation of GS and GP. The study concluded that: 1) Ppp1r3b (GL) had the highest capacity to maintain increased GS activity ratio, independent of insulin stimulation; 2) Ppp1r3c (PTG) maintained the glycogen synthesis capacity induced by insulin in a dose-dependent manner; and 3) GL and GM are responsive to forkskolin-mediated glycogenolysis; however, PTG remains unresponsive because of a lack of inhibition by GP. In summary, it is well established that Ppp1r3a, Ppp1r3b, and Ppp1r3c directly control the phosphorylation of GS.
Although mechanistic studies demonstrate that GL mediates the intracellular scaffolding of PP1 to glycogen in the liver, the in vivo physiology of GL has been largely unexplored prior to this study, in contrast to PTG, for which both whole body knock-out and liver-specific overexpression mouse models have been described (11, 12). To elucidate the role of Ppp1r3b in vivo, we generated liver-specific knock-out mice and first investigated the hepatic glycogen metabolism and whole-body glucose homeostasis. Liver-specific genetic ablation of the Ppp1r3b gene yielded viable mice in which liver glycogen storage and glycogen synthase activity were severely impaired. Despite the absence of Ppp1r3b, there were no compensatory changes in liver mRNA expression of Ppp1r3a and Ppp1r3c, and muscle glycogen content and incorporation of plasma glucose into muscle glycogen were unaffected. It has been previously suggested that liver glycogen is essential for neonatal survival until gluconeogenesis is fully established in the newborn liver (24). The Alb-Cre has been shown to drive incomplete deletion at birth, with progressively complete ablation over the first 6 weeks of life (25, 26), which is presumably what permitted the survival of the Ppp1r3bΔhep pups. The residual signal in livers from Ppp1r3bΔhep mice in biochemical assays and histological stains for glycogen likely is due to the combination of the presence of rare hepatocytes that escaped Cre-deletion of Ppp1r3b, as well as simple glucose polymers in hepatocytes throughout the lobule.
We sought to study the importance of Ppp1r3b in regulation of systemic plasma glucose homeostasis. In many regards, the Ppp1r3bΔhep phenotype resembles that of mice with liver-specific knock-out of glycogen synthase (27) and patients with liver-specific glycogen storage disease 0 (GSD0) because of mutation of the GYS2 gene (28, 29). All three cases are characterized by elevated lactate in the postprandial state, fasting hypoglycemia, and elevated blood ketone bodies. Interestingly, although no mutations in PPP1R3B have been reported in GSD0 patients to date, our findings suggest that human genetic deficiency of PPP1R3B would likely manifest with GSD0. Indeed, the phenotypic similarity of Ppp1r3bΔhep mice to glycogen synthase liver-specific knock-out mice is consistent with the unexpected and novel finding that loss of hepatic Ppp1r3b function impacts not only the phosphorylation state but also the total abundance of GS. We are actively investigating potential mechanisms to explain the effect of Ppp1r3b on GS protein abundance.
Ad libitum fed Ppp1r3bΔhep mice have modestly reduced blood glucose levels compared with controls. In euglycemia, glucose in the liver can be either stored as glycogen or converted to pyruvate and lactate by glycolysis. In Ppp1r3bΔhep mice, synthesis of glycogen is blocked, and this is associated with a reduced ability to dispose of glucose after feeding. Under these conditions, increased lactate in postprandial state is consistent with an increase in glucose flux through glycolysis. Because the expression of Ppp1r3b is restricted mainly to liver in adult mice, the contribution of plasma lactate should presumably be derived mainly from the liver. The fact that human PPP1R3B is also expressed in skeletal muscle (30) may explain the lack of observed PPP1R3B GSD0 patients: the combination of deficiency in both liver and muscle may be incompatible with life.
In the fasted state, when plasma glucose levels are lower, the Ppp1r3bΔhep mice are incapable of liver glycogenolysis and rely on gluconeogenesis. Our understanding, based on the metabolic status of Ppp1r3bΔhep mice, is that even when fed, they are already conditioned toward fasting physiology, which they attain more rapidly than control animals. Blood glucose decreases more rapidly upon fasting but is nonetheless maintained through increased gluconeogenesis, as evidenced by a greater and earlier up-regulation of key gluconeogenic enzymes. Further supporting this interpretation, our results from the pyruvate tolerance test also show that Ppp1r3bΔhep mice depend on utilization of pyruvate as one of the non-carbohydrate precursors to maintain plasma glucose levels. Decreased levels of plasma lactate by 4 h of fasting suggest that liver begins to utilize non-carbohydrate precursors at an earlier onset of fasting in these mice. By 12 h of fasting, blood ketone bodies produced by the liver are already elevated and are sustained compared with control animals.
In reciprocal experiments to determine the effects of activating the glycogen synthesis pathway, we overexpressed mouse Ppp1r3b in liver in WT mice. Many of the phenotypes we observe in the overexpressing mice are reciprocal to mice lacking hepatic expression of the Ppp1r3b gene. Both the protein abundance and activation state of GS were increased, mirroring the effects of Ppp1r3bΔhep. Additionally, with respect to blood glucose, there were no significant differences between AAV-Ppp1r3b mice and controls under fed and short-term fasted conditions, but the overexpressing mice were resistant to reduced plasma glucose upon 36 h of fasting. Reflecting this, the rise in plasma total ketone bodies was significantly delayed in AAV-Ppp1r3b mice, suggesting that the requirement for the liver to shift to utilization of fat and protein for fuel sources was obviated by the larger glycogen reservoir and likely efficient glycogenolysis.
Hepatic overexpression of another glycogen targeting protein, PTG, impaired activation of glycogenolysis by forskolin and glucagon, resulting in a failure to mobilize glycogen stores even during longer-term fasting (31). In contrast, mice overexpressing hepatic Ppp1r3b remained responsive to various nutritional states and have increased capacity for maintenance of plasma glucose under long-term fasting conditions, perhaps because GL is allosterically regulated by binding to glycogen phosphorylase α (32) rather than by post-translational modification. Moreover, mice expressing a Ppp1r3b transgene with a mutated allosteric binding site for glycogen phosphorylase α had improved hepatic glycogen synthesis capacity under fed conditions, but impaired glycogenolysis led to hypoglycemia and weight loss upon longer-term (36 h) fasting (13). Further mechanistic studies will be required to specifically test the hypothesis that increasing hepatic Ppp1r3b might provide the benefit of improved glucose disposal, without inappropriate sequestration of glycogen during fasting.
In summary, when combined with the human genetics pointing to the PPP1R3B locus as compellingly associated with glycemic traits in humans, our studies in mice genetically deficient in or overexpressing hepatic Ppp1r3b indicate the central role of this protein in regulating hepatic glycogen stores and energy homeostasis in the fasting state.
Experimental procedures
Generation of liver-specific Ppp1r3b knock-out mice
The Ppp1r3bflox/flox mice were provided by Merck and were produced for Merck by Taconic. Full details of the design of the Ppp1r3bflox/flox mice may be found on the Taconic website (Model 10482). Briefly, in the conditional allele, two loxP sites were introduced flanking Ppp1r3b exon 2, which contains the entire protein coding sequence of the gene (supplemental S1B). Ppp1r3b liver-specific knock-out mice (Ppp1r3bΔhep) were generated by crossing Ppp1r3bflox/flox mice on a C57BL/6J background with transgenic mice expressing Cre recombinase-expressing under the expression of the hepatocyte-specific albumin promoter (C57BL/6-Cg-Tg (Alb-Cre) 21Mgn/J; Jackson Laboratories). The resultant Ppp1r3bflox/+;Alb-Cre+ progeny were crossed with Ppp1r3bflox/flox mice to obtain tissue-specific knock-out mice (Ppp1r3bflox/flox; Alb-Cre+), termed liver-specific Ppp1r3b knock-out mice (Ppp1r3bΔhep). Mice without the Cre transgene (Ppp1r3bflox/flox) were used as control mice. Genotyping was confirmed for the expression of Cre transgene and Ppp1r3bflox/flox alleles prior to the onset of studies. We also developed a liver-specific knock-out of Ppp1r3b utilizing an AAV-TBG-Cre approach in Ppp1r3bflox/flox mice. The animals were housed under controlled temperature (23 °C) and lighting (12-h light/dark cycle) with free access to water and standard mouse chow diet (LabDiet, 5010).
For 36-h fasting experiments, the indicated mice were initiated on a fasting protocol in the morning (8:00 a.m.), which was considered as the ad libitum fed state. Blood glucose measurements were taken at the time points indicated. The mice were continually fasted for 36 h until the second day evening (8:00 p.m.) at which point food was returned back to their cages. All animal experiments were reviewed and approved by Institutional Animal Care and Use Committees of the University of Pennsylvania (Philadelphia, PA).
AAV vector preparation
AAV vectors, serotype 8, containing either an empty expression cassette (AAV-null), Cre recombinase (AAV-Cre), or mouse Ppp1r3b gene (AAV-Ppp1r3b), were generated by the University of Pennsylvania Vector Core (Philadelphia, PA). The transgene is selectively expressed in hepatocytes these vectors from the thyroxine-binding globulin (TBG/Serpina7) promoter (14). For overexpression experiments utilizing AAV-Ppp1r3b, C57BL/6J wild-type mice were injected with AAV vectors at a dose of 1 × 1012 genome copies via i.p. injection and examined at the indicated time points. For experiments utilizing AAV-TBG-Cre, Ppp1r3bflox/flox mice were injected with AAV vectors at a dose of 1.5 × 1011 genome copies via i.p. injection and examined at the stated time points.
Western blotting analysis
The tissues were isolated from mice both in fed and fasted conditions at different ages and then immediately frozen in liquid nitrogen. Tissue homogenates were prepared with radioimmune precipitation assay buffer containing 50 mm Tris-HCl (pH 7.4), 150 mm NaCl, 1 mm EDTA, 1% Triton-X-100, 1% sodium deoxycholate, and 0.1% SDS with 1× protease and phosphatase inhibitor mixture (catalog no. 5872; Cell Signaling Technology) added. Homogenates were quantified for protein by the BCA method. 30–50 μg of total protein was resolved on 4–12% SDS-PAGE gels and subjected to Western blotting. Immunoblots were performed using the antibodies against the following proteins: GS, P-GS (Ser-641) (CST-3886 and CST-3891S; Cell Signaling Technology), PP1, and GP (AB-53315 and AB-198268; Abcam), and β-actin (SC-81178; Santa Cruz Biotechnology) was used as a loading control.
RNA isolation and quantitative RT-PCR
RNA was isolated from ∼50 mg of flash-frozen liver tissue using TRIzol solution (Invitrogen) according to the manufacturer's instructions. cDNA was synthesized from 2.0–2.5 μg of total RNA using the high-capacity cDNA reverse transcription kit (Applied Biosystems) according to the manufacturer's protocol. Real-time quantitative PCR was performed using SYBR Green Mix (Applied Biosystems) on an ABI7900 RT-PCR machine. The relative quantity of each mRNA was calculated using the ΔCT method with β-actin as the housekeeping gene. The sequences for the primer pairs used in the studies are listed in supplemental Table S1.
Intraperitoneal pyruvate tolerance tests
For the pyruvate tolerance test (PTT) challenge, the mice were overnight fasted for 14–16 h and then administered 2 g/kg body weight of sodium pyruvate (Sigma-Aldrich) by i.p. injection. Blood glucose concentrations were measured using a One-Touch Ultra glucometer (Lifescan, Inc.) at the indicated time points.
Metabolic measurements
Blood glucose, lactate, and ketone bodies were measured using a One-Touch Ultra glucometer (Lifescan, Inc.), a Lactate Plus lactate meter (Nova Biomedical), and a Nova Max Plus Ketone meter, respectively, at the indicated nutritional conditions.
Measurement of tissue glycogen levels
Non-fasting and fasting male mice were sacrificed, and tissues were flash-frozen in liquid nitrogen. Frozen tissue samples (∼50 μg) were homogenized in 300 μl of PBS. Homogenates were quantified for protein by the BCA method (Thermo Scientific). Tissue samples (4 μg of total protein for liver and 50 μg of total protein for skeletal muscle) were used for detection of glycogen levels by colorimetric assay protocol according to manufacturer's instructions (Biovision). Glycogen levels in samples were determined from a standard-curve method generated by the assay.
Incorporation of 2-deoxy-d-[3H] glucose into liver glycogen
Incorporation of glucose into glycogen was accessed as described previously (10). As a tracer, 2-deoxy-d-[3H] glucose (PerkinElmer Life Sciences) was combined with 20% (0.2 g/ml) glucose and then administered 2 g/kg body weight at 1 mCi/mouse by intraperitoneal injection into 6-h fasted mice. Tissues (liver, skeletal muscle, and kidney) were harvested and flash-frozen in liquid nitrogen at 30 and 60 min after glucose and tracer injection. Plasma glucose concentrations were measured at these indicated time points. Frozen tissue samples (100 mg) were digested in 750 μl of 30% KOH at 100 °C for 10 min. Upon digestion, a sample aliquot was used for measurement of protein concentration by the BCA method (ThermoScientific). The samples were then neutralized with 200 μl of 20% Na2SO4, and macromolecules were precipitated with 1 ml of 100% ethanol overnight at −20 °C. Macromolecules were pelleted by centrifugation at 10,000 × g for 15 min and washed twice with 70% ethanol. Sample pellets were air-dried, and then glycogen was digested by heat and acid with 500 μl of 4 n H2SO4 for 10 min at 100 °C. The pellets were then neutralized with an equal volume of 4 n NaOH and radioactivity was determined by liquid scintillation counting. 2-deoxy-d-[3H]glucose incorporation into glycogen was determined by dividing the radioactivity of digested samples by glucose specific activity in plasma (total radioactivity in plasma) over time course and by sample protein content using the protein BCA method.
Primary hepatocyte isolation
Primary hepatocytes were isolated from ad libitum fed male WT and Ppp1r3bΔhep mice by collagenase perfusion, as described previously (33). Briefly, the cells were suspended in DMEM (Life Technologies, Inc.), supplemented with 10% fetal bovine serum, 25 mm glucose, and antibiotics (2 units/ml penicillin and 2 μg/ml streptomycin), and seeded onto collagen-coated plates at a cell density of 6 × 105 cells/well for a standard 6-well plate. After attachment (4 h at 37 °C), hepatocytes were washed and harvested for isolation of protein immunoblotting.
Tissue staining
The liver sections were prepared from mice at the indicated ages, fixed in 4% paraformaldehyde, and embedded in paraffin. To detect polysaccharide content, slices of 5–10 μm were deparaffinized, oxidized with 0.5% periodic acid for 5 min, stained with Schiff reagent for 15 min, and then counterstained with hematoxylin and eosin for 15 min (34).
Image manipulation
All images for the histological sections presented were captured using NIS-Elements-D version 4.12.01, in a single session under identical settings with a Nikon Digital Sight DS-U3 camera, attached to a Nikon Eclipse 80i microscope, using a Plan Fluor 10× objective (0.30 DIC L/N1; 0.17 WD: 16.0). A 500-μm scale bar was digitally added using NIS-Elements-D. Each set of images was incorporated into a single Adobe Photoshop file; the image was flattened, and the white balance was calibrated for the entire field using the lumen of a vein as a reference. A 1-mm scale bar was superimposed in Adobe Illustrator.
Statistical analyses
All results are presented as means ± S.E. The results were analyzed by the unpaired two-tailed Student's t test using Microsoft Excel or GraphPad Prism software (version 7.0, GraphPad Software, Inc.) as appropriate. Statistical significance was defined as follows: *, p < 0.05; **, p < 0.005; and ***, p < 0.0001.
Author contributions
M. B. M. designed and performed experiments, analyzed and interpreted the data, and wrote the manuscript; S. V. S. analyzed and interpreted the data and wrote and edited the manuscript; R. N. S. performed experiments; J. S. M. performed experiments and analyzed and interpreted the data; N. J. H. supervised experiments, analyzed and interpreted the data, and wrote and edited the manuscript; and D. J. R. conceived the study, oversaw all its aspects, and wrote and edited the manuscript.
Supplementary Material
Acknowledgments
We thank Aisha Wilson, Edwige Edouard, and Maosen Sun for excellent technical assistance with animal experiments and husbandry. We also acknowledge thoughtful discussions and suggestions from Dr. Doris A. Stoffers, Dr. Joseph A. Baur, Dr. Charles A. Stanley, Dr. Kendra K. Bence, Dr. Morris J. Birnbaum, Dr. Rexford S. Ahima, Dr. Robert Bauer, Dr. Sumeet Khetarpal, Dr. Salam Ibrahim, Dr. Jeffrey T. Billheimer, Dr. Karthikeyani Chellappa, Dr. Paul Tichenell, and Dr. William Quinn, as well as many members of the Rader lab, past and present.
This work was supported in part by National Institutes of Health Grants RC2HL101864 and R01HL089309 (to D. J. R.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains supplemental Table S1 and Figs. S1 and S2.
- GS
- glycogen synthase
- GP
- glycogen phosphorylase
- PP1
- protein phosphatase 1
- AAV
- adeno-associated virus
- PAS
- periodic acid-Schiff
- GSD0
- glycogen storage disease 0
- PTT
- pyruvate tolerance test.
References
- 1. Bollen M., Keppens S., and Stalmans W. (1998) Specific features of glycogen metabolism in the liver. Biochem. J. 336, 19–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roach P. J. (2002) Glycogen and its metabolism. Curr. Mol. Med. 2, 101–120 [DOI] [PubMed] [Google Scholar]
- 3. Stalmans W., Bollen M., and Mvumbi L. (1987) Control of glycogen synthesis in health and disease. Diabetes Metab. Rev. 3, 127–161 [DOI] [PubMed] [Google Scholar]
- 4. Villar-Palasí C., and Guinovart J. J. (1997) The role of glucose 6-phosphate in the control of glycogen synthase. FASEB J. 11, 544–558 [PubMed] [Google Scholar]
- 5. Newgard C. B., Brady M. J., O'Doherty R. M., and Saltiel A. R. (2000) Organizing glucose disposal: emerging roles of the glycogen targeting subunits of protein phosphatase-1. Diabetes 49, 1967–1977 [DOI] [PubMed] [Google Scholar]
- 6. Roach P. J., Depaoli-Roach A. A., Hurley T. D., and Tagliabracci V. S. (2012) Glycogen and its metabolism: some new developments and old themes. Biochem. J. 441, 763–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brady M. J., Printen J. A., Mastick C. C., and Saltiel A. R. (1997) Role of protein targeting to glycogen (PTG) in the regulation of protein phosphatase-1 activity. J. Biol. Chem. 272, 20198–20204 [DOI] [PubMed] [Google Scholar]
- 8. Gasa R., Jensen P. B., Berman H. K., Brady M. J., DePaoli-Roach A. A., and Newgard C. B. (2000) Distinctive regulatory and metabolic properties of glycogen-targeting subunits of protein phosphatase-1 (PTG, GL, GM/RGl) expressed in hepatocytes. J. Biol. Chem. 275, 26396–26403 [DOI] [PubMed] [Google Scholar]
- 9. Printen J. A., Brady M. J., and Saltiel A. R. (1997) PTG, a protein phosphatase 1-binding protein with a role in glycogen metabolism. Science 275, 1475–1478 [DOI] [PubMed] [Google Scholar]
- 10. Crosson S. M., Khan A., Printen J., Pessin J. E., and Saltiel A. R. (2003) PTG gene deletion causes impaired glycogen synthesis and developmental insulin resistance. J. Clin. Invest. 111, 1423–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. López-Soldado I., Zafra D., Duran J., Adrover A., Calbó J., and Guinovart J. J. (2015) Liver glycogen reduces food intake and attenuates obesity in a high-fat diet-fed mouse model. Diabetes 64, 796–807 [DOI] [PubMed] [Google Scholar]
- 12. Lu B., Bridges D., Yang Y., Fisher K., Cheng A., Chang L., Meng Z. X., Lin J. D., Downes M., Yu R. T., Liddle C., Evans R. M., and Saltiel A. R. (2014) Metabolic crosstalk: molecular links between glycogen and lipid metabolism in obesity. Diabetes 63, 2935–2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kelsall I. R., Rosenzweig D., and Cohen P. T. (2009) Disruption of the allosteric phosphorylase a regulation of the hepatic glycogen-targeted protein phosphatase 1 improves glucose tolerance in vivo. Cell. Signal. 21, 1123–1134 [DOI] [PubMed] [Google Scholar]
- 14. Wang L., Wang H., Bell P., McCarter R. J., He J., Calcedo R., Vandenberghe L. H., Morizono H., Batshaw M. L., and Wilson J. M. (2010) Systematic evaluation of AAV vectors for liver directed gene transfer in murine models. Mol. Ther. 18, 118–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Manning A. K., Hivert M. F., Scott R. A., Grimsby J. L., Bouatia-Naji N., Chen H., Rybin D., Liu C. T., Bielak L. F., Prokopenko I., Amin N., Barnes D., Cadby G., Hottenga J. J ., Ingelsson E., et al. (2012) A genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance. Nat. Genet. 44, 659–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tin A., Balakrishnan P., Beaty T. H., Boerwinkle E., Hoogeveen R. C., Young J. H., and Kao W. H. (2016) GCKR and PPP1R3B identified as genome-wide significant loci for plasma lactate: the Atherosclerosis Risk in Communities (ARIC) study. Diabetic Med. 33, 968–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Teslovich T. M., Musunuru K., Smith A. V., Edmondson A. C., Stylianou I. M., Koseki M., Pirruccello J. P., Ripatti S., Chasman D. I., Willer C. J., Johansen C. T., Fouchier S. W., Isaacs A., Peloso G. M., Barbalic M., et al. (2010) Biological, clinical and population relevance of 95 loci for blood lipids. Nature 466, 707–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hernaez R., McLean J., Lazo M., Brancati F. L., Hirschhorn J. N., Borecki I. B., Harris T. B., Genetics of Obesity-Related Liver Disease (GOLD) Consortium, Nguyen T., Kamel I. R., Bonekamp S., Eberhardt M. S., Clark J. M., Kao W. H., and Speliotes E. K. (2013) Association between variants in or near PNPLA3, GCKR, and PPP1R3B with ultrasound-defined steatosis based on data from the third National Health and Nutrition Examination Survey. Clin. Gastroenterol. Hepatol. 11, 1183–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Palmer N. D., Musani S. K., Yerges-Armstrong L. M., Feitosa M. F., Bielak L. F., Hernaez R., Kahali B., Carr J. J., Harris T. B., Jhun M. A., Kardia S. L., Langefeld C. D., Mosley T. H. Jr., Norris J. M., Smith A. V., et al. (2013) Characterization of European ancestry nonalcoholic fatty liver disease-associated variants in individuals of African and Hispanic descent. Hepatology 58, 966–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ferrer J. C., Favre C., Gomis R. R., Fernández-Novell J. M., García-Rocha M., de la Iglesia N., Cid E., and Guinovart J. J. (2003) Control of glycogen deposition. FEBS Lett. 546, 127–132 [DOI] [PubMed] [Google Scholar]
- 21. Cohen P. T. (2002) Protein phosphatase 1: targeted in many directions. J. Cell Sci. 115, 241–256 [DOI] [PubMed] [Google Scholar]
- 22. Moorhead G., MacKintosh C., Morrice N., and Cohen P. (1995) Purification of the hepatic glycogen-associated form of protein phosphatase-1 by microcystin-Sepharose affinity chromatography. FEBS Lett. 362, 101–105 [DOI] [PubMed] [Google Scholar]
- 23. Hubbard M. J., and Cohen P. (1989) Regulation of protein phosphatase-1G from rabbit skeletal muscle. 1. Phosphorylation by cAMP-dependent protein kinase at site 2 releases catalytic subunit from the glycogen-bound holoenzyme. Eur. J. Biochem. 186, 701–709 [DOI] [PubMed] [Google Scholar]
- 24. Gain K. R., Malthus R., and Watts C. (1981) Glucose homeostasis during the perinatal period in normal rats and rats with a glycogen storage disorder. J. Clin. Invest. 67, 1569–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Postic C., and Magnuson M. A. (2000) DNA excision in liver by an albumin-Cre transgene occurs progressively with age. Genesis 26, 149–150 [DOI] [PubMed] [Google Scholar]
- 26. Postic C., Shiota M., Niswender K. D., Jetton T. L., Chen Y., Moates J. M., Shelton K. D., Lindner J., Cherrington A. D., and Magnuson M. A. (1999) Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J. Biol. Chem. 274, 305–315 [DOI] [PubMed] [Google Scholar]
- 27. Irimia J. M., Meyer C. M., Peper C. L., Zhai L., Bock C. B., Previs S. F., McGuinness O. P., DePaoli-Roach A., and Roach P. J. (2010) Impaired glucose tolerance and predisposition to the fasted state in liver glycogen synthase knock-out mice. J. Biol. Chem. 285, 12851–12861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Szymańska E., Rokicki D., Watrobinska U., Ciara E., Halat P., Ploski R., and Tylki-Szymańka A. (2015) Pediatric patient with hyperketotic hypoglycemia diagnosed with glycogen synthase deficiency due to the novel homozygous mutation in GYS2. Mol. Genet. Metab. Rep. 4, 83–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weinstein D. A., Correia C. E., Saunders A. C., and Wolfsdorf J. I. (2006) Hepatic glycogen synthase deficiency: an infrequently recognized cause of ketotic hypoglycemia. Mol. Genet. Metab. 87, 284–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Montori-Grau M., Guitart M., Lerin C., Andreu A. L., Newgard C. B., García-Martínez C., and Gómez-Foix A. M. (2007) Expression and glycogenic effect of glycogen-targeting protein phosphatase 1 regulatory subunit GL in cultured human muscle. Biochem. J. 405, 107–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. O'Doherty R. M., Jensen P. B., Anderson P., Jones J. G., Berman H. K., Kearney D., and Newgard C. B. (2000) Activation of direct and indirect pathways of glycogen synthesis by hepatic overexpression of protein targeting to glycogen. J. Clin. Invest. 105, 479–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Doherty M. J., Moorhead G., Morrice N., Cohen P., and Cohen P. T. (1995) Amino acid sequence and expression of the hepatic glycogen-binding (GL)-subunit of protein phosphatase-1. FEBS Lett. 375, 294–298 [DOI] [PubMed] [Google Scholar]
- 33. Li W. C., Ralphs K. L., and Tosh D. (2010) Isolation and culture of adult mouse hepatocytes. Methods Mol. Biol. 633, 185–196 [DOI] [PubMed] [Google Scholar]
- 34. Saitoh Y., Terada N., Saitoh S., Ohno N., Fujii Y., and Ohno S. (2010) Histochemical approach of cryobiopsy for glycogen distribution in living mouse livers under fasting and local circulation loss conditions. Histochem. Cell Biol. 133, 229–239 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.