Abstract
Cardiolipin (CL) is a dimeric phospholipid with critical roles in mitochondrial bioenergetics and signaling. Recently, inhibition of the release of oxidized fatty acyl chains from CL by the calcium-independent phospholipase A2γ (iPLA2γ)-selective inhibitor (R)-BEL suggested that iPLA2γ is responsible for the hydrolysis of oxidized CL and subsequent signaling mediated by the released oxidized fatty acids. However, chemical inhibition by BEL is subject to off-target pharmacologic effects. Accordingly, to unambiguously determine the role of iPLA2γ in the hydrolysis of oxidized CL, we compared alterations in oxidized CLs and the release of oxidized aliphatic chains from CL in experiments with purified recombinant iPLA2γ, germ-line iPLA2γ−/− mice, cardiac myocyte-specific iPLA2γ transgenic mice, and wild-type mice. Using charge-switch high mass accuracy LC-MS/MS with selected reaction monitoring and product ion accurate masses, we demonstrated that iPLA2γ is the major enzyme responsible for the release of oxidized aliphatic chains from CL. Our results also indicated that iPLA2γ selectively hydrolyzes 9-hydroxy-octadecenoic acid in comparison to 13-hydroxy-octadecenoic acid from oxidized CLs. Moreover, oxidative stress (ADP, NADPH, and Fe3+) resulted in the robust production of oxidized CLs in intact mitochondria from iPLA2γ−/− mice. In sharp contrast, oxidized CLs were readily hydrolyzed in mitochondria from wild-type mice during oxidative stress. Finally, we demonstrated that CL activates the iPLA2γ-mediated hydrolysis of arachidonic acid from phosphatidylcholine, thereby integrating the production of lipid messengers from different lipid classes in mitochondria. Collectively, these results demonstrate the integrated roles of CL and iPLA2γ in lipid second-messenger production and mitochondrial bioenergetics during oxidative stress.
Keywords: cardiolipin, lipid metabolism, lipid oxidation, lipid signaling, mass spectrometry (MS)
Introduction
Cardiolipin (CL)3 is a unique doubly charged phospholipid that is nearly exclusively present in the mitochondrial membrane where it is synthesized and plays an important role in mitochondrial bioenergetics and signaling (1–7). The importance of CL in mitochondrial function is underscored in Barth Syndrome where alterations in the tafazzin gene result in pathologic changes in CL aliphatic chain composition due to defective CL remodeling precipitating a dilated cardiomyopathy, skeletal muscle weakness, and neutropenia (8–13).
The predominant molecular species of cardiolipin in heart, skeletal muscle, and liver is tetralinoleoyl cardiolipin (TLCL), which is produced from nascent CL (largely 16:0–18:1) by remodeling catalyzed by the transacylase tafazzin as well as other enzymes (14–17). Tetralinoleoyl CL contains four bis-allylic protons that are susceptible to H· radical abstraction producing a resonance-stabilized bis-allylic radical that, in the presence of molecular oxygen, produces a cadre of oxidized cardiolipin hydroperoxides (18–20). Furthermore, cardiolipin content and molecular species composition is markedly altered in both type 1 and type 2 models of diabetes which precipitates mitochondrial bioenergetic dysfunction (21–22).
In the canonical pathway of lipid 2nd messenger generation, polyunsaturated fatty acids such as arachidonic acid (AA) and docosahexaenoic acid (DHA) are released from choline and ethanolamine phospholipids by cytosolic phospholipase A2α (cPLA2α) and subsequently oxidized to bioactive eicosanoids and docosanoids by a diverse array of cyclooxygenases, lipoxygenases, and cytochromes P450s (23–26). Mitochondria occupy >30% of the volume of myocardium (27), and ∼60% of myocardial phospholipids are present in the mitochondrial compartment (28). The close spatial proximity of cardiolipin to mitochondrial sites of generation of reactive oxygen species (ROS) in conjunction with the multiple bis-allylic protons in CL render it susceptible to oxidation in the mitochondrial compartment during oxidative stress (29). Studies by Kagan and co-workers (30) have identified hydrolysis of oxidized CLs (oxCL) as a mitochondrial source of released oxidized fatty acid lipid 2nd messengers from oxCL. The enzyme responsible for the release of the oxidized acyl chains in CL is inhibited by R-BEL. These results suggest that iPLA2γ is a potential mediator of oxidized fatty acid release and signaling from oxCL (30, 31). However, BEL was first identified as a chymotrypsin inhibitor (32) and has been shown to also inhibit other esterases (33). Hydrolysis of BEL by iPLA2 also generates a diffusible bromomethyl keto acid that can alkylate thiol groups present in proteins containing reactive cysteine residues (34). Thus, to establish the role of iPLA2γ in the release of aliphatic chains from CL, it is necessary to use the specificity inherent in genetic ablation. Accordingly, we utilized purified recombinant human iPLA2γ, cardiac myocyte-specific iPLA2γ transgenic mice, and germ-line iPLA2γ knock-out mice we generated to demonstrate that iPLA2γ is the major enzyme responsible for lipid 2nd messenger release from oxidized CLs during oxidative stress by multiple criteria both in vitro and in vivo.
Previously we generated germ-line iPLA2γ−/− mice by eliminating the active site of the enzyme. The phenotype of the iPLA2γ−/− mice is remarkable for 1) growth retardation, 2) decreased exercise endurance, 3) compromised thermal adaption to cold, and 4) inability to compensate for increased cardiac stress after hemodynamic overload by thoracic aortic constriction. Moreover, shotgun lipidomic analysis of myocardial tissue from iPLA2γ KO mice revealed a 15% decrease in total CL content and a 33% decrease in tetra-18:2 CL molecular species in comparison to their WT littermates. These alterations in CL content and molecular species were accompanied by decreased Complex 4 function. Importantly, alterations in CL remodeling were identified in brain, which demonstrated reduced CL content with an increase in nascent CL molecular species (e.g. enrichment of nascent 16:0 and 18:1 fatty acids), again demonstrating the role of iPLA2γ in CL remodeling. The inability of iPLA2γ KO mice to adapt to the absence of iPLA2γ and resultant reduced mitochondrial function identifies the importance of iPLA2γ in maintaining CL content and molecular species alterations in mitochondrial bioenergetics and organismal function. Collectively, these results underscore the pivotal role of iPLA2γ in the remodeling of CL in mitochondria (35).
Previously, we have also generated cardiac myocyte-specific iPLA2γ transgenic mice, which possess multiple remarkable cardiac mitochondrial phenotypes including: 1) a markedly decreased content of total myocardial lipids, 2) a diminished density of mitochondrial cristae, 3) accumulation of triglycerides during caloric restriction, and 4) fasting-induced hemodynamic dysfunction. These results further substantiate the importance of iPLA2γ in maintaining mitochondrial CL content and molecular species to facilitate physiologic adaptations of mitochondria to external perturbations requiring efficient bioenergetic function and energy storage (36).
In early work, we demonstrated that vesicles containing intrinsic negative charge are rapidly hydrolyzed in myocardium in comparison to neutral or positively charged phospholipid bilayers (37). Thus, we hypothesized that the double negatively charged cardiolipin would also activate iPLA2γ-mediated hydrolysis of PC to release free fatty acids such as arachidonic acid, which can then be further metabolized to downstream eicosanoids. This hypothesis was explored through mass spectrometric analyses of PC/CL unilamellar vesicles hydrolyzed by iPLA2γ as a model system to explore the integration of fatty acid signaling emanating from mitochondrial CL and PC pools. Thus, mitochondrial signaling processes can be integrated during normal physiologic function but would be susceptible to maladaptive changes in production of oxidized aliphatic chains during oxidative stress resulting in iPLA2γ-mediated pathologic dysfunction. Herein, we demonstrate that purified recombinant iPLA2γ releases oxidized aliphatic chains from oxCLs, that iPLA2γ is highly selective for the release of 9-HODE over 13-HODE in calcium-stimulated mitochondrial homogenates, and that iPLA2γ is responsible for the release of oxidized linoleic acid lipid mediators in intact mitochondria subjected to oxidative stress. We also demonstrate the important role of CL in promoting the release of arachidonic acid and 2-AA LPC from choline glycerophospholipids emphasizing the importance of iPLA2γ in integrating mitochondrial lipid 2nd messenger signaling from discrete phospholipid classes.
Results
Hydrolysis of cardiolipin by recombinant iPLA2γ
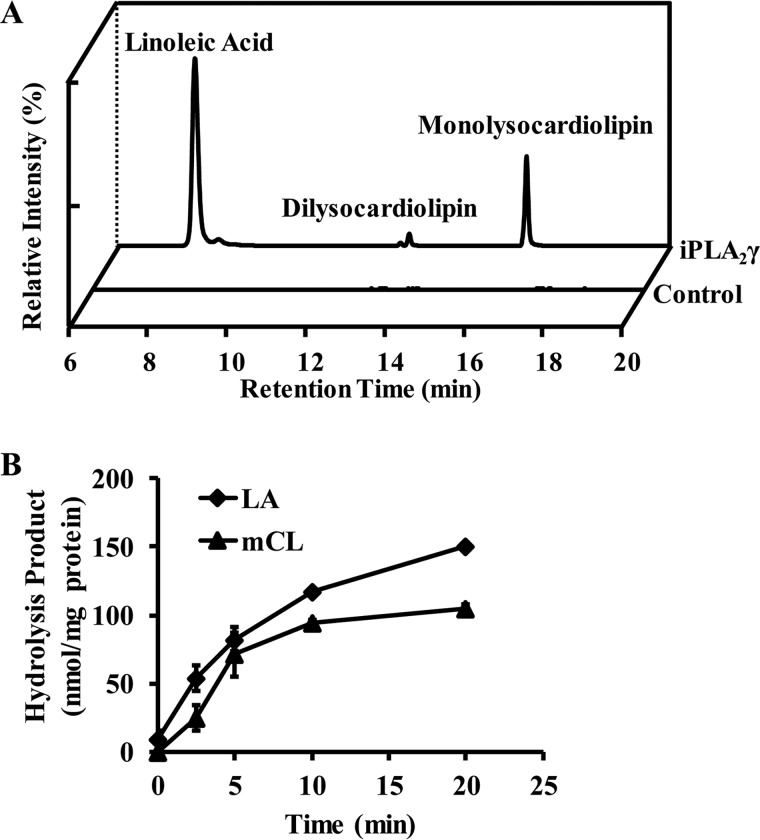
To determine if recombinant human iPLA2γ could hydrolyze cardiolipin, we incubated the purified enzyme with a binary mixture of phospholipids in small unilamellar vesicles. Vesicles composed of TLCL as guest and 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine (PAPC) as host (6 μm and 54 μm, respectively) were prepared by co-sonication. After incubation of purified recombinant iPLA2γ with TLCL/PAPC vesicles, reaction products were extracted with chloroform/methanol/water (1:1:1, v/v/v) and analyzed by LC-MS/MS as described under “Experimental procedures.” Representative extracted ion chromatograms of the reaction products produced during iPLA2γ-catalyzed hydrolysis of TLCL are shown in Fig. 1A. Accurate mass analyses and chromatographic elution profiles led to the assignments of reaction products as linoleic acid, monolysocardiolipin, and dilysocardiolipin. Initial rate analysis (Fig. 1B) demonstrated that the rate of iPLA2γ-catalyzed release of linoleic acid from TLCL guest is ∼18 nmol/mg·min.
Figure 1.
iPLA2γ mediated hydrolysis of cardiolipin to produce free fatty acids, monolysocardiolipin, and dilysocardiolipin. A, extracted ion chromatogram of iPLA2γ-hydrolyzed products from TLCL. Purified recombinant iPLA2γ (6 μg) was incubated with 6 μm TLCL (10 mol%) and 54 μm PAPC at 37 °C for 15 min in 20 mm HEPES, pH 7.2, containing 2 mm EGTA and 1 mm DTT. The reaction was terminated by adding chloroform/methanol (1:1, v/v), and the resultant lipids were extracted in the presence of internal standards (14:0–14:0–14:0 monolysocardiolipin (mCL), d4-16:0-FFA). The chloroform phase was separated and dried under a nitrogen stream. The dried residue was reconstituted in methanol, separated on a C18 HPLC column, and analyzed using a LTQ-Orbitrap mass spectrometer with a mass resolution of 30,000 at m/z = 400 and in the negative ion mode. The extracted ion chromatograms of linoleic acid (279.2325), monolysocardiolipin (592.3632), and dilysocardiolipin (461.2479) are as shown. B, production of linoleic acid (LA) and monolysocardiolipin (mCL) from TLCL hydrolysis by iPLA2γ at different incubation times. Values are the average of three independent preparations ± S.E.
Tandem mass spectrometric analysis of the reaction products generated by iPLA2γ-catalyzed hydrolysis of cardiolipin
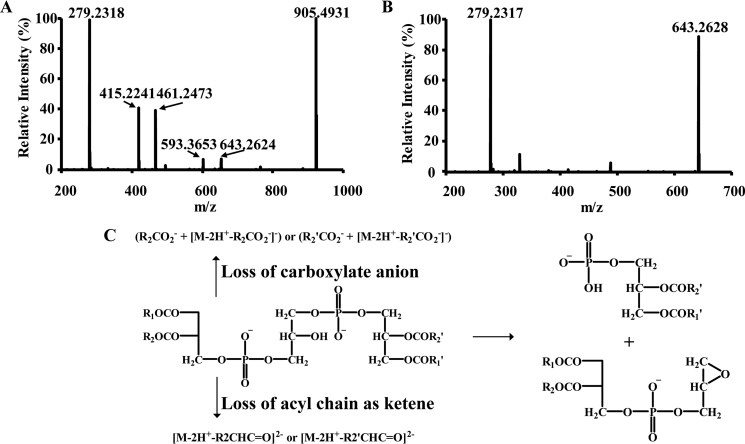
To unambiguously substantiate the identities of products derived from the reaction of iPLA2γ with TLCL, MS2 analyses of the hydrolysis products of TLCL were performed. Fragmentation of the major hydrolytic product present at m/z 592 (corresponding to monolysocardiolipin, the chromatographic peak at 17 min in Fig. 1A) resulted in two major product ions present at m/z 279 (18:2 carboxylate anion) and m/z 905 (18:2 carboxylate anion loss of [M-2H+]2−), arising from the loss of fatty acyl chains as carboxylate anions (Fig. 2A). In addition, tandem mass spectra of the ion at m/z 592 also gave rise to product ions present at m/z 461 (18:2-ketene resulting from the loss of [M-2H+]2−), m/z 643 (18:2-ketene loss plus 18:2 carboxyl anion loss of [M-2H+]2−), and m/z 415 (linoleoylglycerol phosphate) (Fig. 2A). Next, analysis of the fragmentation products of the hydrolysis product at m/z 461 (corresponding to dilysocardiolipin, the chromatographic peak at 14 min in Fig. 1A) was examined. The results identified product ions at m/z 279 (18:2 carboxylate anion), m/z 645 (18:2 carboxyl anion resulting from loss of [M-2H+]2−), and m/z 330 (18:2-ketene loss of [M-2H+]2−) (Fig. 2B). Collectively, these fragmentation patterns are consistent with the previously elucidated major fragmentation pathways for the [M-2H+]2− ions of cardiolipin (Fig. 2C) (38) and substantiate the identities of the products as monolysocardiolipin and dilysocardiolipin.
Figure 2.
Identification of monolysocardiolipin and dilysocardiolipin released from TLCL by purified recombinant human iPLA2γ. The lysocardiolipins generated by iPLA2γ-mediated hydrolysis of TLCL were separated on a C18 HPLC column and analyzed by mass spectrometry. Fragmentations were performed in an LTQ ion trap with a collision energy of 30 eV, and the resultant fragment ions were detected in Orbitrap with a mass resolution of 30,000 at m/z = 400 and a mass accuracy within 5 ppm. A, MS2 spectra of parent ion [M-2H+]2− at m/z 592 (corresponding monolysocardiolipin, the chromatographic peak at 17 min in Fig. 1A). The major fragment ions at m/z 279 and m/z 905 resulting from 18:2 carboxyl anion loss of [M-2H+]2− are characteristic for monolysocardiolipin. B, MS2 spectra of the parent ion [M-2H+]2− at m/z 461 (corresponding dilysocardiolipin, the chromatographic peak at 14 min in Fig. 1A). The major fragment ions at m/z 279 and m/z 643 resulting from 18:2 carboxyl anion loss of [M-2H+]2− are characteristic for dilysocardiolipin. C, scheme of the fragmentation pathways of doubly charged cardiolipin.
Cardiolipin-mediated activation of iPLA2γ
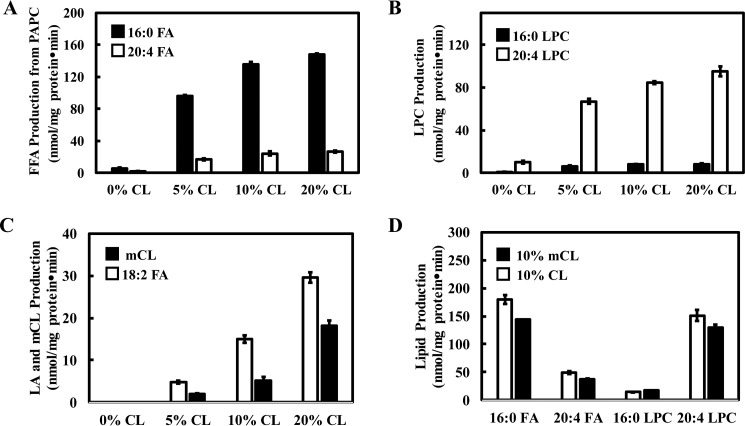
Because iPLA2γ is an important enzymic mediator of the release of fatty acids and lysophospholipids from mitochondrial membrane phospholipids (39), we sought to determine if iPLA2γ activity was modulated by cardiolipin. To this end, the specific activities of iPLA2γ were measured with PAPC vesicle hosts containing incremental mole fractions of cardiolipin guest. Incubations of vesicles composed of 0 μm, 3 μm, 6 μm, or 12 μm TLCL in 60 μm PAPC (representative of the CL content in the mitochondrial inner membrane) were performed with purified recombinant iPLA2γ as described under “Experimental procedures.” The phospholipase activity of iPLA2γ with PAPC substrate was strongly activated by the presence of cardiolipin with substantial effects elicited at 5 mol % CL (10-fold activation), whereas incubations with 20 mol % CL increased iPLA2γ-mediated PAPC hydrolysis by 15-fold (Fig. 3, A and B). In these reactions, cardiolipin was also hydrolyzed as demonstrated by the release of linoleic acid and the concomitant generation of monolysocardiolipin. As the mole fraction of TLCL guest in host PC vesicles increased, the hydrolysis of both TLCL and PC increased (Fig. 3C). In addition, experiments using monolysocardiolipin with its double negative charge as guest in PC vesicles similarly activated iPLA2γ hydrolysis of TLCL guest and PC host vesicles (Fig. 3D). Because both arachidonic acid and 2-arachidonoyl-lysophosphatidylcholine (2-AA-LPC) released by iPLA2γ can serve as substrates for cyclooxygenase-2 (COX-2) and 15-lipoxygenase (15-LOX) oxidation (40), these results identify the potential role of cardiolipin in regulating the synthesis of oxidized lipid 2nd messengers.
Figure 3.
Cardiolipin activated iPLA2γ phospholipase activity resulting in increased release of free fatty acids and lysolipids. A–C, effect of increasing CL content on PAPC and CL hydrolysis. Purified recombinant iPLA2γ (6 μg) was incubated with PAPC SUVs (60 μm) containing either 0, 3, 6, or 12 μm TLCL (0, 5, 10, 20 mol % of PAPC) at 37 °C for 15 min in 20 mm HEPES, pH 7.2, containing 2 mm EGTA and 1 mm DTT. The reaction was terminated by adding chloroform/methanol (1:1, v/v), and the resultant lipids were extracted in the presence of internal standards (17:0-LPC and d4-16:0-FFA). The chloroform phase was separated and dried under nitrogen stream. The dried residue was reconstituted in methanol, separated on a C18 HPLC column, and analyzed by an LTQ-Orbitrap mass spectrometer. The palmitic acid and arachidonic acid released from PAPC (A), lysophosphatidylcholine released from PAPC (B), and linoleic acid and monolysocardiolipin (mCL) released from TLCL (C) were quantified. D, purified recombinant iPLA2γ (6 μg) was incubated with 6 μm TLCL or 6 μm 18:2–18:2–18:2 monolysocardiolipin and 60 μm PAPC at 37 °C for 15 min in 20 mm HEPES, pH 7.2, containing 2 mm EGTA and 1 mm DTT. The lipids released from PAPC were quantified and comparatively shown. Values are the average of three independent preparations ± S.E.
The abundance of oxidized cardiolipins was increased by genetic ablation of iPLA2γ
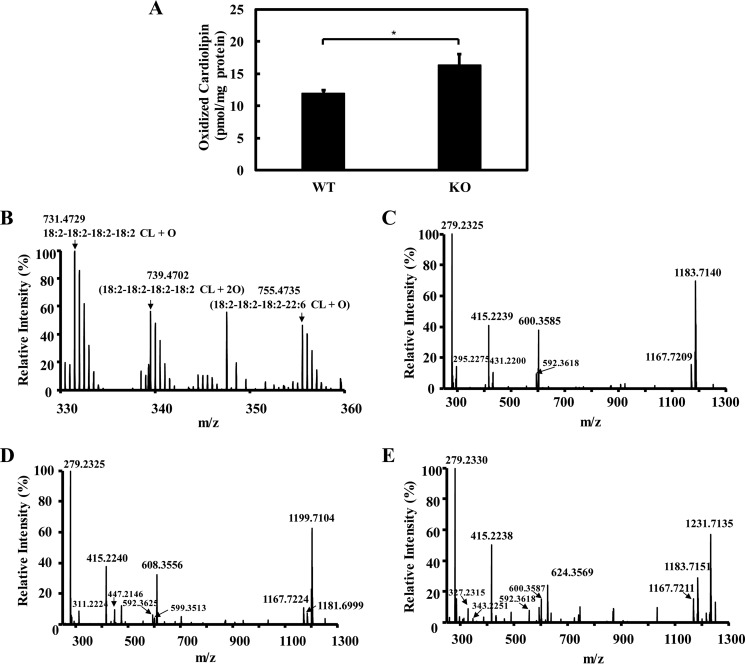
To determine the role of iPLA2γ on the content of oxidized cardiolipins in cardiac myocytes, we quantified the amount of oxidized cardiolipins in WT and iPLA2γ−/− myocardial tissue from 6-month-old mice. To gain access into the extremely low abundance regime of oxidized cardiolipins, crude lipid extracts from WT and KO hearts were initially purified by an aminopropyl solid phase extraction column (to separate acidic phospholipids from total lipids) and analyzed by LC-MS in the negative ion mode as described under “Experimental procedures.” The results demonstrated that iPLA2γ−/− myocardium contains more oxidized cardiolipin in comparisons with wild-type mice (Fig. 4A). Three predominant intrinsic oxidized cardiolipins identified in these analyses include TLCL-OH, TLCL-OOH, and 18:2–18:2–18:2–22:6 CL-OH (Fig. 4B).
Figure 4.
Genetic ablation of iPLA2γ caused the accumulation of oxidized cardiolipin. A, oxidized cardiolipin levels (i.e. the sum of the three predominant oxidized CL species) in wild-type and iPLA2γ−/− myocardium tissue. Freshly isolated heart tissues from wild-type and iPLA2γ−/− mice were flash-frozen in liquid nitrogen, homogenized using a Teflon pestle grinder, and extracted in the presence of TMCL internal standard. The extracts were purified by aminopropyl solid phase extraction column and analyzed by LC-MS/MS in negative ion mode as described under “Experimental procedures.” Values are the average of four independent preparations ± S.E. *, p < 0.05. B, mass spectrum of oxidized cardiolipin from wild-type mouse myocardium tissue. C–E, aminopropyl solid phase extraction purified lipid extract (from two mouse hearts) was separated on a C18 HPLC column, and the fraction containing oxidized cardiolipin was collected and dried. The dried residue was reconstituted in 50 μl of methanol and analyzed by LC-MS/MS. Fragmentations were performed in the LTQ ion trap with collision energy of 30 eV, and the resultant fragment ions were detected in Orbitrap with a mass resolution of 30,000 at m/z = 400 and a mass accuracy within 5 ppm. MS2 spectra of parent ion [M-2H+]2− at m/z 731 (corresponding 18:2–18:2–18:2–18:2-CL-OH) (C), parent ion [M-2H+]2− at m/z 739 (corresponding 18:2–18:2–18:2–18:2-CL-OOH) (D), and parent ion [M-2H+]2− at m/z 755 (corresponding 18:2–18:2–18:2–22:6-CL-OH) (E) are shown here.
To substantiate the proposed molecular species assignments, product ions resulting from the three molecular species were generated by collision-induced dissociation (CID) and analyzed by full mass scanning. As shown in Fig. 4C, the ion present at m/z 731 has a similar tandem mass spectrum as non-oxidized cardiolipin, whereas the only differences present in all fragment pairs are M and M+16 ions for singly charged ions or M and M+8 for doubly charged ions. These results indicate that the ion present at m/z 731 is mono-oxygenated. Accordingly, the presence of these fragment ions substantiates the molecular identity of the ion present at m/z 731 as TLCL-OH. Similarly, the ions present at m/z 739 and m/z 755 were identified as TLCL-OOH and as 18:2–18:2–18:2–22:6 CL-OH, respectively (Fig. 4, D and E). Collectively, the increased content of oxidized molecular species of cardiolipins in iPLA2γ−/−suggests that iPLA2γ is a prominent enzymatic mediator of the hydrolysis of oxidized cardiolipins in myocardium.
Determination of the molecular species of oxidized fatty acyl chains in oxidized cardiolipin
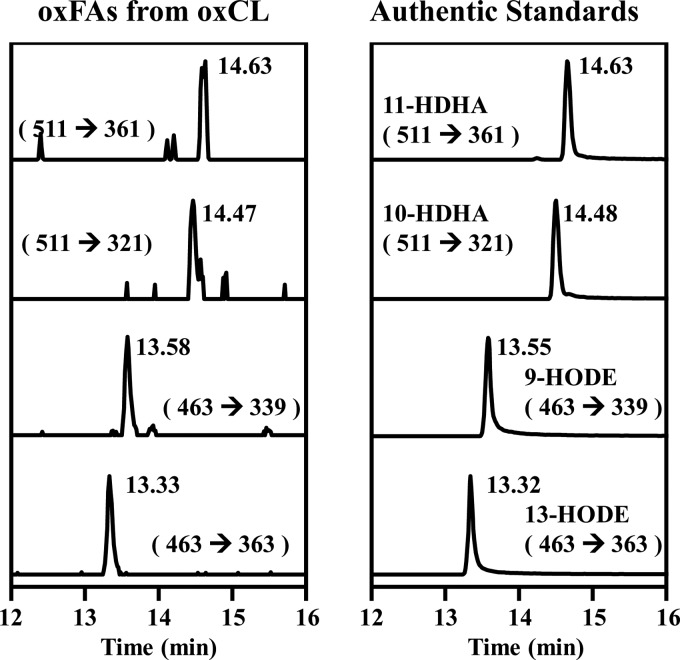
To determine the molecular species of the oxidized fatty acyl chains in oxidized cardiolipins, the oxidized cardiolipins from wild-type mouse myocardium were purified and hydrolyzed by phospholipase A1 from Thermomyces lanuginosus and phospholipase A2 from porcine pancreas. This process hydrolyzed all of the fatty acyl chains from oxidized cardiolipin as described under “Experimental procedures.” The released oxidized polyunsaturated fatty acids were extracted, derivatized with N-(4-aminomethylphenyl)-pyridinium (AMPP), and analyzed by LC-MS/MS. As shown in Fig. 5, the major oxidized fatty acyl chains in myocardial oxidized cardiolipin are 9-HODE, 13-HODE, 10-hydroxydocosahexaenoic acid (10-HDHA), and 11-hydroxydocosahexaenoic acid (11-HDHA). The retention times and selected reaction transitions of the oxidized fatty acids from myocardial oxidized cardiolipin (left panel) are identical to those of authentic standards (right panel) (Fig. 5).
Figure 5.
Identification of the oxidized fatty acyl chains in oxidized cardiolipin from mouse myocardial tissue. Lipids extracts from mouse myocardial tissue were first purified using an aminopropyl solid phase extraction column, and the oxidized cardiolipin was then separated by a C18 HPLC column. The purified oxidized cardiolipin was then completely hydrolyzed by porcine pancreas PLA2 and T. lanuginosus PLA1, derivatized with AMPP, and analyzed by LC-MS/MS. The left panel displays the transitions of the detected oxidized fatty acids in the hydrolyzed oxidized cardiolipin sample, which have identical retention times as authentic standards (right panel).
Hydrolysis of oxidized cardiolipin by purified recombinant iPLA2γ
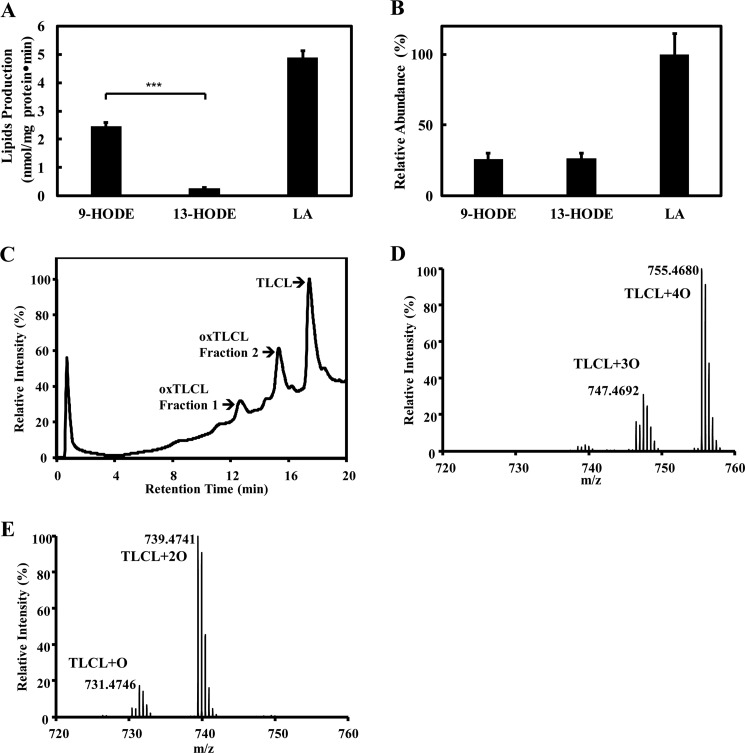
To determine the capacity of iPLA2γ to hydrolyze oxidized cardiolipin, we oxidized TLCL by cytochrome c treatment in vitro as described under “Experimental procedures” and generated vesicles containing oxCL as guest in host PAPC membranes. Incubations with purified recombinant iPLA2γ were performed, and the released oxidized fatty acids were analyzed by charge-switch derivatization, LC-MS/MS, and accurate mass product ion analysis. The oxidized fatty acids released from cardiolipin by iPLA2γ were 9-HODE and 13-HODE (Fig. 6A). Intriguingly, the selectivity of iPLA2γ for 9-HODE release in this system is significantly higher than that of 13-HODE. To determine if this difference is due to differences in substrate content of 9-HODE versus 13-HODE, or due to selectivity of iPLA2γ for discrete oxidized aliphatic chains, each substrate was completely hydrolyzed by phospholipase A1 from T. lanuginosus and phospholipase A2 from porcine pancreas. The released fatty acids were analyzed as described under “Experimental procedures.” That the amounts of 9-HODE and 13-HODE were the same when all aliphatic chains were hydrolyzed (Fig. 6B) indicates that iPLA2γ preferentially hydrolyzes 9-HODE in comparison to 13-HODE from oxCL in bilayer systems.
Figure 6.
Recombinant human iPLA2γ selectively hydrolyzed 9-HODE, but not 13-HODE, from oxidized TLCL. A, specific activity of iPLA2γ hydrolysis of oxidized cardiolipin. Purified recombinant iPLA2γ (2.5 μg) was incubated with 6 μm TLCL, 6 μm oxTLCL (fraction 1 from HPLC purification of oxCL), and 48 μm PAPC at 37 °C for 10 min in 20 mm HEPES, pH 7.2, containing 2 mm EGTA and 1 mm DTT. The products including 9-HODE, 13-HODE, and linoleic acid (LA) were extracted in the presence of internal standards (d4-16:0 FFA, 13-HODE-d4), derivatized with AMPP, and analyzed by LC-MS/MS. B, relative amounts of 9-HODE, 13-HODE, and LA in TLCL/oxTLCL/PAPC vesicles after complete hydrolysis by porcine pancreas PLA2 and T. lanuginosus PLA1, derivatization with AMPP, and analysis by LC-MS/MS. C–E, oxidized cardiolipin used as substrate for iPLA2γ hydrolysis was prepared by cytochrome c-mediated oxidation in the presence of hydrogen peroxide as described previously (52) and purified by reversed-phase HPLC (C). Fraction 1 (D) and fraction 2 (E) containing oxTLCL were collected and analyzed by mass spectrometry as described under “Experimental procedures.” Values are the average of three independent preparations ± S.E. ***, p < 0.001.
Hydrolysis of oxidized cardiolipin by mitochondrial iPLA2γ
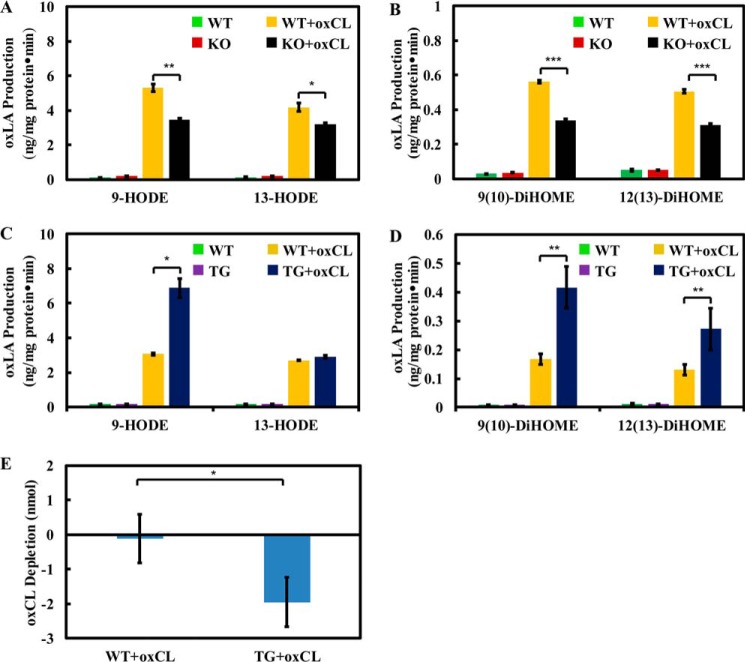
To examine the ability of iPLA2γ to hydrolyze oxidized cardiolipins in mitochondria, we isolated mitochondria from wild-type and iPLA2γ−/− mice liver tissue. Mitochondria were briefly sonicated and incubated with oxidized TLCL (prepared by cytochrome c treatment) or ethanol vehicle-only in the presence of calcium. Although the catalytic activity of purified recombinant human iPLA2γ (63 kDa) is calcium-independent, we have previously demonstrated that mitochondrial membrane-associated iPLA2γ isoforms can be dramatically activated by either calcium or magnesium ion, which we have previously ascribed to the complexing of the divalent cations with the phospholipid acyl chain carbonyl group facilitating hydrolysis (41). The production of oxidized linoleic acids including 9-HODE, 13-HODE, 9(10)-DiHOME, and 12(13)-DiHOME were measured as described under “Experimental procedures.” Compared with wild-type mitochondria homogenate, iPLA2γ−/−mitochondria released significantly less 9-HODE, 9(10)-DiHOME, and 12(13)-DiHOME when incubated with exogenous oxCL (Fig. 7, A and B). In addition, only small amounts of oxidized linoleic acids were released in mitochondrial homogenates without oxCL proving that the production of oxidized linoleic acids was mainly due to the hydrolysis of exogenous oxCL in samples containing oxCL in these in vitro experiments with homogenized mitochondria.
Figure 7.
Oxidized linoleic acid production and oxidized cardiolipin consumption in wild-type, iPLA2γ−/−, and cardiac myocyte-specific iPLA2γ transgenic mitochondrial homogenates in the presence of exogenous oxTLCL. A and B, mitochondria were isolated from wild-type and iPLA2γ−/− mouse liver and homogenized by sonication. Mitochondria homogenates (1 mg protein/ml) were incubated with 20 μm oxTLCL or ethanol vehicle alone at 37 °C for 15 min. The reactions were terminated by the addition of methanol (25% total volume) containing internal standards (13-HODE-d4, 12(13)-DiHOME-d4). The released oxidized fatty acids were purified by reversed phase solid phase extraction, derivatized with AMPP, and finally analyzed by LC-MS/MS. C and D, the same experiments were performed with heart mitochondria isolated from wild-type and cardiac myocyte specific iPLA2γ transgenic mice. E, heart mitochondria were isolated from wild-type and cardiac myocyte-specific iPLA2γ transgenic mice. Mitochondria homogenates (1 mg protein/ml) were incubated with 20 μm oxTLCL or ethanol vehicle alone at 37 °C for 15 min. The reactions were terminated by adding chloroform/methanol 1:1 (v/v) in the presence of TMCL internal standard. The chloroform phase was separated, dried, and redissolved in chloroform. The oxidized cardiolipin was purified by aminopropyl solid phase extraction column and analyzed by LC-MS/MS in the negative ion mode. The results here show the consumption of oxidized cardiolipin per mg of protein in 30-min incubations. Values are the average of three independent preparations ± S.E. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Similar experiments were also performed with mitochondria isolated from wild-type and cardiac myocyte-specific iPLA2γ transgenic mice heart tissue. Compared with wild-type mitochondria homogenates, iPLA2γ transgenic mitochondria homogenates released significantly more 9-HODE, 9(10)-DiHOME, and 12(13)-DiHOME (Fig. 7, C–D). However, the production of 13-HODE was the same between wild-type and transgenic mitochondrial homogenates. This selectivity is consistent with the results obtained with purified recombinant iPLA2γ. In addition, iPLA2γ transgenic mitochondria homogenates also hydrolyzed more oxidized cardiolipin compared with that of wild-type (Fig. 7E).
Genetic ablation of iPLA2γ resulted in the accumulation of oxidized cardiolipins in intact mitochondria subjected to oxidative stress
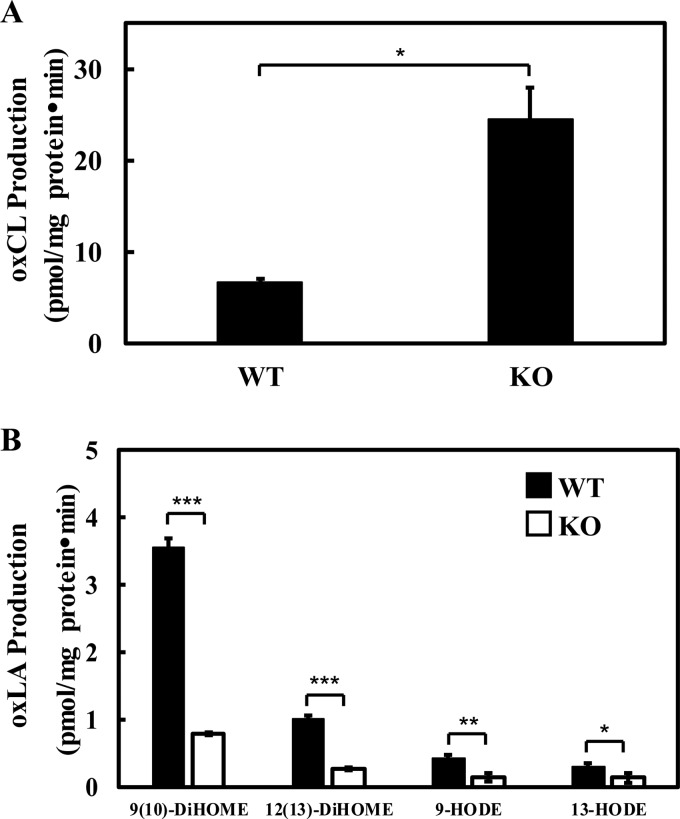
To determine if iPLA2γ is responsible for hydrolyzing the majority of oxidized cardiolipin produced by mitochondria under oxidative stress, we isolated intact mitochondria from WT and iPLA2γ−/− myocardium as described under “Experimental procedures.” Intact mitochondria were subjected to oxidative stress by incubation in buffer containing 2 mm ADP, 0.3 mm NADPH, and 0.012 mm Fe3+ for 15 min. The ADP/NADPH/Fe3+ system has previously been shown to effectively induce oxidative stress and lipid peroxidation (42, 43). Oxidized linoleic acid and oxidized cardiolipins were analyzed as described under “Experimental procedures.” The accumulation of oxidized cardiolipins in iPLA2γ−/− mitochondria during oxidative stress was 4 times greater than that in wild-type mitochondria (Fig. 8A). In contrast, the release of oxidized linoleic acid from wild-type mitochondria is significantly higher than that in iPLA2γ−/− mitochondria during oxidative stress (Fig. 8B). These results are consistent with the notion that iPLA2γ has a major role in hydrolysis of oxidized cardiolipin aliphatic chains during oxidative stress in intact mitochondria to directly release bioactive lipid 2nd messengers.
Figure 8.
Accumulation of oxidized cardiolipin (A) and production of oxidized linoleic acids (B) in intact wild-type and iPLA2γ−/− mitochondria stimulated by oxidative stress. Myocardial mitochondria were isolated from wild-type and iPLA2γ−/− mice and reconstituted in isotonic buffer. Intact mitochondria (0.8 mg/ml) were incubated with 2 mm ADP, 0.3 mm NADPH, 0.012 mm Fe3+, and 2.5 mm phosphate at 37 °C for 15 min. The reactions were terminated by adding chloroform/methanol (1:1, v/v). The chloroform phase was separated and dried under a nitrogen stream. A, for analysis of oxidized cardiolipin, the dried residues were redissolved in chloroform, purified by aminopropyl solid phase extraction, and analyzed by LC-MS/MS in the negative ion mode. B, for analysis of oxidized linoleic acids (oxLAs), the dried residues were redissolved in water/methanol 4:1, purified by reversed phase solid phase extraction, derivatized with AMPP, and finally analyzed by LC-MS/MS in the positive ion mode. Values are the average of four independent preparations ± S.E. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Discussion
The results of the present study identify mitochondrial iPLA2γ as the enzymic mediator of the hydrolysis of oxidized aliphatic chains in oxCL molecular species leading to the direct release of oxidized fatty acid lipid 2nd messengers in the mitochondrial compartment. Moreover, we demonstrate that negatively charged cardiolipin markedly activates iPLA2γ phospholipase activity on arachidonic acid containing choline glycerophospholipids, thereby integrating the production of signaling molecules from discrete phospholipid pools (e.g. choline glycerophospholipids versus oxCLs). Thus, the present study identifies an integrated signaling network in mitochondria mediated by iPLA2γ that participates in the integration of cellular bioenergetics and signaling.
Because CL composes ∼5–20% of the phospholipid content of the inner mitochondria membrane (6), cardiolipin-induced activation of mitochondrial iPLA2γ phospholipid hydrolysis is a likely regulator of the release of polyunsaturated fatty acids (i.e. AA and DHA) and lysophospholipids from glycerophospholipids in the mitochondrial membrane. Both AA and AA-LPC released from phosphatidylcholine by iPLA2γ can serve as substrates for oxidized lipid 2nd messenger synthesis, which is underscored by the work we published previously showing the utilization of 2-AA-LPC by cyclooxygenase-2 to generate 2-eicosanoid lysolipids (40). It has been previously demonstrated that robust losses of cardiolipin content occurs in pathologic conditions including diabetes (21), heart failure (45), and cancer (46). The depletion of cardiolipin in those pathological processes likely reduces the hydrolysis of mitochondrial membrane phospholipid by iPLA2γ followed by decreased synthesis of oxidized lipid 2nd messengers precipitating maladaptive alterations in the interplay of these two integrated signaling pathways.
Through the use of high mass accuracy high resolution mass spectrometry, we identified three types of oxidized cardiolipin molecular species in mouse myocardial tissue. These include hydroxylated linoleic acid, hydroxylated docosahexaenoic acid, and linoleic acid hydroperoxide. We also identified the molecular species of oxidized fatty acids in cardiolipin as 9-HODE, 13-HODE, 10-HDHA, and 11-HDHA by hydrolysis, charge-switch AMPP derivatization, and high mass accuracy analysis of product ions. Furthermore, with purified recombinant iPLA2γ and cardiac myocyte-specific transgenic expression of iPLA2γ, we demonstrated that 9-HODE is preferentially hydrolyzed in comparison to 13-HODE from oxidized CL by iPLA2γ.
To substantiate the role of iPLA2γ in the content of oxidized CL molecular species in vivo, we compared the amount of oxidized CL in WT versus iPLA2γ−/− hearts. Notably, KO of iPLA2γ resulted in the accumulation of oxidized CL molecular species in the heart. The accumulation of oxidized CLs establishes a primary role for iPLA2γ in the release of oxidized aliphatic chains from CLs in vivo. This result is consistent with those of Kagan and co-workers (30) indicating iPLA2γ likely released oxidized aliphatic chains from CL in vivo as determined by use of R-BEL.
To determine the effects of oxidative stress on cardiolipin oxidation and the major role of iPLA2γ, we utilized a well accepted system to produce oxidative stress in intact mitochondria (39, 40). Treatment of WT mitochondria with NADPH, ADP, and Fe3+ resulted in the robust production of 12(13)-DiHOME and 9(10)-DiHOME with lesser amounts of monohydroxylated species including 9-HODE and 13-HODE. Remarkably, mitochondria isolated from iPLA2γ−/− mice exhibited a 70% reduction in both DiHOME and HODE production under oxidative stress, demonstrating the importance of iPLA2γ in the release of oxidized fatty acid lipid 2nd messengers of signal transduction during oxidative stress.
In heart or liver mitochondria, 5–20% of mitochondrial membrane lipids are cardiolipin, and >70% of fatty acyl chains in cardiolipin are linoleic acid (6). Therefore, cardiolipin may be a major source of oxidized linoleic acid second messengers. In this study we have established that the generation of oxidized linoleic acid from oxidized cardiolipin under oxidative stress is regulated by iPLA2γ, substantiating the indispensable role of iPLA2γ in the production of oxidized linoleic acid lipid second messengers from oxCL in mitochondria.
Previous studies have demonstrated that oxidized linoleic acids have pivotal roles in multiple pathological processes, including inflammatory hyperalgesia and the activation of G protein-coupled receptor G2A (47–50). For example, Patwardhan et al. (47), Green et al. (49), and Alsalem et al. (48) have shown that oxidized linoleic acids can activate the TRPA1 receptor and contribute to inflammatory hyperalgesia and allodynia after injury. In addition, Obinata et al. (50) demonstrated that 9-HODE is a ligand for the G protein-coupled receptor G2A that increases mobilization of intracellular calcium in cells expressing this receptor. Because iPLA2γ is a major enzymic mediator of oxidized linoleic acid lipid 2nd messenger release, it seems likely that iPLA2γ is involved in these processes.
In conclusion, this study provides robust evidence that iPLA2γ is the major enzyme mediating the release of oxidized aliphatic chains from oxCLs. This process likely serves multiple roles through 1) the release of lipid 2nd messengers that mediate mitochondrial signaling and bioenergetics and 2) the removal of pathologic products of oxidative stress which compromises mitochondrial bioenergetic function. Moreover, we demonstrate the profound activation of iPLA2γ by cardiolipin, which facilitates the release of polyunsaturated fatty acids from PC for the downstream production of signaling metabolites. Collectively, the direct release of oxidized linoleic acid lipid 2nd messengers from CL in the mitochondrial compartment and iPLA2γ-catalyzed AA and 2-AA LPC release from choline glycerophospholipids are integrated processes for the generation of lipid 2nd messengers in the mitochondrial compartment from discrete phospholipid precursor pools.
Experimental procedures
Materials
Tetralinoleoyl cardiolipin and 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine were purchased from Avanti Polar Lipids (Alabaster, AL). Kinetex 5-μm EVO C18 columns (250 × 4.6 mm and 150 × 2.1 mm) were purchased from Phenomenex (Torrance, CA). Ascentis Express 2.7-μm C18 column (150 × 2.1 mm) was purchased from Supelco (Bellefonte, PA). Strata-X solid phase extraction cartridges (30 mg/1 ml) were purchased from Phenomenex. Aminopropyl solid phase extraction cartridges (50 mg/1 ml) were purchased from Supelco (Bellefonte, PA). HPLC grade acetonitrile and chloroform were purchased from Burdick & Jackson (Muskegon, MI). HPLC grade methanol and water were purchased from Fisher. The AMPP derivatization kit, (±)-9-hydroxy-10E,12Z-octadecadienoic acid, (±)-13-hydroxy-9Z,11E-octadecadienoic acid, (±)9,10-dihydroxy-12Z-octadecenoic acid, (±)12,13-dihydroxy-9Z-octadecenoic acid, (±)10-hydroxy-4Z,7Z,11E,13Z,16Z,19Z-docosahexaenoic acid, (±)11-hydroxy-4Z,7Z,9E,13Z,16Z,19Z-docosahexaenoic acid, 13S-hydroxy-9Z,11E-octadecadienoic-9,10,12,13-d4 acid, and (±)12,13-dihydroxy-9Z-octadecenoic-9,10,12,13-d4 acid were purchased from Cayman Chemical (Ann Arbor, MI). Leupeptin, aprotinin, and glycerol were purchased from Fisher. All other chemicals were purchased from Sigma.
General animal studies
Animal protocols were conducted in strict accordance with the National Institutes of Health guidelines for humane treatment of animals and were reviewed and approved by the Animal Studies Committee of Washington University.
Expression and purification of iPLA2γ(His)6 in Sf9 cells
Recombinant iPLA2γ was purified as previously described (51). Briefly, 100 ml of Sf9 cells (1 × 106 cells/ml) were transfected with recombinant baculovirus encoding iPLA2γ(His)6 at a multiplicity of infection of 1. After 48 h, the cell suspension was centrifuged at 300 × g for 10 min, and the cell pellet was resuspended in 10 ml of lysis buffer (25 mm potassium phosphate, pH 7.8, containing 20% glycerol, 1 mm imidazole, 2 mm 2-mercaptoethanol, 5 μg/ml leupeptin, and 5 μg/ml aprotinin). Cells were lysed by sonication (20 pulses of 1 s at 30% power) and centrifuged at 100,000 × g for 45 min. The supernatant was mixed with an equal volume of dilution buffer (25 mm potassium phosphate, pH 7.8, containing 20% glycerol, 1 mm imidazole, 500 mm sodium chloride, and 2 mm 2-mercaptoethanol) and loaded onto a 5-ml column of HIS Select Cobalt Affinity Gel previously equilibrated with 25 mm potassium phosphate, pH 7.8, containing 20% glycerol, 1 mm imidazole, and 250 mm sodium chloride. The column was washed with 10 column volumes of 25 mm potassium phosphate buffer, pH 7.8, containing 20% glycerol, 10 mm imidazole, 500 mm sodium chloride, and 1 mm 2-mercaptoethanol. Recombinant iPLA2γ(His)6 (63 kDa) was eluted with 25 mm potassium phosphate, pH 7.8, containing 20% glycerol, 200 mm imidazole, 500 mm sodium chloride, and 1 mm 2-mercaptoethanol. Purified iPLA2γ was flash-frozen in liquid N2 and stored at −80 °C.
Preparation of oxidized cardiolipin
Oxidized cardiolipin was prepared as described by Kagan and co-workers (30). Briefly, TLCL (250 μm) was resuspended in buffer (20 mm HEPES, pH 7.4, 100 μm diethylenetriaminepentaacetic acid (DTPA)) by sonication (5 min, 1-s pulse, 30% power). Cytochrome c and H2O2 were added, and samples were incubated for 1 h at 37 °C. Cytochrome c and H2O2 were added at 10-min intervals (6 additions total), and the final concentrations of cytochrome c and H2O2 were 10 μm and 100 μm, respectively. Oxidized CL was extracted by adding two volumes of chloroform/methanol (1:1, v/v). The chloroform phase was separated and dried under N2 stream. The dried residue was dissolved in methanol and loaded on a C18 reverse phase HPLC column (Kinetex EVO C18, 5 μm, 250-mm × 4.6 mm). The column was eluted at a flow rate of 1 ml/min. A gradient of solvent A (acetonitrile/methanol/10 mm ammonium acetate in water, pH 7.5, 2:1:1, v/v/v) and solvent B (methanol) was used as follows: 0 min, 50% B; 5 min, 50% B; 20 min, 100% B; 27 min, 100% B; 27.1 min, 50% B; 35 min, 50% B. Fractions of eluent containing oxCL were collected and dried under a nitrogen stream. The dried residue was extracted by chloroform/methanol/water (1:1:1, v/v/v) to eliminate ammonium acetate and analyzed by mass spectrometry.
Hydrolysis of cardiolipin/phosphatidylcholine by purified recombinant iPLA2γ
Small unilamellar vesicles (SUVs) containing either TLCL or 18:2–18:2–18:2 monolysocardiolipin as guest in PAPC host vesicles were prepared by sonicating (30% power, 1-s pulse, 5 min) TLCL and PAPC in HEPES buffer (20 mm HEPES, pH 7.2, 2 mm EGTA, 1 mm DTT). 18:2–18:2–18:2 monolysocardiolipin was prepared and purified as described previously (52). The concentrations of the phospholipid substrates were specified under “Results.” The reaction was initiated by the addition of purified recombinant iPLA2γ to the SUVs in HEPES buffer and incubated at 37 °C for 10 min. The reaction was terminated by adding 2 volumes of chloroform/methanol (1:1, v/v) containing TMCL (tetramyristoyl cardiolipin) and d4-16:0 fatty acid internal standards. The chloroform phase was separated and dried under a nitrogen stream. The dried residue was resuspended in methanol and used for LC-MS/MS analysis.
For the oxidized cardiolipin hydrolysis reaction, the SUV containing 6 μm TLCL and 6 μm oxTLCL (fraction 1 from HPLC purification of oxCL) was prepared, and the reaction with purified recombinant iPLA2γ was performed the same as for the nonoxidized cardiolipin except that the reaction was terminated by adding methanol (to 20% final volume) containing 13-HODE-d4, 12(13)-DiHOME-d4, and d4-16:0 fatty acid internal standards before solid phase extraction.
Solid phase extraction and AMPP derivatization of oxidized fatty acids
Solid phase extraction of fatty acids and oxidized fatty acids was performed using a Strata-X reversed phase cartridge (Phenomenex, 30 mg/1 ml). The cartridges were prewashed with 2 ml of methanol and then equilibrated with 80% H2O, 20% methanol. The samples were applied to the cartridge, and the cartridges were washed with 2 ml of solvent containing 90% H2O, 10% methanol. Oxidized fatty acids were eluted with 1 ml of methanol and dried under nitrogen stream.
AMPP derivatization was performed as described by Gelb and co-workers (53). In brief, 20 μl of cold acetonitrile/N,N-dimethylformamide (4:1, v/v) was added to the dried residue. The sample tube was vortexed, then 20 μl of cold 640 mm N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide in water, 10 μl of 20 mm 1-hydroxybenzotriazole in acetonitrile/dimethylformamide (99:1 v/v), and 30 μl of N-(4-aminomethylphenyl)-pyridinium (AMPP) in acetonitrile were added and vortexed. After 30 min of incubation at 60 °C, the sample was cooled to room temperature and subjected to LC-MS/MS analysis as previously described (54).
Analysis of oxidized cardiolipin in flash-frozen WT and iPLA2γ−/− myocardium
The iPLA2γ−/− mouse was generated in our laboratory as described previously (35). After euthanasia of wild-type or iPLA2γ−/− mice by cervical dislocation, hearts were removed and quickly rinsed with phosphate-buffered saline, blotted, and flash-frozen in liquid nitrogen. Frozen heart tissue was stored in liquid nitrogen vapor (−180 °C) until extraction. To perform extractions, ∼50 mg of heart tissue were added into the test tubes with 2 ml of chloroform:methanol (2:1 v/v with 1% acetic acid, 2 μl of an antioxidant mixture (0.2 mg/ml butylated hydroxytoluene, 2 mg/ml triphenylphosphine, 0.2 mg/ml EDTA, and 2 mg/ml indomethacin in methanol:ethanol:water, 2:1:1, v/v/v) and 0.1 nmol/mg of protein tetramyristoyl cardiolipin internal standard. The tissue was then homogenized using a Polytron homogenizer followed by the addition of 0.6 ml of 0.9% sodium chloride aqueous solution. The mixture was thoroughly vortexed and then centrifuged at 15,000 × g for 10 min. The chloroform layer was separated, and the aqueous phase was extracted again with 1.2 ml of chloroform. The chloroform phases were combined, dried under a stream of nitrogen, and resuspended in 1.5 ml of chloroform for subsequent aminopropyl solid phase extraction.
The oxidized cardiolipins in the crude extracts were purified using an aminopropyl solid phase extraction column as described before (44). Briefly, the aminopropyl solid phase extraction column was equilibrated with 1 ml of hexane before loading 200 μl of extract in chloroform onto the column. The column was washed with 0.5 ml of chloroform and 2 ml of methanol. Oxidized cardiolipins were eluted by 1 ml of chloroform/methanol (4:1, v/v) containing 0.2 m ammonium acetate. The eluent was dried under a nitrogen stream, resuspended in 0.2 ml of methanol, and analyzed by LC-MS/MS.
For MS2 analysis of oxidized cardiolipin, the lipid extract from two wild-type hearts was first purified by an aminopropyl solid phase extraction column then separated on C18 HPLC column (Kinetex EVO C18, 5 μm, 250 mm × 4.6 mm) as described above. The oxidized cardiolipin fraction was collected, dried under a nitrogen stream, and reconstituted in 50 μl of methanol for LC-MS/MS analysis.
To examine the identities of the oxidized fatty acyl chains in oxidized cardiolipin from mouse myocardium, oxidized cardiolipins from two mouse hearts were purified by C18 HPLC and then resuspended by sonication in 1 ml of HEPES buffer (20 mm HEPES, pH 7.4, 10% glycerol, 2 mm calcium, 1 mm DTT) followed by the addition of 10 μl of phospholipase A2 from porcine pancreas and 10 μl of phospholipases A1 from T. lanuginosus. The reaction was incubated at 37 °C for 30 min. The oxidized fatty acids were purified, derivatized, and analyzed as described above.
LC-MS/MS analysis
LC-MS/MS analysis was performed using an LTQ Orbitrap mass spectrometer connected to a Surveyor HPLC system. AMPP-derivatized oxidized fatty acids were separated with a C18 reverse phase column (Ascentis Express C18 2.7 μm, 150 × 2.1 mm) at 22 °C with a flow rate of 200 μl/min. The linear gradient of solvent A (water with 10 mm ammonium acetate, pH 4.3) and solvent B (acetonitrile) was used as follows: 0 min, 25% B; 5 min, 25% B; 20 min, 95% B; 27 min, 95% B; 27.1 min, 25% B; 35 min, 25% B. The sample injection volume was 10 μl, and the autosampler tray temperature was set as 4 °C. The spray voltage in electrospray ionization source was 4.1 kV. The sheath gas flow rate was 40. The capillary temperature was 270 °C. The AMPP-derivatized oxidized fatty acid was analyzed with selected reaction monitoring. The collision energy used was 30 eV with an isolation width of ±1.5 thomson.
Oxidized cardiolipin molecular species were separated using a C18 reversed phase column (Kinetex EVO C18, 5 μm, 150 × 2.1 mm) at 22 °C with a flow rate of 200 μl/min. A linear gradient of solvent A (acetonitrile/methanol/10 mm ammonium acetate in water, pH 7.5, 2:1:1, v/v/v) and solvent B (methanol) was used as follows: 0 min, 50% B; 5 min, 50% B; 20 min, 100% B; 27 min, 100% B; 27.1 min, 50% B; 35 min, 50% B. The sample injection volume was 10 μl, and the autosampler tray temperature was set as 4 °C. The electrospray ionization conditions were the same as those used in the analysis AMPP-derivatized oxidized fatty acids. For MS2 analysis of oxidized cardiolipin, the collision energy was 30 eV, and the isolation width was ± 2 Th.
Isolation and incubation of mitochondria
Cardiac myocyte-specific iPLA2γ transgenic and iPLA2γ−/− mice were generated in our laboratory as described previously (35, 36). After euthanasia by cervical dislocation, hearts and livers were removed and washed extensively in ice-cold mitochondrial isolation buffer (10 mm HEPES, 0.25 m sucrose, 1 mm EGTA, 0.4% fatty acid-free BSA, pH 7.4) and finely minced. The tissues were then homogenized on ice with a 10-ml Teflon pestle tissue grinder (12 strokes for heart and 8 strokes for liver at speed 15). The homogenates were first centrifuged at 700 × g for 10 min to pellet nuclei and cellular debris. The supernatants were centrifuged at 10,000 × g for 10 min to pellet mitochondria. The mitochondrial pellets were resuspended in isolation buffer and centrifuged again at 10,000 × g. The mitochondrial pellets were briefly rinsed with BSA- and EGTA-free isolation buffer and reconstituted in buffer as indicated under the different experimental conditions.
For exogenous oxidized cardiolipin hydrolysis experiments, liver mitochondria from iPLA2γ−/− and wild-type mice were reconstituted in ice-cold HEPES buffer (10 mm HEPES, 10% glycerol, 2 mm DTT, pH7.4) and sonicated (5 pulses of 1 s at 30% power). The concentration of mitochondrial protein was determined by a Bradford protein assay (Bio-Rad). The oxidized tetralinoleoylcardiolipin was delivered in ethanol (20 nmol of oxCL/mg protein with a final concentration of ethanol at 2%). The mitochondrial homogenate containing oxidized cardiolipins or vehicle alone was sonicated again (5 pulses of 1 s, 30% power). Hydrolysis of oxidized cardiolipins was initiated by the addition of CaCl2 (2 mm) and incubation at 37 °C for 15 min. Reactions were terminated by adding methanol (20% total volume) containing internal standards (13-HODE-d4, 12(13)-DiHOME-d4). The released oxidized fatty acids were purified by reversed phase solid phase extraction, derivatized with AMPP, and finally analyzed by LC-MS/MS as described above. The same experiments were performed with heart mitochondria isolated from wild-type and heart-specific iPLA2γ transgenic mice.
To measure the consumption of oxidized cardiolipin, the reactions were terminated by adding chloroform/methanol (1:1, v/v) in the presence of TMCL internal standard. The chloroform phase was separated, dried, and redissolved in chloroform. The oxidized cardiolipin was purified by aminopropyl solid phase extraction and analyzed by LC-MS/MS in negative ion mode.
For intact mitochondrial oxidation experiments, liver mitochondria from wild-type and iPLA2γ−/− mice were reconstituted in ice-cold isotonic buffer (75 mm Tris-Cl, 5 mm HEPES, pH 7.4, 60 mm KCl, 12.5 mm sucrose, 0.5 mm MgCl2, 2.5 mm KH2PO4). The concentration of mitochondrial protein was determined by a Bradford protein assay and diluted with buffer to a final concentration of 0.8 mg/ml. Oxidative stress was initiated by adding 2 mm ADP, 0.3 mm NADPH, 0.012 mm Fe3+, and subsequently incubation at 37 °C for 15 min. Oxidized fatty acids and oxidized cardiolipins were extracted, purified, and analyzed as described above.
Statistical analyses
Results are expressed as averages ± S.E. The significance of results was determined by Student's t test, and results were considered significant at a level of p < 0.05.
Author contributions
G.-Y. L. and R. W. G. designed the studies. M. L. performed the preliminary experiments. S. G. and H. F. S. generated and provided iPLA2γ−/− and cardiac myocyte-specific iPLA2γ transgenic mice. G.-Y. L. conducted the experiments and performed the mass spectrometric analyses. G.-Y. L., R. W. G., S. H. M., and C. M. J. analyzed the data and prepared the manuscript.
This work was supported by National Institutes of Health Grant R01HL118639. R. W. G.. has financial relationships with LipoSpectrum and Platomics. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- CL
- cardiolipin
- TLCL
- tetralinoleoyl cardiolipin
- TMCL
- tetramyristoyl cardiolipin
- PC
- phosphatidylcholine
- PAPC
- 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine
- DiHOME
- dihydroxyoctadecenoic acid
- HODE
- hydroxyoctadecadienoic acid
- HDHA
- hydroxyldocosahexaenoic acid
- iPLA2γ
- calcium-independent phospholipase A2γ
- AA
- arachidonic acid
- DHA
- docosahexaenoic acid
- oxCL
- oxidized CL
- AA-LPC
- 2-arachidonoyl-lysophosphatidylcholine
- SUV
- small unilamellar vesicle
- AMPP
- N-(4-aminomethylphenyl)-pyridinium.
References
- 1. Patil V. A., and Greenberg M. L. (2013) Cardiolipin-mediated cellular signaling. Adv. Exp. Med. Biol. 991, 195–213 [DOI] [PubMed] [Google Scholar]
- 2. Shen Z., Li Y., Gasparski A. N., Abeliovich H., and Greenberg M. L. (2017) Cardiolipin regulates mitophagy through the PKC pathway. J. Biol. Chem. 292, 2916–2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Raja V., Joshi A. S., Li G., Maddipati K. R., and Greenberg M. L. (2017) Loss of cardiolipin leads to perturbation of acetyl-CoA synthesis. J. Biol. Chem. 292, 1092–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ren M., Phoon C. K., and Schlame M. (2014) Metabolism and function of mitochondrial cardiolipin. Prog. Lipid. Res. 55, 1–16 [DOI] [PubMed] [Google Scholar]
- 5. Acehan D., Malhotra A., Xu Y., Ren M., Stokes D. L., and Schlame M. (2011) Cardiolipin affects the supramolecular organization of ATP synthase in mitochondria. Biophys. J. 100, 2184–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Paradies G., Paradies V., De Benedictis V., Ruggiero F. M., and Petrosillo G. (2014) Functional role of cardiolipin in mitochondrial bioenergetics. Biochim. Biophys. Acta 1837, 408–417 [DOI] [PubMed] [Google Scholar]
- 7. Claypool S. M., and Koehler C. M. (2012) The complexity of cardiolipin in health and disease. Trends. Biochem. Sci. 37, 32–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Claypool S. M. (2009) Cardiolipin, a critical determinant of mitochondrial carrier protein assembly and function. Biochim. Biophys. Acta 1788, 2059–2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schlame M., and Ren M. (2006) Barth syndrome, a human disorder of cardiolipin metabolism. FEBS Lett. 580, 5450–5455 [DOI] [PubMed] [Google Scholar]
- 10. Gaspard G. J., and McMaster C. R. (2015) Cardiolipin metabolism and its causal role in the etiology of the inherited cardiomyopathy Barth syndrome. Chem. Phys. Lipids. 193, 1–10 [DOI] [PubMed] [Google Scholar]
- 11. Raja V., and Greenberg M. L. (2014) The functions of cardiolipin in cellular metabolism-potential modifiers of the Barth syndrome phenotype. Chem. Phys. Lipids. 179, 49–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ye C., Lou W., Li Y., Chatzispyrou I. A., Hüttemann M., Lee I., Houtkooper R. H., Vaz F. M., Chen S., and Greenberg M. L. (2014) Deletion of the cardiolipin-specific phospholipase Cld1 rescues growth and life span defects in the tafazzin mutant: implications for Barth syndrome. J. Biol. Chem. 289, 3114–3125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xu Y., Phoon C. K., Berno B., D'Souza K., Hoedt E., Zhang G., Neubert T. A., Epand R. M., Ren M., and Schlame M. (2016) Loss of protein association causes cardiolipin degradation in Barth syndrome. Nat. Chem. Biol. 12, 641–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schlame M., Rua D., and Greenberg M. L. (2000) The biosynthesis and functional role of cardiolipin. Prog. Lipid. Res. 39, 257–288 [DOI] [PubMed] [Google Scholar]
- 15. Schlame M., and Greenberg M. L. (2017) Biosynthesis, remodeling and turnover of mitochondrial cardiolipin. Biochim. Biophys. Acta 1862, 3–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Houtkooper R. H., Turkenburg M., Poll-The B. T., Karall D., Pérez-Cerdá C., Morrone A., Malvagia S., Wanders R. J., Kulik W., and Vaz F. M. (2009) The enigmatic role of tafazzin in cardiolipin metabolism. Biochim. Biophys. Acta 1788, 2003–2014 [DOI] [PubMed] [Google Scholar]
- 17. Baile M. G., Lu Y. W., and Claypool S. M. (2014) The topology and regulation of cardiolipin biosynthesis and remodeling in yeast. Chem. Phys. Lipids 179, 25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Paradies G., Petrosillo G., Pistolese M., and Ruggiero F. M. (2002) Reactive oxygen species affect mitochondrial electron transport complex I activity through oxidative cardiolipin damage. Gene 286, 135–141 [DOI] [PubMed] [Google Scholar]
- 19. Petrosillo G., Ruggiero F. M., Di Venosa N., and Paradies G. (2003) Decreased complex III activity in mitochondria isolated from rat heart subjected to ischemia and reperfusion: role of reactive oxygen species and cardiolipin. FASEB J. 17, 714–716 [DOI] [PubMed] [Google Scholar]
- 20. Yin H., and Zhu M. (2012) Free radical oxidation of cardiolipin: chemical mechanisms, detection and implication in apoptosis, mitochondrial dysfunction and human diseases. Free Radic. Res. 46, 959–974 [DOI] [PubMed] [Google Scholar]
- 21. Han X., Yang J., Cheng H., Yang K., Abendschein D. R., and Gross R. W. (2005) Shotgun lipidomics identifies cardiolipin depletion in diabetic myocardium linking altered substrate utilization with mitochondrial dysfunction. Biochemistry 44, 16684–16694 [DOI] [PubMed] [Google Scholar]
- 22. Han X., Yang J., Yang K., Zhao Z., Abendschein D. R., and Gross R. W. (2007) Alterations in myocardial cardiolipin content and composition occur at the very earliest stages of diabetes: a shotgun lipidomics study. Biochemistry 46, 6417–6428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rouzer C. A., and Marnett L. J. (2011) Endocannabinoid oxygenation by cyclooxygenases, lipoxygenases, and cytochromes P450: cross-talk between the eicosanoid and endocannabinoid signaling pathways. Chem. Rev. 111, 5899–5921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smith W. L., Urade Y., and Jakobsson P. J. (2011) Enzymes of the cyclooxygenase pathways of prostanoid biosynthesis. Chem. Rev. 111, 5821–5865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brash A. R. (1999) Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. J. Biol. Chem. 274, 23679–23682 [DOI] [PubMed] [Google Scholar]
- 26. Nebert D. W., and Russell D. W. (2002) Clinical importance of the cytochromes P450. Lancet 360, 1155–1162 [DOI] [PubMed] [Google Scholar]
- 27. Kayar S. R., and Banchero N. (1987) Volume density and distribution of mitochondria in myocardial growth and hypertrophy. Respir. Physiol. 70, 275–286 [DOI] [PubMed] [Google Scholar]
- 28. Wheeldon L. W., Schumert Z., and Turner D. A. (1965) Lipid composition of heart muscle homogenate. J. Lipid Res. 6, 481–489 [PubMed] [Google Scholar]
- 29. Pfeiffer K., Gohil V., Stuart R. A., Hunte C., Brandt U., Greenberg M. L., and Schägger H. (2003) Cardiolipin stabilizes respiratory chain supercomplexes. J. Biol. Chem. 278, 52873–52880 [DOI] [PubMed] [Google Scholar]
- 30. Tyurina Y. Y., Poloyac S. M., Tyurin V. A., Kapralov A. A., Jiang J., Anthonymuthu T. S., Kapralova V. I., Vikulina A. S., Jung M.-Y., Epperly M. W., Mohammadyani D., Klein-Seetharaman J., Jackson T. C., Kochanek P. M., Pitt B. R., et al. (2014) A mitochondrial pathway for biosynthesis of lipid mediators. Nat. Chem. 6, 542–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Buland J. R., Wasserloos K. J., Tyurin V. A., Tyurina Y. Y., Amoscato A. A., Mallampalli R. K., Chen B. B., Zhao J., Zhao Y., Ofori-Acquah S., Kagan V. E., and Pitt B. R. (2016) Biosynthesis of oxidized lipid mediators via lipoprotein-associated phospholipase A2 hydrolysis of extracellular cardiolipin induces endothelial toxicity. Am. J. Physiol. Lung Cell. Mol. Physiol. 311, L303–L316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Daniels S. B., Cooney E., Sofia M. J., Chakravarty P. K., and Katzenellenbogen J. A. (1983) Haloenol lactones. Potent enzyme-activated irreversible inhibitors for α-chymotrypsin. J. Biol. Chem. 258, 15046–15053 [PubMed] [Google Scholar]
- 33. Balsinde J., and Dennis E. A. (1996) Bromoenol lactone inhibits magnesium-dependent phosphatidate phosphohydrolase and blocks triacylglycerol biosynthesis in mouse P388D1 macrophages. J. Biol. Chem. 271, 31937–31941 [DOI] [PubMed] [Google Scholar]
- 34. Song H., Ramanadham S., Bao S., Hsu F. F., and Turk J. (2006) A bromoenol lactone suicide substrate inactivates group VIA phospholipase A2 by generating a diffusible bromomethyl keto acid that alkylates cysteine thiols. Biochemistry 45, 1061–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mancuso D. J., Sims H. F., Han X., Jenkins C. M., Guan S. P., Yang K., Moon S. H., Pietka T., Abumrad N. A., Schlesinger P. H., and Gross R. W. (2007) Genetic ablation of calcium-independent phospholipase A2γ leads to alterations in mitochondrial lipid metabolism and function resulting in a deficient mitochondrial bioenergetic phenotype. J. Biol. Chem. 282, 34611–34622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mancuso D. J., Han X., Jenkins C. M., Lehman J. J., Sambandam N., Sims H. F., Yang J., Yan W., Yang K., Green K., Abendschein D. R., Saffitz J. E., and Gross R. W. (2007) Dramatic accumulation of triglycerides and precipitation of cardiac hemodynamic dysfunction during brief caloric restriction in transgenic myocardium expressing human calcium-independent phospholipase A2γ. J. Biol. Chem. 282, 9216–9227 [DOI] [PubMed] [Google Scholar]
- 37. Gross R. W., and Sobel B. E. (1979) Augmentation of cardiac phospholipase activity induced with negative liposomes. Trans. Assoc. Am. Physicians 92, 136–147 [PubMed] [Google Scholar]
- 38. Hsu F. F., Turk J., Rhoades E. R., Russell D. G., Shi Y., and Groisman E. A. (2005) Structural characterization of cardiolipin by tandem quadrupole and multiple-stage quadrupole ion-trap mass spectrometry with electrospray ionization. J. Am. Soc. Mass. Spectrom. 16, 491–504 [DOI] [PubMed] [Google Scholar]
- 39. Moon S. H., Mancuso D. J., Sims H. F., Liu X., Nguyen A. L., Yang K., Guan S., Dilthey B. G., Jenkins C. M., Weinheimer C. J., Kovacs A., Abendschein D., and Gross R. W. (2016) Cardiac myocyte-specific knock-out of calcium-independent phospholipase A2γ (iPLA2γ) decreases oxidized fatty acids during ischemia/reperfusion and reduces infarct size. J. Biol. Chem. 291, 19687–19700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu X., Moon S. H., Jenkins C. M., Sims H. F., and Gross R. W. (2016) Cyclooxygenase-2 mediated oxidation of 2-arachidonoyl-lysophospholipids identifies unknown lipid signaling pathways. Cell Chem. Biol. 23, 1217–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moon S. H., Jenkins C. M., Liu X., Guan S., Mancuso D. J., and Gross R. W. (2012) Activation of mitochondrial calcium-independent phospholipase A2γ (iPLA2γ) by divalent cations mediating arachidonate release and production of downstream eicosanoids. J. Biol. Chem. 287, 14880–14895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pfeifer P. M., and McCay P. B. (1972) Reduced triphosphopyridine nucleotide oxidase-catalyzed alterations of membrane phospholipids. VI. Structural changes in mitochondria associated with inactivation of electron transport activity. J. Biol. Chem. 247, 6763–6769 [PubMed] [Google Scholar]
- 43. Narabayashi H., Takeshige K., and Minakami S. (1982) Alteration of inner-membrane components and damage to electron-transfer activities of bovine heart submitochondrial particles induced by NADPH-dependent lipid peroxidation. Biochem. J. 202, 97–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fauland A., Trötzmüller M., Eberl A., Afiuni-Zadeh S., Köfeler H., Guo X., and Lankmayr E. (2013) An improved SPE method for fractionation and identification of phospholipids. J. Sep. Sci. 36, 744–751 [DOI] [PubMed] [Google Scholar]
- 45. Sparagna G. C., Chicco A. J., Murphy R. C., Bristow M. R., Johnson C. A., Rees M. L., Maxey M. L., McCune S. A., and Moore R. L. (2007) Loss of cardiac tetralinoleoyl cardiolipin in human and experimental heart failure. J. Lipid Res. 48, 1559–1570 [DOI] [PubMed] [Google Scholar]
- 46. Kiebish M. A., Han X., Cheng H., Chuang J. H., and Seyfried T. N. (2008) Cardiolipin and electron transport chain abnormalities in mouse brain tumor mitochondria: lipidomic evidence supporting the Warburg theory of cancer. J. Lipid Res. 49, 2545–2556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Patwardhan A. M., Scotland P. E., Akopian A. N., and Hargreaves K. M. (2009) Activation of TRPV1 in the spinal cord by oxidized linoleic acid metabolites contributes to inflammatory hyperalgesia. Proc. Natl. Acad. Sci. U.S.A. 106, 18820–18824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Alsalem M., Wong A., Millns P., Arya P. H., Chan M. S., Bennett A., Barrett D. A., Chapman V., and Kendall D. A. (2013) The contribution of the endogenous TRPV1 ligands 9-HODE and 13-HODE to nociceptive processing and their role in peripheral inflammatory pain mechanisms. Br. J. Pharmacol. 168, 1961–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Green D., Ruparel S., Gao X., Ruparel N., Patil M., Akopian A., and Hargreaves K. (2016) Central activation of TRPV1 and TRPA1 by novel endogenous agonists contributes to mechanical allodynia and thermal hyperalgesia after burn injury. Mol. Pain. 12, 1744806916661725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Obinata H., Hattori T., Nakane S., Tatei K., and Izumi T. (2005) Identification of 9-hydroxyoctadecadienoic acid and other oxidized free fatty acids as ligands of the G protein-coupled receptor G2A. J. Biol. Chem. 280, 40676–40683 [DOI] [PubMed] [Google Scholar]
- 51. Mancuso D. J., Jenkins C. M., and Gross R. W. (2000) The genomic organization, complete mRNA sequence, cloning, and expression of a novel human intracellular membrane-associated calcium-independent phospholipase A2. J. Biol. Chem. 275, 9937–9945 [DOI] [PubMed] [Google Scholar]
- 52. Kim J., and Hoppel C. L. (2011) Monolysocardiolipin: improved preparation with high yield. J. Lipid Res. 52, 389–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bollinger J. G., Thompson W., Lai Y., Oslund R. C., Hallstrand T. S., Sadilek M., Turecek F., and Gelb M. H. (2010) Improved sensitivity mass spectrometric detection of eicosanoids by charge reversal derivatization. Anal. Chem. 82, 6790–6796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liu X., Moon S. H., Mancuso D. J., Jenkins C. M., Guan S., Sims H. F., and Gross R. W. (2013) Oxidized fatty acid analysis by charge-switch derivatization, selected reaction monitoring, and accurate mass quantitation. Anal. Biochem. 442, 40–50 [DOI] [PMC free article] [PubMed] [Google Scholar]