Abstract
Transglutaminases (TGs) play essential intracellular and extracellular roles by covalently cross-linking many proteins. Drosophila TG is encoded by one gene and has two alternative splicing-derived isoforms, TG-A and TG-B, which contain distinct N-terminal 46- and 38-amino acid sequences, respectively. The TGs identified to date do not have a typical endoplasmic reticulum (ER)-signal peptide, and the molecular mechanisms of their secretion under physiologic conditions are unclear. Immunocytochemistry revealed that TG-A localizes to multivesicular-like structures, whereas TG-B localizes to the cytosol. We also found that TG-A, but not TG-B, was modified concomitantly by N-myristoylation and S-palmitoylation, and N-myristoylation was a pre-requisite for S-palmitoylation. Moreover, TG-A, but not TG-B, was secreted in response to calcium signaling induced by Ca2+ ionophores and uracil, a pathogenic bacteria-derived substance. Brefeldin A and monensin, inhibitors of the ER/Golgi-mediated conventional pathway, did not suppress TG-A secretion, whereas inhibition of S-palmitoylation by 2-bromopalmitate blocked TG-A secretion. Ultracentrifugation, electron microscopy analyses, and treatments with inhibitors of multivesicular body formation revealed that TG-A was secreted via exosomes together with co-transfected mammalian CD63, an exosomal marker, and the secreted TG-A was taken up by other cells. The 8-residue N-terminal fragment of TG-A containing the fatty acylation sites was both necessary and sufficient for the exosome-dependent secretion of TG-A. In conclusion, TG-A is secreted through an unconventional ER/Golgi-independent pathway involving two types of fatty acylations and exosomes.
Keywords: Drosophila, exosome (vesicle), protein myristoylation, protein palmitoylation, protein secretion, transglutaminase
Introduction
N-Myristoylation and S-palmitoylation are two major forms of protein fatty acylations that are 14-carbon- and 16-carbon-saturated fatty acid modifications, respectively (1, 2). These modifications increase the hydrophobicity of proteins; accelerate interactions between the modified proteins and plasma membrane; and control a wide range of cellular processes, such as intracellular trafficking and signaling activities (1, 2). N-Myristoylation occurs co-translationally on the glycine residue after cleavage of the initiation methionine by methionine aminopeptidase with the consensus sequence Met-Gly-X-X-X-Ser/Thr/Cys-, or post-translationally when an internal glycine becomes exposed by caspase-mediated cleavage (1, 2). Protein N-myristoylation is catalyzed by a cytosolic enzyme, N-myristoyltransferase (NMT),2 which is conserved from yeast to mammals and ubiquitously expressed in every cell type (3–5). Unlike N-myristoylation, S-palmitoylation is reversible under physiologic conditions and is dynamically regulated in the presence of thioesterases that depend on extracellular signals (2, 6–8). Therefore, S-palmitoylation and depalmitoylation of proteins promote the switching on and off of membrane trafficking (9). S-Palmitoylation is mediated by membrane-bound protein palmitoyl acyltransferases, whose catalytic site localizes on the cytoplasmic side of the endoplasmic reticulum (ER) or Golgi membranes (10–12). No consensus sequence for S-palmitoylation has been identified in protein substrates. Although many fatty-acylated proteins have been identified and are associated with various cellular phenomena, the physiologic functions and regulation mechanisms of the fatty acylations remain to be explored.
Transglutaminases (TGs) are enzymes that catalyze protein-protein covalent cross-linking between Lys and Gln residues to form ϵ-(γ-glutamyl)lysine bonds and promote blood/hemolymph coagulation and skin/cuticle formation, and control signal transduction (13–15). TGs are present intracellularly and extracellularly, and their substrates are ubiquitously distributed in metazoans (16, 17). Soluble secretory proteins with the N-terminal signal sequence for secretion are secreted into the extracellular space through the conventional ER/Golgi-dependent secretory pathway (18). Mammals have eight TG isoforms and none of the isoforms contain a typical N-terminal or internal signal sequence, indicating that TG isoforms are secreted through an unconventional secretory pathway (16).
Drosophila melanogaster has a single TG gene (FlyBase ID FBgn0031975), and Drosophila TG is involved in hemolymph coagulation (19), cuticle formation (20, 21), and regulation of the immune deficiency pathway in the gut (22). For example, hemolymph proteins, such as fondue and hemolectin, cross-linked by Drosophila TG form clots to stop bleeding and trap invading pathogenic microbes (19, 23), and cross-linked drosocrystallins on the peritrophic matrix, a semi-permeable barrier in insects, form a stabilized fiber structure against toxic proteases released by orally infectious pathogenic bacteria (24). Moreover, Drosophila TG polymerizes and inactivates the nuclear factor-κB-like transcription factor Relish to maintain gut homeostasis by inhibiting the production of antimicrobial peptides induced by commensal bacteria (22). Therefore, Drosophila TG functions intracellularly as well as extracellularly.
Exosomes are extracellular microvesicles 30–120 nm in size that are produced in multivesicular bodies (MVBs) and released into the blood, urine, and other body fluids following fusion of the outer membrane of the MVBs with the plasma membrane (25). Exosomes containing various proteins, such as heat shock proteins and growth factors, and/or mRNAs and microRNAs, are secreted by a variety of cells, and are directly fused with recipient cells or internalized by endocytosis or phagocytosis (26). The exosome-dependent secretion pathway is classified as a non-conventional protein secretion pathway because most exosome-containing proteins do not have the N-terminal signal peptide required for secretion by the ER/Golgi-dependent secretion pathway. The exosome-dependent secretion pathway plays pivotal roles in homeostasis in organisms (25, 27).
In Drosophila, the TG gene is estimated to generate two variant mRNAs by alternative splicing, designated TG-A and TG-B. Here we show that TG-A and TG-B are differentially expressed depending on the developmental stage and tissue, and that the two types of fatty acylations, N-myristoylation and S-palmitoylation, of TG-A are essential for selective recruitment into exosomes and secretion in response to external stimuli under physiologic conditions. To our knowledge, this is the first report in multicellular organisms that a protein synthesized by free ribosomes in the cytosol is secreted through an unconventional transport pathway containing these two types of fatty acid modifications and exosomes.
Results
TG-A is an N-myristoylated protein
The estimated isoforms, TG-A and TG-B, share identical sequences in their C-terminal regions and contain distinct 46- and 38-amino acid sequences, respectively, in their N-terminal regions (Fig. 1A). To examine the expression of these isoforms in vivo, their mRNA amounts were quantitated by real-time reverse transcription polymerase chain reaction (PCR). The amount of TG-B mRNA was 5 to 10 times greater than that of TG-A mRNA at all developmental stages (supplemental Fig. S1A). Moreover, real-time PCR analyses of tissue-specific expression revealed significantly higher levels of both TG-A and TG-B in the tissues examined, with the highest levels in the crop (supplemental Fig. S1B).
Figure 1.
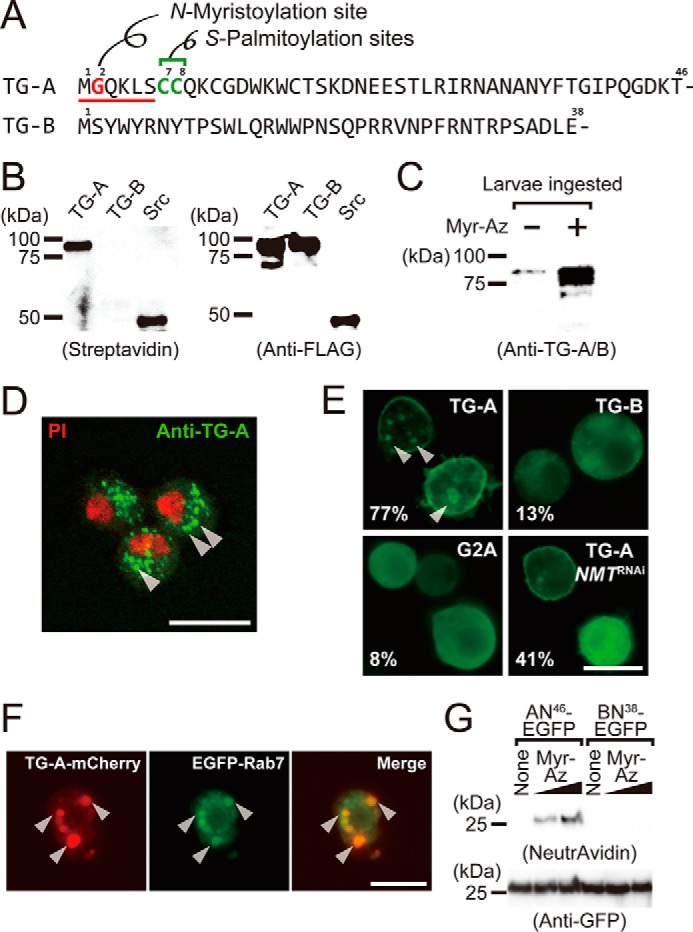
TG-A is a target for N-myristoylation. A, the N-terminal sequences of TG-A and TG-B. The N-myristoyl consensus sequence is underlined, and the N-myristoylated glycine residue and the S-palmitoylation sites are shown in red and green, respectively. B, the N-myristoylated proteins were detected by streptavidin blotting (left) and the expressed proteins were checked by Western blotting using the anti-FLAG antibody (right). C, third instar larvae (w1118) ingested myristic acid-azide and the resulting N-myristoylated proteins were labeled with biotin. Proteins purified on avidin-immobilized agarose were analyzed by Western blotting using the anti-TG-A/B antibody. D, hemocytes of third instar larvae were analyzed by immunocytochemistry using the anti-TG-A-specific antibody (green). PI, propidium iodide (red). Arrowheads indicate puncta structures. The scale bar in white is 10 μm. E, Drosophila S2 cells expressing TG-A, TG-B, or G2A tagged with the C-terminal V5-His6 tag were analyzed by immunocytochemistry using the anti-His6 tag antibody. The percentages of cells with the plasma membrane-localized signal are shown (n = 300). Arrowheads indicate puncta structures. The scale bar in white is 10 μm. F, S2 cells were co-transfected with the C-terminal mCherry (red)-tagged TG-A and the N-terminal EGFP (green)-tagged Rab7 and analyzed by fluorescence microscopy. G, S2 cells expressing AN46-EGFP and BN38-EGFP were incubated with myristic acid-azide (8 or 80 μm) and analog-incorporated proteins were labeled with biotin alkyne using click chemistry. The resulting proteins were purified using anti-GFP-agarose, and detected using NeutrAvidin-horseradish peroxidase. Myr-Az, myristic acid-azide.
TG-A, but not TG-B, had the N-myristoyl consensus sequence Met-Gly-X-X-X-Thr/Ser/Cys- (Fig. 1A). To examine whether TG-A is a potential substrate of N-myristoylation, we expressed TG-A or TG-B in a cell-free protein-expression system prepared from Spodoptera frugiperda (Sf) 21 cells, suitable for analyzing post-translational modifications such as prenylation (28), geranylgeranylation (29), disulfide bond formation (30, 31), and also co-translational modification of N-myristoylation (32). The two isoforms were expressed in the presence of myristic acid-azide, an analog of myristic acid, in a cell-free system, and the resulting proteins incorporating myristic acid-azide were reacted with a biotin-labeled alkyne (tetraethylene glycol carboxamide-propargyl biotin) by click chemistry for detection by streptavidin blotting (supplemental Fig. S2A). TG-A, but not TG-B, was clearly detected by streptavidin-horseradish peroxidase, suggesting that TG-A, but not TG-B, is the N-myristoylation target (Fig. 1B).
To confirm the N-myristoylation of TG-A in vivo, wild-type fly larvae were fed the myristic acid analog, and N-myristoylated proteins in the larval lysates after biotinylation by click chemistry were isolated using avidin-immobilized agarose for Western blotting analysis with a polyclonal antibody that recognizes both TG-A and TG-B (anti-TG-A/B antibody). N-Myristoylation of the TG antigen was detected specifically in a myristic acid analog-dependent manner (Fig. 1C). These findings demonstrated that TG-A, but not TG-B, is a physiologic target of N-myristoylation.
Subcellular localization of TG-A and TG-B
To determine the localization of TG-A in vivo, immunocytochemistry of Drosophila hemocytes was performed using a polyclonal antibody prepared against a synthetic peptide of the N-terminal sequence of TG-A (anti-TG-A-specific antibody). The TG-A antigen was present on vesicle-like puncta in the hemocytes, indicating that TG-A is stored in intracellular vesicles such as MVBs (Fig. 1D, arrowheads). To examine the specificity of the TG-A-specific antibody, an expression vector containing a TG-A or TG-B construct tagged with a C-terminal FLAG was transformed into S2 cells expressing neither TG-A nor TG-B. The anti-TG-A-specific antibody recognized MVB-like structures in S2 cells expressing TG-A-FLAG, but not those in S2 cells expressing TG-B-FLAG, indicating the high specificity of the anti-TG-A-specific antibody against the TG-A antigen (supplemental Fig. S2B).
To confirm the relationship between fatty acid modification and cellular localization of TG-A, an expression vector containing a TG-A or TG-B construct fused with a C-terminal His6 tag was transformed into S2 cells. Immunocytochemistry using an antibody against the C-terminal His6 tag showed TG-A localization in MVB-like structures (Fig. 1E, left upper, arrowheads). In contrast, TG-B was expressed in the cytosol (Fig. 1E, right upper), consistent with the previous observation of the subcellular localization of TG in the Drosophila gut (22). Localization of TG-A in the MVB-like structures was significantly decreased in S2 cells with NMT gene knockdown (Fig. 1E, right lower). In N-myristoylation, the N-terminal Gly residue of synthesizing proteins in the cytosol is essential for co-translational modification by NMT after removal of the initiation Met residue (1, 2). Therefore, TG-A with the Gly-to-Ala replacement at the second position (G2A) was expressed in S2 cells to determine the consensus sequence-dependent N-myristoylation. G2A exhibited subcellular localization in the cytosol, and was not transported to the MVBs (Fig. 1E, left lower).
To examine whether TG-A localizes in MVBs, Rab7 tagged with an N-terminal enhanced green fluorescent protein (EGFP), which is a marker protein for late endosomes/MVBs (33, 34), and TG-A tagged with a C-terminal mCherry were co-expressed in S2 cells. Both EGFP-Rab7 and TG-A-mCherry were co-localized in the vesicle-like puncta in S2 cells, indicating that TG-A is localized in the MVBs (Fig. 1F). Subcellular fractionation of S2 cells expressing TG-A or TG-B using ultracentrifugation also demonstrated major subcellular localization of TG-A in the membrane fraction containing vesicular structures (supplemental Fig. S2C) and major subcellular localization of TG-B in the cytosol fraction (supplemental Fig. S2D).
To examine whether the N-terminal region of TG-A contains information for its subcellular localization, the N-terminal fragment of TG-A (46 residues tagged with EGFP: AN46-EGFP) or that of TG-B (38 residues tagged with EGFP: BN38-EGFP) as a negative control, was expressed in S2 cells. As expected, AN46-EGFP localized in the MVBs (supplemental Fig. S2E, left, arrowheads), and BN38-EGFP localized in the cytosol (supplemental Fig. S2E, middle). Moreover, the N-terminal fragment of TG-A with the Gly-to-Ala replacement (G2A-AN46-EGFP) exhibited cytosolic localization (supplemental Fig. S2E, right). To confirm the N-myristoylation of AN46-EGFP, AN46-EGFP-expressing S2 cells were incubated with myristic acid-azide, and the resulting cell lysates were purified using anti-GFP-agarose and reacted with a biotin-labeled alkyne propargyl biotin by click chemistry for detection by avidin blotting. The myristic acid-azide-dependent band of AN46-EGFP, but not BN38-EGFP, was detected by avidin blotting (Fig. 1G). These findings clearly indicate that the TG-A-specific N-terminal region, consisting of 46 residues, is both necessary and sufficient to determine the subcellular localization of TG-A in the MVBs.
TG-A is also modified by S-palmitoylation
In addition to N-myristoylation, several proteins, such as the α-subunit of G-protein and Src family kinases, are S-palmitoylated (33–35). Multiple S-palmitoylations controlled by both palmitoyl acyl-transferases and thioesterases regulate dynamic cellular processes, including membrane trafficking, lipid raft targeting, and intracellular signaling cascades, through modulation of the protein hydrophobicity (7). To examine the presence of S-acylation in TG-A in vivo, a biotin-switch assay was performed using lysates of adult flies. In this assay, the total proteins were treated sequentially with N-ethylmaleimide to block free sulfhydryl groups and then with hydroxylamine (HA) to remove thioester-linked fatty acids, followed by incubation with a reactive thiol-linked biotinylated reagent (HPDP-biotin; supplemental Fig. S3A). The resulting biotin-labeled TG antigen was detected in the HA-treated sample, but not in the HA-untreated sample (Fig. 2A), indicating that TG-A is a target for not only N-myristoylation, but also S-acylation. To determine whether TG-A is S-acylated in cultured cells, TG-A and TG-B were expressed in S2 cells and Sf-9 cells, and subjected to the biotin-switch assay for S-acylation. The S-acylation of TG-A, but not TG-B, was detected in both S2 cells and Sf-9 cells in the presence of HA (Fig. 2B). Moreover, 2-bromopalmitate, an S-palmitoylation inhibitor, decreased the S-acylation of TG-A in S2 cells (supplemental Fig. S3B), indicating that TG-A is not only N-myristoylated, but also S-palmitoylated. In the same manner, AN46-EGFP, but not BN38-EGFP, was confirmed to be S-acylated in S2 cells (supplemental Fig. S3C). Again, AN46-EGFP or BN38-EGFP-expressing S2 cells were incubated with palmitic acid/azide and analyzed by click chemistry for detection by avidin blotting. The palmitic acid/azide-dependent band of AN46-EGFP, but not BN38-EGFP, was detected by avidin blotting (Fig. 2C).
Figure 2.
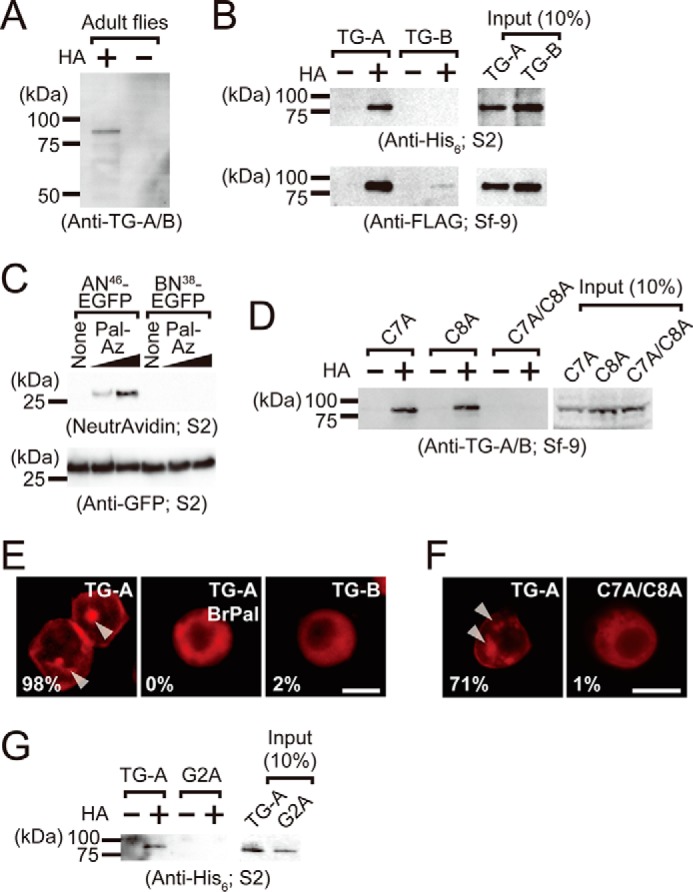
TG-A is a target for S-palmitoylation. A, the biotin-switch assay for lysates of adult w1118 flies. Proteins that precipitated on avidin-immobilized agarose after the biotin-switch assay were detected by Western blotting using the anti-TG-A/B antibody. B, the biotin-switch assay for lysates of cells expressing the C-terminal V5-His6-tagged TG-A and TG-B (S2 cells) and the C-terminal FLAG-tagged TG-A and TG-B (Sf-9 cells). Proteins that precipitated on avidin-immobilized agarose after the biotin-switch assay were detected by Western blotting using the anti-His6 tag and anti-FLAG-tagged antibodies, respectively. C, S2 cells expressing AN46-EGFP and BN38-EGFP were incubated with palmitic acid/azide (8 or 80 μm) and analog-incorporated proteins were labeled with biotin alkyne using click chemistry. The resulting proteins were purified using anti-GFP-agarose, and detected using NeutrAvidin-horseradish peroxidase. Pal-Az, palmitic acid/azide. D, the biotin-switch assay for lysates of Sf-9 cells expressing the C-terminal V5-His6-tagged C7A, C8A, and C7A/C8A. Proteins that precipitated on avidin-immobilized agarose after the biotin-switch assay were detected by Western blotting using the anti-TG-A/B antibody. E and F, the C-terminal FLAG-tagged TG-expressing Sf-9 cells (E) and the C-terminal V5-His6-tagged TG-expressing S2 cells (F) were analyzed by immunocytochemistry using the anti-FLAG antibody and anti-V5 antibody, respectively. The percentage of cells with the MVB-localized signal are shown (E, n = 50; F, n = 100). The scale bar in white is 10 μm. G, The biotin-switch assay for lysates of S2 cells expressing the C-terminal V5-His6-tagged TG-A and G2A. Proteins that precipitated on avidin-immobilized agarose after the biotin-switch assay were detected by Western blotting using the anti-His6 tag antibody.
Cysteine residues at the N-terminal region of protein substrates are potential sites for S-palmitoylation (9), and two cysteine residues exist at the 7 and 8 positions of TG-A, but not TG-B (Fig. 1A). A single amino acid mutation of TG-A with a Cys-to-Ala replacement at position 7 (C7A) or 8 (C8A) was positive for S-acylation by the biotin-switch assay in the presence of HA in Sf-9 cells, but the double mutation at both residues (C7A/C8A) was not, indicating that both Cys7 and Cys8 are S-palmitoylation sites (Fig. 2D). To elucidate the physiologic importance of the S-palmitoylation of TG-A, we examined subcellular localization of TG-A in the presence of 2-bromopalmitate (Fig. 2E). Immunocytochemistry against TG-A or TG-B expressed in Sf-9 cells using the anti-FLAG antibody revealed that 2-bromopalmitate completely blocked the transportation of TG-A to the MVBs (indicated by arrowheads in Fig. 2E). Moreover, a non-palmitoylated mutant, C7A/C8A, localized in the cytosol in S2 cells (Fig. 2F). These findings clearly suggested that both N-myristoylation and S-palmitoylation are essential for TG-A localization in the MVBs.
S-Palmitoylation increases the hydrophobicity of TG-A
To assess whether the hydrophobicity of TG-A is enhanced by S-palmitoylation, the supernatant of cell lysates of Sf-9 cells expressing the C-terminal FLAG-tagged TG-A was mixed with butyl-Sepharose 4B, and the bound fractions were obtained by stepwise elution with several concentrations of NaCl and 1% SDS (supplemental Fig. S3D). Treatment of cell lysates with HA diminished the binding activity of TG-A with butyl-Sepharose 4B (supplemental Fig. S3D), indicating that the hydrophobicity of TG-A is mainly endowed by S-palmitoylation.
N-Myristoylation is a prerequisite for S-palmitoylation
S-Acylated proteins, including the α-subunit of G proteins and Src family kinases, are both N-myristoylated and S-palmitoylated, and N-myristoylation is considered essential for the subsequent S-palmitoylation (33–39). To determine whether N-myristoylation is required for S-palmitoylation in TG-A, the non-myristoylation mutants G2A and G2A-AN46-EGFP were expressed in S2 cells. The biotin-switch assay was negative for both G2A (Fig. 2G) and G2A-AN46-EGFP (supplemental Fig. S3E), indicating that N-myristoylation was pre-requisite for the S-palmitoylation of TG-A. Moreover, S-palmitoylation was significantly decreased in AN46-EGFP-expressing S2 cells treated with NMT-RNA interference, indicating that TG-A requires protein acylation in the correct order: first N-myristoylation and then S-palmitoylation (supplemental Fig. S3F).
Both types of fatty acid modifications are necessary for TG-A secretion
TGs function not only intracellularly, but also extracellularly (23). The TGs identified to date, however, do not have the typical N-terminal or internal signal sequence for secretion, suggesting the presence of an ER/Golgi-independent secretion pathway for TGs. In horseshoe crabs, TGs localized intracellularly in hemocytes are released into the extracellular space in response to stimulation by lipopolysaccharides of Gram-negative bacteria (40). To elucidate whether bacteria stimulate the secretion of TG-A or TG-B from hemocytes in Drosophila, hemocytes were collected from third instar larvae and Gram-negative bacteria Erwinia carotovora carotovora 15 (Ecc15), an opportunistic pathogen for Drosophila, was added to the hemocytes. Western blotting using the anti-TG-A/B antibody showed that the TG antigen was secreted into the extracellular fluid in a time-dependent manner (Fig. 3A, upper and lower). To examine the secretion of TG-A or TG-B in response to stimulation by Ecc15, S2 cells expressing TG-A or TG-B were treated with Ecc15. TG-A, but not TG-B, was secreted from S2 cells in an Ecc15-dependent manner (Fig. 3B, upper and lower). The secretion of TG-A was inhibited by 2-bromopalmitate, indicating that S-palmitoylation is essential for TG-A secretion (Fig. 3C). Notably, heat-killed Ecc15 or microbe-associated molecular patterns, including lipopolysaccharides and peptidoglycans, had no effect on TG-A secretion (Fig. 3, D and E). These findings suggest that a substance(s) derived from living bacteria, but not bacteria cell wall components, plays a key role in the bacteria-dependent TG-A secretion.
Figure 3.
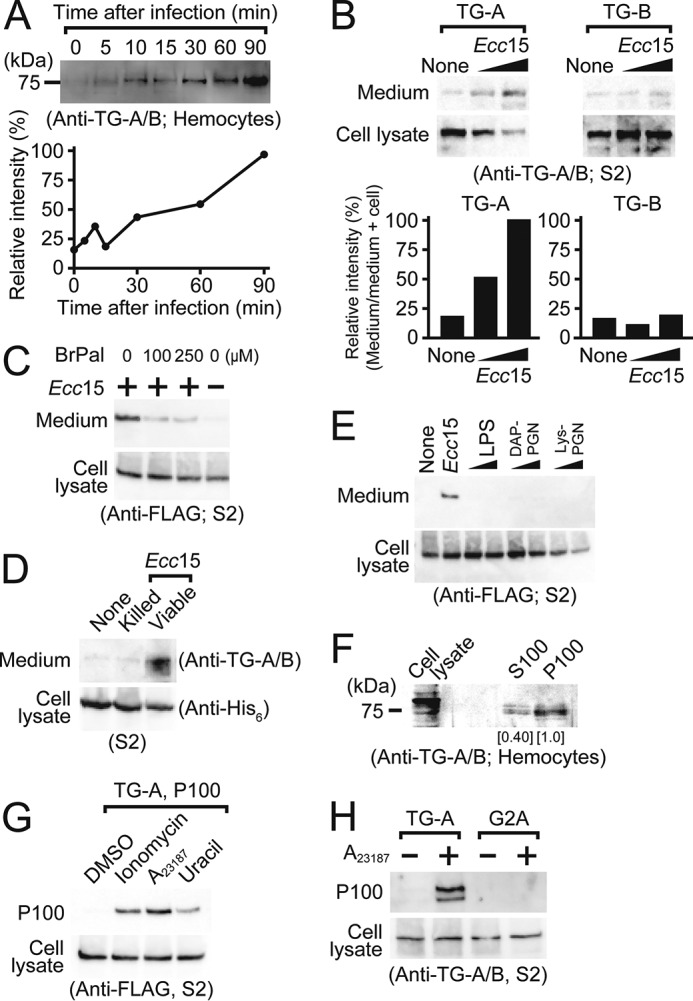
TG is secreted in response to stimulation by Ecc15. A, TG-A or TG-B antigen secreted from hemocytes after treatment with Ecc15 was detected by Western blotting using the anti-TG-A/B antibody. The band intensity at 90 min after infection was defined as 100%. Line graph shows the band intensity analyzed by ImageJ software. B-D, Ecc15 was added to the C-terminal V5-His6-tagged TG-expressing S2 cells (B and D) or FLAG-tagged TG-A-expressing S2 cells (C), and cell lysates or the conditioned media were analyzed by Western blotting using the anti-TG-A/B (B and D), anti-FLAG (C), and anti-His6 tag (D) antibodies, respectively. Bar graph shows the band intensity analyzed by ImageJ software (B). E, lipopolysaccharides or peptidoglycans were added to the C-terminal FLAG-tagged TG-A-expressing S2 cells and cultured supernatants were analyzed by Western blotting using the anti-FLAG antibody. LPS, lipopolysaccharide; DAP-PGN, diaminopimelic acid-type peptidoglycan; Lys-PGN, lysine-type peptidoglycan. One and 10 μg/ml of bacteria cell components were used. F, the supernatant from Ecc15-stimulated hemocytes was separated into the soluble (S100) and precipitated (P100) fractions by ultracentrifugation at 100,000 × g and analyzed by Western blotting using the anti-TG-A/B antibody. Numbers show the band intensity analyzed by ImageJ software, and the intensity of the P100 fraction was set to 1.0. G, A23187, ionomycin (1 μm each), or 20 nm uracil was added to the C-terminal FLAG-tagged TG-A-expressing S2 cells for 1 h, and the resulting P100 fraction was analyzed by Western blotting using the anti-FLAG antibody. H, C-terminal mCherry-tagged TG-A or G2A-expressing S2 cells were treated with A23187 and analyzed by Western blotting using the anti-TG-A/B antibody.
Moreover, to understand whether secreted TG-A continues to interact with lipid vesicles in the extracellular space after induction by bacterial stimulation, the supernatant of hemocytes infected with Ecc15 was analyzed by ultracentrifugation. Most of the secreted TG-A was precipitated in the P100 fraction after centrifugation (Fig. 3F), suggesting that secreted TG-A was associated with extracellular vesicles such as exosomes or microvesicles. To clarify the details of the secretion mechanism of TG-A, S2 cells were treated with Ca2+ ionophores A23187 and ionomycin because an increase in intracellular Ca2+ is involved in the generation and release of exosomes (41). The experiments clearly revealed that TG-A was secreted upon Ca2+ stimulation (Fig. 3G). In addition, TG-A was secreted in response to stimulation with uracil, a pathogenic bacteria-derived substance, to induce a G protein-mediated Ca2+ influx from the ER to the cytosol in Drosophila (Fig. 3G) (42). Moreover, the G2A mutant was not found in the P100 fraction containing exosomes after stimulation with A23187, indicating that the N-myristoylation at the Gly residue is indispensable for the secretion of TG-A (Fig. 3H).
Secretion of TG-A by an unconventional secretion pathway
The Ca2+-dependent secretion of TG-A was not inhibited by inhibitors of ER/Golgi-dependent protein secretion, brefeldin A or monensin (Fig. 4A). Notably, monensin enhanced TG-A secretion because it also induces Ca2+ entry into the cytosol (Fig. 4A) (43). Under the same conditions, these inhibitors blocked the secretion of GFP fused with an authentic Bip-secretion signal peptide at the N terminus (Fig. 4B). Moreover, AN46-EGFP was also secreted and collected in the P100 fraction by the addition of the Ca2+ ionophores, and the secretion was not inhibited by brefeldin A or monensin, indicating that AN46-EGFP is both necessary and sufficient for secretion of TG-A in S2 cells, and that AN46-EGFP is secreted through an unconventional ER/Golgi-independent pathway (Fig. 4, C and D).
Figure 4.
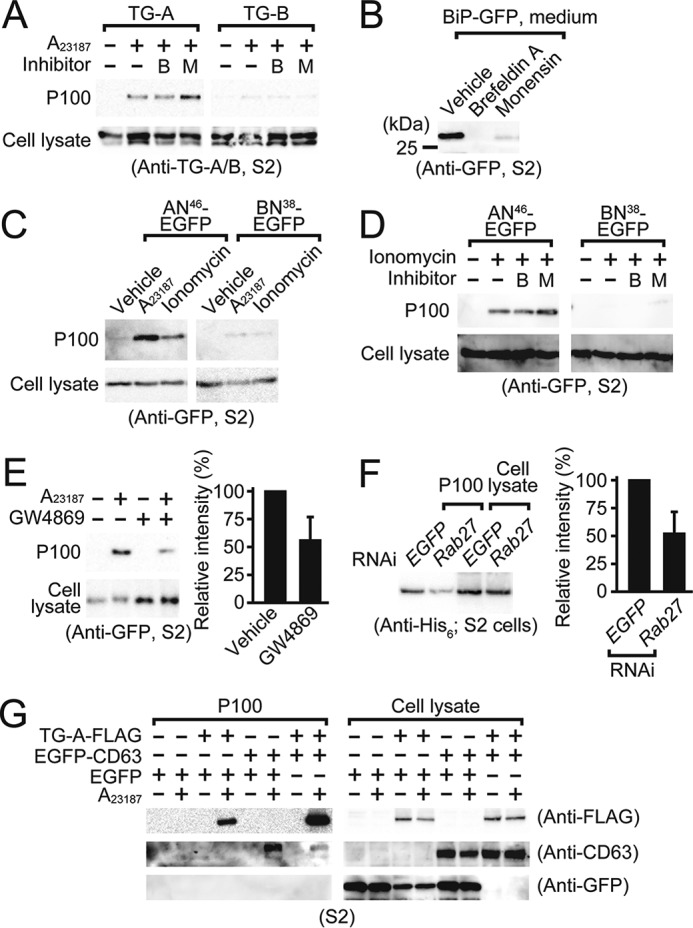
TG-A is secreted in exosomes via an unconventional secretion pathway. A and D, brefeldin A (1 μg/ml, B) or monensin (1 μm, M) were added to the C-terminal FLAG-tagged TG-A, TG-B, AN46-EGFP, or BN38-EGFP-expressing S2 cells, and subsequently treated with A23187 or ionomycin (1 μm each). Cell lysates and the P100 fraction were analyzed by Western blotting using the anti-TG-A/B (A) or anti-GFP tag antibody (D). B, GFP fused with the BiP secretion signal sequence at the N terminus was expressed in S2 cells and treated with brefeldin A (1 μg/ml) or monensin (1 μm). The resulting cultured medium was analyzed by Western blotting using the anti-GFP tag antibody. C, A23187 or ionomycin (1 μm each) were added to the C-terminal EGFP-tagged N-terminal fragment of TG-A and TG-B (AN46-EGFP, BN38-EGFP)-expressing S2 cells for 1 h, and analyzed by Western blotting using the anti-GFP tag antibody. E, the C-terminal EGFP-tagged TG-A-expressing S2 cells were treated with or without 10 μm GW4869 for 40 h, and then with or without 1 μm A23187 for 1 h. Bar graph shows the band intensity analyzed by ImageJ software, and error bars indicate ± S.E. (n = 3). F, the P100 fraction from dsRab27 or dsEGFP (negative control)-treated C-terminal V5-His6-tagged TG-A-expressing S2 cells were analyzed by Western blotting using the anti-His6 tag antibody. Bar graph shows the band intensity analyzed by ImageJ software, and error bars indicate ± S.E. (n = 6). G, the P100 fraction from the C-terminal FLAG-tagged TG-A and/or the C-terminal EGFP-tagged human CD63-expressing S2 cells were analyzed by Western blotting using the anti-FLAG, CD63, or GFP antibody.
TG-A is secreted by exosomes
To determine the vesicle type associated with the secretion of TG-A, we used another reagent, GW4869, which inhibits the ceramide-mediated inward budding of MVBs and the release of mature exosomes from MVBs (44). Treatment of AN46-EGFP or BN38-EGFP-expressing S2 cells with GW4869 inhibited the translocation of AN46-EGFP into the P100 fraction containing exosomes (Fig. 4E). RNA interference of the small GTPase Rab27, which is involved in exosomal release from MVBs (45), decreased the A23187-dependent TG-A secretion from S2 cells (Fig. 4F). Likewise, RNA interference of the small GTPase Rab11, which is involved in exosomal generation in TG-A-expressing S2 cells (46), decreased the A23187-dependent TG-A secretion (supplemental Fig. S4). Moreover, TG-A was secreted together with co-transfected mammalian CD63, an exosomal marker protein (46), concomitant with the absence of any EGFP protein in the P100 fraction (Fig. 4G, right-most lane in the P100 fraction). These findings indicate that TG-A modified with the two types of fatty acids is transported into MVBs to be secreted in exosomes upon an increase in intracellular Ca2+.
Characterization of TG-A-containing exosomes
Exosomes are normally fractionated with densities between 1.13 and 1.19 g/ml by density gradient centrifugation (47). TG-A and CD63-expressing S2 cells were treated with the Ca2+ ionophore A23187 and the P100 fraction of the culture supernatant containing exosomes was further fractionated in a discontinuous iodixanol (OptiPrep) density gradient. Twelve fractions were collected and analyzed by Western blotting. TG-A and CD63 were collected in the fractions with densities between 1.131 and 1.177 (Fig. 5A). To confirm the selective recruitment of TG-A into exosomes, a biotin-switch assay of the TG-A-containing P100 fraction was carried out, which revealed that the secreted TG-A retained the S-acylation modifications (Fig. 5B). These findings suggested that the two types of fatty acid modifications of TG-A remain intact after secretion. Additionally, the TG-A-containing exosome fraction was treated with proteinase K in the presence or absence of Triton X-100 and analyzed by Western blotting using the anti-TG-A/B antibody. The TG-A antigen with a native molecular mass of 80 kDa was retained after treatment with proteinase K in the absence of Triton X-100, but not in the presence of Triton X-100, indicating that TG-A localizes in the inner leaflet of exosomes (Fig. 5C). Moreover, immunoelectron microscopic analysis of TG-A-expressing S2 cells treated with A23187 revealed that TG-A signals were localized in the MVBs, and some gold colloidal signals were observed in the cytosol near the MVBs (Fig. 5D, a and a′). TG-A signals were also observed in ∼100-nm exosomes secreted from the MVBs (Fig. 5D, b and b′). Under these conditions, the intracellular TG-A signals were localized in the MVBs three times more than the cytosol (Fig. 5E).
Figure 5.
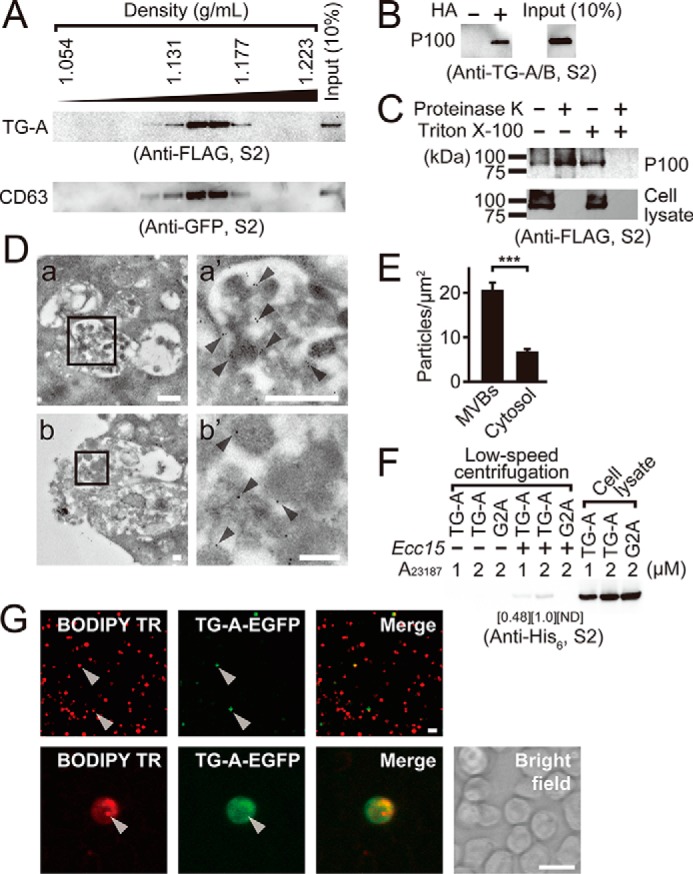
Profiles of TG-A containing exosomes. A, the P100 fraction prepared from A23187-stimulated the C-terminal FLAG tagged-TG-A- and the N-terminal EGFP-tagged-CD63-expressing S2 cells was analyzed by density gradient centrifugation using OptiPrep. B and C, the P100 fraction from A23187-stimulated the C-terminal FLAG tagged-TG-A-expressing S2 cells was analyzed by the biotin-switch assay with or without HA (B), or treated with or without 10 μm proteinase K in the presence or absence of 1% Triton X-100 for 1 h at 37 °C (C). D, immunotransmission electron microscopic analysis of S2 cells expressing the C-terminal FLAG-tagged TG-A. The TG-A-specific antibody was used as a primary antibody and was detected by the anti-rabbit colloidal-gold conjugated secondary antibody. a′ and b′ represent magnified photographs of square area of a and b, respectively. Arrowheads indicate 10-nm colloidal gold signals. The scale bars in white in a and b, or a′ and b′ are 500 and 100 nm, respectively. E, intracellular gold particles from 10 cells in the immune transmission electron microscopic analysis were counted and analyzed by Student's t test. ***, p < 0.001. Error bars indicate ± S.E. (n = 10). F, the P100 fraction prepared from A23187-treated (1 or 2 μm) the C-terminal V5-His6-tagged TG-A-expressing S2 cells were incubated with Ecc15 for 1 h at room temperature. After incubation, bacteria were collected and washed three times, and then bacteria-bound proteins were analyzed by Western blotting using the anti-His6 tag antibody. Numbers show the band intensity of the bacteria-bound fraction analyzed by ImageJ software, and the intensity at the 2 μm A23187 was set to 1.0. ND, not detectable. G, S2 cells expressing the C-terminal EGFP tagged-TG-A were labeled with the exosomal marker BODIPY TR ceramide, and stimulated with A23187, and then the resulting P100 fraction was collected and added to untransfected S2 cells for 1 h. The exosome-treated S2 cells were analyzed under a fluorescence microscope. The scale bars in white are 10 μm.
Exosomes are taken up by other cells to affect the functions of the exosome-captured cells or tissues (48–50). To determine whether there is a direct interaction between exosomes and bacteria, exosome-containing P100 fractions were prepared from S2 cells expressing TG-A or G2A fused with His6 tag in the presence of A23187 by ultracentrifugation. Ecc15 was mixed with the prepared P100 fractions, and the bacterial pellet was collected by low speed centrifugation and subjected to Western blotting. The anti-His6 tag antibody recognized the His6 antigen of TG-A, but not that of G2A, in the bacterial pellet in an A23187-dose-dependent manner, suggesting that exosomes bind to Ecc15 (Fig. 5F).
To determine whether secreted TG-A as a component of exosomes is taken up by other cells in the host, S2 cells expressing TG-A tagged with EGFP were stained with an exosomal marker, BODIPY TR, which is a red fluorescent-labeled analog of ceramides, and treated with the Ca2+ ionophore A23187. The P100 fraction containing exosomes was obtained by ultracentrifugation, suspended in phosphate-buffered saline (PBS), and added to untransfected S2 cells. The untransfected S2 cells with red fluorescent exosomal marker were observed (Fig. 5G, left, arrowheads) and TG-A tagged with EGFP was also taken up into the S2 cells (Fig. 5G, middle, arrowheads). Fig. 5G shows that 19.5% S2 cells took up Bodipy TR-labeled exosomes, and 10.9% of the Bodipy TR-positive cells took up TG-A-containing exosomes.
The 8-residue N-terminal region of TG-A is necessary and sufficient for exosomal secretion
To determine a necessary and sufficient length of the N-terminal sequence of TG-A for exosomal secretion, N-terminal fragments of TG-A including 6, 7, and 8 residues tagged with EGFP (AN6-EGFP, AN7-EGFP, and AN8-EGFP, respectively) were expressed in S2 cells and treated with A23187 for exosomal secretion. AN7-EGFP and AN8-EGFP were recovered in the P100 fraction, and the amount of AN7-EGFP recovered was 50% of the amount of AN8-EGFP that was recovered (supplemental Fig. S5A). In contrast, no AN6-EGFP was recovered in the P100 fraction (supplemental Fig. S5A). In addition, TG-B fused with AN8 (AN8-TG-B), but not TG-B alone, was secreted after the A23187 stimulation (supplemental Fig. S5B).
Discussion
In Drosophila, the TG gene is estimated to generate two mRNA variants, TG-A and TG-B, by alternative splicing. In the present study, we first characterized the mRNA expression profiles of TG-A and TG-B (supplemental Fig. S1, A and B). The amount of TG-B mRNA expressed in the third instar larval and early pupal stages was 5–10-fold greater than that of TG-A. In the late pupal stage, both TG-A and TG-B were highly expressed, indicating a critical need for TG activity intracellularly and extracellularly for morphogenesis at this stage.
We determined that TG-A is contained in vesicular-like structures in hemocytes (Fig. 1D). S2 cells expressing TG-A also contained vesicular-like structures of TG-A (Fig. 1E). Simultaneously, TG-A was localized in the plasma membrane, which may be due to the overexpression of TG-A (Fig. 1E). TG-A and the late endosomal/MVB protein Rab7 were co-localized, indicating that TG-A localizes in MVBs (Fig. 1F). In contrast, TG-B localizes in the cytosol (Fig. 1E). The different localizations of TG-A and TG-B were defined by the two types of intracellular fatty acid modifications, N-myristoylation (Fig. 1, B, C, E, and G, and supplemental Fig. S2, C–E) and S-palmitoylation (Fig. 2, A–F, and supplemental Fig. S3, B and C). Moreover, N-myristoylation of TG-A was necessary for subsequent S-palmitoylation (Fig. 2G and supplemental Fig. S3, E and F).
Analyses of AN46-EGFP-containing sites of fatty acylations clearly indicated that AN46-EGFP was both necessary and sufficient for subcellular localization (supplemental Fig. S2E), fatty acylations (Figs. 1G and 2C and supplemental Fig. S3C), and exosome-dependent secretion in response to calcium signaling (Fig. 4, C and D). Furthermore, we clearly demonstrated that the N-terminal fragment of TG-A comprising eight amino acid residues (MGQKLSCC) is necessary and sufficient for exosomal secretion (supplemental Fig. S5).
Fusion proteins with a short N-terminal tag containing the myristoylation signal are targeted to exosomes (51), indicating that the dual lipid modifications of TG-A are essential for membrane targeting to MVBs and for TG-A secretion through an exosome-dependent pathway. Exosomes are membranous vesicles released by cells, and they are produced in MVBs, endocytic compartments, and released into the extracellular space (52). In Drosophila hemocytes, TG-A was localized in vesicle-like puncta, suggesting that TG-A is stored in MVBs prior to secretion (Fig. 1D).
Twenty-two S-palmitoyl acyltransferase homologs are present in the Drosophila genome: 6 are localized on the Golgi apparatus, 14 are localized on the ER, 1 is localized on the plasma membrane, and the localization of 1 enzyme remains unknown (12). Mammalian ER/Golgi-localized S-palmitoyl acyltransferases are thought to modify de novo synthesized proteins involved in stimulus-independent processes, such as membrane localization and delivery to organelles (11). There is evidence that dually fatty acylated proteins are posttranslationally modified at the cytoplasmic surface of the ER/Golgi, the yeast Ras2 protein is palmitoylated by a Ras protein acyltransferase Erf2/Erf4 in the ER (53, 54); H- and N-Ras are palmitoylated by a human protein palmitoyltransferase DHHC9/GCP16 in the Golgi (55); G-protein α subunits are modified by a Gα palmitoylating enzyme DHHC3 in the Golgi (56). Therefore, S-palmitoyl acyltransferase(s) involved in the S-acylation of TG-A may be localized on the ER/Golgi.
Fig. 6 shows a schematic model of an estimated intracellular transport and secretion pathway of TG-A through an unconventional secretory route, including the two types of fatty acid modifications. At first, TG-A is co-translationally N-myristoylated at the Gly2 residue in the cytosol after cleavage of the initiation Met and subsequently sorted to the cytoplasmic surface of the ER/Golgi, where S-palmitoylation occurs at the Cys7 and Cys8 residues by membrane-bound palmitoyl acyltransferases. Following transient association with the cytoplasmic surface of the ER/Golgi, TG-A modified by both N-myristoylation and S-palmitoylation is recruited into the MVBs. In response to external stimuli, such as uracil derived from pathogenic bacteria, TG-A is secreted in exosomes.
Figure 6.
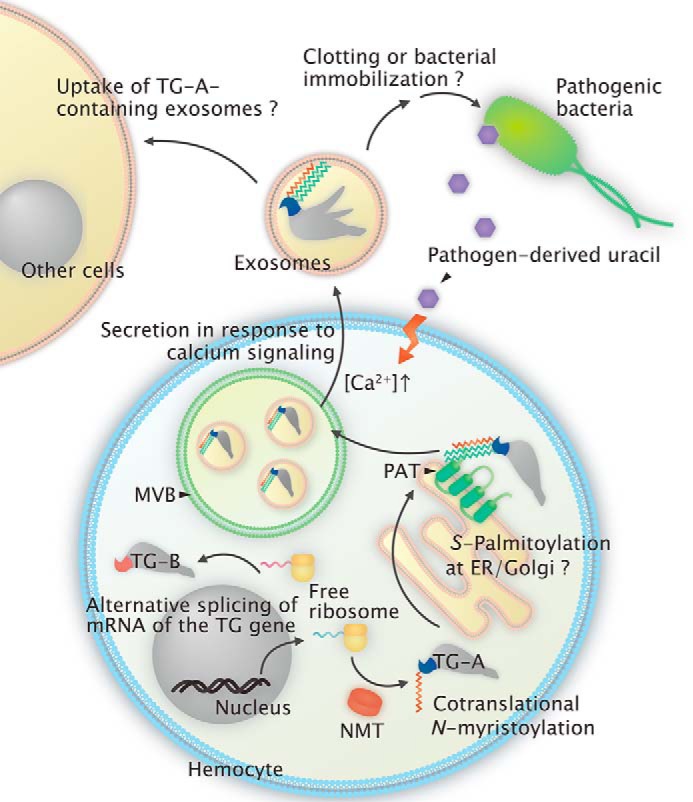
A schematic model of the two types of fatty acid modifications and secretion of TG-A. TG-A is co-translationally N-myristoylated and subsequently S-palmitoylated probably on the cytoplasmic face of the ER/Golgi membrane. The dually fatty acid-modified TG-A is finally transported to the MVBs by unknown mechanisms and secreted as exosomes following activation by external stimuli, such as bacteria-derived uracil. The secreted TG-A retains the fatty acid modification and locates in the inner leaflet of the exosomes.
A secretion system similar to that of TG-A is reported for hydrophilic acylated surface protein B (HASPB) in the protozoa Leishmania (57): HASPB is N-myristoylated at Gly2 next to the initiation Met by a cytosolic NMT and S-palmitoylated at Cys5 by a putative palmitoyl acyltransferase on the Golgi apparatus, and the dual acylation is required for its plasma membrane localization. The transport mechanisms of fatty acid-modified HASPB from the cytoplasmic membrane of the Golgi apparatus to the inner leaflet of the plasma membrane and the subsequent translocation of HASPB across the plasma membrane remain unknown. In 2005, Nickel (58) postulated three options: (i) HASPB is transported to the plasma membrane associated with the cytoplasmic leaflet of secretory vesicles, (ii) HASPB is transported first to endosomal structures followed by translocation to the plasma membrane, and (iii) transportation of HASPB from the Golgi apparatus to the plasma membrane does not rely on transport vesicles. HASPB localizes at the outer leaflet of the plasma membrane with the protein being exposed to the extracellular space, suggesting the translocation of HASPB across the plasma membrane through an unidentified transporter or translocation machinery (58). Interestingly, Chinese hamster ovary cells with exogenous expression of the HASPB gene can secrete HASPB protein, indicating that this fatty acid-mediated protein secretion system in Leishmania is conserved not only in unicellular parasites, but also in mammals (59). Whether HASPB is selectively recruited into exosomes remains unknown; however, an exosome-based secretion pathway is responsible for protein export from Leishmania (60).
Interestingly, TG-A secretion is triggered by uracil derived from Ecc15, but not cellular components such as lipopolysaccharides and peptidoglycans (Fig. 3, E and G). In Drosophila gut, bacteria-derived uracil stimulates the production of reactive oxygen species in a Ca2+-dependent manner through dual oxydase activation to kill pathogenic bacteria (42). The physiologic function of secreted TG-A requires further exploration, but secreted TG-A was bound to Ecc15 (Fig. 5F), suggesting that exosomal-mediated secretion of TG-A is involved in hemolymph coagulation and pathogen entrapment after bacterial infection. Therefore, TG-A is possibly involved in peritrophic matrix formation by cross-linking drosocrystallin, an extracellular substrate for Drosophila TG, to maintain the permeability of the peritrophic matrix (24). On the other hand, in gut epithelial cells, non-acylated cytosolic TG-B participates in immune tolerance to gut commensal bacteria by cross-linking the nuclear factor Relish to its inactive form (22).
Mammalian TG-1, mainly expressed in keratinocytes, is involved in the formation of a cornified cell envelope in terminally differentiating epithelia by cross-linking structural proteins (61). Although there is no consensus sequence for N-myristoylation at the N-terminal region, TG-1 is constitutively N-myristoylated at the Gly3 residue (62). In addition to N-myristoylation, TG-1 is also S-myristoylated in proliferating cells and S-palmitoylated in differentiating cells (62). These multiple fatty acid modifications of TG-1 must control trafficking between the plasma membrane and cytosol. A minor part of mammalian TG-2 in the cytosol is secreted through an unidentified secretion pathway: the majority of TG-2 is localized in the cytosol, and ∼20% of TG-2 is localized on the plasma membrane to regulate cell survival, such as stabilization of the extracellular matrix and cell adhesion by protein-protein cross-linking in the extracellular matrix (63). Zemskov et al. (63) also reported that secretion of TG-2 in the cytosol is mediated by a unique phosphoinositide-dependent pathway to recycle endosomes: (i) TG-2 contains a phospholipid-binding motif, and at the initial stage of secretion, TG-2 binds to phosphoinositide on the recycling endosomes, (ii) recycling endosomes containing TG-2 are targeted to the plasma membrane mediated by Rab11A/Rab11B GTPases, and (iii) the recycling endosomes are fused to the plasma membrane for secretion mediated by VAMP3 and SNAP23 SNAREs (64). In addition, TG-2 is secreted in exosomes from cells under stressful conditions (65).
In invertebrates, TGs are secreted in response to external stimuli such as bacterial infection or injury. In horseshoe crabs, TG is secreted from granular hemocytes into the extracellular space by lipopolysaccharide stimulation to form a cross-linking product of coagulin polymers (40). In crayfish, TG is secreted from hemocytes or muscle cells upon injury to cross-link clottable proteins (66, 67). On the other hand, TGs play important roles in cuticular formation and peritrophic matrix formation (20, 21, 24), indicating that in invertebrates, TGs are secreted if necessary during stationary or developmental stages through unconventional secretory pathways without bacterial infection. This regulated secretion mechanism may be mediated by unknown host factors that exist inside or outside of the cells. The molecular mechanism of the secretion of Drosophila TG requires further investigation, but additional studies of the fatty acid modification-dependent secretion of TG-A will provide new insight into the protein secretion mechanisms involving unconventional secretion pathways.
Experimental procedures
Antibody preparation
The anti-TG-A/B polyclonal antibody, which recognizes both TG-A and TG-B, was described previously (20). Production of the anti-TG-A-specific polyclonal antibody was outsourced to MBL (Nagoya, Japan). A synthetic peptide of the N-terminal of TG-A (amino acids 18 to 32) was conjugated to keyhole limpet hemocyanin and immunized to rabbit. The immunoglobulin G fraction was purified from antiserum using a HiTrap Protein A HP column (GE Healthcare, Little Chalfont, United Kingdom).
Western blotting
Proteins were separated by SDS-PAGE and transferred to a polyvinylidene difluoride membrane. The membrane was blocked with 5% skim milk in Tris-buffered saline (20 mm Tris-HCl, pH 7.5, containing 150 mm NaCl) or Blocking One (Nacalai Tesque, Kyoto, Japan). The blocked membrane was reacted to each antibody at room temperature for 1 h, including the anti-TG/B polyclonal antibody, anti-His tag mAb-HRP-DirecT (catalog number D291-7, MBL), anti-V5 tag HRP-DirecT (catalog number PM003-7, MBL), anti-DDDDK tag mAb-HRP-DirecT (catalog number M185-7, MBL), anti-GFP-HRP (catalog number 120-002-105, Miltenyi Biotec, Bergisch Gladbach, Germany), or anti-CD63 monoclonal antibody TS63 (catalog number 10628D, Life Technologies). Protein bands were visualized by Western Bright Quantum or Sirius (Advansta, Menlo Park, CA). Chemiluminescence was detected using an Omega Lum G fluorescence imager (Aplegen, Pleasanton, CA).
Cell culture
Drosophila S2 cells and Sf-9 cells were maintained at 27 °C in Insect-Xpress protein-free medium (Lonza Japan, Tokyo, Japan) without antibiotics. The insect cells were transfected with FuGENE HD (Promega, Madison, WI) or Cellfectin II reagent (Life Technologies). A pIB/V5-His vector (Thermo Fisher Scientific) was used to express the C-terminal FLAG-tagged or V5 and His6-tagged TG-A, G2A, C7A, C8A, C7A/C8A, and TG-B in S2 and Sf-9 cells. The pIB/V5-His vector was also used to express the N-terminal EGFP-tagged human CD63 and Drosophila Rab7 or the C-terminal EGFP-tagged AN6-EGFP, AN7-EGFP, and AN8-EGFP in S2 cells. A pMT/V5-His A vector (Thermo Fisher Scientific) was used to express C-terminal V5 and His6-tagged TG-A, G2A, and TG-B or the C-terminal EGFP-tagged AN46-EGFP, G2A-AN46-EGFP, BN38-EGFP, and AN8-tagged TG-B (AN8–TG-B) in S2 cells. A pMT/BiP/V5-His/GFP vector was used to express GFP with the N-terminal authentic signal peptide for secretion. The RNA interference of S2 cells using double-stranded RNA was described previously (22). The following primers were used for dsRNA synthesis: NMT, 5′-TAATACGACTCACTATAGGGAGACGCGAATTCGGTCCACACAGCCAGTCACCAA-3′(forward) and 5′-TAATACGACTCACTATAGGGAGAGCGTCTAGACTTCTCCACACGAACGCCAACA-3′ (reverse); Rab27, 5′-TAATACGACTCACTATAGGGTACTCCTCTACCAGTACACGGATGG-3′ (forward) and 5′-TAATACGACTCACTATAGGGTACTGCATCGCGACTGGGTCAGTAG-3′ (reverse); Rab11, 5′-TAATACGACTCACTATAGGGGATGGCAAAACAATTAAAGCGCAAA-3′ (forward) and 5′-TAATACGACTCACTATAGGGGTTGAGTCGAGGGCCGAGGT-3′ (reverse); EGFP, 5′-TAATACGACTCACTATAGGGACCCTCGTGACCACCCTGAC-3′ (forward) and 5′-TAATACGACTCACTATAGGGGGACCATGTGATCGCGCTTC-3′ (reverse).
Cell imaging
For immunocytochemistry, the insect cells were transfected with plasmids expressing TG-A or TG-B for 48 h. The cells were washed with PBS (8.1 mm Na2HPO4, 1.47 mm KH2PO4, 137 mm NaCl, 2.7 mm KCl) and fixed with 4% paraformaldehyde in PBS. After washing with PBS containing 0.1% Triton X-100 (PBS-T), the cells were blocked with 5% goat serum (Sigma) in PBS-T. The cells were then incubated for 1 h with the anti-FLAG M2 monoclonal antibody (catalog number F1804, Sigma), anti-V5 tag monoclonal antibody (catalog number M167-3, MBL), anti-His tag monoclonal antibody (catalog number D291-3, MBL), or anti-TG-A-specific polyclonal antibody. The CF568- or CF488-conjugated goat anti-mouse secondary antibody (Biotium, Hayward, CA) or CF488-conjugated goat anti-rabbit secondary antibody (Biotium) was used to detect the primary antibody. In the case of larval hemocytes, after fixing and permeabilizing, the cells were incubated with the anti-TG-A-specific polyclonal antibody and then incubated with the CF488-conjugated goat anti-rabbit secondary antibody. The cells were imaged with an Olympus fluorescence microscope, model BX-FLA (Olympus Optical Co., Ltd., Tokyo, Japan) or a ZOE fluorescence microscope (Bio-Rad).
Analysis of fatty acid modification in vivo
Fly embryos were transferred to 2-fold diluted Drosophila normal yeast media containing 100 μm Click-iT myristic acid-azide (12-azidododecanoic acid; Thermo Fisher Scientific) and incubated at 25 °C for 5 h. As a negative control, 2-fold diluted Drosophila normal yeast medium without Click-iT myristic acid-azide was used. Third instar larvae were homogenized in Tris-buffered saline (TBS; 20 mm Tris-HCl, pH 7.5, 150 mm NaCl) containing 5 mm EDTA and sonicated. Then, 1% Triton X-100 was added and incubated at 4 °C for 1 h. For biotinylation, the homogenate was mixed with the Click-iT protein reaction buffer (Thermo Fisher Scientific) containing biotin alkyne (Thermo Fisher Scientific). The resulting proteins were precipitated by methanol/chloroform precipitation, and dissolved in 50 mm Tris-HCl, pH 8.0, containing 50 mm NaCl and 1% SDS. After 10-fold dilution with 50 mm Tris-HCl, pH 8.0, containing 50 mm NaCl and 0.02% Triton X-100, avidin-immobilized agarose (NeutrAvidin agarose, Thermo Fisher Scientific) was added and biotinylated proteins were obtained by centrifugation.
Biotin-switch assay
S-Palmitoylation of TG-A or TG-B was assayed using the biotin-switch (acyl-biotin-exchange) assay as previously described (68). Briefly, 15 flies or S2 and Sf-9 cells expressing TG-A or TG-B were homogenized with TBS containing 5 mm EDTA, the protease inhibitor mixture (Nacalai Tesque), and 2 mm phenylmethylsulfonyl fluoride. To block free cysteines, 10 mm N-ethylmaleimide was added. After removing the N-ethylmaleimide by methanol/chloroform precipitation, 0.7 m HA and 1 mm HPDP-biotin (Thermo Fisher Scientific) were added to exchange S-fatty acid with biotin. Biotinylated proteins were purified using avidin-immobilized agarose. The agarose was washed and boiled in 2× SDS sample buffer.
Cell-free expression of TG and detection of N-myristoylation
To express the C-terminal FLAG-tagged TG-A or TG-B, we used the Transdirect insect cell (Shimadzu, Kyoto, Japan) or TnT T7 Insect Cell Extract Protein Expression System (Promega, Madison, WI) according to the manufacturer's instructions with some modifications as follows: 10 μm Z-VAD-fmk (R&D Systems) and 40 μm Click-iT myristic acid-azide were added to the solution and the solution was incubated at 18 °C for 6 h. The resulting samples were desalted with Sephadex G-25 (GE Healthcare), and the C-terminal FLAG-tagged TG-A or TG-B was purified by anti-FLAG M2-agarose (Sigma). For biotinylation, the purified samples bound to the agarose were mixed with the Click-iT protein reaction buffer containing biotin alkyne according to the manufacturer's instructions. After washing with PBS, 2× SDS sample buffer was added to the agarose and the agarose was incubated at 95 °C. The protein samples were then analyzed by SDS-polyacrylamide gel electrophoresis.
Metabolic labeling
Click-iT Myristic acid-azide or Click-iT Palmitic acid-azide (Thermo Fisher Scientific) were added to S2 cells expressing AN46-EGFP or BN38-EGFP for 2 h. The cells were lysed and purified using anti-GFP-agarose (MBL International). After washing the agarose with TBS-T, the click reaction was performed as described above and biotinylated proteins were detected by NeutrAvidin-HRP.
Secretion of TG from hemocytes and S2 cells
Ten third instar larvae were bled in a μ-Slide Chamber (ibidi, Martinsried, Germany) filled with 250 μl of HL3 buffer (5 mm Hepes-NaOH, pH 7.2, containing 70 mm NaCl, 5 mm KCl, 1.5 mm CaCl2, 20 mm MgCl2, 10 mm NaHCO3, 5 mm trehalose, and 115 mm sucrose). The hemocytes were incubated for 1 h at 27 °C in the chamber to induce them to attach to the bottom of the chamber. After washing three times with the HL3 buffer, Ecc15 suspended in the HL3 buffer was added to the chamber (final A600 = 0.5). After incubation at 27 °C, the supernatant was collected by centrifugation and precipitated with trichloroacetic acid. The precipitant was analyzed by Western blotting using the anti-TG-A/B antibody. For ultracentrifugation analysis, the supernatant was cleared at 2,000 × g for 10 min to remove cells. The cleared supernatant was then centrifuged at 10,000 × g for 30 min to remove cell debris. Finally, the supernatant was centrifuged at 100,000 × g for 90 min and separated into soluble (S100) and precipitated (P100) fractions. In the case of S2 cells, the TG-A, TG-B, G2A, AN46-EGFP, or BN38-EGFP-expressing cells were treated with or without 1 μg/ml of brefeldin A or 1 μm monensin for 1 h, and then treated with 1 μm A23187, 1 μm ionomycin, or 20 nm uracil for 90 min. The resulting supernatant was separated into the S100 and P100 fractions, as described above.
Butyl-Sepharose-binding assay
Sf-9 cells expressing the C-terminal FLAG-tagged TG-A were homogenized in 10 mm Tris-HCl, pH 8.0, containing 100 mm NaCl, 0.025% CHAPS, and protease inhibitor mixture, and centrifuged at 18,000 × g for 5 min. The resulting supernatant was incubated with butyl-Sepharose 4B (GE Healthcare) at room temperature for 1 h. Unbound proteins were separated by centrifugation and bound proteins were obtained by stepwise elution from butyl-Sepharose 4B using the homogenizing buffer containing several concentrations of NaCl and 1% SDS.
Exosome labeling
S2 cells expressing the C-terminal EGFP-tagged TG-A were treated with 5 μm BODIPY TR Ceramide (Thermo Fisher Scientific) in PBS for 30 min. After washing with cold PBS, cells were treated with A23187 in Insect Xpress protein-free medium for 1 h at 27 °C. The P100 fraction was collected and suspended in PBS. The suspension was added to untransfected S2 cells and incubated for 1 h at 27 °C. Exosome-incorporated cells were analyzed by ZOE fluorescence microscopy.
Density gradient fractionation using OptiPrep
Solutions (40 (w/v), 20 (w/v), 10 (w/v), and 5% (w/v)) of iodixanol were made by diluting OptiPrep (60% (w/v) aqueous iodixanol, Axis-Shield PoC) with 10 mm Tris-HCl, pH 7.5, containing 0.25 m sucrose, from the bottom to the top of a tube. The P100 fraction was prepared by ultracentrifugation and suspended in PBS. This suspension was separated on a continuous gradient made by centrifugation at 100,000 × g for 18 h. Twelve fractions were collected and each fraction was analyzed by Western blotting.
Immunotransmission electron microscopic analysis
The C-terminal FLAG-tagged TG-A-expressing S2 cells, which were treated with A23187, were fixed with 4% paraformaldehyde and 0.4% glutaraldehyde in 0.1 m cacodylate, pH 7.4, containing 3.4% sucrose, and 3 mm CaCl2, at room temperature. The cell pellets were dehydrated in graded ethanol, and embedded in LR white resin. Thin sections (100 nm) were blocked with 3% bovine serum albumin in PBS for 15 min, and subsequently applied with the anti-TG-A-specific antibody and the secondary anti-rabbit colloidal gold (10 nm)-conjugated goat antibody (BBI Solutions, Cardiff, UK). After washing with PBS, the sections were treated with 0.5% OsO4 in PBS and 2% uranyl acetate, and then examined under a Tecnai electron microscope and Eagle 2k CCD camera (FEI, Hillsboro, OR).
Quantitative real-time reverse transcription-PCR
Total RNA was extracted with RNAiso Plus (Takara Bio, Kusatsu, Japan), treated with deoxyribonuclease I, and used as template (500 ng) for reverse transcription with SuperScript III (Thermo Fisher Scientific) according to the manufacturer's instructions. Real-time PCR was performed with FastStart Essential DNA Green Master (Roche Diagnostics, Basel, Switzerland), and the reactions were performed on a LightCycler Nano (Roche Diagnostics). An absolute quantification method was applied and plasmids containing cloned target sequences are used as a standard curve. The amount of mRNA detected was normalized to control rpL32 mRNA values. The following primers were used: rpL32, 5′-AGATCGTGAAGAAGCGCACCAAG-3′ (forward) and 5′-CACCAGGAACTTCTTGAATCCGG-3′ (reverse); TG-A, 5′-TCGCCAAGGCGAACCGGTTT-3′ (forward) and 5′-CTCCTCGCACGACCAACGCT-3′ (reverse); TG-B, 5′-ACCGGTGTAGCCCCTCTCGT-3′ (forward) and 5′-CTCCTCGCACGACCAACGCT-3′ (reverse). All assays were conducted in biologic triplicates.
Fly stocks
Fly stocks and culturing conditions were described previously (20).
Author contributions
T. S. and S. K. wrote the manuscript. T. S. and S. K. designed the experiments. T. S., J. H., D. K., and X. D. performed the experiments. All authors reviewed and approved this manuscript.
Supplementary Material
Acknowledgments
We are grateful to Youko Ishino (Kyushu University, Japan) for technical assistance, Dr. Keisuke Tagawa (Kyushu University) and Dr. Toshihiko Utsumi (Yamaguchi University) for helpful discussions, and Ryo Ugai (Laboratory for Technical Support, Medical Institute of Bioregulation, Kyushu University, Japan) for the electron microscopy experiments.
This work was supported by Grant-in-Aid for Scientific Research (B) 15H04353 (to S. K.), Grant-in-Aid for Young Scientists (B) 26860333 (to T. S.), and the Qdai-jump Research Program (TT type) Grant 26701 (to T. S.). The authors declare that they have no conflicts of interest with the contents of this article.
This article contains supplemental Figs. S1–S5.
- NMT
- N-myristoyltransferase
- ER
- endoplasmic reticulum
- TG
- transglutaminase
- MVB
- multivesicular body
- Sf
- S. frugiperda
- EGFP
- enhanced green fluorescent protein
- HA
- hydroxylamine
- HPDP-biotin
- N-[6-(biotinamido)hexyl]-3′-(2′-pyridyldithio)propionamide
- Ecc15
- Erwinia carotovora carotovora 15
- HASPB
- hydrophilic acylated surface protein B
- Z
- benzyloxycarbonyl
- fmk
- fluoromethyl ketone.
References
- 1. Resh M. D. (2006) Trafficking and signaling by fatty-acylated and prenylated proteins. Nat. Chem. Biol. 2, 584–590 [DOI] [PubMed] [Google Scholar]
- 2. Resh M. D. (1999) Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim. Biophys. Acta 1451, 1–16 [DOI] [PubMed] [Google Scholar]
- 3. Maurer-Stroh S., Eisenhaber B., and Eisenhaber F. (2002) N-terminal N-myristoylation of proteins: prediction of substrate proteins from amino acid sequence. J. Mol. Biol. 317, 541–557 [DOI] [PubMed] [Google Scholar]
- 4. Maurer-Stroh S., Eisenhaber B., and Eisenhaber F. (2002) N-terminal N-myristoylation of proteins: refinement of the sequence motif and its taxon-specific differences. J. Mol. Biol. 317, 523–540 [DOI] [PubMed] [Google Scholar]
- 5. Farazi T. A., Waksman G., and Gordon J. I. (2001) The biology and enzymology of protein N-myristoylation. J. Biol. Chem. 276, 39501–39504 [DOI] [PubMed] [Google Scholar]
- 6. Smotrys J. E., and Linder M. E. (2004) Palmitoylation of intracellular signaling proteins: regulation and function. Annu. Rev. Biochem. 73, 559–587 [DOI] [PubMed] [Google Scholar]
- 7. Greaves J., and Chamberlain L. H. (2007) Palmitoylation-dependent protein sorting. J. Cell Biol. 176, 249–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aicart-Ramos C., Valero R. A., and Rodriguez-Crespo I. (2011) Protein palmitoylation and subcellular trafficking. Biochim. Biophys. Acta 1808, 2981–2994 [DOI] [PubMed] [Google Scholar]
- 9. Conibear E., and Davis N. G. (2010) Palmitoylation and depalmitoylation dynamics at a glance. J. Cell Sci. 123, 4007–4010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fukata M., Fukata Y., Adesnik H., Nicoll R. A., and Bredt D. S. (2004) Identification of PSD-95 palmitoylating enzymes. Neuron 44, 987–996 [DOI] [PubMed] [Google Scholar]
- 11. Ohno Y., Kihara A., Sano T., and Igarashi Y. (2006) Intracellular localization and tissue-specific distribution of human and yeast DHHC cysteine-rich domain-containing proteins. Biochim. Biophys. Acta 1761, 474–483 [DOI] [PubMed] [Google Scholar]
- 12. Bannan B. A., Van Etten J., Kohler J. A., Tsoi Y., Hansen N. M., Sigmon S., Fowler E., Buff H., Williams T. S., Ault J. G., Glaser R. L., and Korey C. A. (2008) The Drosophila protein palmitoylome: characterizing palmitoyl-thioesterases and DHHC palmitoyl-transferases. Fly (Austin) 2, 198–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Theopold U., Krautz R., and Dushay M. S. (2014) The Drosophila clotting system and its messages for mammals. Dev. Comp. Immunol. 42, 42–46 [DOI] [PubMed] [Google Scholar]
- 14. Eckert R. L., Kaartinen M. T., Nurminskaya M., Belkin A. M., Colak G., Johnson G. V., and Mehta K. (2014) Transglutaminase regulation of cell function. Physiol. Rev. 94, 383–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Demény M., Korponay-Szabó I., and Fésüs L. (2015) Structure of transglutaminases: unique features serve diverse functions. in Transglutaminases-Multiple Functional Modifiers and Targets for New Drug Discovery (Hitomi K., Kojima S., and Fesus L., eds) pp. 1–41, Springer, Tokyo, Japan [Google Scholar]
- 16. Lorand L., and Graham R. M. (2003) Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat. Rev. Mol. Cell Biol. 4, 140–156 [DOI] [PubMed] [Google Scholar]
- 17. Furutani Y., and Kojima S. (2015) Control of TG functions depending on their localization. in Transglutaminases-Multiple Functional Modifiers and Targets for New Drug Discovery (Hitomi K., Kojima S., and Fesus L., eds) pp. 43–62, Springer, Tokyo, Japan [Google Scholar]
- 18. Rothman J. E. (1994) Mechanisms of intracellular protein transport. Nature 372, 55–63 [DOI] [PubMed] [Google Scholar]
- 19. Lindgren M., Riazi R., Lesch C., Wilhelmsson C., Theopold U., and Dushay M. S. (2008) Fondue and transglutaminase in the Drosophila larval clot. J. Insect Physiol. 54, 586–592 [DOI] [PubMed] [Google Scholar]
- 20. Shibata T., Ariki S., Shinzawa N., Miyaji R., Suyama H., Sako M., Inomata N., Koshiba T., Kanuka H., and Kawabata S. (2010) Protein crosslinking by transglutaminase controls cuticle morphogenesis in Drosophila. PLoS ONE 5, e13477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ichikawa A., Yamada A., Sakamoto H., Umehara M., Yoshioka Y., Yamaguchi M., and Ikura K. (2010) Overexpression of transglutaminase in the Drosophila wing imaginal disc induced an extra wing crossvein phenotype. Biosci. Biotechnol. Biochem. 74, 2494–2496 [DOI] [PubMed] [Google Scholar]
- 22. Shibata T., Sekihara S., Fujikawa T., Miyaji R., Maki K., Ishihara T., Koshiba T., and Kawabata S. (2013) Transglutaminase-catalyzed protein-protein cross-linking suppresses the activity of the NF-κB-like transcription factor relish. Sci. Signal. 6, ra61. [DOI] [PubMed] [Google Scholar]
- 23. Wang Z., Wilhelmsson C., Hyrsl P., Loof T. G., Dobes P., Klupp M., Loseva O., Mörgelin M., Iklé J., Cripps R. M., Herwald H., and Theopold U. (2010) Pathogen entrapment by transglutaminase: a conserved early innate immune mechanism. PLoS Pathog. 6, e1000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shibata T., Maki K., Hadano J., Fujikawa T., Kitazaki K., Koshiba T., and Kawabata S. (2015) Crosslinking of a peritrophic matrix protein protects gut epithelia from bacterial exotoxins. PLoS Pathog. 11, e1005244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Johnstone R. M. (2006) Exosomes biological significance: A concise review. Blood Cells Mol. Dis. 36, 315–321 [DOI] [PubMed] [Google Scholar]
- 26. Bastos-Amador P., Pérez-Cabezas B., Izquierdo-Useros N., Puertas M. C., Martinez-Picado J., Pujol-Borrell R., Naranjo-Gómez M., and Borràs F. E. (2012) Capture of cell-derived microvesicles (exosomes and apoptotic bodies) by human plasmacytoid dendritic cells. J. Leukoc. Biol. 91, 751–758 [DOI] [PubMed] [Google Scholar]
- 27. Raposo G., and Stoorvogel W. (2013) Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 200, 373–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Suzuki T., Ito M., Ezure T., Shikata M., Ando E., Utsumi T., Tsunasawa S., and Nishimura O. (2007) Protein prenylation in an insect cell-free protein synthesis system and identification of products by mass spectrometry. Proteomics 7, 1942–1950 [DOI] [PubMed] [Google Scholar]
- 29. Moriya K., Tsubota T., Ishibashi N., Yafune A., Suzuki T., Kobayashi J., Shiotsuki T., and Utsumi T. (2010) Bombyx mori Ras proteins BmRas1, BmRas2 and BmRas3 are neither farnesylated nor palmitoylated but are geranylgeranylated. Insect Mol. Biol. 19, 291–301 [DOI] [PubMed] [Google Scholar]
- 30. Ezure T., Suzuki T., Shikata M., Ito M., Ando E., Nishimura O., and Tsunasawa S. (2007) Expression of proteins containing disulfide bonds in an insect cell-free system and confirmation of their arrangements by MALDI-TOF MS. Proteomics 7, 4424–4434 [DOI] [PubMed] [Google Scholar]
- 31. Phan H. P., Ezure T., Ito M., Kadowaki T., Kitagawa Y., and Niimi T. (2008) Expression and chain assembly of human laminin-332 in an insect cell-free translation system. Biosci. Biotechnol. Biochem. 72, 1847–1852 [DOI] [PubMed] [Google Scholar]
- 32. Suzuki T., Moriya K., Nagatoshi K., Ota Y., Ezure T., Ando E., Tsunasawa S., and Utsumi T. (2010) Strategy for comprehensive identification of human N-myristoylated proteins using an insect cell-free protein synthesis system. Proteomics 10, 1780–1793 [DOI] [PubMed] [Google Scholar]
- 33. Méresse S., Gorvel J. P., and Chavrier P. (1995) The rab7 GTPase resides on a vesicular compartment connected to lysosomes. J. Cell Sci. 108, 3349–3358 [DOI] [PubMed] [Google Scholar]
- 34. Chavrier P., Parton R. G., Hauri H. P., Simons K., and Zerial M. (1990) Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell 62, 317–329 [DOI] [PubMed] [Google Scholar]
- 35. Alland L., Peseckis S. M., Atherton R. E., Berthiaume L., and Resh M. D. (1994) Dual myristylation and palmitylation of Src family member p59fyn affects subcellular localization. J. Biol. Chem. 269, 16701–16705 [PubMed] [Google Scholar]
- 36. Zlatkine P., Mehul B., and Magee A. I. (1997) Retargeting of cytosolic proteins to the plasma membrane by the Lck protein tyrosine kinase dual acylation motif. J. Cell Sci. 110, 673–679 [DOI] [PubMed] [Google Scholar]
- 37. van't Hof W., and Resh M. D. (1997) Rapid plasma membrane anchoring of newly synthesized p59fyn: selective requirement for NH2-terminal myristoylation and palmitoylation at cysteine-3. J. Cell Biol. 136, 1023–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hallak H., Brass L. F., and Manning D. R. (1994) Failure to myristoylate the α subunit of Gz is correlated with an inhibition of palmitoylation and membrane attachment, but has no affect on phosphorylation by protein kinase C. J. Biol. Chem. 269, 4571–4576 [PubMed] [Google Scholar]
- 39. Berthiaume L., and Resh M. D. (1995) Biochemical characterization of a palmitoyl acyltransferase activity that palmitoylates myristoylated proteins. J. Biol. Chem. 270, 22399–22405 [DOI] [PubMed] [Google Scholar]
- 40. Osaki T., Okino N., Tokunaga F., Iwanaga S., and Kawabata S. (2002) Proline-rich cell surface antigens of horseshoe crab hemocytes are substrates for protein cross-linking with a clotting protein coagulin. J. Biol. Chem. 277, 40084–40090 [DOI] [PubMed] [Google Scholar]
- 41. Savina A., Furlán M., Vidal M., and Colombo M. I. (2003) Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J. Biol. Chem. 278, 20083–20090 [DOI] [PubMed] [Google Scholar]
- 42. Lee K. A., Kim S. H., Kim E. K., Ha E. M., You H., Kim B., Kim M. J., Kwon Y., Ryu J. H., and Lee W. J. (2013) Bacterial-derived uracil as a modulator of mucosal immunity and gut-microbe homeostasis in Drosophila. Cell 153, 797–811 [DOI] [PubMed] [Google Scholar]
- 43. Nassar-Gentina V., Rojas E., and Luxoro M. (1994) Rise in cytoplasmic Ca2+ induced by monensin in bovine medullary chromaffin cells. Cell Calcium 16, 475–480 [DOI] [PubMed] [Google Scholar]
- 44. Kosaka N., Iguchi H., Yoshioka Y., Takeshita F., Matsuki Y., and Ochiya T. (2010) Secretory mechanisms and intercellular transfer of microRNAs in living cells. J. Biol. Chem. 285, 17442–17452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ostrowski M., Carmo N. B., Krumeich S., Fanget I., Raposo G., Savina A., Moita C. F., Schauer K., Hume A. N., Freitas R. P., Goud B., Benaroch P., Hacohen N., Fukuda M., Desnos C., et al. (2010) Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 12, 19–30 [DOI] [PubMed] [Google Scholar]
- 46. Beckett K., Monier S., Palmer L., Alexandre C., Green H., Bonneil E., Raposo G., Thibault P., Le Borgne R., and Vincent J. P. (2013) Drosophila S2 cells secrete wingless on exosome-like vesicles but the wingless gradient forms independently of exosomes. Traffic 14, 82–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Thery C., Amigorena S., Raposo G., and Clayton A. (2006) Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. Chapter 3, Unit 3.22 [DOI] [PubMed] [Google Scholar]
- 48. Pan B. T., and Johnstone R. M. (1983) Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell 33, 967–978 [DOI] [PubMed] [Google Scholar]
- 49. Harding C., Heuser J., and Stahl P. (1983) Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J. Cell Biol. 97, 329–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Raposo G., Nijman H. W., Stoorvogel W., Liejendekker R., Harding C. V., Melief C. J., and Geuze H. J. (1996) B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 183, 1161–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shen B., Wu N., Yang J. M., and Gould S. J. (2011) Protein targeting to exosomes/microvesicles by plasma membrane anchors. J. Biol. Chem. 286, 14383–14395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Simons M., and Raposo G. (2009) Exosomes: vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 21, 575–581 [DOI] [PubMed] [Google Scholar]
- 53. Lobo S., Greentree W. K., Linder M. E., and Deschenes R. J. (2002) Identification of a Ras palmitoyltransferase in Saccharomyces cerevisiae. J. Biol. Chem. 277, 41268–41273 [DOI] [PubMed] [Google Scholar]
- 54. Bartels D. J., Mitchell D. A., Dong X., and Deschenes R. J. (1999) Erf2, a novel gene product that affects the localization and palmitoylation of Ras2 in Saccharomyces cerevisiae. Mol. Cell. Biol. 19, 6775–6787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Swarthout J. T., Lobo S., Farh L., Croke M. R., Greentree W. K., Deschenes R. J., and Linder M. E. (2005) DHHC9 and GCP16 constitute a human protein fatty acyltransferase with specificity for H- and N-Ras. J. Biol. Chem. 280, 31141–31148 [DOI] [PubMed] [Google Scholar]
- 56. Tsutsumi R., Fukata Y., Noritake J., Iwanaga T., Perez F., and Fukata M. (2009) Identification of G protein α subunit-palmitoylating enzyme. Mol. Cell. Biol. 29, 435–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Denny P. W., Gokool S., Russell D. G., Field M. C., and Smith D. F. (2000) Acylation-dependent protein export in Leishmania. J. Biol. Chem. 275, 11017–11025 [DOI] [PubMed] [Google Scholar]
- 58. Nickel W. (2005) Unconventional secretory routes: direct protein export across the plasma membrane of mammalian cells. Traffic 6, 607–614 [DOI] [PubMed] [Google Scholar]
- 59. Stegmayer C., Kehlenbach A., Tournaviti S., Wegehingel S., Zehe C., Denny P., Smith D. F., Schwappach B., and Nickel W. (2005) Direct transport across the plasma membrane of mammalian cells of Leishmania HASPB as revealed by a CHO export mutant. J. Cell Sci. 118, 517–527 [DOI] [PubMed] [Google Scholar]
- 60. Silverman J. M., Clos J., Horakova E., Wang A. Y., Wiesgigl M., Kelly I., Lynn M. A., McMaster W. R., Foster L. J., Levings M. K., and Reiner N. E. (2010) Leishmania exosomes modulate innate and adaptive immune responses through effects on monocytes and dendritic cells. J. Immunol. 185, 5011–5022 [DOI] [PubMed] [Google Scholar]
- 61. Kalinin A., Marekov L. N., and Steinert P. M. (2001) Assembly of the epidermal cornified cell envelope. J. Cell Sci. 114, 3069–3070 [DOI] [PubMed] [Google Scholar]
- 62. Steinert P. M., Kim S. Y., Chung S. I., and Marekov L. N. (1996) The transglutaminase 1 enzyme is variably acylated by myristate and palmitate during differentiation in epidermal keratinocytes. J. Biol. Chem. 271, 26242–26250 [DOI] [PubMed] [Google Scholar]
- 63. Zemskov E. A., Janiak A., Hang J., Waghray A., and Belkin A. M. (2006) The role of tissue transglutaminase in cell-matrix interactions. Front. Biosci. 11, 1057–1076 [DOI] [PubMed] [Google Scholar]
- 64. Zemskov E. A., Mikhailenko I., Hsia R. C., Zaritskaya L., and Belkin A. M. (2011) Unconventional secretion of tissue transglutaminase involves phospholipid-dependent delivery into recycling endosomes. PLoS ONE 6, e19414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Diaz-Hidalgo L., Altuntas S., Rossin F., D'Eletto M., Marsella C., Farrace M. G., Falasca L., Antonioli M., Fimia G. M., and Piacentini M. (2016) Transglutaminase type 2-dependent selective recruitment of proteins into exosomes under stressful cellular conditions. Biochim. Biophys. Acta 1863, 2084–2092 [DOI] [PubMed] [Google Scholar]
- 66. Kopáček P., Hall M., and Söderhäll K. (1993) Characterization of a clotting protein, isolated from plasma of the freshwater crayfish Pacifastacus leniusculus. Eur. J. Biochem. 213, 591–597 [DOI] [PubMed] [Google Scholar]
- 67. Hall M., Wang R., van Antwerpen R., Sottrup-Jensen L., and Söderhäll K. (1999) The crayfish plasma clotting protein: a vitellogenin-related protein responsible for clot formation in crustacean blood. Proc. Natl. Acad. Sci. U.S.A. 96, 1965–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wan J., Roth A. F., Bailey A. O., and Davis N. G. (2007) Palmitoylated proteins: purification and identification. Nat. Protoc. 2, 1573–1584 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


