Abstract
Utero-placental (Ut-Pl) angiogenesis and blood flow are fundamental for successful outcome of pregnancy. They are controlled by numerous vasodilator and vasoconstrictor systems such as endothelins (EDNs) and the renin angiotensin system. Dogs possess an invasive type of placentation, classified as endotheliochorial. Despite increasing knowledge regarding canine Ut-Pl function, little information exists on uterine and placental vascular activity during initiation, maintenance and termination of pregnancy in this species. The current study investigated expression of EDNs and their receptors (EDNRA and EDNRB) in the pre-implantation uterus and Ut-Pl compartments during gestation and at normal parturition, as well as in mid-pregnant dogs treated with the antigestagen aglepristone. The Ut-Pl mRNA expression of EDN1 and EDNRA was constant until mid-gestation and increased significantly during prepartum luteolysis. In contrast, EDN2 was highest pre-implantation and decreased following placentation, remaining low thereafter. Expression of the EDN-activating enzyme ECE1 and mRNA of EDNRB increased towards mid-gestation and was further elevated at prepartum luteolysis. Antigestagen treatment resulted in increased levels of EDN1 and EDNRA. At the cellular level, the uterine expression of EDN1, ECE1 and EDNRB was found predominantly in the endometrial surface and glandular epithelial cells; uterine signals for EDNRA were weak. In Ut-Pl all targets were mainly localized in the placenta fetalis, with syncytiotrophoblast staining stronger for ECE1 and EDNRB. In contrast, EDNRA stained strongly at the base of the placental labyrinth. Expression and localization of EDNs (EDN1, -2), EDN receptors and ECE1 in the placenta fetalis suggests their involvement in the trophoblast invasion and proliferation.
Keywords: Domestic dog, Endothelin (EDN)-system, Placenta, Uterus
The endothelins (EDNs) are 21 amino acid peptides which are known for their strong vasoactive properties, and they are mainly produced in response to hypoxia [1]. Their active forms are converted from a 212-amino acid precursor by the membrane-bound metalloproteinase, endothelin-converting enzyme-1 (ECE1) [2]. Three different isoforms of EDNs have been identified so far, designated as EDN1, -2 and -3 [3]. They are mainly derived from endothelium, and by acting through their EDNRA and EDNRB receptors can invoke arterial and venous vasoconstriction or vasodilatation, respectively. Whereas all EDNs show similar affinity to EDNRB, EDN1 and 2 have higher affinity to EDNRA than EDN3 [4, 5].
Although EDNs are mostly known for their vasomodulatory properties, numerous paracrine and/or autocrine effects have been demonstrated, e.g., induction of mitosis in placental fibroblasts and vascular smooth muscle cells [6,7,8]. In addition, analysis of pre-implantation lumen flushes and demonstration of receptor expression by uterine and conceptus cells in sheep suggest that EDN1 might play an important role in paracrine regulation of embryo development, implantation and early placentation [9]. Importantly, acting through EDNRA and EDNRB, EDN1 also stimulates in vitro proliferation of human trophoblast cells isolated from first trimester placenta, whereas its stimulatory properties on trophoblast invasion are EDNRB-dependent [10, 11]. Being less abundant during the first trimester, expression of EDN1 increases gradually towards term [7]. It is localized in the endothelium of fetal vessels, syncytiotrophoblast and extravillous cytotrophoblast in the human term placenta [12]. The concomitant presence of ECE1 in these cells supports the autocrine and paracrine function of EDN1 during placentation [13]. Less is known about the cellular utero-placental expression and function of EDN2 and -3.
Other than these data, knowledge is very limited regarding the potential role of EDNs and other vasomodulatory factors in the uterus and utero-placental (Ut-Pl) units during implantation and placentation in the canine species. However, especially when the invasive, endotheliochorial type of canine placentation is taken into consideration, adequate proliferation and invasion of trophoblasts must be critical for the species-specific decidualization, implantation and placentation in dogs [14,15,16].
Furthermore, in addition to the physiological importance of the EDN-system in trophoblast invasion and proliferation, it is believed to be involved in the pathophysiology of pre-eclampsia [17, 18]. As a pregnancy-specific, multisystem disorder, pre-eclampsia is characterized by high blood pressure frequently accompanied by severe functional damage to other organ systems, like the kidneys or liver. It is thought to originate in dysregulated trophoblast invasion and is now widely regarded as a systemic endothelial cell disease [19,20,21]. In patients suffering from pre-eclampsia, elevated levels of EDN1 were reported in blood serum [22]. Importantly, application of EDNRA receptor blockers to a pre-eclamptic rat model reduced hypertension [23, 24]. No such information regarding the potential role of vasoactive factors in placental function is available for the canine species, in which abnormal trophoblast invasion, possibly connected to aberrant vascularisation and tissue oxygenation, may result in severe conditions like subinvolution of placental sites (SIPS) [25, 26].
Therefore, in order to better understand the processes of implantation and placentation in the dog and, thereby, fill the existing knowledge gap, in this study we investigated the expression and localization patterns of EDNs (EDN-1, -2, -3) and EDN receptors (EDNRA and EDNRB), as well as of their activator ECE1, in the canine uterus and Ut-Pl compartments during pregnancy and at term. Potential progesterone-mediated effects were investigated in animals in which preterm parturition/abortion was induced by an antigestagen (aglepristone).
Materials and Methods
Tissue collection and preservation
Uterine and Ut-Pl samples from healthy, cross-breed pregnant bitches (2–8 years of age) used in our previous studies were also used here [27,28,29]. Stages of pregnancy were classified as pre-implantation (days 8–12, n = 5), post-implantation (days 18–25, n = 5), mid-gestation (days 35–40, n = 5) and prepartum luteolysis (n = 3). In addition, the progesterone (P4) receptor blocker (antigestagen) aglepristone (Alizine®, Virbac, Bad Oldesloe, Germany; 10 mg/kg bw; 2x/24 h apart) was applied to mid-pregnant dogs (days 40–45 of pregnancy; n = 10) to induce abortions and Ut-Pl units were obtained 24 h (n = 5) and 72 h (n = 5) after the second treatment.
Dogs were mated 2 days after ovulation (P4 > 5 ng/ml in peripheral blood), which is the time needed for oocyte maturation. The day of mating was defined as day 0 of pregnancy and the pre-implantation stage was detected by observation of embryos in uterine flushings under a stereomicroscope. Pre-partum luteolysis was defined based on measurement of P4 concentrations in serum every 6 h beginning from day 58 of pregnancy. When P4 concentrations continued to decline below 3 ng/ml in 3 consecutive measurements, ovariohysterectomy (OHE) was performed (the respective P4 concentrations are presented in [30]). The animal experiments and use of all tissue samples were approved by the respective authorities of the Justus-Liebig University, Giessen (permit no. II 25.3-19c20-15c GI 18/14 and VIG3-19c-20/15c GI 18,14), Giessen, Germany and the University of Ankara (permit no. Ankara 2006/06), Ankara, Turkey.
Following OHE, all uterine samples (pre-implantation) and Ut-Pl units (following implantation) were collected, rinsed in phosphate-buffered saline (PBS) and cleared of surrounding connective tissues as described before [29]. The Ut-Pl units (full-thickness, all anatomical tissue layers comprising the placenta and the adjacent uterus) were sampled from the middle part of the placental girdle avoiding marginal hematoma. For non-radioactive in situ hybridization (ISH) and immunohistochemistry (IHC) experiments, samples were immediately fixed in 10% neutral PBS-buffered formalin for 24 h at + 4°C, incubated in PBS for one week, dehydrated through ethanol series and embedded in paraffin wax. In order to isolate RNA, tissue samples were immersed in RNAlater® (Ambion Biotechnology GmbH, Wiesbaden, Germany) for 24 h at + 4°C and then stored at –80°C until analysis.
Total RNA extraction, reverse transcription and Real Time (TaqMan) PCR
Total RNA was extracted from the pre-implantation uterus and Ut-Pl compartments using TRIzol® reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocol. The quality and quantity of isolated RNA were measured using a NanoDrop 2000C® spectrophotometer (Thermo Fisher Scientific AG, Reinach, CH). In the next step, DNase-treatment was performed with RQ1 RNase-free DNase (Promega, Dübendorf, CH, Switzerland), followed by cDNA synthesis by reverse transcription (RT) with random hexamers used as starters and other RT reagents purchased from Applied Biosystems, Foster City, CA, USA, following the manufacturer’s instructions and in accordance with our previously published protocol [31, 32]. Semi-quantitative Real Time (TaqMan) PCR for all EDN-system members was performed on an ABI PRISM 7500 Sequence Detection System (Applied Biosystems) as described previously [32]. All reactions were run in duplicate and containing 200 nM TaqMan Probe, 300 nM of each primer, 12.5 μl Fast Start Universal Probe Master (ROX)® (Roche Diagnostics, Mannheim, Germany) and 5 μl cDNA corresponding to 100 ng total RNA for each target. As a control, experiments were run in the absence of the enzyme during reverse transcription step to check for any potential genomic DNA contamination (the so-called minus-RT control). Additionally, water was used instead of cDNA. The amplification conditions were as follows: denaturation at 95°C for 10 min, followed by 40 cycles: 95°C for 15 sec and 60°C for 60 sec. Selected PCR products from all targets were sent for sequencing (Microsynth, Balgach, CH, Switzerland). Results were quantified with the comparative CT (ΔΔCT) method using three reference genes (GAPDH, 18SrRNA and CYCLOPHILIN A) as normalizers. The sample with the lowest concentration of the target gene was used as the calibrator. The reaction efficiency was calculated with the CT slope method and was set up for approximately 100%. The following primers and TaqMan Probes were used: GAPDH forward: 5’-GCT GCC AAA TAT GAC GAC ATC A-3’, reverse: 5’-GTA GCC CAG GAT GCC TTT GAG-3’, TaqMan probe: 5’-TCC CTC CGA TGC CTG CTT CAC TAC CTT-3’ (GenBank: AB028142, amplicon length: 75bp); 18SrRNA forward: 5’-GTC GCT CGC TCC TCT CCT ACT-3’, reverse: 5’-GGC TGA CCG GGT TGG TTT-3’, TaqMan probe: 5’-ACA TGC CGA CGG GCG CTG AC-3’ (GenBank: FJ797658, amplicon length: 125bp); EDNRA forward: 5’-GGC CCCAAC GCA CTG ATA-3’, reverse: 5’-CCC GCC AGA AGC TTA AAC AC-3’, TaqMan probe: 5’-CCA GCC TTG CCC TTG GAG ACC TTA TC-3’ (GenBank: NM_001031632.1, amplicon length: 92bp); EDNRB forward: 5’-CAT CAT CGG GAA CTC CAC ACT-3’, reverse: 5’-CAG AGC CAG GCT GGC TAT CA-3’, TaqMan probe: 5’-CAA GAA CAA GTG CAT GCG AAA CGG C-3’ (GenBank: NM_001010943.2, amplicon length: 91bp). The following commercially available TaqMan Gene Expression Assays from Applied Biosystems were used: EDN1 (prod. no. Cf02622421_m1), EDN2 (prod. no. Cf02622240-m1), EDN3 (prod. no. Cf02622419-g1), ECE1 (prod. no. Cf02627515-m1) and CYCLOPHILIN A (prod. no. Cf03986523-gH).
Immunohistochemistry
Our standard immunoperoxidase IHC assay was performed [31, 33] in order to identify the localization of EDN1, ECE1 and the two EDN receptors (EDNRA and EDNRB) in the uterus and Ut-Pl compartments. Briefly: tissues were sectioned at 2–3 μm thickness, mounted onto SuperFrost microscope slides (Menzel-Glaeser, Braunschweig, Germany), deparaffinized with xylene and rehydrated in an ethanol series. This was followed by antigen retrieval in a microwave oven at 560 W for 15 min in 10 mM citrate buffer pH 6.0. Then, sections were cooled down and treated with 0.3% hydrogen peroxide in methanol for 30 min, blocked with 10% normal serum from the same species in which the secondary antibody was produced, and overlaid with primary antibodies overnight at 4°C. These were: affinity purified goat polyclonal anti-EDNRA (sc-21194; dilution 1:50), affinity purified goat polyclonal anti-EDNRB (sc-21196; dilution 1:200), affinity purified goat polyclonal anti-ECE1 (sc-27558; dilution 1:300), all from Santa Cruz Biotechnology, CA, USA; and mouse monoclonal anti-EDN1 (E166; dilution 1:300) purchased from Sigma-Aldrich, St. Louis, MO, USA. No commercial anti-EDN2 canine-specific antibody was available for the present study. In addition, monoclonal mouse anti-VIMENTIN IgG2a (M7020; Clone 3B4), dilution 1:100 (Dako Schweiz AG, Basel, Switzerland) and affinity-purified polyclonal rabbit anti-CYTOKERATIN, wide spectrum screening, dilution 1:300 (Dako Schweiz AG) antibodies were used. The specificity of all antibodies was checked by replacing the primary antibody with non-immune IgGs of the same species instead of the primary antibody (serving as the so-called isotype control), applied at the same protein concentration. In the next step, sections were washed with IHC buffer/0.3% Triton X pH 7.2–7.4 (0.8 mM Na2HPO4, 1.47 mM KH2PO4, 2.68 mM KCl, 137 mM NaCl), and incubated with the following biotin-labeled secondary antibodies at 1:100 dilution: horse anti-goat IgG BA-9500, horse anti-mouse IgG BA2000 and goat anti-rabbit IgG BA1000 (all purchased from Vector Laboratories, Burlingame, CA, USA). The intensity of signals was enhanced by applying the streptavidin-peroxidase Vectastain ABC kit (Vector Laboratories) for 30 min. Immune reactions were visualized with the Liquid DAB+ substrate kit (Dako Schweiz AG). Finally, slides were counter-stained in hematoxylin, dehydrated in a graded ethanol series and xylene, and mounted in Histokit® (Assistant, Osterode, Germany).
In situ hybridization
Non-radioactive ISH was performed on paraffin-embedded tissue slides in order to localize EDN1, -2, ECE1, EDNRA and EDNRB mRNA based on our previously published protocol [33, 34]. Briefly, RT-PCRs were performed for complementary RNA (cRNA) probe synthesis using the following canine-specific primers:
EDN1 forward: 5`-CCA CAG GAA GAG ATG CCA GT-3`;
EDN1 reverse: 5`-GAT GGC GTC CAA CCT TCT TA-3` (amplicon length 205bp);
EDN2 forward: 5`-ACA TCA TCT GGG TGA ACA CT-3`;
EDN2 reverse: 5`-CCT AGG AAA GCG GAT CTT-3` (amplicon length 281bp) ;
ECE1 forward: 5`-CCC CTG ATG GAG CTA ATT GA-3`;
ECE1 reverse: 5`-GCC CAG TTG GAC CAT GTA GT-3` (amplicon length 276bp) ;
EDNRA forward: 5`-AGG GTG AAC AGC ACA AAA CC-3`;
EDNRA reverse: 5`-CTT AAG TGA AGC GGG AAC CA-3` (amplicon length 280bp)
EDNRB forward: 5`-ACG TTA CCT GCG AAT CTG CT-3`;
EDNRB reverse: 5`-GCT TCA AAA TCC TGC TGA GG-3` (amplicon length 293bp).
Following PCR, amplicons were separated on ethidium bromide-stained 2% agarose gel, cut from the gel, purified using the Qiaex II gel extraction system (Qiagen GmbH, Hilden, Germany) and ligated into the pGEM-T vector (Promega, Duebendorf, CH, Switzerland).
The incorporation of PCR products into the pGEM-T vector was verified by control digestion with NcoI and NotI restriction enzymes (New England Biolabs, Frankfurt, Germany). Plasmids carrying the amplicons were sent for sequencing (Microsynth). Then, selected clones were linearized with NcoI (antisense, positive probe) and NotI (sense probe, negative control) restriction enzymes and labeled using the DIG-RNA labelling kit according to the manufacturer’s protocols (Roche Diagnostics).
Tissue sections (2–3 μm) were cut, mounted on SuperFrost Plus microscope slides (Menzel-Glaeser), deparaffinized in xylene, rehydrated in an ethanol series and digested with proteinase K (70 μg/ml, Boehringer, Mannheim, Germany), followed by hybridization overnight at 37°C using cRNA probes as described previously [34]. Then, following incubation of the probed slides with alkaline phosphatase-conjugated, sheep anti-DIG Fab Fragments (Roche Diagnostics, dilution 1:5000), signals were detected with the substrate 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium (NBT/BCIP; Roche Diagnostics).
Statistics
The effects of the observation group on target gene expression were assessed by applying a parametric one-way ANOVA. In case of P < 0.05, either the Tukey-Kramer multiple comparisons post-test was performed in order to detect the effect of pregnancy stage on expression of EDN system members at different time points during pregnancy, or Dunnett’s multiple comparison test in experiments investigating the effects of aglepristone treatment on target gene expression; results show the n-fold change in gene expression compared with its expression at mid-gestation (non-treated control). The statistical software program GraphPad 3.06 (GraphPad Software) was used for all tests. Numerical data are presented as the mean ± S.D.
Results
Expression profile of the EDN system in canine uterus and Ut-Pl compartments throughout gestation and at normal prepartum luteolysis.
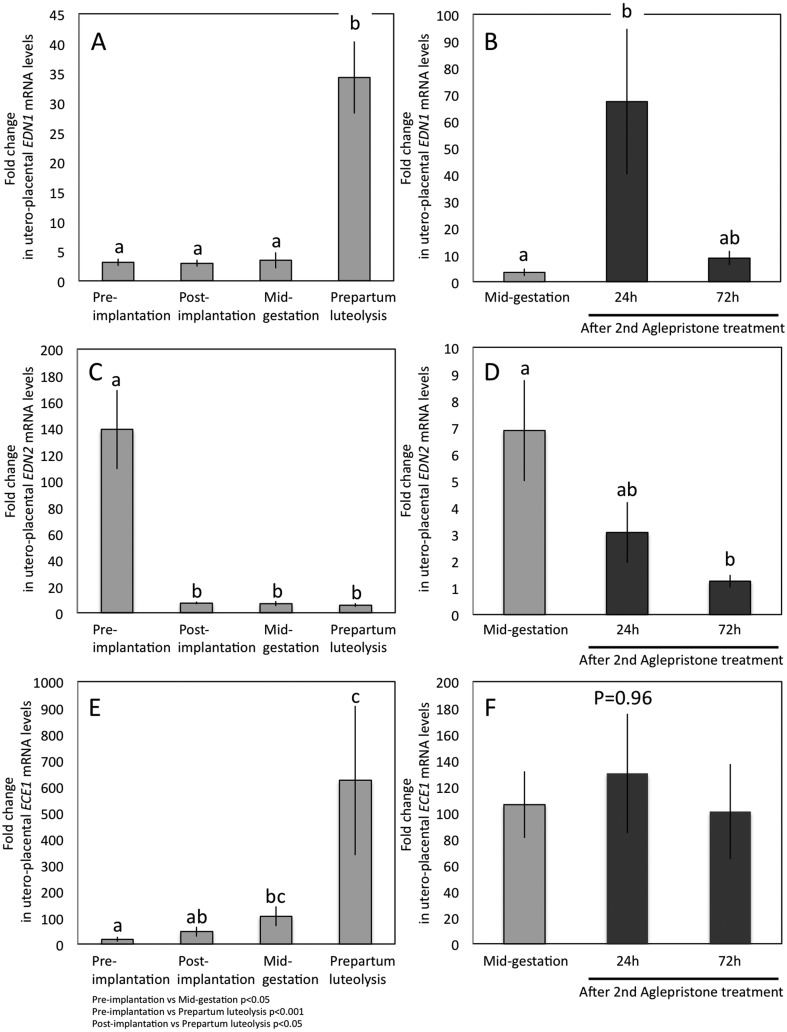
A time-dependent expression profile of EDN1, -2, ECE1 and both EDN receptors (EDNRA and EDNRB) was detectable in all samples (Fig. 1A, C, E and Fig. 2A, C). Specifically, EDN1 mRNA was expressed constantly from pre-implantation until mid-gestation, and increased strongly (P < 0.001) during prepartum luteolysis compared with previous gestational stages (Fig. 1A). The opposite expression pattern was observed for EDN2 mRNA: it was highest at the pre-implantation stage of pregnancy and was significantly suppressed (P < 0.001) following implantation and placentation, remaining low until prepartum luteolysis (Fig. 1C). The expression of EDN3 was generally low and even frequently below the detection limit in both uterine and Ut-Pl samples throughout pregnancy, normal and induced parturition, precluding quantification of its expression (data not shown). The expression of mRNA encoding for ECE1, responsible for converting and thereby activating EDNs, showed a gradual increase following placentation towards mid-gestation (P < 0.05) (Fig. 1E). It reached the highest levels at prepartum luteolysis compared with pre-implantation and post-implantation stages (P < 0.001 and P < 0.05, respectively), resembling the expression pattern observed for EDN1.
Fig. 1.
Expression of EDN1, -2 and ECE1-mRNA as determined by Real Time (TaqMan) PCR (mean ± SD) in the canine pre-implantation uterus and utero-placental (Ut-Pl) compartments during pregnancy (A, C, E) and at aglepristone-induced parturition/abortion in mid-pregnant dogs (B, D, F). (B, D and F; compared with the mid-pregnancy group as a non-treated control). Bars with different letters differ at P < 0.001 in (A), P < 0.05 in (B) or at P < 0.01 in (D).
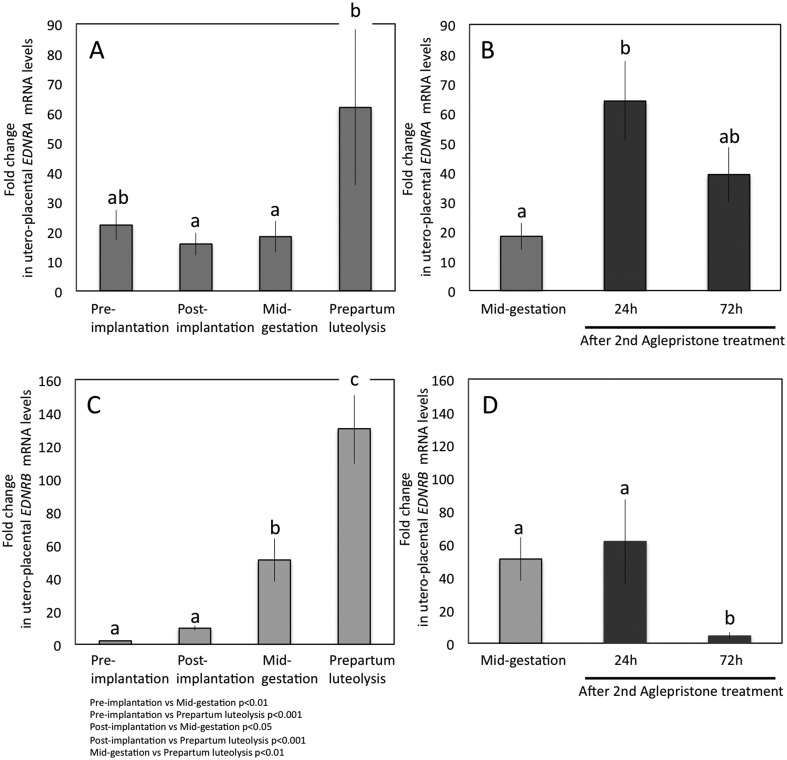
Fig. 2.
Expression of EDNRA and EDNRB-mRNA as determined by Real Time (TaqMan) PCR (mean ± SD) in canine pre-implantation uterus and utero-placental (Ut-Pl) compartments during pregnancy (A, C) and at aglepristone-induced parturition/abortion in mid-pregnant dogs (B, D). (B and D; compared with the mid-pregnancy group as a non-treated control). Bars with different letters differ at P < 0.05 in (A, B) or at P < 0.001 in (D).
Regarding the EDN receptors, like EDN1, EDNRA mRNA was constantly expressed during pregnancy and was strongly upregulated at prepartum luteolysis, compared with all other gestational time points (P < 0.05) (Fig. 2A). A similar expression pattern was observed for EDNRB, the expression of which was, however, strongly elevated at mid-gestation (P < 0.01 and P < 0.001 compared with pre-implantation and post-implantation stages, respectively) (Fig. 2C). There was a further, significant strong increase of EDNRB towards prepartum luteolysis (P < 0.01) (Fig. 2C).
Effects of antigestagen treatment on expression of the EDN system in canine Ut-Pl units during mid-gestation
In order to examine the effects of the antigestagen aglepristone treatment on target gene expression during mid-gestation, corresponding samples from mid-pregnant dogs were used as non-treated controls. The expression levels of EDN1 and EDNRA increased significantly (P < 0.05 and P < 0.05, respectively) 24 h after the second treatment. Their mRNA levels were, however, diminished 48 h later, i.e., at the 72 h time point, and did not differ significantly from controls (Fig. 1B and Fig. 2B). Whereas EDN2 decreased gradually but significantly (P < 0.01) towards 72 h after the second application of aglepristone, the expression of EDNRB was significantly suppressed at 72 h compared with the control and the 24 h time point (P < 0.01) (Fig. 1D and Fig. 2D). The expression of ECE1 remained unaffected by treatment with antigestagen (P > 0.05) (Fig. 1F).
Localization of EDN system in the canine uterus and placenta
Localization of EDN1, ECE1 and EDN receptors (EDNRA and EDNRB) was determined in canine pre-implantation uterus and Ut-Pl compartments at the protein and transcript levels by IHC and ISH. Due to the lack of canine-specific and/or cross-reacting antibody, expression of EDN2 was localized only at the mRNA level. During pre-implantation, strong positive signals for EDN1 were observed in the endometrial surface epithelium, superficial and deep uterine glands and myometrium (Fig. 3A, B). Relatively weaker staining was observed in the connective tissues of stromal compartments. A similar localization pattern of EDN1 in the uterine part of the Ut-Pl compartments was also observed following implantation, however, with signals appearing stronger at prepartum luteolysis (Fig. 3F, G), compared, e.g., with mid-gestation (Fig. 3C, D). The placental localization of EDN1 was observed in fetal trophoblast cells, predominantly in cytotrophoblast, throughout the placentation period, with signals noticeably stronger at prepartum luteolysis (Fig. 3E, H).
Fig. 3.
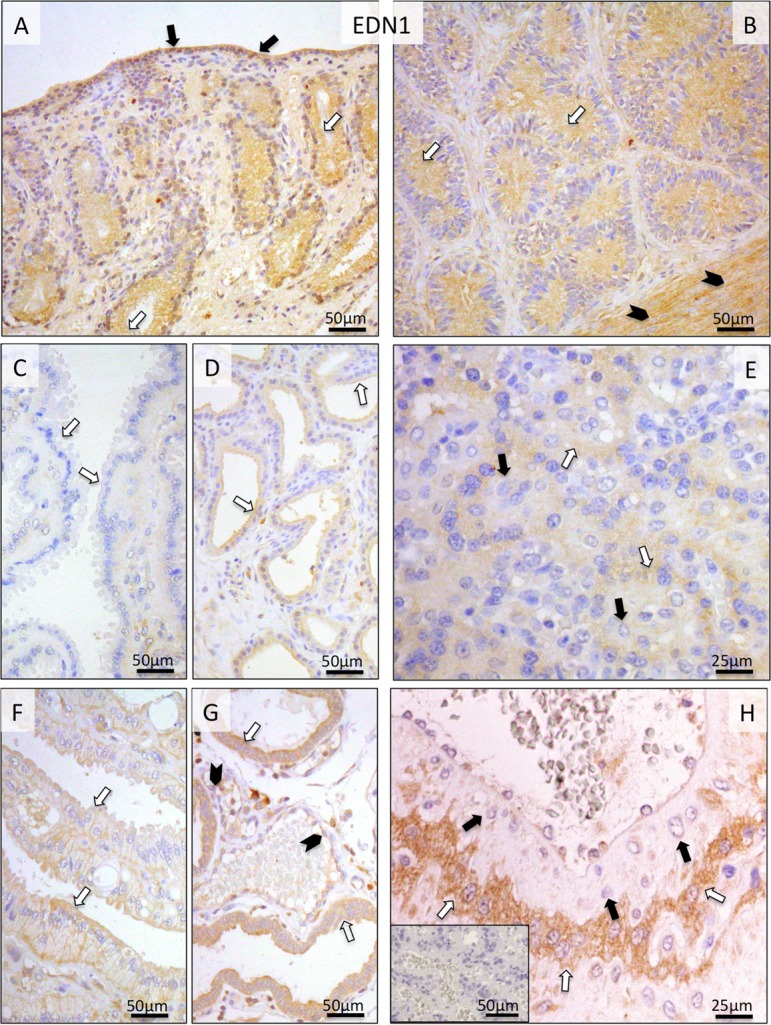
Immunohistochemical (IHC) localization of EDN1 in the canine uterus and utero-placental (Ut-Pl) compartments at selected time points during pregnancy; at the pre-implantation stage (A and B), in the Ut-Pl units during mid-gestation (C, D and E), and in the Ut-Pl compartments at prepartum luteolysis (F, G and H). (A, B) At pre-implantation, EDN1 is localized to the endometrial luminal (surface) epithelial cells (solid arrows in A), glandular epithelial cells of the superficial (open arrows in A) and deep uterine glands (open arrows in B) and myocytes (solid arrowheads in B). During mid-gestation, within the Ut-Pl units endometrial EDN1 expression appears weaker and is detected in the superficial glands (the so-called glandular chambers) and deep uterine glands (open arrows in C and D, respectively). During prepartum luteolysis, endometrial EDN1 expression is detected in the superficial glands (the so-called glandular chambers), deep uterine glands (open arrows in F and G, respectively) and endothelial cells (solid arrowheads in G). In the placental labyrinth during mid-gestation, signals are localized in fetal trophoblast cells (cytotrophoblast) (open arrows in E). At prepartum luteolysis, fetal trophoblast (cytotrophoblast) stains strongly (open arrows in H). No or only weak signals were detected in maternal stroma-derived decidual cells (solid arrows in E and H). There is no background staining in the isotype control (inserted in H).
As for ECE1, during pre-implantation its uterine endometrial signals were localized to the epithelial cells of the endometrial surface, superficial and deep uterine glands, as well as to the myometrium and media of the vessels (Fig. 4A, B). The uneven distribution pattern of signals in the surface epithelium can be most probably attributed to the relatively low protein expression level in this compartment or to the sensitivity limits of the antibody. Following implantation until prepartum luteolysis, no or only weak signals were detected in the uterus (Fig. 4C, D, H, I). In order to facilitate characterization of the localization pattern of ECE1 within the specific cellular components of the placenta (i.e., in endothelial cells, fetal trophoblast and maternal decidual cells), differential staining was performed on consecutive sections using anti-VIMENTIN or anti-(pan)CYTOKERATIN antibodies (Fig. 4E and F, respectively). Whereas during earlier stages of gestation (post-implantation and mid-gestation) both cyto- and syncytiotrophoblast stained intensely for ECE1, during pre-partum luteolysis the signals were mainly localized in the cytotrophoblast (Fig. 4G, J). Much weaker staining was observed in maternal stroma-derived decidual cells (Fig. 4G, J).
Fig. 4.
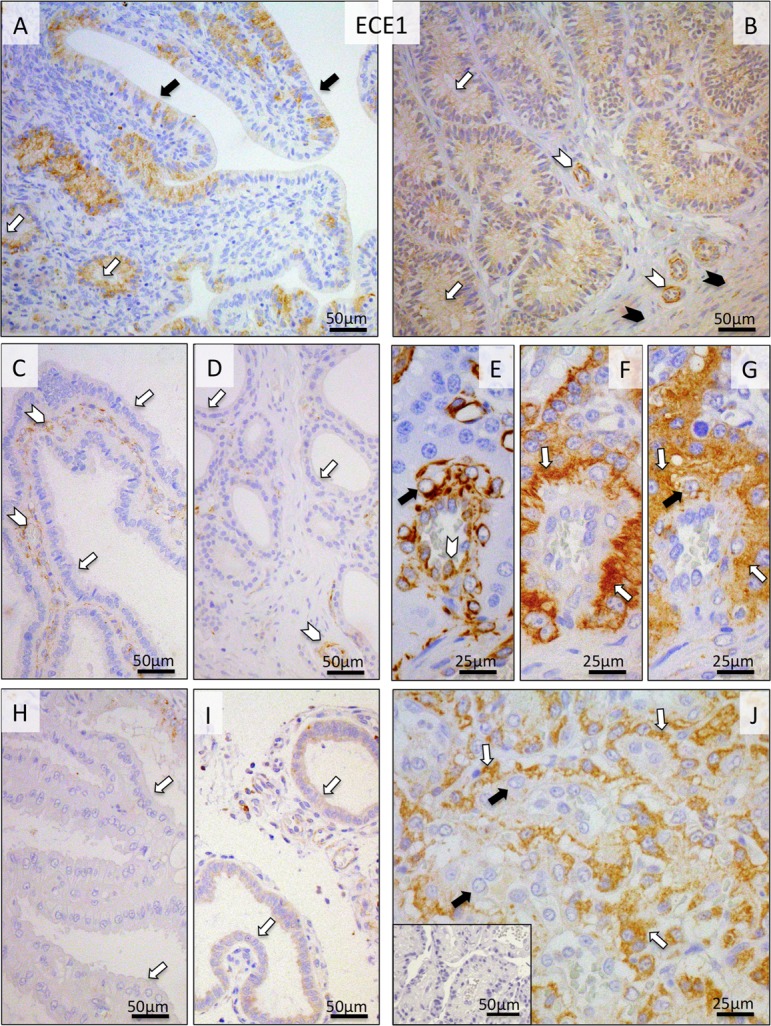
Immunohistochemical (IHC) localization of ECE1 in the canine uterus and utero-placental (Ut-Pl) compartments at selected time points during pregnancy and at prepartum luteolysis; at the pre-implantation stage (A and B), in the Ut-Pl units during mid-gestation (C, D and G), and in the Ut-Pl compartments at prepartum luteolysis (H, I, J). (A, B) At pre-implantation, signals are predominantly localized to the endometrial luminal (surface) epithelial cells (solid arrows in A), glandular epithelial cells of the superficial and deep uterine glands (open arrows in A and B), in media of the vessels (open arrowheads in B) and myocytes (solid arrowheads in B). During mid-gestation and at prepartum luteolysis, within the Ut-Pl units, endometrial expression of ECE1 appears weaker and is detected in tunica media of the vessels (open arrowheads in C and D), the superficial glands (so-called glandular chambers) and deep uterine glands (open arrows in C, H, and D, I, respectively). In the placental labyrinth during mid-gestation, signals are localized in fetal trophoblast cells (i.e., syncytio- and cytotrophoblast) (open arrows in G). In order to distinguish between fetal and maternal cell types within the canine placenta, i.e., endothelial, trophoblast and decidual cells, (pan) CYTOKERATIN (wide spectrum) and VIMENTIN staining was applied to consecutive sections following those used for ECE1. Trophoblast cells stain positively for CYTOKERATIN (open arrows in F), whereas endothelial cells (open arrowhead in E) and decidual cells (solid arrow in E) stain positively for VIMENTIN. At prepartum luteolysis, fetal cytotrophoblasts stain strongly (open arrows in J) for ECE1. No or only weak signals can be seen in maternal decidual cells (solid arrows in G and J). There is no background staining in the isotype control (inserted in J).
The uterine expression of EDNRA receptor was generally weak throughout pregnancy, with some signals localized to uterine epithelial compartments (Fig. 5A, B, C, D). This strongly contrasted with its placental expression during prepartum luteolysis. Following relatively low expression during earlier stages of pregnancy, strong EDNRA signals were detected prepartum in the fetal cytotrophoblast, especially at the base of the placental labyrinth (Fig. 5E, F).
Fig. 5.
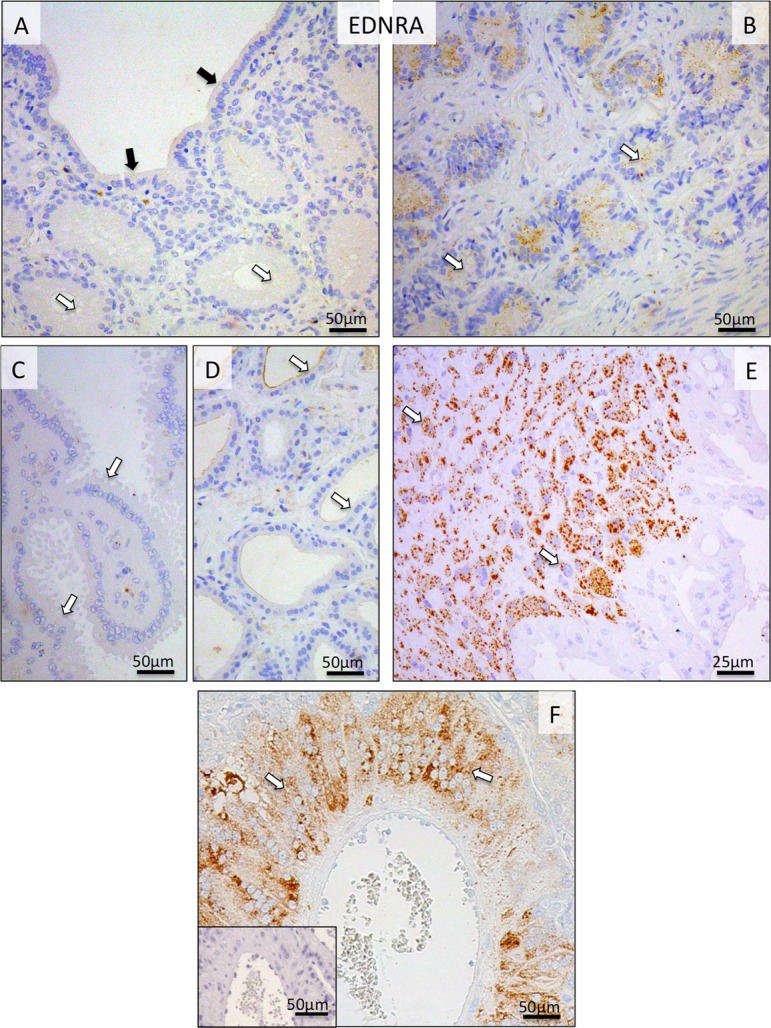
Immunohistochemical (IHC) localization of EDNRA in the canine uterine and utero-placental (Ut-Pl) compartments at selected time points during pregnancy; at the pre-implantation stage (A and B), and in the Ut-Pl compartments at prepartum luteolysis (C, D, E and F). (A, B) At pre-implantation, the signals for EDNRA in endometrial surface epithelial cells (solid arrows in A), superficial and deep uterine glands are weak (open arrows in A and B, respectively). During prepartum luteolysis, within the Ut-Pl units, only weak endometrial EDNRA expression is observed in the superficial glands (the so-called glandular chambers) and deep uterine glands (open arrows in C and D, respectively). In the placental labyrinth during prepartum luteolysis, cytotrophoblast cells at the base of the placental labyrinth stain strongly (open arrows in E and F). There is no background staining in the isotype control (inserted in F).
Regarding EDNRB, its uterine expression and cellular localization patterns were similar to those observed for EDN1 and ECE1. Thus, signals were detected in luminal epithelial cells, superficial and deep uterine glands and myometrium (Fig. 6A, B). However, after placentation (Fig. 6C, D) and during prepartum luteolysis, no or relatively weak signals were observed in the uterine part of Ut-Pl units. In the placental labyrinth, strong EDNRB signals were detected predominantly in syncytiotrophoblast cells from post-implantation until mid-gestation (Fig. 6E). Interestingly, however, the signals were spread over the entire trophoblast (i.e., syncytio- and cytotrophoblast) at the time of prepartum luteolysis (Fig. 6F). Anti-VIMENTIN staining was used on consecutive sections for easier differentiation of cells (Fig. 6G).
Fig. 6.
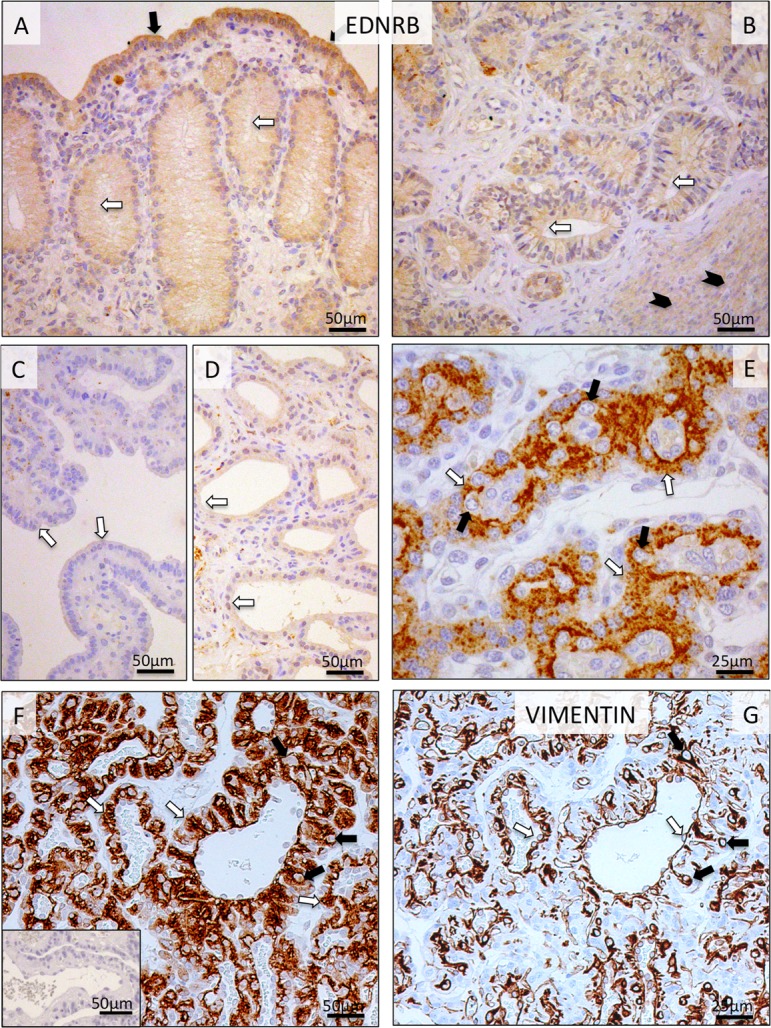
Immunohistochemical (IHC) localization of EDNRB in the canine uterine and utero-placental (Ut-Pl) compartments at selected time points during pregnancy; at the pre-implantation stage (A and B), in the Ut-Pl units during mid-gestation (C, D and E), and in the Ut-Pl compartments at prepartum luteolysis (F). (A, B) At pre-implantation, staining of EDNRB is predominantly localized to the endometrial luminal (surface) epithelial cells (solid arrows in A), glandular epithelial cells of the superficial and deep uterine glands (open arrows in A and B) and myocytes (solid arrowheads in B). During mid-gestation, within the Ut-Pl units, weak endometrial EDNRB expression is detected in the superficial glands (the so-called glandular chambers) and deep uterine glands (open arrows in C and D, respectively). In the placental labyrinth during mid-gestation, strong signals are localized in fetal syncytiotrophoblast cells (open arrows in E). At prepartum luteolysis, only fetal trophoblast (i.e., syncytio- and cytotrophoblast) stains strongly (open arrows in F). No, or sporadically only weak signals, can be identified in maternal decidual cells (solid arrows in E and F). In order to distinguish between fetal and maternal cell types within the canine placenta, i.e., endothelial, trophoblast and decidual cells, VIMENTIN staining was performed on consecutive sections following those used for EDNRB. Endothelial cells (open arrows in G) and decidual cells (solid arrows in G) stain positively for VIMENTIN. There is no background staining in the isotype control (inserted in F).
Similar localization patterns of EDN1, ECE1, EDNRA and EDNRB were observed in placental labyrinth by non-radioactive ISH (Fig. 7).
Fig. 7.
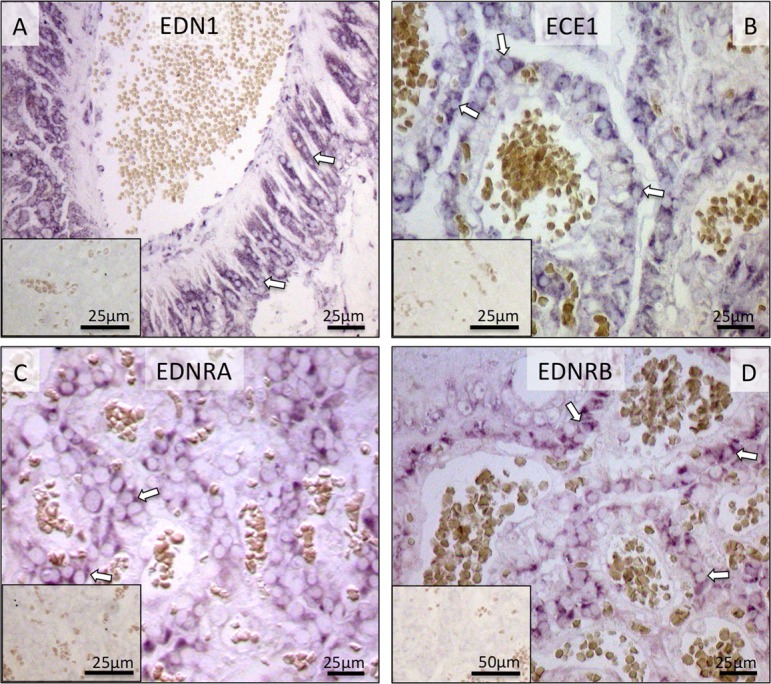
Localization of EDN1, ECE1, EDNRA and EDNRB mRNA in the canine utero-placental (Ut/Pl) units during prepartum luteolysis as determined by in situ Hybridization (ISH). All factors are localized in trophoblast cells (open arrows; A–D). There is no background staining in the negative controls (insert to A–D).
Due to the lack of commercially available canine-specific antibody, localization of EDN2 was determined at the transcript level. The localization pattern of EDN2 mRNA greatly resembled that of EDN1. In the uterus, it was localized predominantly in epithelial compartments (Fig. 8A, B). It was clearly detectable during pre-implantation, whereas during prepartum luteolysis it was barely detectable (Fig. 8C, D). In Ut-Pl units, the placental localization of EDN2 was targeted to the fetal trophoblast cells (Fig. 8E).
Fig. 8.
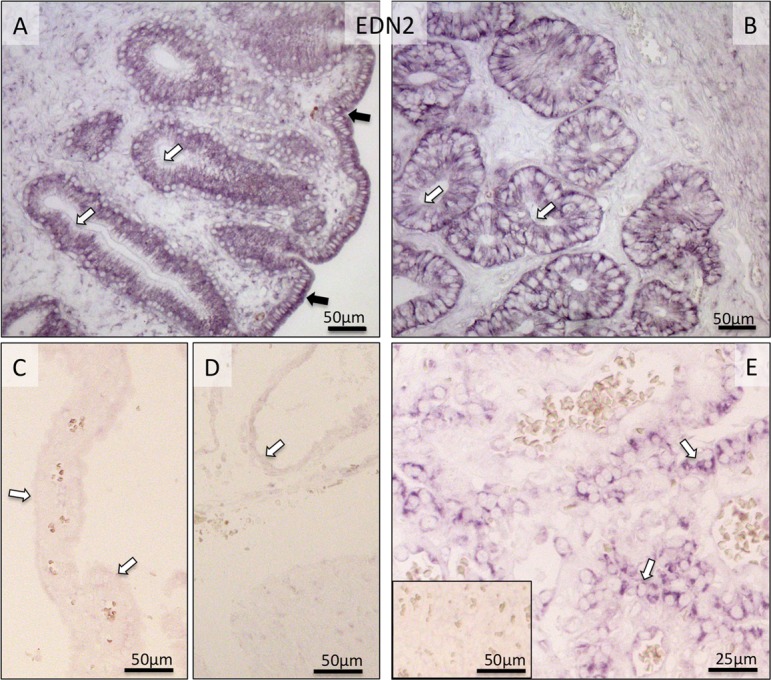
Localization of EDN2-mRNA as determined by in situ Hybridization (ISH) in the canine uterus at the pre-implantation stage (A and B) and in the utero-placental (Ut-Pl) compartments during prepartum luteolysis (C, D and E). (A, B) At pre-implantation, EDN2-mRNA is localized to the endometrial luminal (surface) epithelial cells (solid arrows in A), glandular epithelial cells of the superficial (open arrows in A) and deep uterine glands (open arrows in B). During prepartum luteolysis, only weak or no signals are visible for EDN2 expression in the superficial glands (the so-called glandular chambers) and deep uterine glands (open arrows in C and D, respectively). In the placental labyrinth during prepartum luteolysis, fetal trophoblast cells stain strongly (open arrows in E). There is no background staining in the negative control (inserted in E).
Discussion
Unequivocally, angiogenesis and vasculogenesis are critical steps responsible for successful decidualization, implantation and placentation. While they have been intensively investigated in other mammalian species, so far, little is known about these processes in the canine species. Therefore, in the current study, we felt prompted to investigate the expression and localization patterns of the members of the EDN family, i.e., EDN1, -2, -3, as well as of the EDN-activating enzyme ECE1 and the two EDN receptors, EDNA and EDNB, in the canine uterus and Ut-Pl compartments during selected time points of gestation (pre-implantation, post-implantation, mid-gestation), and during normal parturition (prepartum luteolysis) and antigestagen-induced abortion.
In agreement with previously published data from other species, e.g., humans [35, 36] or sheep [37] , there was clearly detectable protein expression of EDN1 and -2, the converting enzyme (ECE1) and one of the receptors, EDNRB, particularly in the epithelial uterine compartments during the pre-implantation stage of pregnancy. Interestingly, EDN2 seems to be the major isoform represented during the early stages of pregnancy in the dog. This finding corresponds positively with observations made in mice, in which EDN2 was highly represented in some organs like the uterus, ovary or intestine [38]. It appears plausible that also in the dog, prior to implantation, EDN2, which is colocalized with EDNRB, might be involved in facilitating the release of NO [39]. This, in turn, would imply the indirect involvement of EDN2 in regulation of uterine proliferation, vascular permeabilization and edema that are important for uterine remodeling, embryo attachment and implantation. It is also interesting that EDN2 was absent from the ovaries of knockout mice lacking expression of the P4 receptor (PGR), indicating a functional relationship between expression and function of these two entities [40]. Taking into account the high uterine content of this peptide prior to implantation and its significantly decreased expression in UT-Pl compartments of mid-pregnant dogs in response to aglepristone-treatment, such relationship might also apply in the dog uterus.
Furthermore, the expression and colocalization of EDN-family members, i.e., the receptors, their ligands and the EDN-activating ECE1, within the placenta fetalis (trophoblast) of the Ut/Pl compartments, suggests a possible functional role of this system in trophoblast proliferation and invasion. If proved, this would resemble the situation described previously for human trophoblasts, whose proliferative and invasive properties are mediated by the EDNs receptor [10, 11].
EDNRB is known as a potent vasodilator [41], whereas EDNRA exhibits strong vasoconstrictor properties [42]. Thus, the gradual increase of EDNRB expression towards mid-gestation, paralleling the progression of canine gestation, and its abundant presence in the fetal trophoblast, implies the possible involvement of EDNRB in modulating vascular permeability and thereby facilitating blood flow in feto-maternal units. It is noteworthy that its placental expression shown herein is colocalized with cellular localization of another potent vascular permeability factor, which is VEGFA [43].
During prepartum luteolysis, placental expression of the EDN receptors, as well as ECE1 and EDN1, being predominantly associated with trophoblast cells, strongly resembles the placental localization of prostaglandin (PG) family members regulating the availability of prepartum prostaglandins in the dog, e.g., cyclooxygenase (COX2/PTGS2), PTGFS/AKR1C3 or PTGES. The functional involvement of EDNs in PG synthesis has been shown previously [27,28,29], implying that EDNs play a role in the regulation of utero-placental hemodynamics affecting the physiological mechanisms of parturition. Whether this could also apply in the canine species remains to be elucidated. Nevertheless, the upregulation of EDN1 and EDNRA expression not only during normal prepartum luteolysis but also during antigestagen-induced parturition/abortion, which in both situations is associated with increased PG output, implies a temporal association between PGs and EDNs during prepartum luteolysis in the dog.
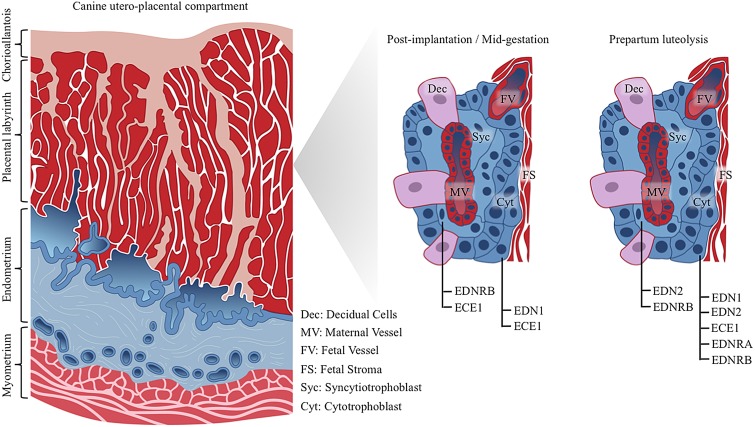
It is noteworthy that the spatial expression of ECE1 and EDNRB changed during gestation. Whereas ECE1 was initially expressed both in syncytio- and cytotrophoblast, its expression prepartum was predominantly restricted to the cytotrophoblast and was colocalized with the increased availability of EDN1 and EDNRA in the same cells. In contrast, the expression of EDNRB, which was initially localized mostly in the syncytiotrophoblast, spread over the entire trophoblast at the time of its increased expression during prepartum luteolysis. Even if this implies spatio-temporal differences in the biological effects evoked by EDNs during the maintenance and termination of canine gestation, the functional meaning of these observation remains to be further investigated. Schematic representation of placental EDN system distribution within canine utero-placental compartments is shown in Fig. 9.
Fig. 9.
Schematic representation of placental EDN system distribution within canine utero-placental compartments (placenta endotheliochorialis). Post-implantation and at mid-gestation similar localization patterns are observed for EDNRB, ECE1 and EDN1. Whereas ECE1 is localized both, in syncytiotrophoblast and cytotrophoblast cells, EDN1 targets only to cytotrophoblast cells, and EDNRB stains positively in syncytiotrophoblast. During prepartum luteolysis, both types of fetal trophoblast cells stain positively for EDN2 and EDNRB. EDN1, ECE1 and EDNRA are localized only in cytotrophoblast cells. As determined by in situ hybridization (ISH), localization patterns of EDN1, ECE1, EDNRA and EDNRB mRNA were similar with expression profiles of the respective proteins (investigated by immunohistochemistry). Due to the lack of commercially available canine-specific antibody, localization of EDN2 was determined at the transcript level by ISH.
The time-dependent uterine and placental expression of the EDN system, and in particular its strong abundance in fetal trophoblast cells throughout pregnancy, implies its involvement in the functional proliferative and invasive properties of these cells. The functional approach presented here, consisting of antigestagen-treated animals, leading to the upregulation of fetal placental EDN1 and its vasoconstrictor receptor EDNRA, resembling thereby the situation observed at normal parturition, suggests that they are involved in initiating the signaling cascade of PG synthesis leading to the induction of parturition in the dog.
Acknowledgments
Authors are grateful to Prof Dr Selim Aslan, Near East University, Cyprus (formerly of the University of Ankara, Turkey), and Prof Dr Bernd Hoffmann, Justus-Liebig University Giessen, Germany, and their teams for provision of the tissue material, and to Dr Barry Bavister for careful editing of the manuscript. The technical expertise and contributions of Elisabeth Högger are greatly appreciated. Part of the laboratory work was performed using the logistics at the Center for Clinical Studies, Vetsuisse Faculty, University of Zurich. This research was supported by the Swiss National Science Foundation (SNSF); research grant number 31003A_160251. The authors declare that they have no conflict of interests. All authors read and approved the final version of the manuscript.
References
- 1.Kourembanas S, Marsden PA, McQuillan LP, Faller DV. Hypoxia induces endothelin gene expression and secretion in cultured human endothelium. J Clin Invest 1991; 88: 1054–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988; 332: 411–415. [DOI] [PubMed] [Google Scholar]
- 3.Inoue A, Yanagisawa M, Kimura S, Kasuya Y, Miyauchi T, Goto K, Masaki T. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci USA 1989; 86: 2863–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature 1990; 348: 730–732. [DOI] [PubMed] [Google Scholar]
- 5.Clouthier DE, Hosoda K, Richardson JA, Williams SC, Yanagisawa H, Kuwaki T, Kumada M, Hammer RE, Yanagisawa M. Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice. Development 1998; 125: 813–824. [DOI] [PubMed] [Google Scholar]
- 6.Fant ME, Nanu L. Human placental endothelin: expression of endothelin-1 mRNA by human placental fibroblasts in culture. Mol Cell Endocrinol 1995; 109: 119–123. [DOI] [PubMed] [Google Scholar]
- 7.Fant ME, Nanu L, Word RA. A potential role for endothelin-1 in human placental growth: interactions with the insulin-like growth factor family of peptides. J Clin Endocrinol Metab 1992; 74: 1158–1163. [DOI] [PubMed] [Google Scholar]
- 8.Goldie RG. Endothelins in health and disease: an overview. Clin Exp Pharmacol Physiol 1999; 26: 145–148. [DOI] [PubMed] [Google Scholar]
- 9.Riley SC, Findlay JK, Salamonsen LA. Endothelin-1 and endothelin receptors are present in the sheep uterus and conceptus at implantation. J Endocrinol 1995; 147: 235–244. [DOI] [PubMed] [Google Scholar]
- 10.Chakraborty C, Gleeson LM, McKinnon T, Lala PK. Regulation of human trophoblast migration and invasiveness. Can J Physiol Pharmacol 2002; 80: 116–124. [DOI] [PubMed] [Google Scholar]
- 11.Cervar-Zivkovic M, Dieber-Rotheneder M, Barth S, Hahn T, Kohnen G, Huppertz B, Lang U, Desoye G. Endothelin-1 stimulates proliferation of first-trimester trophoblasts via the A- and B-type receptor and invasion via the B-type receptor. J Clin Endocrinol Metab 2011; 96: 3408–3415. [DOI] [PubMed] [Google Scholar]
- 12.Graf AH, Hütter W, Hacker GW, Steiner H, Anderson V, Staudach A, Dietze O. Localization and distribution of vasoactive neuropeptides in the human placenta. Placenta 1996; 17: 413–421. [DOI] [PubMed] [Google Scholar]
- 13.Ahmad Z, Reznik SE. Immunohistochemical localization of ECE-1 in the human placenta. Placenta 2000; 21: 226–233. [DOI] [PubMed] [Google Scholar]
- 14.Finn CA. Implantation. In: Lamming GE (ed.), Marshall’s physiology of reproduction. Volume 3: pregnancy and lactation. Part one: ovulation and early pregnancy. Fourth edition. London, Glasgow etc: Chapman & Hall; 1994: 157−231.
- 15.Wooding FBP, Flint APF. Placentation. In: Lamming GE (ed.), Marshall’s physiology of reproduction. Volume 3: pregnancy and lactation. Part one: ovulation and early pregnancy. Fourth edition. London, Glasgow etc: Chapman & Hall; 1994: 233−460.
- 16.Austin CR. Pre-implantation development. In: Lamming GE (ed.), Marshall’s physiology of reproduction. Volume 3: pregnancy and lactation. Part one: ovulation and early pregnancy. Fourth edition. London, Glasgow etc: Chapman & Hall; 1994: 93−155.
- 17.Noris M, Perico N, Remuzzi G. Mechanisms of disease: Pre-eclampsia. Nat Clin Pract Nephrol 2005; 1: 98–114, quiz :120. [DOI] [PubMed] [Google Scholar]
- 18.George EM, Granger JP. Endothelin: key mediator of hypertension in preeclampsia. Am J Hypertens 2011; 24: 964–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uzan J, Carbonnel M, Piconne O, Asmar R, Ayoubi JM. Pre-eclampsia: pathophysiology, diagnosis, and management. Vasc Health Risk Manag 2011; 7: 467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Redman CW, Sargent IL. Placental stress and pre-eclampsia: a revised view. Placenta 2009; 30Suppl A: S38−42. [DOI] [PubMed] [Google Scholar]
- 21.Roberts JM. Endothelial dysfunction in preeclampsia. Semin Reprod Endocrinol 1998; 16: 5–15. [DOI] [PubMed] [Google Scholar]
- 22.Dekker GA, Kraayenbrink AA, Zeeman GG, van Kamp GJ. Increased plasma levels of the novel vasoconstrictor peptide endothelin in severe pre-eclampsia. Eur J Obstet Gynecol Reprod Biol 1991; 40: 215–220. [DOI] [PubMed] [Google Scholar]
- 23.LaMarca B, Parrish M, Ray LF, Murphy SR, Roberts L, Glover P, Wallukat G, Wenzel K, Cockrell K, Martin JN, Jr, Ryan MJ, Dechend R. Hypertension in response to autoantibodies to the angiotensin II type I receptor (AT1-AA) in pregnant rats: role of endothelin-1. Hypertension 2009; 54: 905–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy SR, LaMarca BB, Cockrell K, Granger JP. Role of endothelin in mediating soluble fms-like tyrosine kinase 1-induced hypertension in pregnant rats. Hypertension 2010; 55: 394–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Bassam MA, Thomson RG, ODonnell L. Involution abnormalities in the postpartum uterus of the bitch. Vet Pathol 1981; 18: 208–218. [DOI] [PubMed] [Google Scholar]
- 26.Weydert JA, Benda JA. Subinvolution of the placental site as an anatomic cause of postpartum uterine bleeding: a review. Arch Pathol Lab Med 2006; 130: 1538–1542. [DOI] [PubMed] [Google Scholar]
- 27.Gram A, Büchler U, Boos A, Hoffmann B, Kowalewski MP. Biosynthesis and degradation of canine placental prostaglandins: prepartum changes in expression and function of prostaglandin F2α-synthase (PGFS, AKR1C3) and 15-hydroxyprostaglandin dehydrogenase (HPGD). Biol Reprod 2013; 89: 2. [DOI] [PubMed] [Google Scholar]
- 28.Gram A, Fox B, Büchler U, Boos A, Hoffmann B, Kowalewski MP. Canine placental prostaglandin E2 synthase: expression, localization, and biological functions in providing substrates for prepartum PGF2alpha synthesis. Biol Reprod 2014; 91: 154. [DOI] [PubMed] [Google Scholar]
- 29.Kowalewski MP, Beceriklisoy HB, Pfarrer C, Aslan S, Kindahl H, Kücükaslan I, Hoffmann B. Canine placenta: a source of prepartal prostaglandins during normal and antiprogestin-induced parturition. Reproduction 2010; 139: 655–664. [DOI] [PubMed] [Google Scholar]
- 30.Kowalewski MP, Beceriklisoy HB, Aslan S, Agaoglu AR, Hoffmann B. Time related changes in luteal prostaglandin synthesis and steroidogenic capacity during pregnancy, normal and antiprogestin induced luteolysis in the bitch. Anim Reprod Sci 2009; 116: 129–138. [DOI] [PubMed] [Google Scholar]
- 31.Kowalewski MP, Schuler G, Taubert A, Engel E, Hoffmann B. Expression of cyclooxygenase 1 and 2 in the canine corpus luteum during diestrus. Theriogenology 2006; 66: 1423–1430. [DOI] [PubMed] [Google Scholar]
- 32.Kowalewski MP, Meyer A, Hoffmann B, Aslan S, Boos A. Expression and functional implications of peroxisome proliferator-activated receptor gamma (PPARγ) in canine reproductive tissues during normal pregnancy and parturition and at antiprogestin induced abortion. Theriogenology 2011; 75: 877–886. [DOI] [PubMed] [Google Scholar]
- 33.Kowalewski MP, Mason JI, Howie AF, Morley SD, Schuler G, Hoffmann B. Characterization of the canine 3beta-hydroxysteroid dehydrogenase and its expression in the corpus luteum during diestrus. J Steroid Biochem Mol Biol 2006; 101: 254–262. [DOI] [PubMed] [Google Scholar]
- 34.Gram A, Boos A, Kowalewski MP. Uterine and placental expression of canine oxytocin receptor during pregnancy and normal and induced parturition. Reprod Domest Anim 2014; 49(Suppl 2): 41–49. [DOI] [PubMed] [Google Scholar]
- 35.Cameron IT, Davenport AP, van Papendorp C, Barker PJ, Huskisson NS, Gilmour RS, Brown MJ, Smith SK. Endothelin-like immunoreactivity in human endometrium. J Reprod Fertil 1992; 95: 623–628. [DOI] [PubMed] [Google Scholar]
- 36.Salamonsen LA, Butt AR, Macpherson AM, Rogers PA, Findlay JK. Immunolocalization of the vasoconstrictor endothelin in human endometrium during the menstrual cycle and in umbilical cord at birth. Am J Obstet Gynecol 1992; 167: 163–167. [DOI] [PubMed] [Google Scholar]
- 37.Riley SC, Butt AR, Doughton BW, Li SX, Zheng SH, Findlay JK, Salamonsen LA. Endothelin in the ovine uterus during the oestrous cycle and early pregnancy. J Reprod Fertil 1994; 100: 451–459. [DOI] [PubMed] [Google Scholar]
- 38.Uchide T, Masuda H, Mitsui Y, Saida K. Gene expression of vasoactive intestinal contractor/endothelin-2 in ovary, uterus and embryo: comprehensive gene expression profiles of the endothelin ligand-receptor system revealed by semi-quantitative reverse transcription-polymerase chain reaction analysis in adult mouse tissues and during late embryonic development. J Mol Endocrinol 1999; 22: 161–171. [DOI] [PubMed] [Google Scholar]
- 39.Hirata Y, Emori T, Eguchi S, Kanno K, Imai T, Ohta K, Marumo F. Endothelin receptor subtype B mediates synthesis of nitric oxide by cultured bovine endothelial cells. J Clin Invest 1993; 91: 1367–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palanisamy GS, Cheon YP, Kim J, Kannan A, Li Q, Sato M, Mantena SR, Sitruk-Ware RL, Bagchi MK, Bagchi IC. A novel pathway involving progesterone receptor, endothelin-2, and endothelin receptor B controls ovulation in mice. Mol Endocrinol 2006; 20: 2784–2795. [DOI] [PubMed] [Google Scholar]
- 41.Verhaar MC, Strachan FE, Newby DE, Cruden NL, Koomans HA, Rabelink TJ, Webb DJ. Endothelin-A receptor antagonist-mediated vasodilatation is attenuated by inhibition of nitric oxide synthesis and by endothelin-B receptor blockade. Circulation 1998; 97: 752–756. [DOI] [PubMed] [Google Scholar]
- 42.Black CE, Huang N, Neligan PC, Forrest CR, Lipa JE, Pang CY. Vasoconstrictor effect and mechanism of action of endothelin-1 in human radial artery and vein: implication of skin flap vasospasm. J Cardiovasc Pharmacol 2003; 41: 460–467. [DOI] [PubMed] [Google Scholar]
- 43.Gram A, Hoffmann B, Boos A, Kowalewski MP. Expression and localization of vascular endothelial growth factor A (VEGFA) and its two receptors (VEGFR1/FLT1 and VEGFR2/FLK1/KDR) in the canine corpus luteum and utero-placental compartments during pregnancy and at normal and induced parturition. Gen Comp Endocrinol 2015; 223: 54–65. [DOI] [PubMed] [Google Scholar]