Abstract
Follicle growth in the mammalian ovary is coordinately controlled by multiple factors to sustain periodic ovulation. In this study, we investigated the role of progesterone on follicle growth in the mouse ovary. As the concentration of progesterone changes during the estrus cycle, we cultured the sliced mouse ovary in a medium containing 10 ng/ml, 100 ng/ml, and 1 μg/ml progesterone. Progesterone promoted the growth of primordial to primary follicles at 100 ng/ml, while it suppressed the growth of secondary follicles at 1 μg/ml. Follicles at other developmental stages in the cultured ovary were unaffected with different concentrations of progesterone. The number of ovulated oocytes increased in the medium containing 100 ng/ml progesterone but decreased in the presence of 1 μg/ml progesterone. Follicles expressed two types of progesterone receptors, progesterone receptor (PGR) and PGR membrane component 1 (PGRMC1). While PGR shows transient expression on granulosa cells of Graafian follicles, PGRMC1 expresses in granulosa cells of developing follicles. These results suggest that progesterone controls the growth of developing follicles through PGRMC1. Our study shows that the effect of progesterone on ovulation and follicle growth in mouse ovary is dependent on the concentration of progesterone and the follicle stage.
Keywords: Follicle growth, Ovary culture, Progesterone, Progesterone receptor membrane component 1 (PGRMC1)
Follicle growth in the ovary is controlled by multiple factors and physiological events. While follicle-stimulating hormone (FSH) and luteinizing hormone (LH) are known to regulate the growth of antral follicles, the regulation of early stages of follicle growth is complex and unclear [1,2,3]. The early stage in follicle growth is independent of FSH and LH and controlled by many factors such as the kit ligand, growth differentiation factor 9 (GDF-9), leukemia inhibitor factor (LIF), insulin-like growth factor 1 (IGF-1), and bone morphogenetic protein-4 (BMP-4) [4, 5, 6, 7, 8, 9, 10, 11]. The development of follicles in the ovary is regulated by several mechanisms; understanding of these mechanisms necessitates simultaneous observation of the growth dynamics of various follicles. Our previous study reported a new method for culturing mouse ovarian tissue to observe the dynamics of follicle growth [11]. This method allows simultaneous observation of dynamics of many follicles to evaluate the effect of a given factor at the tissue level. In this study, we focused on the effect of progesterone (P4) on follicle growth in the mouse ovary.
Follicles and corpus luteum (CL) in the ovary synthesize and secrete P4 [12, 13]. P4 is involved in the maintenance of pregnancy and controls follicle growth and ovulation [14,15,16,17,18]. The progesterone receptor (PGR) is a nuclear receptor that is temporally expressed on granulosa cells of Graafian follicles during the periovulatory period [19, 20]. Ovulation is inhibited in mutant mice lacking functional PGR and in mice treated with PGR antagonist. Thus, P4-mediated stimulation of the PGR on granulosa cells is essential for ovulation [21,22,23,24]. Granulosa cells contain other progesterone-binding proteins, one of which is the PGR membrane component 1 (PGRMC1) [18]. PGRMC1 binds P4 with high affinity and mediates anti-mitotic and anti-apoptosis functions of P4 in granulosa cells [25,26,27,28]. The effect of P4 on follicles in the ovary varies with the presence of these binding proteins.
The concentration of P4 in blood changes during the estrus cycle [29]. This change affects ovarian dynamics, follicle growth, ovulation, CL formation, and pregnancy. We hypothesized that P4 may play an important role in the regulation of follicle growth at tissue level. Changes in serum P4 concentration occur at three stages: phase 1, before ovulation; phase 2, periovulation; and phase 3, pregnant period. The concentration of P4 in blood transiently increases during phase 2 and is the highest during phase 3 [29]. We tested our hypothesis by treating cultured mouse ovarian tissues with different concentrations of P4 (10 ng/ml, 100 ng/ml, and 1 μg/ml) and analyzed the effect of P4 on follicle growth [11]. Our study revealed new effects of P4 on the follicle growth and ovulation at tissue level.
Materials and Methods
Animals
Ovaries were isolated from 4-week-old female ICR mice (Japan SLC, Shizuoka, Japan). Mice were housed in an environmentally controlled room maintained at 23 ± 1°C with a 12-h light/12-h dark cycle. Animal care and experiments were conducted in accordance with the Guidelines for Animal Experimentation of Aichi Medical University. Experiments in this study were approved by The Animal Care and Use Committee of Aichi Medical University.
Culture of ovarian tissue
Ovaries from 4-week-old mice were sliced at a thickness of several hundred micrometers using a microtome blade (Leica Biosystems, Nussloch, Germany) under a stereoscopic microscope (Leica Biosystems). Ovarian tissue slices were placed in a 30-mm cell culture insert (Merck Millipore, Darmstadt, Germany), which was subsequently placed in a 3.5-cm culture dish (AGC Techno Glass, Shizuoka, Japan). Ovarian slices were cultured in the minimum essential medium alpha (MEM-alpha) GlutaMax (Gibco, Carlsbad, CA, USA) supplemented with 5% (v/v) fetal bovine serum (FBS, Gibco), 100 mIU/ml FSH from human pituitary gland (Sigma-Aldrich, St Louis, MO, USA), and 10 mIU/ml LH from equine pituitary gland (Sigma-Aldrich) under conditions of 5% CO2 and 37°C. The cultured ovarian tissue slices were treated with 100 mIU/ml of LH for 12 h every 4 days to reproduce the physiological LH surge. Concentrations of FSH and LH used were based on previous experiments [10, 11]. The effect of P4 on follicle growth was evaluated after adding P4 (10 ng/ml, 100 ng/ml, and 1 μg/ml) (Sigma-Aldrich) to the culture medium, followed by culture for 18 days. In this experiment, one ovary was cut into four slices; two of these slices were treated with dimethyl sulfoxide (DMSO) alone and the other two, with P4 dissolved in DMSO. Four or five ovaries were cultured with each concentration of P4.
Imaging of cultured ovarian slices
Cultured ovarian slices were imaged at intervals of 24 h using a confocal microscope (LSM 710, Carl Zeiss Microimaging, Oberkochen, Germany), with a Z-step size of 5 μm and Z-stack thickness approximately 150 μm.
Analysis of follicle growth in cultured ovarian tissue slices
The area of each follicle observed in cultured ovarian tissue slices was measured using ImageJ software (http://rsbweb.nih.gov/ij/). The outline of the follicle in captured images was traced with a tablet pen (Intuos, Wacom, Saitama, Japan); the number of pixels in each follicle was measured and converted into area (μm2). The follicle area was measured at 24-h intervals to track follicle growth in cultured ovarian tissue slices. The development of follicles was classified into three stages —primordial-primary, secondary, and antral— based on their area on culture day 1. In the previous experiment, we observed cultured ovaries using Hoechst staining and counted the number of granulosa cell layer of each follicle. We classified the follicle stage based on the number of granulosa cell layer and measured the area of follicles. Based on these results, we established the criteria for the classification of follicles based on the area. The detailed information is presented in our previous report [11].
Measurement of 17β-estradiol in the cultured ovary and culture medium
After sliced ovaries were cultured for 2 days, P4 (100 ng/ml) was added into the culture medium; equal volume of DMSO was used as a control. Following incubation for 12 and 24 h, cultured ovaries and the culture medium were collected. The right and left ovary from the same mouse was used as the control and test (P4-treated), respectively. Proteins from the ovary tissue were extracted using Tissue Extraction Reagent I (Invitrogen, Carlsbad, CA, USA) containing protease inhibitor cocktail (Clontech, Mountain View, CA, USA). The concentration of 17β-estradiol in the ovary extract and culture medium was measured using 17β-estradiol high-sensitivity enzyme-linked immunosorbent assay (ELISA) kit (Enzo Life Science, Farmingdale, NY, USA) following the manufacturer’s protocol. Three ovaries were cultured in each condition and used in this experiment.
Staining of ovarian sections for PGRMC1
Ovaries of 4-week-old female mice were fixed with SUPER FIX (KURABO, Osaka, Japan) and embedded in paraffin. The embedded ovary was sliced into 6-μm thick serial sections. Endogenous peroxidase activity was blocked by treatment with 0.6% hydrogen peroxide/methanol at room temperature for 40 min. Sections were treated with anti-PGRMC1 antibody (2 μg/ml; Abcam, Cambridge, UK) or rabbit IgG (2 μg/ml; Vector Laboratories, Burlingame, CA, USA) and subjected to immunoperoxidase staining using the VECTASTAIN ABC Kit (Vector Laboratories) according to the standard protocol. All sections were counterstained with hematoxylin (MUTO PURE CHEMICALS, Tokyo, Japan).
Measurement of progesterone in serum of mice
The estrus cycle of female mice was checked by vaginal smear method at 0900 h and 50 μl blood at proestrus, estrus, metestrus, diestrus, and pregnant day 7 was collected from the caudal vein of the mouse at 1700 h. Blood serum was collected by centrifugation at 25,000 × g for 10 min at 4°C. The concentration of P4 in the serum of three mice at each stage was measured using Progesterone ELISA kit (Enzo Life Science) following the manufacturer’s protocol.
Statistical analyses
Statistical analyses were performed using the software R (http://www.r-project.org/). First, the normality of all data was evaluated using the Shapiro-Wilk normality test (P < 0.05). We then analyzed the normally distributed data using the unpaired t-test/one-way analysis of variance (Figs. 2, 3, 4 and 6) and non-normally distributed data using the Mann-Whitney U test (Fig. 2). The normality of the data for follicular area differed between the culture days, the test chosen (unpaired t-test/one-way ANOVA, or Mann‑Whitney U test) (Fig. 2).
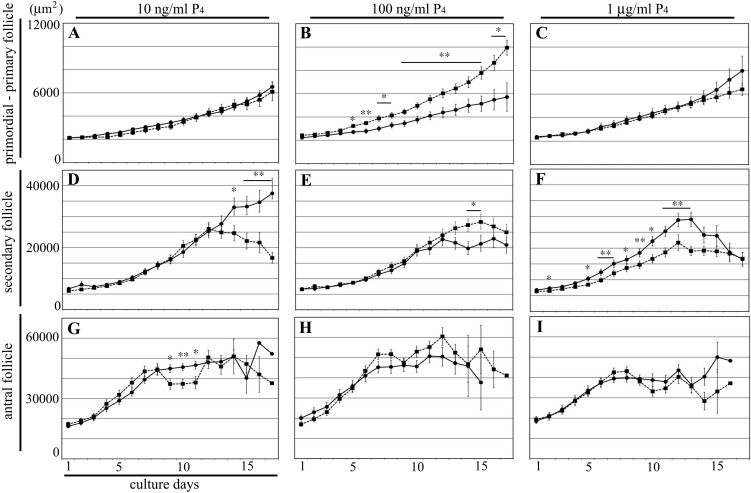
Fig. 2.
Tracking follicle growth in the cultured ovary. Change in the average follicle area within the cultured ovary. Panels A, B and C: area of primordial-primary follicles. Panels D, E and F: area of secondary follicles. Panels G, H and I: area of antral follicles. Panels A, D and G: control and follicles treated with 10 ng/ml P4. Panels B, E and H: control and follicles treated with 100 ng/ml P4. Panels C, F and I: control and follicles treated with 1 μg/ml P4. Black circles and lines, control; black squares and dash lines, follicles treated with P4. Data are presented as the mean ± SD. * P < 0.05; ** P < 0.01.
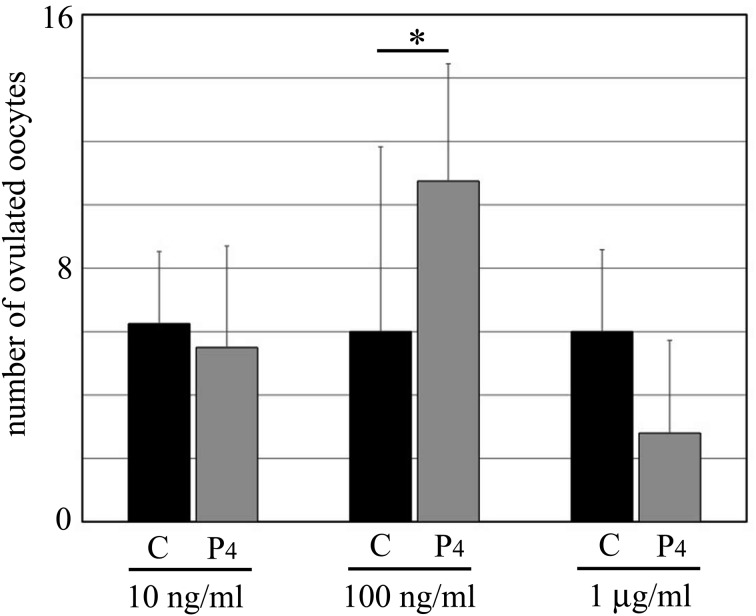
Fig. 3.
The number of ovulated oocytes during the culture period. Black bars, control; gray bars, ovaries treated with P4. C: control, P4: progesterone. 10 ng/ml, 100 ng/ml, and 1 μg/ml: concentrations of P4. Data are presented as the mean ± SD. * P < 0.05.
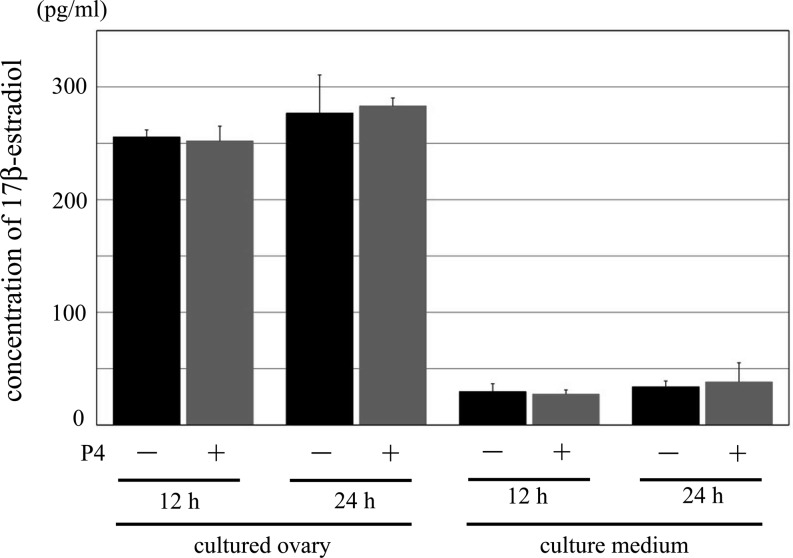
Fig. 4.
The concentration of 17β-estradiol in the cultured ovaries and the culture medium. Comparison of the concentration of 17β-estradiol between normal culture condition and treatment group with P4. 12 h and 24 h indicate the time of treatment with DMSO (control) or P4. Black bar: control group, gray bar: treatment group with P4. There was no significant difference between each control and treatment group. Data are presented as the mean ± SD.
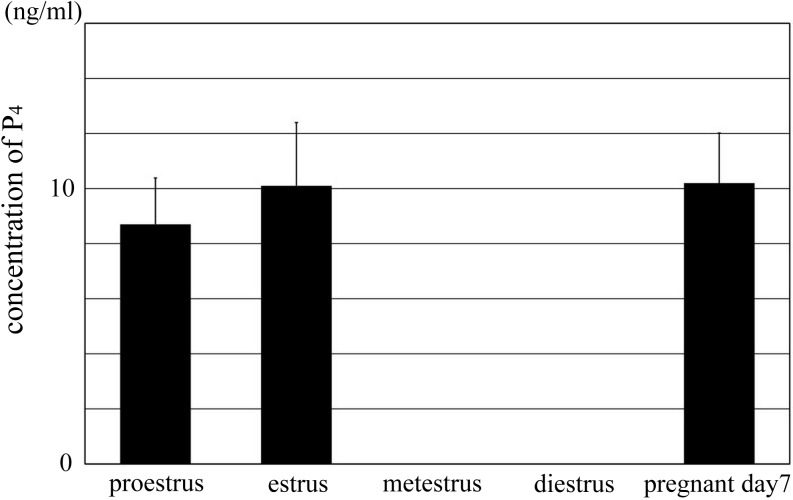
Fig. 6.
The concentration of P4 in the serum of mouse during estrus cycle and pregnancy. The change in the concentration of P4 in the serum of mouse during estrus cycle. The concentration of P4 at metestrus and diestrus was undetected in this experiment; therefore, the concentration at these stages was presented as 0 ng/ml in this graph. There was no significant difference between proestrus, estrus, and pregnant day 7. There was significant difference between metestrus/diestrus and other stages (P < 0.01). Data are presented as the mean ± SD.
Results
The effect of progesterone on follicle growth in the ovary
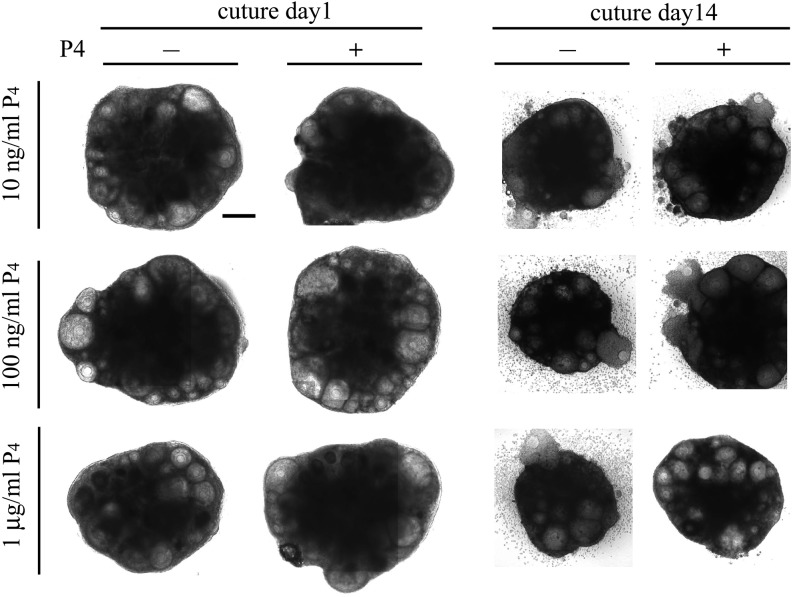
We determined the effect of P4 on follicle growth in the ovary by adding P4 to the culture medium at 10 ng/ml, 100 ng/ml, and 1 μg/ml concentrations (Fig. 1). These P4 concentrations reproduced the change in blood P4 concentration during estrus cycle. We analyzed the effect of each P4 concentration by tracking the growth of each follicle in the cultured ovary during 18 days in culture. We measured the follicle area at 24-h intervals as the index of follicle growth (Fig. 2). Based on the area of each follicle on culture day 1, the growth of follicle was divided into three stages as follows: primordial-primary follicle, < 3000 μm2; secondary follicle, 3000–10000 μm2; antral follicle, > 10000 μm2 (Fig. 2). Details of follicle classification were described in our previous report [11]. At 10 ng/ml, P4 showed no effect on follicle growth (Fig. 2A, D, and G). While P4 promoted growth of primordial-primary follicles at 100 ng/ml (Fig. 2B), it suppressed the growth of secondary follicles at 1 μg/ml (Fig. 2F). At 100 ng/ml and 1 μg/ml, P4 exhibited no effect on other follicle stages (Fig. 2C, E, H and I). Significant differences were observed in the growth of secondary and antral follicles between 10 and 100 ng/ml P4 at some time points (Fig. 2D, E and G). At these time points, some of the follicles ovulated or degenerated and hence were excluded from the measurement, thereby decreasing the average follicle area. Therefore, these significant differences are not contributed by the effect of P4 on follicle growth. In addition, we cultured ovarian tissues in presence of 10 μg/ml P4, but its effect on the follicle growth and ovulation was same as seen with 1 μg/ml P4 (data not shown). These results indicate that the effect of P4 on follicle growth is dependent on its concentration.
Fig. 1.
Images of the cultured ovaries. Images of cultured ovaries of culture day 1 and culture day 14 are presented. Except the cultured ovary containing 1 μg/ml P4, each cultured ovary ovulated some oocytes before culture day 14. Scale bar: 200 μm.
The effect of progesterone on ovulation
During the 18-day culture period, we counted the number of ovulated oocytes in the culture medium at each P4 concentration (Fig. 3). At 100 ng/ml, P4 significantly increased the average number of ovulated oocytes (control, 6 oocytes; 100 ng/ml P4, 10.8 oocytes). On the other hand, P4 treatment at 1 μg/ml concentration tended to decrease the number of ovulated oocytes (control, 6 oocytes; 1 μg/ml P4, 2.8 oocytes; no significant difference, P = 0.12). At 10 ng/ml, P4 showed no effect on ovulation (control, 6.3 oocytes; 10 ng/ml P4, 5.5 oocytes).
The effect of progesterone on the concentration of 17β-estradiol in the cultured tissue and culture medium
Progesterone is converted to 17β-estradiol in follicles. Thus, the growth of follicles and process of ovulation may be promoted by P4 either directly or through 17β-estradiol. We measured the concentration of 17β-estradiol in the cultured ovary and culture medium with ELISA. The concentration of 17β-estradiol recorded was as follows: cultured ovary of a 12-h culture, P4(–): 255.5 ± 6.3 pg/ml, P4(+): 252 ± 13.0 pg/ml; culture medium of a 12-h culture, P4(–): 30.0 ± 7.0 pg/ml, P4(+): 27.3 ± 3.8 pg/ml; cultured ovary of a 24-h culture, P4(–): 276.8 ± 33.8 pg/ml, P4(+): 283.1 ± 7.1 pg/ml; and culture medium of a 24-h culture, P4(–): 33.9 ± 5.1 pg/ml, P4(+): 38.3 ± 17.0 pg/ml (Fig. 4). P4 exhibited no effect on the concentration of 17β-estradiol in the cultured ovary and culture medium, indicative of its direct action on the follicle growth.
The expression of PGRMC1 in follicles
Progesterone receptors are expressed in Graafian follicles only during the periovulatory period. We, therefore, evaluated the expression of PGRMC1 in follicles (Fig. 5). The expression of PGRMC1 was detected in granulosa cells of some primordial follicles (Fig. 5A) and all of the primary, secondary, and antral follicles (Fig. 5C and E). The level of expression increased with the growth of follicles. Staining with rabbit IgG revealed weak signals in granulosa cells of secondary and antral follicles (Fig. 5D and F); however, these signals were much weaker than those recorded with anti-PGRMC1 antibody (Fig. 5C–F). As shown in Figs. 5C and D, the expression of PGRMC1 was detected in granulosa cells of secondary and antral follicles. In addition, the expression of PGRMC1 was detected in ovarian surface cells and some stromal cells (Fig. 5A).
Fig. 5.
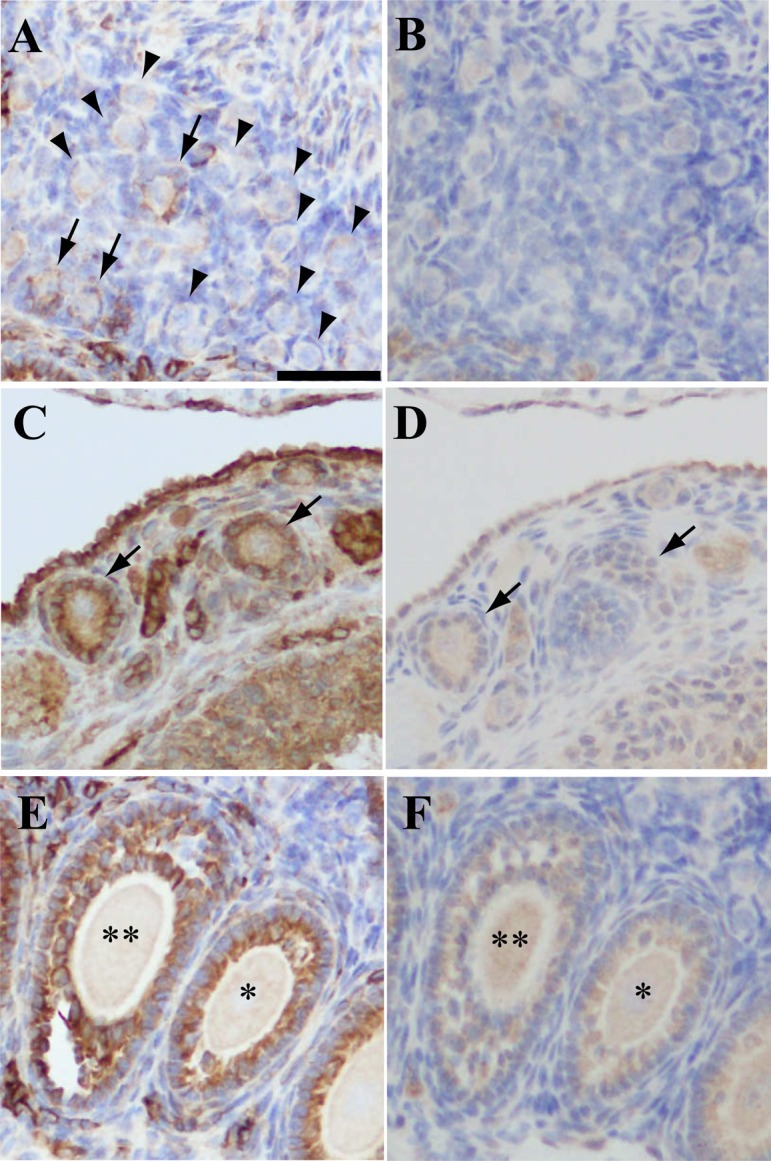
Immunohistochemistry of PGRMC1 in mouse ovary. Panels A and B, C and D, and E and F are images of adjacent mouse ovary sections. Panels A, C and D: stained with anti-PGRMC1 antibody. Panels B, D and F: stained with rabbit IgG. Panel A: arrows, primordial follicles expressing PGRMC1 in granulosa cells; arrowheads, primordial follicles without PGRMC1 expression. Panel C: arrows, primary follicles expressing PGRMC1 in granulosa cells; Panel D: arrows, same follicles indicated in panel C. Panel E and F: * secondary follicle; ** antral follicle expressing PGRMC1 in granulosa cells. Scale bar: 50 μm.
The change in the concentration of P4 in mouse blood during estrus cycle and pregnancy
In the cultured ovary, the effect of P4 was concentration dependent. We investigated the change in the concentration of P4 in the mouse blood by measuring the serum concentration of P4 at each estrus stage and pregnant day 7. The concentration of P4 detected at each stage was as follows: proestrus, 8.7 ± 1.7 ng/ml; estrus, 10.1 ± 2.3 ng/ml; metestrus, undetected; diestrus, undetected; and pregnant day 7, 10.2 ± 1.8 ng/ml (Fig. 6). The level of P4 at proestrus, estrus, and pregnant day 7 was almost similar. On the other hand, P4 was undetected at metestrus and diestrus, owing to its low concentration. Blood was collected from same mice at proestrus and estrus; blood collection at estrus was 24 h following blood collection at proestrus. These results indicate that the concentration of P4 in blood of the mouse varies during estrus cycle and pregnancy.
Discussion
In this study, we evaluated the effect of P4 on follicle growth and ovulation in the mouse ovary and found that the P4 effect differed between follicle stages in a concentration-dependent manner (Figs. 1, 2 and 3). In the ovary, P4 is produced in both follicular and CL cells [12, 30]. The concentration of P4 in blood regularly changed based on the dynamics of ovarian tissue, follicular development, ovulation, follicle atresia, as well as the formation and atresia of CL during estrus cycles [29]. Previous studies showed that ovulation was disturbed by treatment with PGR antagonist or in mice with mutated PGR [19, 21, 23, 24]. Furthermore, PGR expresses in granulosa cells of Graafian follicles only during periovulatory period [20], suggestive of the regulatory role of P4 in ovulation; however, some studies showed that P4 affected the growth of developing follicles except Graafian follicles [31, 32]. Using immortalized and isolated primary rat granulosa cells, Peluso et al. found that P4 inhibited both mitosis and apoptosis of granulosa cells [33, 34]. While these reports clearly showed that P4 controls follicle growth, others address the effect of P4 on follicular growth in vivo [35]. We aimed to evaluate the effect of P4 at each stage of follicle growth in vivo using ovarian tissue culture. We used varying P4 concentration to reproduce in vivo conditions and observed that the effect of P4 on follicle growth differs at every follicle stage (Fig. 2). P4 showed no effect at 10 ng/ml, while it promoted the growth of primordial-primary follicles at 100 ng/ml. At 1 μg/ml, P4 suppressed the growth of secondary follicles (Fig. 2B and F). These results indicate that the blood concentration of P4 coordinates with the follicle growth in the ovary to prepare for the next ovulation. The blood concentration of P4 increases together with an LH surge and further increases after ovulation [29]. Based on these changes in blood concentration, P4 is thought to promote ovulation and growth of primordial and primary follicles for future ovulation in response to LH surge. Furthermore, at high concentration P4 suppressed ovulation and growth of secondary follicles to prepare for fertilization and pregnancy (Figs. 2 and 3). Previous reports indicated the change in the concentration of P4 in blood of rats, but there is no data available in mice. It is difficult to measure the concentration of P4 in the mice, owing to the low volume of blood in mice than in rats. In this experiment, we tried to measure the concentration of P4 in 50 μl blood. While P4 was detected in blood at proestrus, estrus, and pregnant stage, its concentration at metestrus and diestrus was undetected. These results indicate that the concentration of P4 surged during the periovulatory stage and pregnancy (Fig. 6). The concentration of P4 may be higher than that detected in our study at proestrus and pregnant stage and same as that in rats, as we measured P4 level only at one time point at each stage. As blood was withdrawn from the same mouse at proestrus and estrus, the concentration of P4 around ovulation must be sustained to be higher level than other stage during mouse estrus cycle. These results show that the concentration of P4 in mouse blood varied during the estrus cycle, as reported in rats. The relationship between the blood concentration of P4 and results observed in our study is consistent with the physiology of the ovary during the estrus cycle. A previous study reported that intraovarian insertion of a P4 implant into monkey ovary suppressed follicle growth [36]. Therefore, the effect of P4 on follicle growth may not be exerted directly through blood but through follicles and CLs in the ovary. The follicle environment in the ovary is important for the regulation of follicle growth because P4 production is higher in CLs than in follicles and P4 production in follicles varies with the follicle stage [12, 30]. Therefore, the localization and developmental stage of follicles may determine the growth speed of each follicle. In any case, P4 is one of the key factors controlling follicle growth based on the physiological condition of the ovary.
P4 is metabolized to estrogens and androgens in follicles of rodents [37,38,39]. Estrogens have some roles in the follicle development [40, 41]. We evaluated whether P4 was converted into 17β-estradiol in the cultured ovary and studied its ability to bind to receptors on follicles. The treatment with P4 failed to alter the concentration of 17β-estradiol (Fig. 4), indicating the direct effect of P4 on follicle growth through its receptor.
P4 transduces signal to granulosa cells through PGR. However, PGR is not expressed in granulosa cells of developing follicles [42,43,44]. A recent study revealed high-level expression of PGRMC1 in granulosa cells of developing follicles [25]. PGRMC1 can transduce the P4 signal into granulosa cells [26]. Studies have shown that P4 suppresses the proliferation and apoptosis of cultured granulosa cells [17, 34, 45], consistent with our observation that P4 suppressed the growth of secondary follicles at high concentration. However, this is contradictory to our observation that P4 promoted the growth of primordial-primary follicle at 100 ng/ml (Fig. 2B and F). The contrasting result may be attributed to two factors. First, the effect of P4 varied between the granulosa cells from the primordial-primary follicles and secondary follicles. Previous reports used granulosa cells from various follicle stages or large follicles rather than secondary follicles [46, 47]. Our result suggests variation in the effect of P4 between granulosa cells of the primordial-primary and secondary-antral follicles. Second, the interaction between follicles in the ovary may alter the effect of P4. The growth of follicles must be coordinated within the ovary; the interaction between follicles is important in controlling the growth of each follicle. Secondary or antral follicles may influence the effect of P4 on primordial-primary follicles. In this case, the actual in vivo effect of P4 on follicular growth at each stage is not observed in cultured granulosa cells. We observed the expression of PGRMC1 only in a few primordial follicles (Fig. 5A). Therefore, P4 may mainly promote the growth of primary follicles (Fig. 2B).
Our results show that the P4 concentration in blood or ovary controls the follicular growth at each stage. The concentration-dependent effect of P4 is important for sustaining long-term periodic ovulation. The P4-PGRMC1 pathway is thought to be involved in premature ovarian failure [35]. Thus, it is important to understand the regulatory mechanism of follicle growth through the P4-PGRMC1 pathway not only in the cultured granulosa cells but also in the ovary containing follicles at various developmental stages, CLs, and other many tissues. Culture methods that mimic the in vivo conditions may help us study the effect of factors controlling the follicle growth in the ovary. Our culture method is useful to reveal the regulatory mechanism of the follicle growth in the ovary.
Acknowledgments
This work was supported by JSPS KAKENHI Grant Number JP15H06275.
References
- 1.Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet 1997; 15: 201–204. [DOI] [PubMed] [Google Scholar]
- 2.Abel MH, Wootton AN, Wilkins V, Huhtaniemi I, Knight PG, Charlton HM. The effect of a null mutation in the follicle-stimulating hormone receptor gene on mouse reproduction. Endocrinology 2000; 141: 1795–1803. [DOI] [PubMed] [Google Scholar]
- 3.Picton HM, Harris SE, Muruvi W, Chambers EL. The in vitro growth and maturation of follicles. Reproduction 2008; 136: 703–715. [DOI] [PubMed] [Google Scholar]
- 4.Nilsson EE, Skinner MK. Bone morphogenetic protein-4 acts as an ovarian follicle survival factor and promotes primordial follicle development. Biol Reprod 2003; 69: 1265–1272. [DOI] [PubMed] [Google Scholar]
- 5.Nilsson EE, Skinner MK. Growth and differentiation factor-9 stimulates progression of early primary but not primordial rat ovarian follicle development. Biol Reprod 2002; 67: 1018–1024. [DOI] [PubMed] [Google Scholar]
- 6.Nilsson EE, Kezele P, Skinner MK. Leukemia inhibitory factor (LIF) promotes the primordial to primary follicle transition in rat ovaries. Mol Cell Endocrinol 2002; 188: 65–73. [DOI] [PubMed] [Google Scholar]
- 7.Nilsson EE, Skinner MK. Kit ligand and basic fibroblast growth factor interactions in the induction of ovarian primordial to primary follicle transition. Mol Cell Endocrinol 2004; 214: 19–25. [DOI] [PubMed] [Google Scholar]
- 8.Kezele PR, Nilsson EE, Skinner MK. Insulin but not insulin-like growth factor-1 promotes the primordial to primary follicle transition. Mol Cell Endocrinol 2002; 192: 37–43. [DOI] [PubMed] [Google Scholar]
- 9.Nilsson EE, Schindler R, Savenkova MI, Skinner MK. Inhibitory actions of Anti-Müllerian Hormone (AMH) on ovarian primordial follicle assembly. PLoS ONE 2011; 6: e20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiomi-Sugaya N, Komatsu K, Wang J, Yamashita M, Kikkawa F, Iwase A. Regulation of secondary follicle growth by theca cells and insulin-like growth factor 1. J Reprod Dev 2015; 61: 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Komatsu K, Koya T, Wang J, Yamashita M, Kikkawa F, Iwase A. Analysis of the Effect of Leukemia Inhibitory Factor on Follicular Growth in Cultured Murine Ovarian Tissue. Biol Reprod 2015; 93: 18. [DOI] [PubMed] [Google Scholar]
- 12.Roy SK, Greenwald GS. In vitro steroidogenesis by primary to antral follicles in the hamster during the periovulatory period: effects of follicle-stimulating hormone, luteinizing hormone, and prolactin. Biol Reprod 1987; 37: 39–46. [DOI] [PubMed] [Google Scholar]
- 13.Monniaux D, Huet C, Besnard N, Clément F, Bosc M, Pisselet C, Monget P, Mariana JC. Follicular growth and ovarian dynamics in mammals. J Reprod Fertil Suppl 1997; 51: 3–23. [PubMed] [Google Scholar]
- 14.Peluso JJ, Pappalardo A. Progesterone mediates its anti-mitogenic and anti-apoptotic actions in rat granulosa cells through a progesterone-binding protein with gamma aminobutyric acidA receptor-like features. Biol Reprod 1998; 58: 1131–1137. [DOI] [PubMed] [Google Scholar]
- 15.Peluso JJ, Pappalardo A. Progesterone maintains large rat granulosa cell viability indirectly by stimulating small granulosa cells to synthesize basic fibroblast growth factor. Biol Reprod 1999; 60: 290–296. [DOI] [PubMed] [Google Scholar]
- 16.Peluso JJ, Pappalardo A. Progesterone regulates granulosa cell viability through a protein kinase G-dependent mechanism that may involve 1433sigma. Biol Reprod 2004; 71: 1870–1878. [DOI] [PubMed] [Google Scholar]
- 17.Peluso JJ. Progesterone receptor membrane component 1 and its role in ovarian follicle growth. Front Neurosci 2013; 7: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peluso JJ, Pru JK. Non-canonical progesterone signaling in granulosa cell function. Reproduction 2014; 147: R169–R178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robker RL, Russell DL, Espey LL, Lydon JP, OMalley BW, Richards JS. Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases. Proc Natl Acad Sci USA 2000; 97: 4689–4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ismail PM, Li J, DeMayo FJ, OMalley BW, Lydon JP. A novel LacZ reporter mouse reveals complex regulation of the progesterone receptor promoter during mammary gland development. Mol Endocrinol 2002; 16: 2475–2489. [DOI] [PubMed] [Google Scholar]
- 21.Lydon JP, DeMayo FJ, Conneely OM, O’Malley BW. Reproductive phenotpes of the progesterone receptor null mutant mouse. J Steroid Biochem Mol Biol 1996; 56: 67–77. [DOI] [PubMed] [Google Scholar]
- 22.Robker RL, Russell DL, Yoshioka S, Sharma SC, Lydon JP, OMalley BW, Espey LL, Richards JS. Ovulation: a multi-gene, multi-step process. Steroids 2000; 65: 559–570. [DOI] [PubMed] [Google Scholar]
- 23.Svensson EC, Markström E, Andersson M, Billig H. Progesterone receptor-mediated inhibition of apoptosis in granulosa cells isolated from rats treated with human chorionic gonadotropin. Biol Reprod 2000; 63: 1457–1464. [DOI] [PubMed] [Google Scholar]
- 24.Conneely OM, Mulac-Jericevic B, DeMayo F, Lydon JP, OMalley BW. Reproductive functions of progesterone receptors. Recent Prog Horm Res 2002; 57: 339–355. [DOI] [PubMed] [Google Scholar]
- 25.Peluso JJ, Pappalardo A, Losel R, Wehling M. Progesterone membrane receptor component 1 expression in the immature rat ovary and its role in mediating progesterones antiapoptotic action. Endocrinology 2006; 147: 3133–3140. [DOI] [PubMed] [Google Scholar]
- 26.Peluso JJ, Liu X, Gawkowska A, Johnston-MacAnanny E. Progesterone activates a progesterone receptor membrane component 1-dependent mechanism that promotes human granulosa/luteal cell survival but not progesterone secretion. J Clin Endocrinol Metab 2009; 94: 2644–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peluso JJ, Liu X, Gawkowska A, Lodde V, Wu CA. Progesterone inhibits apoptosis in part by PGRMC1-regulated gene expression. Mol Cell Endocrinol 2010; 320: 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peluso JJ, Lodde V, Liu X. Progesterone regulation of progesterone receptor membrane component 1 (PGRMC1) sumoylation and transcriptional activity in spontaneously immortalized granulosa cells. Endocrinology 2012; 153: 3929–3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Butcher RL, Collins WE, Fugo NW. Altered secretion of gonadotropins and steroids resulting from delayed ovulation in the rat. Endocrinology 1975; 96: 576–586. [DOI] [PubMed] [Google Scholar]
- 30.Roy SK, Greenwald GS. Methods of separation and in-vitro culture of pre-antral follicles from mammalian ovaries. Hum Reprod Update 1996; 2: 236–245. [DOI] [PubMed] [Google Scholar]
- 31.Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology 1974; 94: 1704–1708. [DOI] [PubMed] [Google Scholar]
- 32.Hirshfield AN. Stathmokinetic analysis of granulosa cell proliferation in antral follicles of cyclic rats. Biol Reprod 1984; 31: 52–58. [DOI] [PubMed] [Google Scholar]
- 33.Peluso JJ, Fernandez G, Pappalardo A, White BA. Characterization of a putative membrane receptor for progesterone in rat granulosa cells. Biol Reprod 2001; 65: 94–101. [DOI] [PubMed] [Google Scholar]
- 34.Peluso JJ, Fernandez G, Pappalardo A, White BA. Membrane-initiated events account for progesterones ability to regulate intracellular free calcium levels and inhibit rat granulosa cell mitosis. Biol Reprod 2002; 67: 379–385. [DOI] [PubMed] [Google Scholar]
- 35.Mansouri MR, Schuster J, Badhai J, Stattin EL, Lösel R, Wehling M, Carlsson B, Hovatta O, Karlström PO, Golovleva I, Toniolo D, Bione S, Peluso J, Dahl N. Alterations in the expression, structure and function of progesterone receptor membrane component-1 (PGRMC1) in premature ovarian failure. Hum Mol Genet 2008; 17: 3776–3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.diZerega GS, Hodgen GD. The interovarian progesterone gradient: a spatial and temporal regulator of folliculogenesis in the primate ovarian cycle. J Clin Endocrinol Metab 1982; 54: 495–499. [DOI] [PubMed] [Google Scholar]
- 37.Young JM, McNeilly AS. Theca: the forgotten cell of the ovarian follicle. Reproduction 2010; 140: 489–504. [DOI] [PubMed] [Google Scholar]
- 38.Andersen CY, Ezcurra D. Human steroidogenesis: implications for controlled ovarian stimulation with exogenous gonadotropins. Reprod Biol Endocrinol 2014; 12: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gervásio CG, Bernuci MP, Silva-de-Sá MF, Rosa-E-Silva AC. The role of androgen hormones in early follicular development. ISRN Obstet Gynecol 2014; 2014: 818010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Emmen JM, Couse JF, Elmore SA, Yates MM, Kissling GE, Korach KS. In vitro growth and ovulation of follicles from ovaries of estrogen receptor (ER)alpha and ERbeta null mice indicate a role for ERbeta in follicular maturation. Endocrinology 2005; 146: 2817–2826. [DOI] [PubMed] [Google Scholar]
- 41.Shirwalkar H, Modi DN, Maitra A. Exposure of adult rats to estradiol valerate induces ovarian cyst with early senescence of follicles. Mol Cell Endocrinol 2007; 272: 22–37. [DOI] [PubMed] [Google Scholar]
- 42.Park OK, Mayo KE. Transient expression of progesterone receptor messenger RNA in ovarian granulosa cells after the preovulatory luteinizing hormone surge. Mol Endocrinol 1991; 5: 967–978. [DOI] [PubMed] [Google Scholar]
- 43.Natraj U, Richards JS. Hormonal regulation, localization, and functional activity of the progesterone receptor in granulosa cells of rat preovulatory follicles. Endocrinology 1993; 133: 761–769. [DOI] [PubMed] [Google Scholar]
- 44.Shao R, Markström E, Friberg PA, Johansson M, Billig H. Expression of progesterone receptor (PR) A and B isoforms in mouse granulosa cells: stage-dependent PR-mediated regulation of apoptosis and cell proliferation. Biol Reprod 2003; 68: 914–921. [DOI] [PubMed] [Google Scholar]
- 45.Peluso JJ, Pappalardo A, Losel R, Wehling M. Expression and function of PAIRBP1 within gonadotropin-primed immature rat ovaries: PAIRBP1 regulation of granulosa and luteal cell viability. Biol Reprod 2005; 73: 261–270. [DOI] [PubMed] [Google Scholar]
- 46.Stein LS, Stoica G, Tilley R, Burghardt RC. Rat ovarian granulosa cell culture: a model system for the study of cell-cell communication during multistep transformation. Cancer Res 1991; 51: 696–706. [PubMed] [Google Scholar]
- 47.Rao IM, Mills TM, Anderson E, Mahesh VB. Heterogeneity in granulosa cells of developing rat follicles. Anat Rec 1991; 229: 177–185. [DOI] [PubMed] [Google Scholar]