Abstract
Casein kinase 2 (CK2) is a highly conserved, ubiquitously expressed serine/threonine protein kinase with hundreds of substrates. The role of CK2 in the G2/M transition of oocytes, zygotes, and 2-cell embryos was studied in mouse by enzyme activity inhibition using the specific inhibitor 4, 5, 6, 7-tetrabromobenzotriazole (TBB). Zygotes and 2-cell embryos were arrested at G2 phase by TBB treatment, and DNA damage was increased in the female pronucleus of arrested zygotes. Further developmental ability of arrested zygotes was reduced, but that of arrested 2-cell embryos was not affected after releasing from inhibition. By contrast, the G2/M transition in oocytes was not affected by TBB. These results indicate that CK2 activity is essential for mitotic G2/M transition in early embryos but not for meiotic G2/M transition in oocytes.
Keywords: Casein kinase 2 (CK2); G2/M transition; Meiosis; 4, 5, 6, 7-tetrabromobenzotriazole (TBB)
Casein kinase 2 (CK2), a ubiquitous serine/threonine protein kinase, is a tetramer composed of two catalytic (α or α’) and two regulatory (β) subunits. CK2 is highly pleiotropic because of its broad cellular substrates. It is reported to be involved in cancer [1, 2], proliferation [3], apoptosis [4, 5], DNA damage repair [6, 7], cell cycle progression [8, 9] and other cellular processes. Gene knockout analysis of each subunit shows that disruption of the α or β subunit leads to lethality after implantation [10, 11], while disruption of the α’ subunit is not lethal, but results in globozoospermia [12] in mouse. CK2 plays an important role in cell cycle regulation. CK2 phosphorylates p34cdc2 at Ser39 during G1 phase in Hela cells [13], and is demonstrated to be required for G0/G1, early G1, and G1/S transition in human primary fibroblasts [14]. CK2 is also involved in cell cycle progression during G1 and G2/M in yeast [15]. Subsequent research identified several substrates for CK2 in cell cycle regulation, such as Bdp1 [16], geminin [17], p27KIP1 [18], and eIF5 [9]. A recent study indicated that inhibition of CK2 activates p53 function and induces p53-dependent cell cycle arrest and apoptosis in cancer cells [8]. Despite these findings for CK2 during cell cycle progression in somatic and tumor cells, its role in early embryo development or oocyte meiosis remains unknown.
The G2/M transition in cell cycle is mainly regulated by maturation promoting factor (MPF), which is composed of cyclin-dependent kinase 1 (Cdk1) and cyclin B. Cyclin B-Cdk1 activation results in nuclear envelope breakdown, which is a characteristic of the initiation of mitosis. DNA damage is checked before the transition to ensure that cells with DNA damage are arrested at the G2 phase until the damaged DNA is repaired.
In this work, we examined the effect of CK2 activity on the G2/M transition in oocytes and early embryos in mouse using a highly specific CK2 inhibitor tetrabromobenzotriazole (TBB) [19]. TBB acts in an ATP/GTP-competitive manner. It may adopt different modes of binding to the active site of CK2 (catalytic subunits)[20]; therefore, ATP/GTP binding is competitively blocked and CK2 activity is inhibited. We show that CK2 activity is essential for mitotic G2/M transition in zygotes and 2-cell embryos, but not for meiotic G2/M transition in oocytes.
Materials and Methods
ICR mice care and manipulations were handled according to the Ethics Committee of the Nanjing Drum Tower Hospital and Institute of Zoology, Chinese Academy of Sciences.
Oocyte collection and culture
Immature oocytes were collected from the ovaries of 8-week-old female ICR mice into M2 medium (Sigma, St. Louis, MO, USA) with 2.5 μM milrinone (Sigma), which was used to maintain oocytes at the GV (germinal vesicle) stage. Only fully grown oocytes with a GV were further cultured in M2 medium in a humidified incubator of 5% CO2 at 37°C. The rate of GVBD (germinal vesicle break down) was measured after 2 h in culture, according to disappearance of the GV.
In vitro fertilization (IVF) and embryo culture
For IVF, spermatozoa were obtained from the epididymis of male ICR mice, and incubated at 37°C in HTF medium for 30 min to capacitate. Female ICR mice were superovulated via administration of 5 IU PMSG and hCG. Mature oocytes were collected from ampullae of the oviduct 14–15 h after hCG injection and inseminated with capacitated sperm in HTF medium. Two hours after insemination, embryos were washed and cultured in KSOM medium in a humidified incubator of 5% CO2 at 37°C. TBB (100 mM) dissolved in DMSO was diluted with KSOM and used to treat the embryos. Developmental rates of 2-cell embryos, 3/4-cell embryos, and morulae were measured 24, 48, and 72 h after IVF, respectively.
DNA duplication examination by BrdU
DNA duplication in zygotes was determined by measuring the incorporation of BrdU into the duplicated DNA. Zygotes were transferred into KSOM medium containing 100 μM BrdU (Sigma) at 6 h after IVF with or without TBB (Tocris bioscience, Bristol, UK) treatment. Both groups of zygotes were fixed at 12 h after IVF, and BrdU incorporation was detected by immunostaining with an anti-BrdU antibody (Sigma), together with a FITC-conjugated secondary antibody.
DNA damage examination by immunocytochemistry
Embryos were fixed in 3.7% paraformaldehyde for 30 min, permeabilized in 0.5% Triton X-100 for 20 min and blocked in 1% BSA for 1 h in PBS at 25°C. Embryos were incubated overnight at 4°C with an antibody against phosphorylated H2A X, followed by a FITC-conjugated secondary antibody for 2 h at 25°C. Hoechst 33342 was used to label the nucleus.
Statistical analysis
Statistical analysis was performed by an independent-sample t-test to compare two groups, and one-way analysis of variance (ANOVA) was applied to multi-groups. LSD test was used for multiple comparisons. P < 0.05 was considered statistically significant. All results were expressed as mean ± SE.
Results
TBB inhibited mouse zygotes at the pronuclear stage
CK2β gene expression in zygotes, 2-cell embryos, and GV oocytes was assayed using immunocytochemistry. It was shown to be expressed in these cells, and specifically located in the nuclei (Fig. 1). Mouse zygotes obtained by IVF developed normally to the 2-cell stage 24 h after insemination. In this experiment, one-cell mouse embryos were treated with TBB at 6 h after IVF. Although 10 and 20 μM TBB did not reduce the developmental rate of 2-cell embryos, 40 μM did completely disrupt their development (Fig. 2A). Hoechst 33342 staining confirmed this inhibitory effect. TBB-treated embryos were arrested at the pronuclear stage, while control embryos had reached the 2-cell stage 24 h after IVF (Fig. 2B).
Fig. 1.
CK2β gene expression in the zygote, 2-cell embryo, and GV oocyte. CK2β was used to indicate CK2 expression. CK2β protein in zygotes, 2-cell embryos, and GV oocytes was assayed by immunocytochemistry. CK2β was expressed and located in the nuclei of these cells.
Fig. 2.
Inhibition of zygotes at pronuclear phase by TBB. (A) Zygotes derived form IVF were cultured in KSOM medium and treated with 10, 20, or 40 μM TBB at 6 h. The developmental rate of 2-cell embryos was measured 24 h after IVF. Approximately 60 zygotes in 3 replicates were counted in each group. TBB (40 μM) completely abolished 2-cell development. Double asterisks show highly significant differences compared to the control (Mock) group (P < 0.01). (B) Embryos 24 h after IVF were stained with Hoechst 33342 to confirm existence of the pronucleus in the TBB group.
TBB inhibited G2/M transition in mouse zygotes
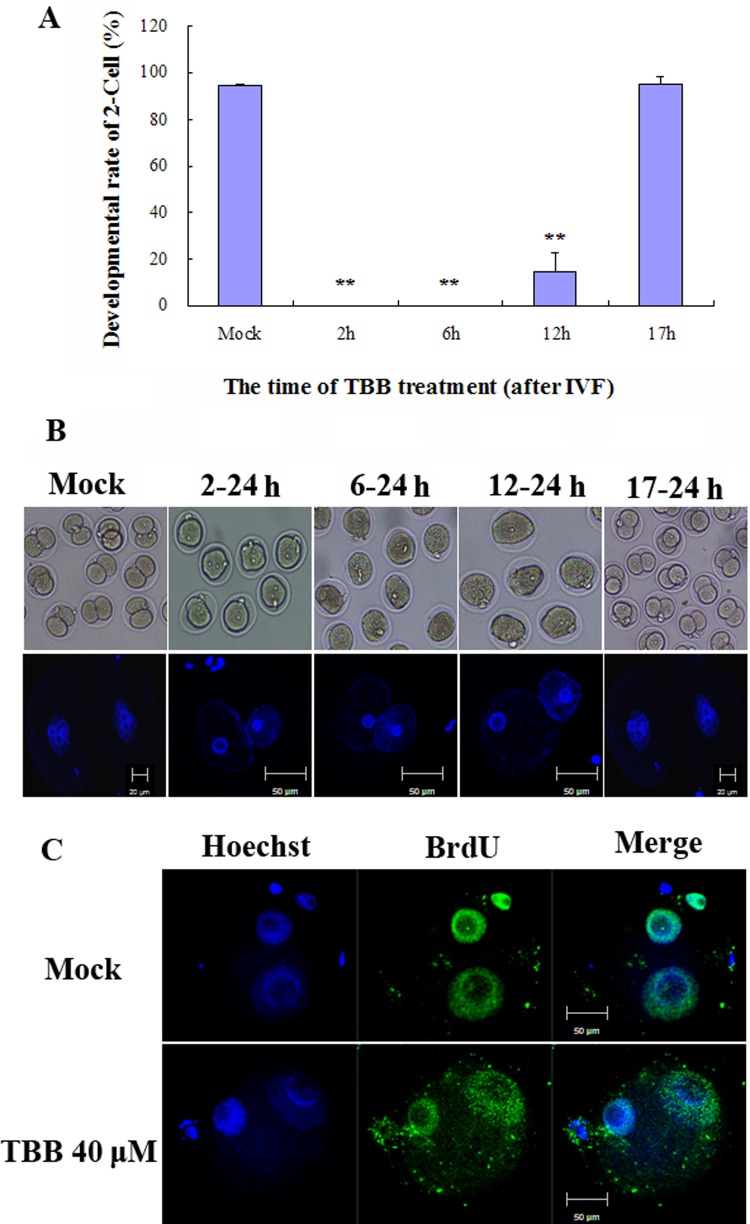
To further determine the stage at which TBB is involved, we treated zygotes with 40 μM TBB at different time points: 2, 6, 12, and 17 h after IVF, corresponding to the pre-pronuclear stage, early pronuclear stage, late pronuclear stage, and pronuclear break down stage, respectively. Embryos treated with TBB at 2 h and 6 h did not develop into 2-cell embryos, and only a small number (27.5%) from the 12 h group developed to the 2-cell stage. However, the developmental rate of 2-cell embryos treated with TBB at 17 h was similar to that of controls (Figs. 3A and B). These results indicate that TBB plays a role before pronuclear break down and blocks progression of the cell cycle into M phase. The results also indicate that TBB did not inhibit formation of the pronucleus. To define the exact interphase stage at which TBB inhibits zygote development, BrdU was employed to determine whether the DNA had been duplicated. Results showed that DNA was duplicated in embryos (20/20) 12 h after IVF (Fig. 3C). This also showed that TBB had no affect on DNA duplication in the first mitosis and embryos were arrested at the G2 phase. Thus, TBB inhibited the G2/M transition of the first mitosis.
Fig. 3.
Inhibition of the zygote at G2 phase by TBB treatment. (A) Zygotes were transferred into KSOM medium containing 40 μM TBB at specific time points, and the developmental rate of 2-cell embryos was measured 24 h after IVF. Approximately 90 zygotes in 3 replicates were counted in each group. Double asterisks show highly significant differences compared to the control (Mock) group (P < 0.01). (B) Embryos from each group were stained with Hoechst 33342. (C) DNA duplication was examined by BrdU incorporation. Zygotes treated with TBB at 6 h were fixed and immunostained with an anti-BrdU antibody at 12 h after IVF, while zygotes collected 12 h after IVF without TBB treatment were used as controls.
TBB decreased the developmental potential of zygotes and increased DNA damage in female pronucleus
To observe the developmental potential of zygotes treated with TBB, the developmental rates of 2-cell embryos, 3/4-cell embryos, and morulae were measured at 24, 48, and 72 h after IVF, respectively. Zygotes treated with 40 μM TBB 6 h after IVF were washed and cultured in normal medium at 12, 17, and 24 h after IVF. Because of the fact that zygotes treated with TBB at 6 to 24 h remained at the pronuclear stage (G2 phase), an additional 24 h was allowed for embryo development; then, the developmental rate was measured in this group. It was found that development of zygotes in the groups washed at 12 and 17 h was indistinguishable that of controls, whereas the developmental ability of zygotes washed at 24 h was dramatically reduced (Fig. 4A). To investigate the reason for this reduced developmental ability, DNA damage was assayed by immunostaining for phosphorylated H2A X, since DNA damage is the main event at the G2/M check point. Interestingly, these results indicate that DNA damage was increased only in the female pronucleus of these zygotes (Fig. 4B).
Fig. 4.
Decreased developmental potential and increased DNA damage in female pronucleus of the blocked zygotes. (A) Zygotes treated with 40 μM TBB 6 h after IVF were washed and cultured in normal medium at different time points, and developmental rates of 2-cell embryos, 3/4-cell embryos, and morulae were measured. Approximately 90 zygotes in 3 replicates were counted in each group. Double asterisks show highly significant differences compared to the control (Mock) group (P < 0.01). (B) DNA damage was examined by immunostaining for phosphorylated H2A X. Zygotes treated with TBB at 6 h were fixed and immunostained at 24 h after IVF, while zygotes 12 h after IVF without TBB treatment were used as controls.
TBB inhibited G2/M transition in 2-cell embryos
In addition to the G2/M transition in zygotes, the effect of TBB on G2/M transition in 2-cell embryos was studied. Development of 2-cell embryos treated with 40 μM TBB was blocked at the 2-cell stage 48 h after IVF, while the control group developed to the 3/4-cell stage (Figs. 5A and B). This result suggests that TBB may also affect the G2/M transition in 2-cell embryos. TBB-treated 2-cell embryos were washed 48 h after IVF, and their subsequent development was not affected. This is different from zygotes treated with TBB. The developmental rate of 3/4-cell embryos and morulae reached control levels 24 h later (Fig. 5C).
Fig. 5.
Effect of CK2 inhibition by TBB on 2-cell embryo development. (A) 2-cell embryos were treated with 40 μM TBB 24 h after IVF, and the developmental rate of 3/4-cell embryos was measured 48 h after IVF. Double asterisks show highly significant differences compared to the control (Mock) group (P < 0.01). (B) Embryos were stained with Hoechst 33342. (C) 2-cell embryos treated with TBB at 24 h were washed and cultured in normal medium 48 h after IVF, and developmental rates of 3/4-cell embryos and morulae were measured 24 h later than that of the control group. Approximately 90 2-cell embryos in 3 replicates were counted in each group for (A) and (C).
TBB had no effect on G2/M transition in oocytes
The effect of TBB on the G2/M transition in oocytes was examined. Oocytes usually undergo GVBD within 2 h of in vitro culture in M2 medium (Fig. 6A). Contrary to the results for mitosis in zygotes and 2-cell embryos, TBB did not block the G2/M transition in oocytes, since the rate of GVBD was not reduced as the concentration of TBB increased (Fig. 6B).
Fig. 6.
Effect of CK2 inhibition by TBB on GVBD of oocytes. (A) Oocytes were cultured in vitro in M2 medium; GV oocytes undergo GVBD in 2 h. (B) TBB was added to M2 dedium in a series of concentrations, and the rate of GVBD was measured after 2 h. Approximately 150 oocytes in 3 replicates were counted in each group.
Discussion
CK2 exists as tetrameric complex consisting of three subunits (CK2α, CK2α’, and CK2β) that are encoded by independent genes. Each subunit may exist both within the CK2 tetrameric complex and as a free subunit [21], and may function dependently or independently of CK2. For example, knockdown of CK2β by RNA interference results in delayed cell cycle progression at the onset of mitosis by regulating CDK1 activity through the PLK-Wee1 complex, and this is independent of its role as a CK2 regulatory subunit [22]. Consequently, TBB, a selective inhibitor of CK2, was used to investigate the role of CK2 in mitosis and meiosis. Three kinds of G2/M transitions were selected for the present study: zygotes, 2-cell embryos, and oocytes, which represent the G2/M transition of the first mitosis, general mitosis, and meiosis, respectively. Results show that CK2 plays different roles in these three kinds of G2/M transition. First, CK2 activity is essential for mitosis in early embryogenesis but not for meiosis of oocytes. This indicates different regulatory mechanisms between mitosis and meiosis. Since inhibition of CK2 can cause DNA damage, this could be explained in terms of the DNA damage checkpoint. Fully grown GV oocytes fail to launch a robust DNA damage checkpoint during the G2 phase [23]; therefore, DNA damage in oocytes does not prevent G2/M transition. However, DNA damage does cause cell cycle arrest at the G2 stage in mitotic cells. Another reasonable explanation is that accumulation of CK2 in the nucleus seems to be higher in zygotes and 2-cell stage embryos than in oocytes. On the other hand, CK2 inhibition reduces further development of zygotes but not of 2-cell embryos, indicating an important role during the first mitosis of embryo development.
Studies have demonstrated that CK2 is required for G0/G1 and G1/S transition during mitosis [14, 24]. However, in our studies, DNA duplication examination confirmed that the zygote is blocked at the G2 phase by CK2 inhibition, which indicates a mitosis entry defect. This observation is consistent with phenotypes observed in yeast [15] and 3T3 L1 cells [25]. Studies in human primary lung fibroblasts and plant cells revealed that destruction of CK2 activity at different phases of the cell cycle leads to different effects on cell cycle progression. Blocking CK2 activity before S phase results in significant inhibition of growth in human primary lung fibroblasts, but neither DNA synthesis nor cell division is affected if it is blocked during S phase [24]. Inhibition of CK2 at G1 phase in tobacco BY-2 cells leads to premature chromatin condensation; however, the nuclear membrane does not undergo break down. On the other hand, inhibition of CK2 at S or G2 phase results in cell death with abolished DNA synthesis or a block in mitosis entry, respectively [26]. In our experiments, addition of TBB before nuclear envelope break down (NEBD) resulted in a mitosis entry block in the zygote, but TBB addition after NEBD did not affect cell cycle progression. Thus, NEBD seems to be a watershed in the mouse zygote for CK2 function, and not S phase as in human primary lung fibroblasts. Restrained zygotes display a rounded, shrunken morphology, consistent with the phenomenon observed in other studies [25, 27], suggesting that CK2 is involved in normal cytoskeletal maintenance in the mouse zygote. This provides a possible explanation for the low developmental ability of these zygotes. DNA damage in the female pronucleus partially unveils the mechanism of inhibition of G2/M transition because it can activate the G2/M checkpoint. TBB also inhibits the G2/M transition in 2-cell embryos. Thus, our data indicate that CK2 is essential for early embryogenesis. Following the knockout assay of CK2α and CK2β that caused embryo death [10, 11], our results provide additional novel evidence for the role of CK2 in early embryogenesis in mouse. Although a 3–4 fold activity increase at day 12 of gestation has been reported for CK2 [28], our results show that CK2 functions as early as the zygote stage in mice.
During meiosis, CK2α’ is reported to be involved in spermatogenesis in mouse [12], while CK2β is involved in oogenesis in Xenopus [29]. It is well documented that CK2β binds to Mos and inhibits its activity directly, thus, repressing Mos-mediated mitogen-activated protein kinase (MAPK) activation [30, 31]. CK2β knock down by RNAi stimulates GVBD in Xenopus oocytes [29]. However, in our experiment, inhibition of CK2 by TBB did not affect GVBD in mouse oocytes. This difference could be owing to different animal species or different experimental methods. Another reasonable explanation is that CK2β may play a separate role, independent of CK2.
In conclusion, our results indicate that CK2 activity is essential for early embryogenesis in mouse, however, further research is required to elucidate the molecular mechanisms of the G2/M transition inhibition, the reason for DNA damage in only the female and not male pronucleus, and the reason for decrease in developmental ability in blocked zygotes but not in 2-cell embryos. The underlying reason for CK2 inhibition affecting G2/M transition in mitosis but not in meiosis also needs to be elucidated.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (NO.31501204) and the Fundamental Research Funds for the Central Universities (NO.021414380059). We are grateful to Yi Hou and Hua Qin for technical assistance.
References
- 1.Scaglioni PP, Yung TM, Cai LF, Erdjument-Bromage H, Kaufman AJ, Singh B, Teruya-Feldstein J, Tempst P, Pandolfi PP. A CK2-dependent mechanism for degradation of the PML tumor suppressor. Cell 2006; 126: 269–283. [DOI] [PubMed] [Google Scholar]
- 2.Chatterjee A, Chatterjee U, Ghosh MK. Activation of protein kinase CK2 attenuates FOXO3a functioning in a PML-dependent manner: implications in human prostate cancer. Cell Death Dis 2013; 4: e543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lebrin F, Chambaz EM, Bianchini L. A role for protein kinase CK2 in cell proliferation: evidence using a kinase-inactive mutant of CK2 catalytic subunit alpha. Oncogene 2001; 20: 2010–2022. [DOI] [PubMed] [Google Scholar]
- 4.Zhao T, Jia H, Li L, Zhang G, Zhao M, Cheng Q, Zheng J, Li D. Inhibition of CK2 enhances UV-triggered apoptotic cell death in lung cancer cell lines. Oncol Rep 2013; 30: 377–384. [DOI] [PubMed] [Google Scholar]
- 5.Ahmad KA, Wang G, Unger G, Slaton J, Ahmed K. Protein kinase CK2a key suppressor of apoptosis. Adv Enzyme Regul 2008; 48: 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loizou JI, El-Khamisy SF, Zlatanou A, Moore DJ, Chan DW, Qin J, Sarno S, Meggio F, Pinna LA, Caldecott KW. The protein kinase CK2 facilitates repair of chromosomal DNA single-strand breaks. Cell 2004; 117: 17–28. [DOI] [PubMed] [Google Scholar]
- 7.Ghavidel A, Schultz MC. TATA binding protein-associated CK2 transduces DNA damage signals to the RNA polymerase III transcriptional machinery. Cell 2001; 106: 575–584. [DOI] [PubMed] [Google Scholar]
- 8.Dixit D, Sharma V, Ghosh S, Mehta VS, Sen E. Inhibition of Casein kinase-2 induces p53-dependent cell cycle arrest and sensitizes glioblastoma cells to tumor necrosis factor (TNFα)-induced apoptosis through SIRT1 inhibition. Cell Death Dis 2012; 3: e271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Homma MK, Wada I, Suzuki T, Yamaki J, Krebs EG, Homma Y. CK2 phosphorylation of eukaryotic translation initiation factor 5 potentiates cell cycle progression. Proc Natl Acad Sci USA 2005; 102: 15688–15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lou DY, Dominguez I, Toselli P, Landesman-Bollag E, OBrien C, Seldin DC. The alpha catalytic subunit of protein kinase CK2 is required for mouse embryonic development. Mol Cell Biol 2008; 28: 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchou T, Vernet M, Blond O, Jensen HH, Pointu H, Olsen BB, Cochet C, Issinger OG, Boldyreff B. Disruption of the regulatory beta subunit of protein kinase CK2 in mice leads to a cell-autonomous defect and early embryonic lethality. Mol Cell Biol 2003; 23: 908–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu X, Toselli PA, Russell LD, Seldin DC. Globozoospermia in mice lacking the casein kinase II alpha catalytic subunit. Nat Genet 1999; 23: 118–121. [DOI] [PubMed] [Google Scholar]
- 13.Russo GL, Vandenberg MT, Yu IJ, Bae YS, Franza BR, Jr, Marshak DR. Casein kinase II phosphorylates p34cdc2 kinase in G1 phase of the HeLa cell division cycle. J Biol Chem 1992; 267: 20317–20325. [PubMed] [Google Scholar]
- 14.Pepperkok R, Lorenz P, Ansorge W, Pyerin W. Casein kinase II is required for transition of G0/G1, early G1, and G1/S phases of the cell cycle. J Biol Chem 1994; 269: 6986–6991. [PubMed] [Google Scholar]
- 15.Hanna DE, Rethinaswamy A, Glover CV. Casein kinase II is required for cell cycle progression during G1 and G2/M in Saccharomyces cerevisiae. J Biol Chem 1995; 270: 25905–25914. [DOI] [PubMed] [Google Scholar]
- 16.Hu P, Samudre K, Wu S, Sun Y, Hernandez N. CK2 phosphorylation of Bdp1 executes cell cycle-specific RNA polymerase III transcription repression. Mol Cell 2004; 16: 81–92. [DOI] [PubMed] [Google Scholar]
- 17.Kulartz M, Hiller E, Kappes F, Pinna LA, Knippers R. Protein kinase CK2 phosphorylates the cell cycle regulatory protein Geminin. Biochem Biophys Res Commun 2004; 315: 1011–1017. [DOI] [PubMed] [Google Scholar]
- 18.Tapia JC, Bolanos-Garcia VM, Sayed M, Allende CC, Allende JE. Cell cycle regulatory protein p27KIP1 is a substrate and interacts with the protein kinase CK2. J Cell Biochem 2004; 91: 865–879. [DOI] [PubMed] [Google Scholar]
- 19.Sarno S, Reddy H, Meggio F, Ruzzene M, Davies SP, Donella-Deana A, Shugar D, Pinna LA. Selectivity of 4,5,6,7-tetrabromobenzotriazole, an ATP site-directed inhibitor of protein kinase CK2 (casein kinase-2). FEBS Lett 2001; 496: 44–48. [DOI] [PubMed] [Google Scholar]
- 20.Makowska M, Łukowska-Chojnacka E, Wińska P, Kuś A, Bilińska-Chomik A, Bretner M. Design and synthesis of CK2 inhibitors. Mol Cell Biochem 2011; 356: 91–96. [DOI] [PubMed] [Google Scholar]
- 21.Martel V, Filhol O, Nueda A, Cochet C. Dynamic localization/association of protein kinase CK2 subunits in living cells: a role in its cellular regulation? Ann N Y Acad Sci 2002; 973: 272–277. [DOI] [PubMed] [Google Scholar]
- 22.Yde CW, Olsen BB, Meek D, Watanabe N, Guerra B. The regulatory beta-subunit of protein kinase CK2 regulates cell-cycle progression at the onset of mitosis. Oncogene 2008; 27: 4986–4997. [DOI] [PubMed] [Google Scholar]
- 23.Marangos P, Carroll J. Oocytes progress beyond prophase in the presence of DNA damage. Curr Biol 2012; 22: 989–994. [DOI] [PubMed] [Google Scholar]
- 24.Lorenz P, Pepperkok R, Pyerin W. Requirement of casein kinase 2 for entry into and progression through early phases of the cell cycle. Cell Mol Biol Res 1994; 40: 519–527. [PubMed] [Google Scholar]
- 25.Li D, Dobrowolska G, Aicher LD, Chen M, Wright JH, Drueckes P, Dunphy EL, Munar ES, Krebs EG. Expression of the casein kinase 2 subunits in Chinese hamster ovary and 3T3 L1 cells provides information on the role of the enzyme in cell proliferation and the cell cycle. J Biol Chem 1999; 274: 32988–32996. [DOI] [PubMed] [Google Scholar]
- 26.Espunya MC, Combettes B, Dot J, Chaubet-Gigot N, Martínez MC. Cell-cycle modulation of CK2 activity in tobacco BY-2 cells. Plant J 1999; 19: 655–666. [DOI] [PubMed] [Google Scholar]
- 27.Carneiro AC, Fragel-Madeira L, Silva-Neto MA, Linden R. A role for CK2 upon interkinetic nuclear migration in the cell cycle of retinal progenitor cells. Dev Neurobiol 2008; 68: 620–631. [DOI] [PubMed] [Google Scholar]
- 28.Schneider HR, Reichert GH, Issinger OG. Enhanced casein kinase II activity during mouse embryogenesis. Identification of a 110-kDa phosphoprotein as the major phosphorylation product in mouse embryos and Krebs II mouse ascites tumor cells. Eur J Biochem 1986; 161: 733–738. [DOI] [PubMed] [Google Scholar]
- 29.Chen M, Cooper JA. The beta subunit of CKII negatively regulates Xenopus oocyte maturation. Proc Natl Acad Sci USA 1997; 94: 9136–9140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen M, Li D, Krebs EG, Cooper JA. The casein kinase II beta subunit binds to Mos and inhibits Mos activity. Mol Cell Biol 1997; 17: 1904–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lieberman SL, Ruderman JV. CK2 beta, which inhibits Mos function, binds to a discrete domain in the N-terminus of Mos. Dev Biol 2004; 268: 271–279. [DOI] [PubMed] [Google Scholar]