Abstract
Tuberculosis (TB), although a curable disease, is still one of the most difficult infections to treat. Mycobacterium tuberculosis infects 10 million people worldwide and kills 1.5 million people each year. Reactivation of a latent infection is the major cause of TB. Cholesterol is a critical carbon source during latent infection. Catabolism of cholesterol contributes to the pool of propionyl‐CoA, a precursor that is incorporated into lipid virulence factors. The M. tuberculosis genome contains a large regulon of cholesterol catabolic genes suggesting that the microorganism can utilize host sterol for infection and persistence. The protein products of these genes present ideal targets for rational drug discovery programmes. This review summarizes the development of enzyme inhibitors targeting the cholesterol pathway in M. tuberculosis. This knowledge is essential for the discovery of novel agents to treat M. tuberculosis infection.
Linked Articles
This article is part of a themed section on Drug Metabolism and Antibiotic Resistance in Micro‐organisms. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.14/issuetoc
Abbreviations
- 2,3‐DHB
2,3‐dihydroxybiphenyl
- 3,4‐DHSA
3,4‐dihydroxy‐9,10‐seconandrost‐1,3,5(10)‐triene‐9,17‐dione
- 3β‐HSD
3β‐hydroxysteroid dehydrogenase
- 4,9‐DSHA
4,5–9,10‐diseco‐3‐hydroxy‐5,9,17‐tri‐oxoandrosta‐1(10),2‐diene‐4‐oic acid
- ACAD
acetyl‐CoA dehydrogenases
- Ac‐CoA
acetyl‐CoA
- BCG
Bacillus Calmette–Guérin
- BSPPA
2‐(benzo[d]‐2,1,3‐thiadiazole‐4‐sulfonyl)‐2‐amino‐2‐phenyl‐N‐(pyridinyl‐4)‐acetamide
- CYP450
cytochrome P450
- DHEA
dehydroepiandrosterone
- EPBA
α‐ethyl‐N‐4‐pyridinyl‐benzeneacetamide
- HOPDA
2‐hydroxy‐6‐oxo‐6‐phenylhexa‐2,4‐dienoic acid
- HOPODA
8‐(2‐chlorophenyl)‐2‐hydroxy‐5‐methyl‐6‐oxoocta‐2,4‐dienoic acid
- HTS
high throughput screening
- MCP
meta‐cleavage product
- MIC
minimum inhibitory concentration
- NAT
arylamine N‐acetyltransferase
- PDIM
phthiocerol dimycocerosate
- Pr‐CoA
propionyl‐CoA
- SAR
structure activity relationship
- TB
Tuberculosis
Tables of Links
| TARGETS |
|---|
| Enzymes |
| CYP51 |
These Tables list key protein targets and ligands in this article that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander et al., 2015).
Introduction
Tuberculosis (TB) remains one of the leading causes of death from bacterial infection. Mycobacterium tuberculosis infects one‐third of the human population and is responsible for approximately 1.5 million deaths annually (WHO, 2015). The mycobacterium bacillus is characterized by its inherent resistance to antibiotics, because of its extremely slow growth rate and the complex lipid composition of its cell wall (Figure 1). Thus, treatment with currently available anti‐TB therapies (Figure 2) is of long duration and usually leads to problems related to patient non‐compliance, high frequency of side effects, relapses and emergence of resistance. The treatment of infections caused by multi drug‐resistant (MDR) strains is one of the biggest challenges of TB therapy, causing around 190 000 deaths in 2014 (WHO, 2015). Moreover, the untreatable extensively drug‐resistant TB is spreading at an alarming rate and was reported by 105 countries in 2015 (WHO, 2015).
Figure 1.
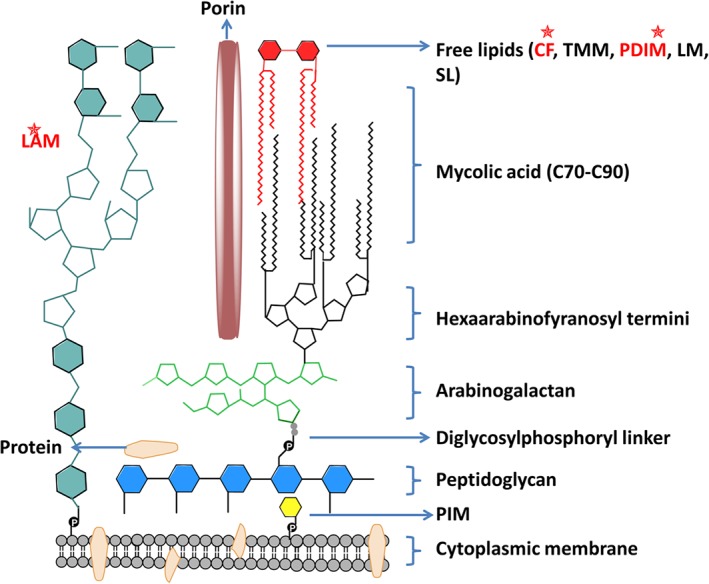
Diagram of the mycobacterial cell envelope. The cytoplasmic membrane of mycobacteria is encapsulated by a layer of peptidoglycans. The peptidoglycan backbone is attached to arabinogalactan through an unusual disaccharide‐phosphate‐linker region. The arabinogalactan is a branched‐chain polysaccharide consisting of a proximal galactose chain linked to a distal arabinose chain. Mycolic acids are covalently linked to the arabinogalactan‐peptidoglycan co‐polymer and are an essential component of the cell wall. Extractable lipids (free lipids) are shown in red. Another major component non‐covalently associated to the mycobacterial cell wall is the immunogenic lipoarabinomannan (LAM), which is attached to the cytoplasmic membrane by a phosphatidylinositol anchor. The porin MspA mediates the uptake of small and hydrophilic nutrients such as sugars and phosphates, whereas hydrophobic compounds diffuse directly across the cell wall. PIM, phosphatidylinositol mannoside; CF, cord factor; TMM, trehalose monomycolate; PDIM, phthiocerol dimycocerosates; LM, lipomannan, SL: sulfolipids. Virulence factors that could be affected by the cholesterol pathway are shown in red and marked with stars.
Figure 2.
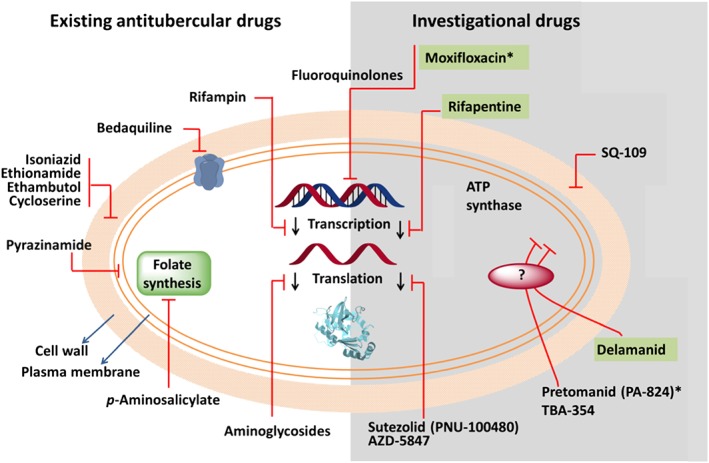
Mechanisms of action for current and investigational tuberculosis drugs. Targets of current drugs include cell‐wall synthesis (isoniazid, ethionamide, ethambutol and cycloserine), folate synthesis (p‐aminosalicylate), transcription (rifampin), translation (aminoglycosides), DNA metabolism (fluoroquinolones) and the cell membrane (pyrazinamide). New compounds in clinical trials target other bacterial functions. TMC207 seems to inhibit the ATP synthase complex. SQ‐109 inhibits cell‐wall synthesis, and linezolid and PNU‐100480 affect protein synthesis. Delamanid (OPC‐67863) and pretomanid (PA‐824) are prodrugs, the activation of which depends on the same cellular enzyme. TBA‐354, a nitroimidazole that is part of the same class of drugs as delamanid and pretomanid. The ultimate targets of these compounds remain unknown. Moxifloxacin, rifapentine and delamanid (green background) are in Phase III clinical trials, whereas the rest are either in Phase I or II. The asterisks denote projects that are being developed or co‐developed by the GTB Alliance.
Luckily, there has been a resurgence in drug discovery for TB in the last 10 years. These efforts culminated in the approval of two new drugs for the treatment of MDR‐TB as part of a combination regimen: the ATP synthase inhibitor bedaquiline (Andries et al., 2005; Cohen, 2013; Pym et al., 2015) and delamanid (Blair and Scott, 2015; Gupta et al., 2015; WHO, 2015). Furthermore, great efforts have been made to understand the biology of TB. Despite this the TB drug pipeline is still far below the level needed. Eight novel compounds are now in clinical development for the treatment of drug‐susceptible, MDR‐TB or latent TB infection (WHO, 2015) (Figure 2).
Latent TB
According to the WHO reports, latent infection represents the major pool of worldwide TB cases, thus making the treatment of latent TB an important strategy towards eradicating the disease (Young et al., 2009; Getahun et al., 2015; WHO, 2015).
Latent infection with M. tuberculosis is associated with the absence of clinical symptoms or the detection of any isolated bacteria from the patient (Vernon, 2013). In latent M. tuberculosis infection, the bacteria are usually contained within a well‐organized granuloma by the cell‐mediated immune response (Gideon and Flynn, 2011). Despite the role of the granuloma as a host‐defence mechanism, it also provides a shelter that houses and protects the mycobacteria from antibiotics and from further destruction by the immune responses of the host (Pieters, 2008). M. tuberculosis can persist in a dormant state (i.e. in a state of low metabolic activity but not forming colonies) in the macrophage for decades despite the acidic hypoxic environment (Muttucumaru et al., 2004; Pieters, 2008). The exact mechanisms by which M. tuberculosis enters the host cell and avoids host defences are not completely understood. It has been suggested that M. tuberculosis uses a variety of mechanisms to survive the hostile environment of the macrophage (Figure 3) (Meena and Rajni, 2010). Interestingly, it has been suggested that the accumulation of cholesterol and other lipids within granulomas contributes to the development of a non‐replicating state of mycobacteria (Brzostek et al., 2009; Caceres et al., 2009; Ehrt and Rhee, 2013; Lovewell et al., 2016).
Figure 3.
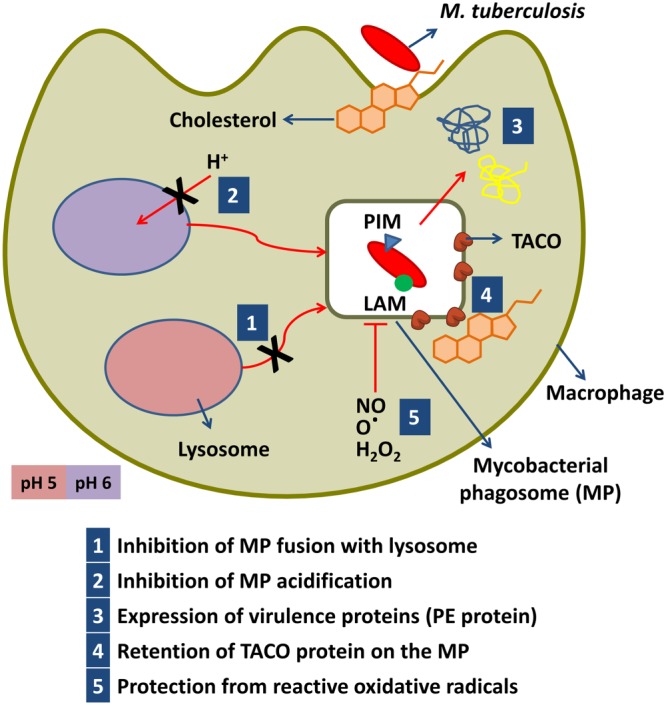
Strategies used by M. tuberculosis to modulate phagosome maturation. After internalization, the bacterium uses an array of effector molecules, including the lipids phosphatidylinositol mannoside (PIM) and lipoarabinomannan (LAM) to arrest phagosome maturation. Retention of the TACO protein prevents bacterial delivery to the lysosome. TACO is a host protein that is known to be associated with cholesterol. Cholesterol has also been shown to play a role in the entry of mycobacteria into the macrophage. PE‐proteins are (Pro‐Glu) repetitive glycine‐rich proteins. MP, mycobacterial phagosome.
The role of cholesterol
Cholesterol has a pivotal role in the infectivity and virulence of M. tuberculosis (Larrouy‐Maumus, 2015; Kumar et al., 2016; Lovewell et al., 2016). Host cholesterol has been shown to facilitate the entry of mycobacteria into macrophages (Kaul et al., 2004; Pandey and Sassetti, 2008; Kumar et al., 2016). Furthermore, M. tuberculosis is capable of using cholesterol as a carbon source (Russell et al., 2010). The cholesterol degradation pathway in mycobacteria has been proposed based on the determination of the genes involved in cholesterol catabolism in the Rhodococcus species (Van der Geize et al., 2007). Most of these genes in M. tuberculosis have been found to be under the control of kstR regulons, which encode a TetR‐like transcriptional repressor (Kendall et al., 2007; Kendall et al., 2010). Several genes in the kstR cholesterol regulons are induced in macrophages or are essential for infection, emphasizing the role of cholesterol catabolism in intracellular survival (Kendall et al., 2007; Kendall et al., 2010).
There are three main putative operons that affect cholesterol entry and catabolism in mycobacteria (Figure 4). The mammalian cell entry mce4 operon (Rv3492c‐Rv3501c) is involved in cholesterol uptake and utilization in M. tuberculosis (Pandey and Sassetti, 2008). Deletion of the mce4 operon in M. tuberculosis resulted in a growth defect when cholesterol was used as the primary source of carbon (Pandey and Sassetti, 2008). It also attenuated infection in both activated macrophages and mouse models (Senaratne et al., 2008). The intracellular growth igr operon (Rv3545c to Rv3540c) was essential for growth and virulence in macrophages and in mice and was involved in cholesterol metabolism (Schnappinger et al., 2003; Chang et al., 2009). Deletion of this operon resulted in defective growth on cholesterol due to toxic metabolite accumulation (Chang et al., 2009). The third operon contains the hsaACDB cluster (Rv3566‐Rv3570c), which is involved in the cholesterol sterol‐ring degradation (Anderton et al., 2006; Van der Geize et al., 2007). This is also essential for the intracellular survival of mycobacteria within the macrophage, although the gene‐knockout strain retains the ability to grow on rich media (Bhakta et al., 2004; Anderton et al., 2006).
Figure 4.
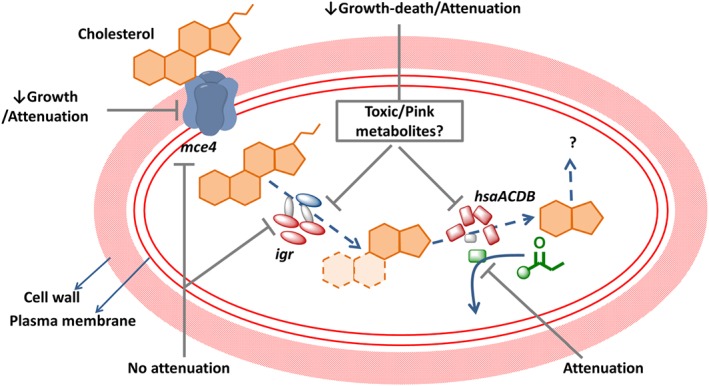
The role of essential putative operons in the intracellular survival of M. tuberculosis in macrophages (mce4, igr and the hsaACDB) in cholesterol metabolism. The diagram shows the established roles of the mce4, igr and the nat operons in cholesterol uptake and metabolism. The effect of deleting each of these operons or genes within the operon on the growth of M. tuberculosis in cholesterol and/or the effect on attenuation of infection in macrophage or in a mouse model is shown in black text. Cholesterol is transported into M. tuberculosis via the Mce4 transport system. The igr operon consists of six genes, the most important of which is cytochrome P450 (cyp125). It also comprises two acyl‐CoA dehydrogenases (fadE28 and fadE29), two conserved hypothetical proteins (Rv3541‐2c) and a putative lipid carrier protein (ltp2). Inactivation of the igr operon resulted in growth defects in cholesterol attenuation in a mouse model, an effect that is prevented by mutating the sterol uptake Mce4 system. The nat operon consists of six genes, including a NAT, four hsaACDB genes and a hypothetical protein. The nat gene product NAT utilizes Pr‐CoA, a cholesterol degradation product. Products of uncertain identity are shown as question marks. The dashed arrows indicate a multistep process. Gene products with enzymic activity are shown in red, the NAT enzyme is shown in green, hypothetical proteins in grey and transport proteins in blue.
Furthermore, cholesterol makes a potentially important contribution to the metabolic pool of propionyl‐CoA (Pr‐CoA) in mycobacteria (Yang et al., 2009). Pr‐CoA is converted to methylmalonyl‐CoA, which is considered to be the building block of multimethyl‐branched mycolic acids such as phthiocerol dimycocerosate (PDIM) (Yang et al., 2009). Cholesterol metabolism in mycobacteria has been shown to increase the average mass of the lipid virulence factor PDIM.
The cholesterol catabolic pathway in M. tuberculosis involves two major phases, the initial degradation of the aliphatic side chain and the subsequent degradation of the sterol A‐D rings (Figure 5) (Ouellet et al., 2011a). The putative degradation of the aliphatic side chain, by β‐oxidation, results in the formation of one acetyl‐CoA (Ac‐CoA) and two Pr‐CoA molecules (Figure 5). The Pr‐CoA derived from the side chain has been shown to be incorporated into PDIM (Pandey and Sassetti, 2008). However, the sterol ring degradation is more complicated, as it involves a large number of enzymes and is not yet completely understood (Figure 5). The products of the hsaACDB operon play important role in the degradation of rings A and B (Ouellet et al., 2011a).
Figure 5.
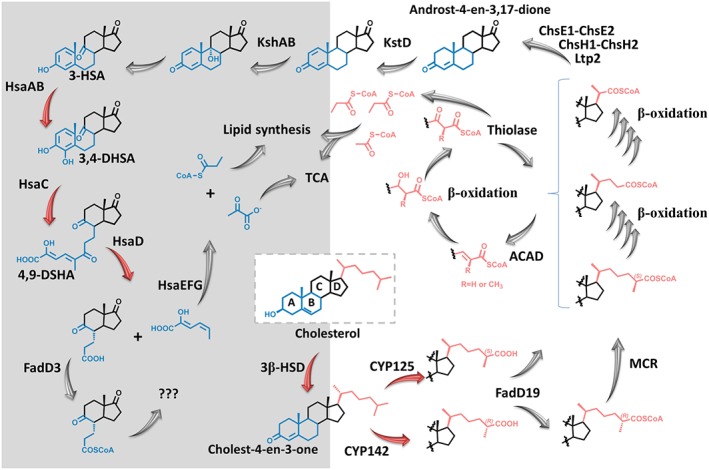
Proposed degradation pathways of the cholesterol aliphatic side chain and ring nucleus. The carbon atoms derived from the ring nucleus are converted to CO2 via the tricarboxylic acid cycle (TCA), whereas the Pr‐CoA produced from the degradation of the side chain is assimilated into mycobacterial lipids (e.g. PDIM). The enzymes and the cholesterol metabolites are described in the text. The aliphatic side chain (pink) degradation is shown with no background while the sterol ring (blue) degradation is shown with a grey background. The fate of rings C and D (black) is still unknown. Numbers correspond to the intermediates mentioned in the text. Steps catalysed by enzymes discussed in this review are shown in red arrows.
Targeting these putative enzymes involved in the cholesterol pathway in mycobacteria hold promise for the development of novel therapeutic approaches for TB. Particularly important is that most of these targets lack human homologues and have low risk of cross resistance with current TB drugs.
This review summarizes the current progress in targeting cholesterol metabolism as a novel strategy for generating effective and safe short‐term antitubercular drugs that would provide new therapeutic options for TB, MDR‐TB and potentially latent TB, and might limit the development of multidrug‐resistant strains.
Targeting key enzymes in the cholesterol pathway
3β‐hydroxysteroid dehydrogenase
In M. tuberculosis, the first step in the cholesterol metabolism pathway is catalysed by 3β‐hydroxysteroid dehydrogenase (3β‐HSD) (Rv1106c), converting cholesterol to the 4‐en‐3‐one (Yang et al., 2007) (Figure 5). M. tuberculosis 3β‐HSD is a bifunctional enzyme that oxidizes and isomerises dehydroepiandrosterone (DHEA) and pregnenolone to their respective α,β‐unsaturated ketones. Such activity indicates that the steroid skeleton could undergo oxidative degradation simultaneously with side chain truncation. Homologues of this enzyme are present in humans and are required for the biosynthesis of steroid hormones. M. tuberculosis 3β‐HSD shares 29% amino acid sequence identity with type I and type II human 3β‐HSD and catalyses a similar reaction. Both the active site catalytic triad S131, Y158, K162 and the Rossman fold motif for NAD+ cofactor binding are conserved. Yang et al. (2011) showed that mutant strains lacking this gene have impaired growth on cholesterol, but normal or hyper‐virulence in infection models. This observation led to the conclusion that cholesterol is not an essential nutrition source during infection (Yang et al., 2011). These studies contrast with others indicating that 3β‐hsd does not have an essential role in cholesterol catabolism. For example, in deep‐sequencing studies of a transposon mutant library of strain H37Rv, 3β‐hsd was not among the 96 genes predicted to be specifically required for growth on cholesterol (Griffin et al., 2011). Beste et al. (2013) have shown that 3β‐hsd is not necessary for nutrition acquisition. Therefore, additional studies are required to resolve the contradictions in published work and to identify the actual role of this enzyme in the cholesterol pathway. Although the inhibition of 3β‐HSD may not be suitable for drug development, it can help improve our understanding of the biological role of this enzyme.
It is worth noting that another enzyme was also suggested to catalyse the first step in the cholesterol pathway. Cholesterol oxidase is important for virulence and growth of M. tuberculosis in peritoneal macrophages and lungs of mice (Brzostek et al., 2007). However, the enzyme does not appear to be essential for cholesterol degradation by mycobacteria and was not explored as a drug target (Klink et al., 2013; Gao and Sampson, 2014).
Azasteroid 3β‐HSD inhibitors
Azasteroids are validated therapeutic agents that inhibit steroid biosynthetic pathways and can be used in some prostate cancers (Frye et al., 1995; Frye, 2006). Among these, the 6‐azasteroid series are transition state analogues and thus are potent inhibitors against human adrenal 3β‐HSD (Frye et al., 1993; Frye et al., 1994).
Thomas et al. (2011) studied the inhibition of the M. tuberculosis 3β‐HSD using a set of 20 azasteroids. Their study aimed at understanding the structure‐activity relationship (SAR) of inhibition of this enzyme by 6‐azasteroids (Thomas et al., 2011) and they identified a cholestenone analogue that exhibited the most potent inhibitory activity (compound 7; Figure 6). Kinetics studies showed competitive behaviour versus the sterol substrate DHEA and uncompetitive behaviour versus NAD+, suggesting that the azasteroid binds most effectively to the E‐NAD+ complex. (Thomas et al., 2011).
Figure 6.
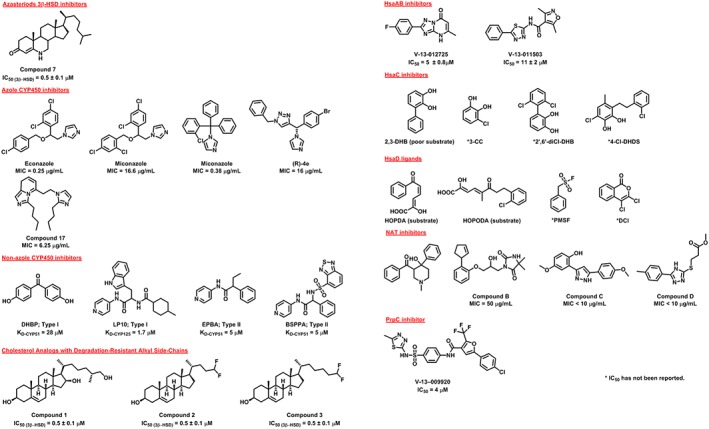
Identified inhibitors of key enzymes in the cholesterol pathway in M. tuberculosis.
Inhibitors of M. tuberculosis 3β‐HSD are important for targeting the cholesterol metabolic pathway. However, further development is needed before in vivo analysis of enzyme inhibition could be undertaken to avoid interference with human analogues.
Cytochrome P450: CYP125/CYP142 and CYP151
One of the most unexpected findings from the M. tuberculosis H37Rv genome sequence was the presence of 20 different CYP genes encoding cytochrome P450 (CYP450) enzymes (McLean et al., 2006a). Characterization of these enzymes has confirmed novel functions in the cholesterol pathway and has pointed to their potential for exploitation as therapeutic targets (McLean and Munro, 2008; Hudson et al., 2012).
The mycobacterial CYP51 was the first identified prokaryotic sterol demethylase form of CYP450. CYP51 catalyses the removal of the 14α‐methyl group from sterol precursors such as lanosterol, an essential step in sterol biosynthesis in eukaryotes (McLean et al., 2006a). Targeting CYP51 has turned out to be highly successful in developing broad spectrum antifungal and anti‐Chagas drugs (Sheehan et al., 1999; Lepesheva et al., 2011; Choi et al., 2014). By contrast, this enzyme is not essential for viability or pathogenicity of M. tuberculosis (Podust et al., 2001). A complete sterol biosynthetic pathway is not present in the tubercular bacilli (McLean et al., 2010), and thus the in vivo function of CYP51 in mycobacteria is unknown.
The most important CYP450 drug target in M. tuberculosis is CYP125 (Rv3545c). This enzyme catalyses the 27‐hydroxylation of cholest‐4‐en‐3‐one on its side chain to generate oxidized sterols, preferentially forming the (25S)‐26‐hydroxy product (Figure 5) (Capyk et al., 2009). CYP125 lies in the igr operon (Figure 4) and thus is induced in macrophages and has been identified as essential for virulence during macrophage infection in mice (Schnappinger et al., 2003; Kendall et al., 2004). These findings suggest that drugs inhibiting CYP125 could be important for eliminating non‐replicating latent M. tuberculosis. However, CYP125 redundancy has been found in M. tuberculosis H37Rv, as a mutant strain lacking this enzyme can metabolize cholesterol and grows using this substrate as the only source of carbon (Capyk et al., 2009). Interestingly, CYP142 (Rv3518c) was identified as the likely compensatory enzyme in the absence of CYP125 function (Ouellet et al., 2010). CYP142 catalyses either 27‐hydroxylation of cholesterol/cholest‐4‐en‐3‐one (25R isomer) or generates 5‐cholestenoic acid/cholest‐4‐en‐3‐one‐27‐oic acid from these substrates by successive sterol oxidations (Figure 5) (Driscoll et al., 2010; Johnston et al., 2010). CYP142 is a product of a cluster of genes involved in the metabolism of host lipids, including cholesterol (Johnston et al., 2010). Hence, it may also present an additional secondary target for the latent M. tuberculosis (Johnston et al., 2010).
Both CYP125 and CYP142 have been investigated as potential M. tuberculosis drug targets (McLean et al., 2009; Ouellet et al., 2010). McLean et al. (2009) solved the structure of the CYP125 in complex with cholest‐4‐en‐3‐one via X‐ray crystallography. Ouellet et al. (2010) have also determined the structure of this enzyme in a ligand‐free state and in complex with inhibitors. The structures reveal an active site cavity with dimensions appropriate for the binding of steroid‐like molecules and a ‘letterbox’‐like hydrophobic entry cavity that narrows in a funnel‐like manner on approach to the haem centre (McLean et al., 2009; Ouellet et al., 2011b). Driscoll et al. (2010) determined the crystal structure of CYP142 and , although this revealed a similar active site organization to CYP125, it has also shown an identical organization of distal pocket residues to CYP124. M. tuberculosis CYP124 is a branched fatty acid‐oxidizing enzyme (Johnston et al., 2009).
Azole CYP450 inhibitors
Antifungal azoles such as econazole, miconazole and clotrimazole have potent antimycobacterial activity in vitro (Figure 6) (McLean et al., 2002). Ahmad et al. (2006b, 2006c) have shown that clotrimazole and econazole are effective against persistent and MDR M. tuberculosis strains. They have also shown that econazole is effective in reducing bacterial burden by 90% in lungs and spleen of infected mice (Ahmad et al., 2006a) and that econazole and clotrimazole have synergistic anti‐TB activity when combined with rifampicin and isoniazid (Ahmad et al., 2005).
The CYP450s of M. tuberculosis bind azoles with high affinity and hence were proposed as the primary target for their antitubercular activity (McLean et al., 2002; McLean et al., 2006b; McLean et al., 2009). Deletion of Cyp125 gene in M. tuberculosis renders the bacterium more sensitive to growth inhibition by clotrimazole suggesting a marginal role in azoles activity (Carroll and Parish, 2015). The main target for azole action in M. tuberculosis has yet to be found (Carroll and Parish, 2015).
Attempts have been made to optimize the azole scaffolds (Castagnolo et al., 2009; Pandey et al., 2009). Castagnolo et al. (2009) synthesized a group of novel enantiomerically pure azole derivatives and managed to obtain an active compound with minimum inhibitory concentration (MIC) against M. tuberculosis of 16 μg·mL−1. Pandey et al. (2009) synthesized a series of imidazole based compounds and identified a bis‐imidazolyl derivative (compound 17; Figure 6) with an MIC value of 6.25 μg·mL−1 against M. tuberculosis (Pandey et al., 2009). However, neither of these studies established the contribution of CYP450 inhibition to the anti‐mycobacterial activity of those inhibitors.
Although they represent a good starting point for medicinal chemistry, current azole antifungals are unsuitable as front‐line anti‐TB drugs, because of their poor bioavailability and cross‐reactivity with human CYP450s (McLean et al., 2010). Furthermore, resistance to azoles in M. tuberculosis has been observed and linked to the MmpS5‐MmpL5 efflux system (Milano et al., 2009).
Therefore, further research is still needed to understand fully the molecular mechanisms of inhibition by azoles. Extensive optimization of the current antifungal azoles is needed to establish azole‐based inhibitors selective for M. tuberculosis CYP125 or CYP142. Such efforts would benefit from the crystallographic studies on the enzyme‐azole complexes (Podust et al., 2001; McLean et al., 2009).
Non‐azole CYP450 inhibitors
Podust et al. (2007) identified two non‐azole CYP450 inhibitory scaffolds by conducting a high throughput screening (HTS) using a 20 000‐compound chemical library against M. tuberculosis CYP51. Two HTS hits, 4,4′‐dihydroxybenzophenone and α‐ethyl‐N‐4‐pyridinyl‐benzeneacetamide (EPBA) (Figure 6), were found to bind tightly to CYP51, but neither of them was a potent M. tuberculosis inhibitor (Podust et al., 2007). Another compound, 2‐(benzo[d]‐2,1,3‐thiadiazole‐4‐sulfonyl)‐2‐amino‐2‐phenyl‐N‐(pyridinyl‐4)‐acetamide (BSPPA) was also found to bind to CYP51B1 with high affinity (Figure 6) (Podust et al., 2007). These compounds showed higher selectivity for CYP51 when compared to CYP125 or other CYP450s (Podust et al., 2007). The crystal structures of CYP51 in complex with EPBA and BSPPA were determined revealing the binding mode of these compounds in the active site (Podust et al., 2007).
Ouellet et al. (2011b) explored the interactions of CYP125 with LP10, a reverse type I inhibitor. This compound was previously identified as a potent type II inhibitor of Trypanosoma cruzi CYP51 (Doyle et al., 2010). When tested against M. tuberculosis CYP450s, it showed higher affinity for CYP125 (KD = 1.7 μM) than for CYP51 (K D = 18 μM) (Ouellet et al., 2011b). Crystallographic studies revealed that LP10 exhibits an unusual reverse type‐I binding mode with CYP125. The pyridine ring of this compound did not co‐ordinate directly with the haem iron but instead interacted with a three water hydrogen‐bond network around the iron (Ouellet et al., 2011b).
Each of these novel scaffolds represents a starting point for medicinal chemistry optimization to improve their activity against CYP125 and CYP142.
Cholesterol analogues with degradation‐resistant alkyl side‐chains
Ouellet et al. (2010) have shown that cholest‐4‐en‐3‐one inhibits the growth of M. tuberculosis in defined media containing glycerol. However, cholest‐4‐en‐3‐one is rapidly degraded by wild‐type mycobacteria and only causes transient inhibition (Ouellet et al., 2010).
Schmidt et al. (2013) identified 3β‐hydroxysterol‐(25R)‐cholest‐5‐en‐3β,16β,26‐triol (compound 1; Figure 6) as an inhibitor of M. tuberculosis growth without causing toxicity to the mammalian cell‐line. Frank et al. (2016) demonstrated that growth inhibition of M. tuberculosis by the 16‐hydroxy derivatives of cholesterol is due to the inhibition of sterol side‐chain oxidation. This structural modification renders the compound a poor substrate that binds to CYP125, but not CYP142, in a non‐productive manner (Frank et al., 2016). They concluded that cholesterol analogues with non‐degradable side‐chains represent a novel class of anti‐mycobacterial agents. Frank et al. (2016) have recently identified two cholesterol analogues with truncated, fluorinated side‐chains (compounds 2 and 3; Figure 6) that are resistant to degradation and similarly inhibit M. tuberculosis growth.
Further exploration of these compounds might lead to a new paradigm for the development of therapeutically useful inhibitors (Frank et al., 2016).
HsaAB
The protein HsaAB (hsaA (Rv3570c) and hsaB (RV3567c)) has been predicted to be a two‐component flavin‐dependent monooxygenase (TC‐FDM) (Van der Geize et al., 2007). In this type of monooxygenase, the reductase utilizes NADH to reduce a flavin, which is then transferred to the oxygenase (Galán et al., 2000). HsaAB converts 3‐HSA to 3,4‐DHSA (3,4‐dihydroxy‐9,10‐seconandrost‐1,3,5(10)‐triene‐9,17‐dione) in the catabolism of the A/B rings of cholesterol (Figure 5) (Dresen et al., 2010). In transposon mutagenesis studies, hsaA was identified as essential for survival of M. tuberculosis in macrophages while hsaB is not (Rengarajan et al., 2005). It is likely that the flavin‐reducing function of HsaB could be compensated for by other reductases (Dresen et al., 2010).
Dresen et al. (2010) solved the crystal structure of HsaA, showing a substrate‐binding pocket that is well‐adapted to the steroid metabolite.
HsaAB inhibitors
VanderVen et al. (2015) identified inhibitors of the HsaAB enzyme complex by utilizing a novel screening approach. They carried out an extensive, unbiased chemical screen to identify low MW compounds that inhibited M. tuberculosis metabolism within macrophages and in medium containing cholesterol as the principal carbon source. They then developed a chemical‐rescue strategy to identify compounds that target metabolic enzymes involved in cholesterol metabolism (VanderVen et al., 2015). Surprisingly, many of the identified inhibitors require the presence of cholesterol in the media, although some of these compounds do not appear to target cholesterol utilization directly.
Thus, VanderVen et al. (2015) identified the first known inhibitors (V‐13‐011503 and V‐13‐012725; Figure 6) of cholesterol catabolism in M. tuberculosis, that target HsaAB. These hits represent new classes of inhibitors with novel modes of action. Chemical inhibition of HsaAB limits M. tuberculosis replication in macrophages and is consistent with the prediction that these genes are required for growth in macrophages. Efforts are currently underway to optimize these compounds, determine their molecular mechanism of HsaAB inhibition, and to establish whether HsaAB inhibitors alone or in combination with front‐line anti‐TB drugs could improve treatment outcomes in murine chemotherapy models in vivo.
HsaC
HsaC (Rv3567c) has been identified as type I iron‐dependent extradiol dioxygenase that cleaves 2,3‐dihydroxybiphenyl (2,3‐DHB) (Figure 6) (Van der Geize et al., 2007). In the cholesterol degradation pathway in M. tuberculosis, HsaC catalyses the meta‐cleavage of 3,4‐DHSA to produce 4,5–9,10‐diseco‐3‐hydroxy‐5,9,17‐tri‐oxoandrosta‐1(10),2‐diene‐4‐oic acid (4,9‐DSHA). Transposon site hybridization analysis has found that hsaC is essential for survival of the bacilli within macrophages (Rengarajan et al., 2005). Deleting the hsaC gene in M. tuberculosis resulted in cell death in the presence of cholesterol, due to the blockade of a catabolic pathway and to the accumulation of toxic catechol metabolites (Figure 4) (Pandey and Sassetti, 2008; Van der Geize et al., 2007; Yam et al., 2009). Furthermore, the hsaC‐knockout strains showed attenuated growth in immunocompromised mice and reduction in granuloma in guinea pigs infected with the knockout mutants (Yam et al., 2009). Humans have dioxygenase analogues such as human 3‐hydroxy‐anthranilate‐3,4‐dioxygenase, which are essential for the conversion of tryptophan to quinolinate (Zhang et al., 2005; Yam et al., 2009). Yam et al. (2009) solved the structure of the enzyme in ligand‐free form and in complex with 3,4‐DHSA revealing the large hydrophobic substrate‐binding pocket and an unexpected monodentate‐bound ligand.
HsaC inhibitors
Anderton et al. (2006) studied the kinetics of the HsaC homologue in M. bovis Bacillus Calmette–Guérin (BCG), which is >99.95% identical at the nucleotide level to M. tuberculosis (Garnier et al., 2003), using a synthetic catechol substrate 2,3‐DHB (Figure 6). They observed that M. bovis BCG growth was inhibited with either 2,3‐DHB (poor substrate) or 3‐chlorocatechol (Figure 6), which is an extradiol dioxygenase inhibitor (Anderton et al., 2006). In addition, incubation of M. bovis BCG with 2,3‐DHB decreased the levels of mycolates in the cell wall of the bacilli (Anderton et al., 2006). Yam et al. (2009) showed that HsaC was inactivated by halogenated substrate analogues. Interestingly, two halogenated compounds 2′,6′‐dichloro‐DHB and 4‐chloro‐3‐dihydroxy‐6‐methyl‐7,8‐dihydro‐10‐Cl‐stilbene (Figure 6), efficiently inactivated HsaC via different modes of action. They explained these findings by the presence of steric and electronic factors contributing to ligand binding (Yam et al., 2009). Full understanding of such factors will facilitate the optimization of these inhibitors.
HsaD
HsaD (Rv3569c) was identified as a meta‐cleavage product (MCP) hydrolase. It catalyses the hydrolysis of a carbon–carbon bond in the cholesterol MCP, 4,9‐DSHA (Figure 6). TarSH analysis has indicated that HsaD is critical for the intracellular survival of M. tuberculosis (Rengarajan et al., 2005). Humans have a structural homologue of HsaD, known as monoglyceride lipase (Bertrand et al., 2010). It also shares the same fold and the serine‐based catalytic triad with human acetylcholinesterases.
Lack et al. (2010) have characterized the enzyme using the putative product of HsaC catalysed oxidation of 2,3‐DHB, known as 2‐hydroxy‐6‐oxo‐6‐phenylhexa‐2,4‐dienoic acid (HOPDA) and the synthetic substrate analogue 8‐(2‐chlorophenyl)‐2‐hydroxy‐5‐methyl‐6‐oxoocta‐2,4‐dienoic acid (HOPODA) (Figure 6). They have also determined the structure of the enzyme in a ligand free form and in complex with HOPDA, HOPODA, or 4,9‐DSHA. The structure revealed a high structural similarity to the α/β‐hydrolase superfamily, a conserved Ser‐His‐Asp catalytic triad as well as an ‘oxyanion’ hole (Lack et al., 2008; Lack et al., 2010). The structures also showed that the substrate binding‐site consists of two sub‐sites: a hydrophilic pocket where the dienoate moiety binds and a large nonpolar pocket that can accommodates the hydrophobic part of cholesterol MCP.
HsaD inhibitors
Ryan et al. (2014) screened a range of serine‐protease and esterase inhibitors for their effects on M. tuberculosis HsaD activity. They have identified two irreversible inhibitors, phenylmethylsulfonyl fluoride and 3,4‐dichloroisocoumarin, that covalently modify HsaD. The majority of the noncovalent protease/esterase inhibitors (e.g. benzamidine) either did not or only weakly affected the enzyme activity. They attributed these findings to the relatively small size of these inhibitors and to lack of selectivity. HsaD has a large open active site, which is considerably larger and more hydrophobic than the active site of either serine proteases or acetylcholinesterases (Ryan et al., 2014). This study was proposed as the first step towards fragment‐based ligand discovery efforts.
Arylamine N‐acetyl transferase
Arylamine N‐acetyltransferase (NAT) (Rv3566c) is a cytosolic enzyme, found in M. tuberculosis and humans, as well as in many other organisms (Sim et al., 2008; Sim et al., 2014). This enzyme catalyses the transfer of an acyl group from Ac‐CoA or Pr‐CoA to an arylamine substrate through a conserved cysteine residue by a Ping‐Pong bi‐bi mechanism (Sinclair et al., 2000; Fullam et al., 2009). However, neither the biological substrate, nor an endogenous role for the N‐acetylation activity of NAT has been reported. The nat genes from M. tuberculosis and M. bovis BCG are identical and are encoded in highly similar gene clusters (HsaACBD cluster) in both organisms (Anderton et al., 2006). Deleting the nat gene in M. bovis BCG resulted in depleted mycolic acids and virulence‐lipid content (PDIM and cord factor; Figure 1) (Bhakta et al., 2004; Westwood et al., 2010). Bhakta et al. (2004) showed that the nat is essential for intracellular survival, although the gene‐knockout strain retains the ability to grow on rich media. The ability of the enzyme to utilize Pr‐CoA, the intermediate cofactor in virulence lipid synthesis explains these findings and relates the enzyme to the cholesterol pathway (Yang et al., 2009; Sim et al., 2014). The part this enzyme plays in the virulence of mycobacteria, as well as intracellular survival, has made it an attractive target for anti‐TB drug development. Most of the studies conducted on this enzyme relied on the structure of homologous enzymes from other mycobacterial species, in particular the M. marinum (Fullam et al., 2008; Abuhammad et al., 2010). The M. tuberculosis NAT enzyme proved to be very recalcitrant to crystallization (Abuhammad et al., 2011). The structure of this enzyme has only recently been solved using a novel cross‐microseeding method after prolonged and varied attempts (Abuhammad et al., 2013).
NAT inhibitors
NAT inhibitors have been identified by a HTS of a 5000‐compound chemical library using the homologous NAT enzymes that were available at the time (Westwood et al., 2011). Several hits that inhibited the mycobacterial NATs selectively, also showed promising antimycobacterial activity (Westwood et al., 2011) (compounds A–D; Figure 6). All the identified inhibitors have been modified by structure‐based medicinal chemistry efforts to improve their potency and antimycobacterial activity as well as to study their SAR (Westwood et al., 2010; Fullam et al., 2011; Abuhammad et al., 2012; Fullam et al., 2013; Abuhammad et al., 2014).
Abuhammad et al. (2012) studied NAT inhibition by compound A (Figure 6). This piperidinol derivative was a time‐dependent irreversible inhibitor of NAT activity when tested against NAT from M. marinum. Compound A and five of its analogues exert potent antimycobacterial activity against M. tuberculosis with MIC values of 2.3–16.9 μM. Based on their structural studies and mass spectroscopy analysis of the NAT‐inhibitor adducts, Abuhammad et al. (2012) proposed a covalent mechanism of NAT inhibition that involves the formation of a reactive intermediate and selective cysteine residue modification. Although the SAR of this set of NAT inhibitors has been established (Abuhammad et al., 2014), these findings do not exclude the presence of an off‐target mechanism of antimycobacterial activity. However, these piperidinols present a unique class of antimycobacterial compounds that have a novel mode of action.
Fullam et al. (2011) explored NAT inhibition by compound B (a,β‐amino alcohol; Figure 6). Although these studies led to an improved understanding of the SAR, they were not successful in identifying compounds with enhanced activity (Fullam et al., 2011). Fullam et al. (2013) have also carried out similar studies on compound C (Figure 6). Detailed study of NAT inhibition by compound D (Figure 6) and its analogues has also been performed and resulted in a more thorough understanding of the SAR of these compounds (Westwood et al., 2010). The general observation from these SAR studies was that introducing a large lipophilic substituent in the structure would result in improved inhibition. Interestingly, NAT inhibition by compound D resulted in similar changes in cell‐wall lipids, morphology and intracellular survival to those observed upon deleting the nat gene (Westwood et al., 2010). Furthermore, compound D acted synergistically with gentamycin, mimicking the effects of the nat knockout in the same mycobacteria (Bhakta et al., 2004).
2‐Methylcitrate synthase. 2‐Methylcitrate synthase (prpC; Rv1131) is a key enzyme in gating the methylcitrate cycle (MCC). This enzyme catalyses the Claisen condensation of Pr‐CoA with oxaloacetate, a step required for assimilation of cholesterol‐derived Pr‐CoA into the tricarboxylic acid cycle in mycobacteria and other organisms (Uchiyama and Tabuchi, 1976; Textor et al., 1997; Brock et al., 2000; Claes et al., 2002; Muñoz‐Elías et al., 2006; Upton and McKinney, 2007). Pr‐CoA is a key precursor in several lipid biosynthetic pathways in M. tuberculosis (Kolattukudy et al., 1997).
Griffin et al. (2012) showed that deletion of the prpC gene impaired the bacterium's ability to grow in media containing cholesterol as a primary carbon source. They supported these findings by showing that Δmce4/ΔprpDC double mutants grew significantly more rapidly than ΔprpDC single mutants (Griffin et al., 2012). These observations highlight the importance of Pr‐CoA metabolism during growth on cholesterol and suggest that M. tuberculosis obtains a significant amount of its carbon requirements from cholesterol (Yang et al., 2009). Further studies showed that ΔprpDC mutants were unable to grow on propionate (Muñoz‐Elías and McKinney, 2005) or on fatty acids in vitro (Savvi et al., 2008).
In many bacteria, including mycobacteria, the inability to assimilate Pr‐CoA inhibits growth, even in the presence of other carbon sources (Muñoz‐Elías et al., 2006; Savvi et al., 2008). This is likely to be due to the accumulation of toxic intermediates that inhibit other essential pathways (Rocco and Escalante‐Semerena, 2010).
PrpC was also found to be required for intracellular growth in macrophages (Muñoz‐Elías and McKinney, 2005; Muñoz‐Elías et al., 2006; Griffin et al., 2012), indicating that Pr‐CoA metabolism is critical in intracellular infection. Addition of vitamin B12 resulted in a dose dependent reversal of Pr‐CoA‐related toxicity and thus of the growth defect.
Although essential for intracellular growth in macrophages, the prpDC genes are dispensable for bacterial growth in the mouse model of TB (Muñoz‐Elías et al., 2006). These findings support the central role for propionate metabolism in the growth and persistence of M. tuberculosis in vivo.
More recently, using an unbiased chemical screen to identify chemical compounds that inhibit M. tuberculosis metabolism within macrophages VanderVen et al. (2015) isolated a compound that inhibits PrpC with an IC50 of 4.0 μM. (VanderVen et al., 2015).
Promising targets on the horizon
Efforts to elucidate the role of the putative enzymes responsible for the ability of M. tuberculosis to utilize cholesterol during latent infection have revealed several candidate targets that are potentially amenable to small‐molecule drug discovery approaches (Nesbitt et al., 2010; Thomas and Sampson, 2013; Wipperman et al., 2013; Lu et al., 2015; Schaefer et al., 2015; Ho et al., 2016).
For example, M. tuberculosis possesses number of acetyl‐CoA dehydrogenases (ACAD) that are distinct from typical ACADs known to date. These mycobacterial ACADs are assembled from two adjacent gene products and form obligate α2β2‐heterotetrameric structure. This particular structural architecture has been found only in bacteria known to metabolize sterols. Examples of these enzymes are ChsE1‐ChsE2 and ChsE4‐ChsE5 (Yang et al., 2015). Interestingly, the expression of all of the α2β2‐ACAD enzymes from M. tuberculosis is regulated by cholesterol. The binding‐site features of the α2β2‐ACAD distinguish it from the mammalian host homotetrameric structure and will provide guidance for rational inhibitor design (Thomas and Sampson, 2013).
The basics for targeting these enzymes have been established very recently by characterizing them structurally and functionally and, in many instances, revealing unusual architecture and/or mechanisms of catalysis. Inhibitors for such targets have not been reported yet, although further research on such targets is expected.
Interestingly, VanderVen et al. (2015) have identified three structurally‐diverse compounds that limit cholesterol utilization indirectly by perturbing cAMP levels. It is likely that cAMP plays a role in the regulation of cholesterol utilization. However, since these inhibitors act indirectly and the exact targets have not been identified yet, they do not fit the scope of this review.
Summary and future perspectives
The discovery of novel anti‐TB drugs has been hampered by several obstacles (Van den Boogaard et al., 2009; Kaneko et al., 2011). The combination of the special nature of M. tuberculosis and the complicated pathophysiology of the disease represent the inherent challenges facing TB drug development (Mdluli et al., 2015). The greatest burden of TB is among poor, developing countries which presentg insufficient profit opportunity to encourage big pharma to develop new drugs. This trend has led to the domination of not‐for‐profit foundations investing on TB drug research and filling up the TB drug discovery pipeline (Table 1). However, many of the forthcoming investigational compounds are either derivatives of currently used drugs or modulate the same cellular pathways as drugs presently in use and thus are subject to cross‐resistance.
Table 1.
International partner organizations involved in tuberculosis research and control programsa
| Organization | Website |
|---|---|
| Bill and Melinda Gates Foundation | www.gatesfoundation.org/ |
| CDC Foundation | www.cdcfoundation.org/ |
| Centres for Disease Control and Prevention | www.cdc.gov/ |
| European & Developing Countries Clinical Trials Partnership (EDCTP) | www.edctp.org/ |
| European Centre for Disease Prevention and Control | http://ecdc.europa.eu/ |
| Global Alliance for TB Drug Development | www.tballiance.org/ |
| International Union Against Tuberculosis and Lung Disease | www.theunion.org/ |
| KNCV Tuberculosis Foundation | www.kncvtbc.org/en/ |
| Lilly Not‐For‐Profit Partnership for TB Early Phase Drug Discovery | www.tbdrugdiscovery.org/ |
| Stop TB Partnership | www.stoptb.org/ |
| TB Drug UK Consortium | www.tbd‐uk.org.uk/ |
| TB Structural Genomics Consortium | www.webtb.org / |
| Tuberculosis Network European Study Group Clinical Trials (Tbnet) | www.tb‐net.org/ |
| Tuberculosis Trials Consortium (TBTC) | www.cdc.gov/tb/topic/research/tbtc/ |
| TB Drug Accelerator | http://partnerships.ifpma.org/partnership/tb‐drug‐accelerator‐program |
The list is not intended to be exhaustive but rather to provide examples of active entities and initiatives contributing to anti‐TB drug discovery.
Proper understanding of the complex interaction between M. tuberculosis and its human host that leads to dormancy, persistence or eventually resistance, is key to development of effective anti‐TB agents. Ideal TB therapy should kill both the growing mycobacteria and possess sterilizing activity against the persisting tubercle bacilli. Ample evidence exists to support the role of cholesterol in latent TB infection. Extensive efforts of several research groups have elucidated the activity of many putative enzymes within the TB cholesterol pathway, yet there are still significant gaps in understanding and an incomplete knowledge of the biological role of these enzymes.
Targeting the cholesterol pathway will disrupt the ability of TB to lurk beyond the reach of the immune system and thus will have a real impact on eradicating the disease. This has driven the quest for selective inhibitors and has been based primarily on the development of cholesterol mimetics. However, such agents might have limitations in terms of their pharmacokinetics, oral bioavailability and possible off‐target/side effects. The urgent need to find new treatments for TB has stimulated the search for new strategies in hit‐selection and lead identification, compared to other therapeutic uses (Kaneko et al., 2011; Katsuno et al., 2015). The further development of selective inhibitors against the cholesterol pathway will undoubtedly change the treatment of latent TB.
Conflict of interest
The author declares no conflicts of interest.
Acknowledgements
I would like to thank Professor Edith Sim and Dr. Ali Ryan for the invitation to write this review.
Abuhammad, A. (2017) Cholesterol metabolism: a potential therapeutic target in Mycobacteria . British Journal of Pharmacology, 174: 2194–2208. doi: 10.1111/bph.13694.
References
- Abuhammad A, Fullam E, Bhakta S, Russell AJ, Morris GM, Finn PW et al. (2014). Exploration of piperidinols as potential antitubercular agents. Molecules 19: 16274–16290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abuhammad A, Fullam E, Lowe ED, Staunton D, Kawamura A, Westwood IM et al. (2012). Piperidinols that show anti‐tubercular activity as inhibitors of arylamine N‐acetyltransferase: an essential enzyme for mycobacterial survival inside macrophages. PLoS One 7: e52790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abuhammad A, Lack N, Schweichler J, Staunton D, Sim RB, Sim E (2011). Improvement of the expression and purification of Mycobacterium tuberculosis arylamine N‐acetyltransferase (TBNAT) a potential target for novel anti‐tubercular agents. Protein Expr Purif 80: 246–252. [DOI] [PubMed] [Google Scholar]
- Abuhammad A, Lowe ED, McDonough MA, Shaw Stewart PD, Kolek SA, Sim E et al. (2013). Structure of arylamine N‐acetyltransferase from Mycobacterium tuberculosis determined by cross‐seeding with the homologous protein from M. marinum: triumph over adversity. Acta Crystallogr D Biol Crystallogr 69: 1433–1446. [DOI] [PubMed] [Google Scholar]
- Abuhammad AM, Lowe ED, Fullam E, Noble M, Garman EF, Sim E (2010). Probing the architecture of the Mycobacterium marinum arylamine N‐acetyltransferase active site. Protein Cell 1: 384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad Z, Sharma S, Khuller GK (2005). In vitro and ex vivo antimycobacterial potential of azole drugs against Mycobacterium tuberculosis H37Rv. FEMS Microbiol Lett 251: 19–22. [DOI] [PubMed] [Google Scholar]
- Ahmad Z, Sharma S, Khuller GK (2006a). Azole antifungals as novel chemotherapeutic agents against murine tuberculosis. FEMS Microbiol Lett 261: 181–186. [DOI] [PubMed] [Google Scholar]
- Ahmad Z, Sharma S, Khuller GK (2006b). The potential of azole antifungals against latent/persistent tuberculosis. FEMS Microbiol Lett 258: 200–203. [DOI] [PubMed] [Google Scholar]
- Ahmad Z, Sharma S, Khuller GK, Singh P, Faujdar J, Katoch VM (2006c). Antimycobacterial activity of econazole against multidrug‐resistant strains of Mycobacterium tuberculosis . Int J Antimicrob Agents 28: 543–544. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderton MC, Bhakta S, Besra GS, Jeavons P, Eltis LD, Sim E (2006). Characterization of the putative operon containing arylamine N‐acetyltransferase (nat) in Mycobacterium bovis BCG. Mol Microbiol 59: 181–192. [DOI] [PubMed] [Google Scholar]
- Andries K, Verhasselt P, Guillemont J, Gohlmann HW, Neefs JM, Winkler H et al. (2005). A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis . Science 307: 223–227. [DOI] [PubMed] [Google Scholar]
- Bertrand T, Augé F, Houtmann J, Rak A, Vallée F, Mikol V et al. (2010). Structural basis for human Monoglyceride lipase inhibition. J Mol Biol 396: 663–673. [DOI] [PubMed] [Google Scholar]
- Beste DJ, Nöh K, Niedenführ S, Mendum TA, Hawkins ND, Ward JL et al. (2013). 13 c‐flux spectral analysis of host‐pathogen metabolism reveals a mixed diet for intracellular Mycobacterium tuberculosis . Chem Biol 20: 1012–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakta S, Besra GS, Upton AM, Parish T, Sholto‐Douglas‐Vernon C, Gibson KJ et al. (2004). Arylamine N‐acetyltransferase is required for synthesis of mycolic acids and complex lipids in Mycobacterium bovis BCG and represents a novel drug target. J Exp Med 199: 1191–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair HA, Scott LJ (2015). Delamanid: a review of its use in patients with multidrug‐resistant tuberculosis. Drugs 75: 91–100. [DOI] [PubMed] [Google Scholar]
- Brock M, Fischer R, Linder D, Buckel W (2000). Methylcitrate synthase from Aspergillus nidulans: implications for propionate as an antifungal agent. Mol Microbiol 35: 961–973. [DOI] [PubMed] [Google Scholar]
- Brzostek A, Dziadek B, Rumijowska‐Galewicz A, Pawelczyk J, Dziadek J (2007). Cholesterol oxidase is required for virulence of Mycobacterium tuberculosis . FEMS Microbiol Lett 275: 106–112. [DOI] [PubMed] [Google Scholar]
- Brzostek A, Pawelczyk J, Rumijowska‐Galewicz A, Dziadek B, Dziadek J (2009). Mycobacterium tuberculosis is able to accumulate and utilize cholesterol. J Bacteriol 191: 6584–6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres N, Tapia G, Ojanguren I, Altare F, Gil O, Pinto S et al. (2009). Evolution of foamy macrophages in the pulmonary granulomas of experimental tuberculosis models. Tuberculosis (Edinb) 89: 175–182. [DOI] [PubMed] [Google Scholar]
- Capyk JK, Kalscheuer R, Stewart GR, Liu J, Kwon H, Zhao R et al. (2009). Mycobacterial cytochrome P450 125 (Cyp125) catalyzes the terminal hydroxylation of C27 steroids. J Biol Chem 284: 35534–35542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll P, Parish T (2015). Deletion of cyp125 Confers Increased Sensitivity to Azoles in Mycobacterium tuberculosis . PLoS One 10: e0133129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagnolo D, Radi M, Dessì F, Manetti F, Saddi M, Meleddu R et al. (2009). Synthesis and biological evaluation of new enantiomerically pure azole derivatives as inhibitors of Mycobacterium tuberculosis. Bioorg Med Chem Lett 19: 2203–2205. [DOI] [PubMed] [Google Scholar]
- Chang JC, Miner MD, Pandey AK, Gill WP, Harik NS, Sassetti CM et al. (2009). igr Genes and Mycobacterium tuberculosis cholesterol metabolism. J Bacteriol 191: 5232–5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JY, Podust LM, Roush WR (2014). Drug strategies targeting CYP51 in neglected tropical diseases. Chem Rev 114: 11242–11271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes WA, Pühler A, Kalinowski J (2002). Identification of two prpDBC gene clusters in Corynebacterium glutamicum and their involvement in propionate degradation via the 2‐methylcitrate cycle. J Bacteriol 184: 2728–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (2013). Approval of novel TB drug celebrated‐‐with restraint. Science 339: 130–130. [DOI] [PubMed] [Google Scholar]
- Doyle PS, Chen C‐K, Johnston JB, Hopkins SD, Leung SSF, Jacobson MP et al. (2010). A nonazole CYP51 inhibitor cures chagas' disease in a mouse model of acute infection. Antimicrob Agents Chemother 54: 2480–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresen C, Lin LY, D'Angelo I, Tocheva EI, Strynadka N, Eltis LD (2010). A flavin‐dependent monooxygenase from Mycobacterium tuberculosis involved in cholesterol catabolism. J Biol Chem 285: 22264–22275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll MD, McLean KJ, Levy C, Mast N, Pikuleva IA, Lafite P et al. (2010). Structural and biochemical characterization of Mycobacterium tuberculosis CYP142: evidence for multiple cholesterol 27‐hydroxylase activities in a human pathogen. J Biol Chem 285: 38270–38282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrt S, Rhee K (2013). Mycobacterium tuberculosis metabolism and host interaction: mysteries and paradoxes In: Pieters J, McKinney JD. (eds). Pathogenesis of Mycobacterium tuberculosis and its interaction with the host organism. Berlin Heidelberg: Springer, pp. 163–188. [DOI] [PubMed] [Google Scholar]
- Frank DJ, Zhao Y, Wong SH, Basudhar D, De Voss JJ, de Montellano PRO (2016). Cholesterol analogs with degradation‐resistant alkyl side‐chains are effective Mycobacterium tuberculosis growth inhibitors. J Biol Chem 291: 7325–7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye SV (2006). Discovery and clinical development of dutasteride, a potent dual 5alpha‐reductase inhibitor. Curr Top Med Chem 6: 405–421. [DOI] [PubMed] [Google Scholar]
- Frye SV, Haffner CD, Maloney PR, Hiner RN, Dorsey GF, Noe RA et al. (1995). Structure–activity relationships for inhibition of type 1 and 2 human 5 alpha‐reductase and human adrenal 3 beta‐hydroxy‐delta 5‐steroid dehydrogenase/3‐keto‐delta 5‐steroid isomerase by 6‐azaandrost‐4‐en‐3‐ones: optimization of the C17 substituent. J Med Chem 38: 2621–2627. [DOI] [PubMed] [Google Scholar]
- Frye SV, Haffner CD, Maloney PR, Mook RA, Dorsey GF, Hiner RN et al. (1993). 6‐Azasteroids: potent dual inhibitors of human type 1 and 2 steroid 5.alpha.‐reductase. J Med Chem 36: 4313–4315. [DOI] [PubMed] [Google Scholar]
- Frye SV, Haffner CD, Maloney PR, Mook RA Jr, Dorsey GF Jr, Hiner RN et al. (1994). 6‐Azasteroids: structure–activity relationships for inhibition of type 1 and 2 human 5 alpha‐reductase and human adrenal 3 beta‐hydroxy‐delta 5‐steroid dehydrogenase/3‐keto‐delta 5‐steroid isomerase. J Med Chem 37: 2352–2360. [DOI] [PubMed] [Google Scholar]
- Fullam E, Abuhammad A, Wilson DL, Anderton MC, Davies SG, Russell AJ et al. (2011). Analysis of beta‐amino alcohols as inhibitors of the potential anti‐tubercular target N‐acetyltransferase. Bioorg Med Chem Lett 21: 1185–1190. [DOI] [PubMed] [Google Scholar]
- Fullam E, Kawamura A, Wilkinson H, Abuhammad A, Westwood I, Sim E (2009). Comparison of the arylamine N‐acetyltransferase from Mycobacterium marinum and Mycobacterium tuberculosis . Protein J 28: 281–293. [DOI] [PubMed] [Google Scholar]
- Fullam E, Talbot J, Abuhammed A, Westwood I, Davies SG, Russell AJ et al. (2013). Design, synthesis and structure–activity relationships of 3,5‐diaryl‐1H‐pyrazoles as inhibitors of arylamine N‐acetyltransferase. Bioorg Med Chem Lett 23: 2759–2764. [DOI] [PubMed] [Google Scholar]
- Fullam E, Westwood IM, Anderton MC, Lowe ED, Sim E, Noble ME (2008). Divergence of cofactor recognition across evolution: coenzyme A binding in a prokaryotic arylamine N‐acetyltransferase. J Mol Biol 375: 178–191. [DOI] [PubMed] [Google Scholar]
- Galán B, Díaz E, Prieto MA, García JL (2000). Functional analysis of the small component of the 4‐hydroxyphenylacetate 3‐monooxygenase of Escherichia coli W: a prototype of a new flavin:NAD(P)H reductase subfamily. J Bacteriol 182: 627–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Sampson NS (2014). A GMC oxidoreductase homologue is required for acetylation of glycopeptidolipid in Mycobacterium smegmatis. Biochemistry 53: 611–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier T, Eiglmeier K, Camus J‐C, Medina N, Mansoor H, Pryor M et al. (2003). The complete genome sequence of Mycobacterium bovis . Proc Natl Acad Sci 100: 7877–7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getahun H, Matteelli A, Abubakar I, Aziz MA, Baddeley A, Barreira D et al. (2015). Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur Respir J 46: 1563–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gideon HP, Flynn JL (2011). Latent tuberculosis: what the host “sees”? Immunol Res 50: 202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JE, Gawronski JD, DeJesus MA, Ioerger TR, Akerley BJ, Sassetti CM (2011). High‐resolution phenotypic profiling defines genes essential for mycobacterial growth and cholesterol catabolism. PLoS Pathog 7: e1002251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JE, Pandey Amit K, Gilmore Sarah A, Mizrahi V, McKinney John D, Bertozzi Carolyn R et al. (2012). Cholesterol catabolism by mycobacterium tuberculosis requires transcriptional and metabolic adaptations. Chem Biology 19: 218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R, Gao M, Cirule A, Xiao H, Geiter LJ, Wells CD (2015). Delamanid for extensively drug‐resistant tuberculosis. N Engl J Med 373: 291–292. [DOI] [PubMed] [Google Scholar]
- Ho NAT, Dawes SS, Crowe AM, Casabon I, Gao C, Kendall SL et al. (2016). The structure of the transcriptional repressor Kstr in complex with CoA thioester cholesterol metabolites sheds light on the regulation of cholesterol catabolism in Mycobacterium tuberculosis . J Biol Chem 291: 7256–7266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson SA, McLean KJ, Munro AW, Abell C (2012). Mycobacterium tuberculosis cytochrome P450 enzymes: a cohort of novel TB drug targets. Biochem Soc Trans 40: 573–579. [DOI] [PubMed] [Google Scholar]
- Johnston JB, Kells PM, Podust LM, Ortiz de Montellano PR (2009). Biochemical and structural characterization of CYP124: A methyl‐branched lipid ω‐hydroxylase from Mycobacterium tuberculosis . Proc Natl Acad Sci U S A 106: 20687–20692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JB, Ouellet H, Ortiz de Montellano PR (2010). Functional redundancy of steroid C26‐monooxygenase activity in Mycobacterium tuberculosis revealed by biochemical and genetic analyses. J Biol Chem 285: 36352–36360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T, Cooper C, Mdluli K (2011). Challenges and opportunities in developing novel drugs for TB. Future Med Chem 3: 1373–1400. [DOI] [PubMed] [Google Scholar]
- Katsuno K, Burrows JN, Duncan K, van Huijsduijnen RH, Kaneko T, Kita K et al. (2015). Hit and lead criteria in drug discovery for infectious diseases of the developing world. Nat Rev Drug Discov 14: 751–758. [DOI] [PubMed] [Google Scholar]
- Kaul D, Anand PK, Verma I (2004). Cholesterol‐sensor initiates M. tuberculosis entry into human macrophages. Mol Cell Biochem 258: 219–222. [DOI] [PubMed] [Google Scholar]
- Kendall SL, Burgess P, Balhana R, Withers M, ten Bokum A, Lott JS et al. (2010). Cholesterol utilization in mycobacteria is controlled by two TetR‐type transcriptional regulators: kstR and kstR2. Microbiology 156: 1362–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall SL, Rison SCG, Movahedzadeh F, Frita R, Stoker NG (2004). What do microarrays really tell us about M. tuberculosis . Trends Microbiol 12: 537–544. [DOI] [PubMed] [Google Scholar]
- Kendall SL, Withers M, Soffair CN, Moreland NJ, Gurcha S, Sidders B et al. (2007). A highly conserved transcriptional repressor controls a large regulon involved in lipid degradation in Mycobacterium smegmatis and Mycobacterium tuberculosis . Mol Microbiol 65: 684–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klink M, Brzezinska M, Szulc I, Brzostek A, Kielbik M, Sulowska Z et al. (2013). Cholesterol oxidase is indispensable in the pathogenesis of Mycobacterium tuberculosis . PLoS One 8: e73333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolattukudy PE, Fernandes ND, Azad AK, Fitzmaurice AM, Sirakova TD (1997). Biochemistry and molecular genetics of cell‐wall lipid biosynthesis in mycobacteria. Mol Microbiol 24: 263–270. [DOI] [PubMed] [Google Scholar]
- Kumar GA, Jafurulla M, Chattopadhyay A (2016). The membrane as the gatekeeper of infection: Cholesterol in host–pathogen interaction. Chem Phys Lipids 199: 179–185. [DOI] [PubMed] [Google Scholar]
- Lack N, Lowe ED, Liu J, Eltis LD, Noble ME, Sim E et al. (2008). Structure of HsaD, a steroid‐degrading hydrolase, from Mycobacterium tuberculosis . Acta Crystallogr Sect F Struct Biol Cryst Commun 64: 2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lack NA, Yam KC, Lowe ED, Horsman GP, Owen RL, Sim E et al. (2010). Characterization of a carbon–carbon hydrolase from Mycobacterium tuberculosis involved in cholesterol metabolism. J Biol Chem 285: 434–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrouy‐Maumus G (2015). Cholesterol acquisition by Mycobacterium tuberculosis . Virulence 6: 412–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepesheva GI, Villalta F, Waterman MR (2011). Chapter 4 ‐ Targeting Trypanosoma cruzi sterol 14α‐Demethylase (CYP51) In: Weiss LM, Tanowitz HB, Louis VK. (eds). Advances in parasitology. London: Academic Press, pp. 65–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovewell RR, Sassetti CM, VanderVen BC (2016). Chewing the fat: lipid metabolism and homeostasis during M. tuberculosis infection. Curr Opin Microbiol 29: 30–36. [DOI] [PubMed] [Google Scholar]
- Lu R, Schmitz W, Sampson NS (2015). α‐Methyl acyl CoA racemase provides Mycobacterium tuberculosis catabolic access to cholesterol esters. Biochemistry 54: 5669–5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean KJ, Belcher J, Driscoll MD, Fernandez CC, Le Van D, Bui S et al. (2010). The Mycobacterium tuberculosis cytochromes P450: physiology, biochemistry & molecular intervention. Future Med Chem 2: 1339–1353. [DOI] [PubMed] [Google Scholar]
- McLean KJ, Clift D, Lewis DG, Sabri M, Balding PR, Sutcliffe MJ et al. (2006a). The preponderance of P450s in the Mycobacterium tuberculosis genome. Trends Microbiol 14: 220–228. [DOI] [PubMed] [Google Scholar]
- McLean KJ, Lafite P, Levy C, Cheesman MR, Mast N, Pikuleva IA et al. (2009). The Structure of Mycobacterium tuberculosis CYP125: molecular basis for cholesterol binding in a P450 needed for host infection. J Biol Chem 284: 35524–35533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean KJ, Marshall KR, Richmond A, Hunter IS, Fowler K, Kieser T et al. (2002). Azole antifungals are potent inhibitors of cytochrome P450 mono‐oxygenases and bacterial growth in mycobacteria and streptomycetes. Microbiology 148: 2937–2949. [DOI] [PubMed] [Google Scholar]
- McLean KJ, Munro AW (2008). Structural biology and biochemistry of cytochrome P450 systems in Mycobacterium tuberculosis . Drug Metab Rev 40: 427–446. [DOI] [PubMed] [Google Scholar]
- McLean KJ, Warman AJ, Seward HE, Marshall KR, Girvan HM, Cheesman MR et al. (2006b). Biophysical characterization of the sterol demethylase P450 from Mycobacterium tuberculosis, its cognate ferredoxin, and their interactions. Biochemistry 45: 8427–8443. [DOI] [PubMed] [Google Scholar]
- Mdluli K, Kaneko T, Upton A (2015). The tuberculosis drug discovery and development pipeline and emerging drug targets. Cold Spring Harb Perspect Med 5: a021154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meena LS, Rajni (2010). Survival mechanisms of pathogenic Mycobacterium tuberculosis H37Rv. FEBS J 277: 2416–2427. [DOI] [PubMed] [Google Scholar]
- Milano A, Pasca MR, Provvedi R, Lucarelli AP, Manina G, Luisa de Jesus Lopes Ribeiro A et al. (2009). Azole resistance in Mycobacterium tuberculosis is mediated by the MmpS5–MmpL5 efflux system. Tuberculosis 89: 84–90. [DOI] [PubMed] [Google Scholar]
- Muñoz‐Elías EJ, McKinney JD (2005). Mycobacterium tuberculosis isocitrate lyases 1 and 2 are jointly required for in vivo growth and virulence. Nat Med 11: 638–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz‐Elías EJ, Upton AM, Cherian J, McKinney JD (2006). Role of the methylcitrate cycle in Mycobacterium tuberculosis metabolism, intracellular growth, and virulence. Mol Microbiol 60: 1109–1122. [DOI] [PubMed] [Google Scholar]
- Muttucumaru DN, Roberts G, Hinds J, Stabler RA, Parish T (2004). Gene expression profile of Mycobacterium tuberculosis in a non‐replicating state. Tuberculosis 84: 239–246. [DOI] [PubMed] [Google Scholar]
- Nesbitt NM, Yang X, Fontán P, Kolesnikova I, Smith I, Sampson NS et al. (2010). A thiolase of Mycobacterium tuberculosis is required for virulence and production of androstenedione and androstadienedione from cholesterol. Infect Immun 78: 275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet H, Guan S, Johnston JB, Chow ED, Kells PM, Burlingame AL et al. (2010). Mycobacterium tuberculosis CYP125A1, a steroid C27 monooxygenase that detoxifies intracellularly generated cholest‐4‐en‐3‐one. Mol Microbiol 77: 730–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet H, Johnston JB, de Montellano PR (2011a). Cholesterol catabolism as a therapeutic target in Mycobacterium tuberculosis . Trends Microbiol 19: 530–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet H, Kells PM, Ortiz de Montellano PR, Podust LM (2011b). Reverse type I inhibitor of Mycobacterium tuberculosis CYP125A1. Bioorg Med Chem Lett 21: 332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey AK, Sassetti CM (2008). Mycobacterial persistence requires the utilization of host cholesterol. Proc Natl Acad Sci U S A 105: 4376–4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey J, Tiwari VK, Verma SS, Chaturvedi V, Bhatnagar S, Sinha S et al. (2009). Synthesis and antitubercular screening of imidazole derivatives. Eur J Med Chem 44: 3350–3355. [DOI] [PubMed] [Google Scholar]
- Pieters J (2008). Mycobacterium tuberculosis and the macrophage: maintaining a balance. Cell Host Microbe 3: 399–407. [DOI] [PubMed] [Google Scholar]
- Podust LM, Poulos TL, Waterman MR (2001). Crystal structure of cytochrome P450 14α‐sterol demethylase (CYP51) from Mycobacterium tuberculosis in complex with azole inhibitors. Proc Natl Acad Sci 98: 3068–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podust LM, von Kries JP, Eddine AN, Kim Y, Yermalitskaya LV, Kuehne R et al. (2007). Small‐molecule scaffolds for CYP51 inhibitors identified by high‐throughput screening and defined by X‐ray crystallography. Antimicrob Agents Chemother 51: 3915–3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pym AS, Diacon AH, Tang S‐J, Conradie F, Danilovits M, Chuchottaworn C et al. (2015). Bedaquiline in the treatment of multidrug‐and extensively drug‐resistant tuberculosis. Eur Respir J 47: 564–574. [DOI] [PubMed] [Google Scholar]
- Rengarajan J, Bloom BR, Rubin EJ (2005). Genome‐wide requirements for Mycobacterium tuberculosis adaptation and survival in macrophages. Proc Natl Acad Sci U S A 102: 8327–8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocco CJ, Escalante‐Semerena JC (2010). In Salmonella enterica, 2‐Methylcitrate Blocks Gluconeogenesis. J Bacteriol 192: 771–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell DG, VanderVen BC, Lee W, Abramovitch RB, Kim M‐J, Homolka S et al. (2010). Mycobacterium tuberculosis wears what it eats. Cell Host Microbe 8: 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan A, Keany S, Eleftheriadou O, Ballet R, Cheng H‐Y, Sim E (2014). Mechanism‐based inhibition of HsaD: a CC bond hydrolase essential for survival of Mycobacterium tuberculosis in macrophage. FEMS Microbiol Lett 350: 42–47. [DOI] [PubMed] [Google Scholar]
- Savvi S, Warner DF, Kana BD, McKinney JD, Mizrahi V, Dawes SS (2008). Functional characterization of a vitamin B12‐dependent methylmalonyl pathway in Mycobacterium tuberculosis: implications for propionate metabolism during growth on fatty acids. J Bacteriol 190: 3886–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer CM, Lu R, Nesbitt NM, Schiebel J, Sampson NS, Kisker C (2015). FadA5 a Thiolase from Mycobacterium tuberculosis: a steroid‐binding pocket reveals the potential for drug development against tuberculosis. Structure 23: 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AW, Choi TA, Theumer G, Franzblau SG, Knölker H‐J (2013). Inhibitory effect of oxygenated cholestan‐3β‐ol derivatives on the growth of Mycobacterium tuberculosis. Bioorg Med Chem Lett 23: 6111–6113. [DOI] [PubMed] [Google Scholar]
- Schnappinger D, Ehrt S, Voskuil MI, Liu Y, Mangan JA, Monahan IM et al. (2003). Transcriptional adaptation of Mycobacterium tuberculosis within macrophages: insights into the phagosomal environment. J Exp Med 198: 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senaratne RH, Sidders B, Sequeira P, Saunders G, Dunphy K, Marjanovic O et al. (2008). Mycobacterium tuberculosis strains disrupted in mce3 and mce4 operons are attenuated in mice. J Med Microbiol 57: 164–170. [DOI] [PubMed] [Google Scholar]
- Sheehan DJ, Hitchcock CA, Sibley CM (1999). Current and emerging azole antifungal agents. Clin Microbiol Rev 12: 40–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim E, Abuhammad A, Ryan A (2014). Arylamine N‐acetyltransferases: from drug metabolism and pharmacogenetics to drug discovery. Br J Pharmacol 171: 2705–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim E, Sandy J, Evangelopoulos D, Fullam E, Bhakta S, Westwood I et al. (2008). Arylamine N‐acetyltransferases in mycobacteria. Curr Drug Metab 9: 510–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair JC, Sandy J, Delgoda R, Sim E, Noble ME (2000). Structure of arylamine N‐acetyltransferase reveals a catalytic triad. Nat Struct Biol 7: 560–564. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44 (Database Issue): D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Textor S, Wendisch FV, Graaf DAA, Müller U, Linder IM, Linder D et al. (1997). Propionate oxidation in Escherichia coli: evidence for operation of a methylcitrate cycle in bacteria. Arch Microbiol 168: 428–436. [DOI] [PubMed] [Google Scholar]
- Thomas ST, Sampson NS (2013). Mycobacterium tuberculosis utilizes a unique heterotetrameric structure for dehydrogenation of the cholesterol side chain. Biochemistry 52: 2895–2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas ST, Yang X, Sampson NS (2011). Inhibition of the M. tuberculosis 3β‐hydroxysteroid dehydrogenase by azasteroids. Bioorg Med Chem Lett 21: 2216–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama H, Tabuchi T (1976). Properties of Methylcitrate Synthase from Candida lipolytica . Agric Biol Chem 40: 1411–1418. [Google Scholar]
- Upton AM, McKinney JD (2007). Role of the methylcitrate cycle in propionate metabolism and detoxification in Mycobacterium smegmatis. Microbiology 153: 3973–3982. [DOI] [PubMed] [Google Scholar]
- Van den Boogaard J, Kibiki GS, Kisanga ER, Boeree MJ, Aarnoutse RE (2009). New Drugs against tuberculosis: problems, progress, and evaluation of agents in clinical development. Antimicrob Agents Chemother 53: 849–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Geize R, Yam K, Heuser T, Wilbrink MH, Hara H, Anderton MC et al. (2007). A gene cluster encoding cholesterol catabolism in a soil actinomycete provides insight into Mycobacterium tuberculosis survival in macrophages. Proc Natl Acad Sci U S A 104: 1947–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderVen BC, Fahey RJ, Lee W, Liu Y, Abramovitch RB, Memmott C et al. (2015). Novel inhibitors of cholesterol degradation in Mycobacterium tuberculosis reveal how the bacterium's metabolism is constrained by the intracellular environment. PLoS Pathog 11: e1004679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon A (2013) Treatment of latent tuberculosis infection. Seminars in respiratory and critical care medicine. 34: 67–86. [DOI] [PubMed] [Google Scholar]
- Westwood I, Bhakta S, Russell A, Fullam E, Anderton M, Kawamura A et al. (2010). Identification of aryalmine N‐acetyltransferase inhibitors as an approach towards novel anti‐tuberculars. Protein Cell 1: 82–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westwood IM, Kawamura A, Russell AJ, Sandy J, Davies SG, Sim E (2011). Novel small‐molecule inhibitors of arylamine N‐acetyltransferases: drug discovery by high throughput screening. Comb Chem High Throughput Screen 14: 117–124. [DOI] [PubMed] [Google Scholar]
- WHO (2015).Global tuberculosis report 2015 . [Online] Available at http://apps.who.int/iris/bitstream/10665/191102/1/9789241565059_eng.pdf (accessed 15/4/2016).
- Wipperman MF, Yang M, Thomas ST, Sampson NS (2013). Shrinking the FadE proteome of Mycobacterium tuberculosis: insights into cholesterol metabolism through identification of an α2β2 heterotetrameric acyl coenzyme A dehydrogenase family. J Bacteriol 195: 4331–4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam KC, D'Angelo I, Kalscheuer R, Zhu H, Wang JX, Snieckus V et al. (2009). Studies of a ring‐cleaving dioxygenase illuminate the role of cholesterol metabolism in the pathogenesis of Mycobacterium tuberculosis . PLoS Path 5: e1000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Lu R, Guja KE, Wipperman MF, St. Clair JR, Bonds AC et al. (2015). Unraveling cholesterol catabolism in Mycobacterium tuberculosis: ChsE4‐ChsE5 α2β2 acyl‐CoA dehydrogenase initiates β‐oxidation of 3‐oxo‐cholest‐4‐en‐26‐oyl CoA. ACS Infect Dis 1: 110–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Dubnau E, Smith I, Sampson NS (2007). Rv1106c from Mycobacterium tuberculosis Is a 3β‐Hydroxysteroid Dehydrogenase. Biochemistry 46: 9058–9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Gao J, Smith I, Dubnau E, Sampson NS (2011). Cholesterol is not an essential source of nutrition for Mycobacterium tuberculosis during infection. J Bacteriol 193: 1473–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Nesbitt NM, Dubnau E, Smith I, Sampson NS (2009). Cholesterol metabolism increases the metabolic pool of propionate in Mycobacterium tuberculosis . Biochemistry (Mosc) 48: 3819–3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young DB, Gideon HP, Wilkinson RJ (2009). Eliminating latent tuberculosis. Trends Microbiol 17: 183–188. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Colabroy KL, Begley TP, Ealick SE (2005). Structural studies on 3‐hydroxyanthranilate‐3,4‐dioxygenase: The catalytic mechanism of a complex oxidation involved in NAD biosynthesis. Biochemistry 44: 7632–7643. [DOI] [PubMed] [Google Scholar]


