Abstract
Bacterial infection remains a major challenge to healthcare and is responsible for significant morbidity and mortality. This situation is becoming complicated by an increasingly ageing and susceptible population and large numbers of bacterial isolates, which have developed resistance to antibiotics. Bacteria that form biofilms and colonize or infect medical devices or wounds are particularly hard to treat as biofilms are inherently highly antibiotic resistant. Most infections have a component where bacteria exist as a biofilm and as a result, prevention or treatment of biofilm‐associated infections is highly important. A number of novel strategies to kill biofilms have been in development; these include the use of weak organic acids, photo irradiation and the application of bacteriophage. All have promise and are able to effectively kill biofilms in model systems, but for each there are still unanswered questions. This review summarizes the main features of biofilm infections, each of these novel approaches and the evidence that is still lacking before these potential treatments can be incorporated into clinical usage.
Linked Articles
This article is part of a themed section on Drug Metabolism and Antibiotic Resistance in Micro‐organisms. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.14/issuetoc
Abbreviations
- ATR
acid tolerance response
- ESBLs
extended spectrum β‐lactamases
- GRAS
generally regarded as safe
- MIC
minimum inhibitory concentration
- OM
outer membrane
- MRSA
methicillin resistant Staphylococcus aureus
- WOA
weak organic acids
Table of Links
| LIGANDS | |
|---|---|
| Glucose | Acetic acid (acetate) |
| Peptidoglycan | Lactic acid |
| Citric acid | Benzoic acid (benzoate) |
This Table lists key ligands in this article that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016).
Introduction
Bacterial infections are becoming more difficult to treat due to higher numbers of patients with complex underlying conditions and the increase in pathogens, which are resistant to current antimicrobial therapies (Piddock, 2016). This is complicated by a paucity of new antibiotics in development and has prompted renewed interest in various novel antimicrobial therapies. Part of the difficulty in treating bacterial and fungal infections is the fact that many involve the formation of a biofilm at some stage. When bacteria exist as a biofilm, they are significantly less susceptible to antibiotics; this is a result of metabolic changes to cells within the biofilm and structural features influencing drug permeability (Jolivet‐Gougeon and Bonnaure‐Mallet, 2014). Biofilms are particularly problematic in the contamination of medical devices and in the infection of wounds (Mihai et al., 2015). This article aims to review the current literature in terms of existing strategies for treating biofilm infections with a specific interest in treatments, which are being developed to prevent and treat biofilm infection of medical devices and wounds.
Bacterial resistance to antibiotics
Bacteria can exhibit resistance to antibiotics through a variety of mechanisms. These can be divided into intrinsic (which are innate properties of a species and often result from a lack of target site for the antibiotic in question or differences in the cell wall) and extrinsic or acquired mechanisms (including development of specific mutations or horizontal transfer of resistance genes from other organisms). Of the intrinsic mechanisms, the difference in cell wall structure between Gram‐positive and Gram‐negative organisms is significant (Blair et al., 2015). The outer membrane (OM) layer in Gram‐negative bacteria limits their susceptibility to a number of different antibiotics and renders many traditional antibiotics ineffective against Gram‐negative cells. The impact of this permeability barrier has been demonstrated where permeabilizing the OM of Escherichia coli increased antibiotic susceptibility significantly (Randall et al., 2013). Reducing drug access to the target site is also mediated by active export; insertional inactivation of multidrug efflux pumps, which help define the baseline level of drug accumulation within the cell (e.g. AcrAB‐TolC), also increases antibiotic susceptibility. Not all antibiotics are effective against Gram‐positive and Gram‐negative cells; the synthetic monobactam aztreonam has a spectrum of activity limited to aerobic Gram‐negative organisms. This is due to its affinity for penicillin binding protein (PBP) 3 of susceptible Gram‐negative organisms. It does not bind well to the specific PBPs found in Gram‐positive organisms (Westley‐Horton and Koestner, 1991).
Acquired resistance can result from multiple mechanisms and includes those which can be inherited either via vertical transmission of genetic material or plasmid mediated horizontal transmission. Acquired resistance mechanisms can be broadly categorized into three distinct classes. Firstly, bacteria can mutate to change or protect the structure of the target for the antibiotic whilst maintaining function. There are numerous examples of this, for example, fluoroquinolone antibiotics target DNA gyrase in Gram‐negative cells, and mutation or decoration of the target site reduces drug binding and provides resistance (Redgrave et al., 2014). Secondly, bacteria are able to produce enzymes, which can inactivate or modify the antibiotic rendering it ineffective. Extended spectrum β‐lactamases (ESBLs) are an important example of this. CTX‐M enzymes are one of the most commonly isolated ESBLs found in Gram‐negative species globally reducing the efficacy of penicillins, cephalosporins and aztreonam (Zhao and Hu, 2013). Thirdly, bacteria can prevent access of the antibiotics to their target by up‐regulating the normal level of efflux activity of the cell or reducing the permeability of the cell membrane by repressing porin production. MexAB‐OprM in Pseudomonas aeruginosa or AcrAB‐TolC in E. coli are important examples of efflux pumps able to export multiple drugs (Webber and Piddock, 2003).
Biofilm formation
Biofilm formation has been observed in almost all bacterial species studied, and whilst there are large variations in the processes involved, there are a number of generalized distinct recognized steps.
Firstly, bacteria in a freely moving, planktonic state adhere to a surface (Høiby et al., 2010) whether it be tissue in the case of endocarditis (Presterl et al., 2005) and burns (Halstead et al., 2015) or prosthetic material in the case of urinary catheters (Getliffe, 2012), vascular catheters (Grudzinski et al., 2015) or orthopaedic fixation devices (McConoughey et al., 2014). Once they have adhered to a surface, they begin multiplication of the individual bacteria to form micro‐colonies. Development and maturation then occurs. Bacteria produce an extracellular polymeric matrix, which is the hallmark of a biofilm and may contain proteins, DNA and polysaccharides (Høiby et al., 2010). The biofilm can expand and mature. Once nutrients are diminished and waste products have accumulated, cells begin to be released as biofilms disperse. Quorum sensing is a mechanism to measure cell density and is reliant on the production of communication molecules by cells. This occurs between cells within many biofilms and helps recognize when a population is at high density and can trigger dispersal (Solano et al., 2014). The host response to biofilm infection can inadvertently increase biofilm mass as platelets and fibrin migrate and attach to the site of infection (Hall‐Stoodley et al., 2004), particularly in Streptococcal and Staphylococcal endocarditis. Figure 1 depicts routes of biofilm colonization of devices and wounds.
Figure 1.
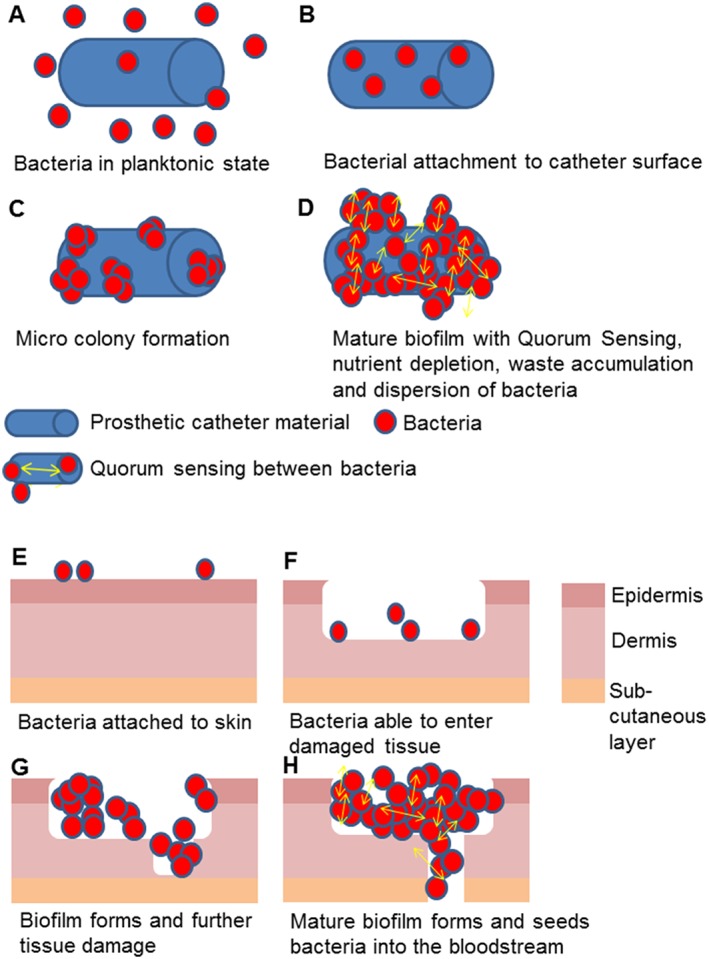
Biofilm contamination of catheters and wounds can lead to bacteraemia. Panels A–D show the stages of colonization of a catheter where planktonic cells attach, form micro colonies then a mature biofilm. Panels E–H depict a normal skin barrier colonized with bacteria from the flora before an injury allowing bacteria to enter lower layers, cause damage to deeper tissues and eventually enter the bloodstream.
The clinical challenge posed by biofilms enhanced bacterial resistance
As mentioned above, growth as a biofilm allows bacteria to demonstrate up to 1000 fold reduced susceptibility to antibiotics (Nickel et al., 1985). It was previously thought that this may be primarily due to reduced penetration of the antibiotics, so they could not reach the bacteria living within the biofilm. We now know that whilst biofilm does play a role in limiting diffusion of the antibiotic, this is not the only mechanism of reduced susceptibility as antibiotic agents are generally able to penetrate within biofilms (Dunne et al., 1993). The restricted diffusion gradient of oxygen, glucose and other nutrients to the bacteria deep within a biofilm may further explain this observation (Anderl et al., 2003; Evans and Holmes, 1987). Firstly, due to the altered environment, the growth kinetics of bacteria within a biofilm are altered. Those at the lower end of the gradient exist in a stationary phase limiting the efficacy of any antibiotic, which is able to penetrate down to this deeper level (Anderl et al., 2000). For example, β‐lactam antibiotics target peptidoglycan in the bacterial cell wall (Strominger and Tipper, 1965) during the growth phase and it has been shown that their efficacy is directly proportional to the rate of growth (Tuomanen et al., 1986). Secondly, as the environment is different, the antibiotic mode of action can be adversely affected. Aminoglycosides for example rely on aerobic bacterial respiration creating an electrical potential for uptake into the cell (Vakulenko and Mobashery, 2003), so an anaerobic environment deep in the biofilm strata reduces their efficacy too. Finally, biofilms contain large proportions of ‘persister’ cells, which are metabolically dormant and highly antibiotic resistant; these can survive antibiotic exposure and occasionally come out of dormancy and act to re‐populate the biofilm (Conlon et al., 2015).
Organic acids as antibacterials
Organic acids are often described as ‘weak acids’. They contain a carboxyl group COOH, and unlike ‘strong’ mineral/inorganic acids such as hydrochloric or sulphuric acid, they do not completely dissociate in water (Hirshfield et al., 2003). Interestingly, inorganic acids appear to be less effective antimicrobials than weak organic acids (WOA) when concentrations are equivalent. The stronger antimicrobial effect of WOA is thought to rely on their relatively higher hydrophobicity and lipid permeability allowing them to diffuse into the bacterial cell cytoplasm before dissociation occurs (Hirshfield et al., 2003). Whilst the use of WOA in medicine as a treatment for infection, disinfectant or antiseptic has been documented for the past 6000 years, large powered methodologically sound clinical trials have been lacking (Ryssel et al., 2009). The surgeon John Hunter noted over 200 years ago that the patency of a urinary catheter was prolonged when he dipped them into a solution containing acetic acid before insertion into the bladder (Quist, G. 1981). This is one of the earliest documented medical observations of using an organic acid to prevent a urinary biofilm in a catheter setting.
Clinical use of acetic acid to treat infection has been widely employed, although on a sporadic basis, reports exist of clinical success for the treatment of infections caused by P. aeruginosa, particularly infection of burn wounds (Nagoba et al., 2013) and chronic ulcers (Bjarnsholt et al., 2015). In these contexts, the acid is applied topically within dressings (at concentrations between 2 and 5%) and appears to be well tolerated. There have been some small scale clinical trials of the use of acetic acid to treat wound infection. One study with 16 patients suffering from infection of wounds with P. aeruginosa reported successful elimination of the pathogen for 15 of the 16 patients within 2 weeks (Sloss et al., 1993). However, there were wide variations in the treatment protocols between the patients and no control arm in the study. A lack of consistency in application method and concentration of the acids used has been a hallmark of the clinical reports of the uses of WOA to treat infection to date; also the microbiological reporting has used a wide range of methodologies and end points. These factors together ensure there is no clear picture of clinical utility although the anecdotal evidence is promising. Table 1 summarizes the current clinical uses of organic acids.
Table 1.
Current clinical uses of organic acids
| Specialty | Use and applied concentration | Acid used | References |
|---|---|---|---|
| Plastics/dermatology | Topical application in burn infections (2.5%) | Acetic acid | (Halstead et al., 2015) |
| Urology | Catheter and bladder irrigation solution to maintain patency (2–5%) | Citric acid | (Getliffe, 2012) |
| – | – | ||
| Acetic acid | (Doles et al., 2015) | ||
| Haematology/oncology/renal | Antibiotic Lock Technique in vascular catheter infections (5%) | Citric acid | (Grudzinski et al., 2015) |
| Gynaecology | Colposcopy examination of dyskaryotic cells in cervical cancer (N/A) | Acetic acid | (Sankaranarayanan et al., 1998) |
| Hepatology/oncology | Percutaenous injection for small hepatocellular carcinomas (2–4%) | Acetic acid | (Resistance and Usage, 2007) |
Given the target state of a pathogen within a wound or device infection is a biofilm, a couple of studies have assessed the activity of WOA in vitro against biofilms of relevant pathogens. Both have reported good activity against a range of common wound pathogens including P. aeruginosa and Acinetobacter baumannii and indicated that effective concentrations in vitro are much lower than those in current clinical usage (Halstead et al., 2015; Bjarnsholt et al., 2015). These reports suggest that there is work still to be done to determine the optimal formulation of WOA in this context.
Interestingly, whilst the study of organic acids in a medical setting has only recently become formalized, their study and use as antimicrobials in the food and drink industry has been active for some time. A wide range of food and drink labels will list organic acids such as citric acid, ascorbic acid, acetic acid, lactic acid and benzoic acid to name a few. Spoilage of food and drink secondary to microbial contamination is a major concern for the industry. It is estimated that roughly £3 billion of food is wasted each year in the UK hospitality and food service of which 21% arises from spoilage (WRAP, 2013). Their use as preservatives has shown long‐term efficacy in prevention of contamination is achievable by incorporating WOA into products and suggests analogous uses may be possible in medical settings.
Mechanism of action of WOA
Previous articles have suggested a number of potential modes of antimicrobial action for WOA (Brul and Coote, 1999; Salmond et al., 1984). As WOA are relatively hydrophobic, they can diffuse across bacterial cell membranes at which point they dissociate and lower the internal cytoplasmic pH of the bacteria in a process known as ion trapping (Figure 2 gives an overview of potential mechanisms of action of WOA). This can then lead to a disruption of metabolic activities, and as the dissociated acid components do not readily pass across membranes, there is an intracellular accumulation of the breakdown products of the WOA. Whilst initial studies focused on changes to intracellular pH as being key to understanding the toxicity of organic acids, it is now evident that many bacteria can efficiently deal with low‐pH environments (Roe et al., 1998). In addition, differences between antimicrobial activity of different WOA when pH matched are still seen, for example with Listeria monocytogenes, which suggests there are antimicrobial effects unrelated to pH alone (Young and Foegeding, 1993). Disruption of membrane barrier function is another suggested mechanism of action for WOA. The lipophilic nature of some organic acids means they can migrate and intercalate in the lipid membrane of the bacterial or fungal cell envelope, potentially with toxic consequences. To support this idea, there is a correlation in antifungal activity and lipid solubility with the minimum inhibitory concentration (MIC) of sorbic acid being lower than acetic acid with sorbic acid being more lipid soluble than acetic acid (Stratford and Anslow, 1998). It has also been suggested that metabolic energy expenditure to restore homeostasis after the damaging effects of WOA is costly to the bacterial cell, which inhibits bacterial growth (Slonczewski et al., 2009). Some acids such as acetic acid have also been proposed as interfering with central metabolism – dissociation of acetic acid within the cell results in an increase in acetate levels, which can disrupt normal flux rates through the tricarboxylic acid cycle (Hirshfield et al., 2003). A final potential mechanism of action has been suggested to follow cytoplasmic WOA accumulation, which can have an osmotic effect on the bacterial cells causing an increase in turgor pressure. As already discussed, organic acids can diffuse across a cell membrane where they can then dissociate. Whilst this can decrease the internal cytoplasmic pH, it can also increase the osmolality resulting in an influx of water (Roe et al., 1998). Given all the above data, it is likely that a combination of factors leads to a bacteriostatic and bactericidal effect of WOA.
Figure 2.
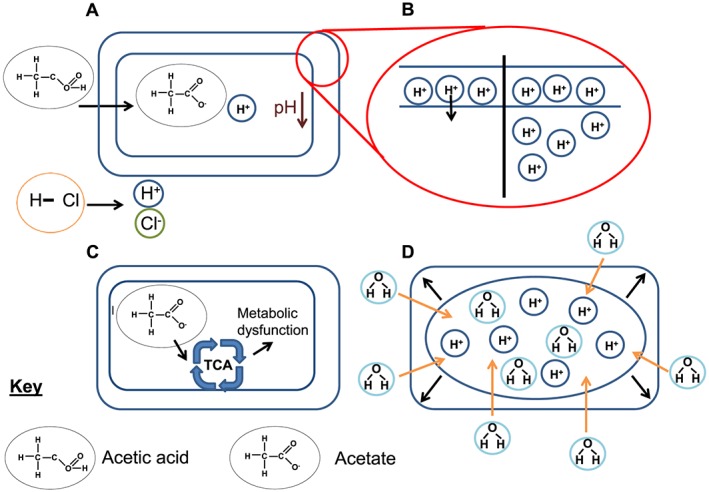
Possible mechanisms of action of WOA. Panel A shows the ability of acetic acid to pass into a cell compared with hydrochloric acid and the subsequent ion trapping when it dissociates inside the cell. Panel B shows the loss of the proton motive force, which results from increasing acidity of the cytoplasm (the right side of the cell). Panel C shows the impact of the dissociated anion, here acetate, which can enter the tricarboxylic acid (TCA) cycle and cause abnormal flux of metabolites. Panel D shows the possible impact from osmotic shock on the cell.
Mode of resistance
Acid tolerance has been studied in some enteric species including E. coli and Salmonella, but very little data exist on how other bacteria, in particular Pseudomonas, may be able to develop resistance to organic acids and if so by which mechanisms (De Biase and Lund, 2015). The acid tolerance response (ATR) described in Salmonella enterica serovar Typhimurium is important in the ability of this species to pass through the stomach before colonizing the gastrointestinal tract, which is part of its life cycle. Cells are exposed to environments with a range in pH values during this journey (Foster and Hall, 1990). Salmonella primed by being exposed to an acidic environment of pH 5.8 were able to subsequently resist a strong acid challenge of pH 3.3 (100–1000 fold more than naïve cells). This observation showed this response is inducible. The ATR is governed by a number of different processes. Regulator proteins including Fur, RpoS and PhoP are involved in maintaining a pH homeostasis. We know that mutations in the Fur protein for example can render a cell acid sensitive highlighting its importance in the ATR (Bearson et al., 2006).
Whilst the ATR has mainly been studied in relation to ability to survive in very low pH after strong acid exposure, it is logical to think that the same mechanisms may be relevant for WOA (Baik et al., 1996). One study described how applying an acid shock was then able to induce resistance to WOAs. Interestingly, when they pre‐exposed bacteria to sub lethal concentrations of WOAs at 5–10 fold below the MIC it did not, however, appear to induce the same response, highlighting a potential difference in its mechanism of action and resistance (Baik et al., 1996).
One other mechanism relevant to acid survival is the formation of endospores, which allow bacteria of some species to survive in unfavourable environments until conditions have improved. These spores are highly resistant to environmental stresses including pH shock and can survive prolonged periods of stress (Cano and Borucki, 1995).
Safety
The use of WOAs to treat wounds has been reported to be well tolerated in the various case studies available to date and some evidence has suggested improved wound healing and granulation following WOA application (Nagoba et al., 2013). There has, however, been no systematic clinical trial organized to test both the efficacy and tolerability of WOAs in humans to date; such work is required before large scale use of WOAs could be recommended.
Many questions still remain unanswered in relation to how WOAs may be clinically useful including how to apply the acid and which acids will be most effective against which target species. We also do not fully understand the mechanisms of action or the potential for resistance to develop. Finally, possible synergies between WOA and other agents including antibiotics have not been explored.
Phage
Bacteriophage are viruses, which target bacteria and can cause cell death; they are found in all environments on earth where bacteria are present and represent a diverse group of different virus families. Bacteriophage have been studied as a potential therapy for bacterial infection for over a hundred years and were used clinically after the First World War to treat various infections before antibiotics were discovered and became widespread (Kutter et al., 2015). Phage have been used clinically to treat infections caused by E. coli , Klebsiella pneumoniae , Staphylococcus aureus , P. aeruginosa and S. Typhimurium with a wide range of concentrations of phage particles, types of phage and routes of application reported although the majority of this usage was not in a controlled manner and has been reported in anecdotal case reports (Bai et al., 2016). Given the increase in antibiotic resistance, there has been a recent resurgence in interest in phage therapy as conventional mechanisms of antibiotic resistance are not relevant to phage sensitivity. A number of phage products are licensed for use in the production of fruit and vegetables and are specifically aimed to eliminate specific pathogens including Listeria, Salmonella or E. coli O157. The use in the food industry has progressed faster than human use as licensing requirements are simpler with materials regarded as ‘Generally Regarded as Safe’ (GRAS) being suitable for usage in this context (Bai et al., 2016).
In addition to the potential for use of phage as a novel antimicrobial therapy there is evidence for phage being active against biofilms, which represents a possible therapeutic niche not well covered by antibiotics as discussed earlier.
Mechanism of action
Lytic phage recognize target cells via their tail fibres with high specificity and bind to the membrane of the cell (Salmond and Fineran, 2015). Once the phage is bound, the nucleic acid is injected into the cell in a process that can require both enzymatic degradation of cell wall components and mechanical injection. Once within the cell, the phage genome is replicated and phage particle proteins produced and assembled using the host cells machinery as per any virus. Release of newly produced phage particles results in lysis of the host cell (Salmond and Fineran, 2015). Phage have the advantages of being specific to the species level or below, which is beneficial in terms of destroying undesirable strains from a specific community without removing potentially beneficial and normal flora – this is especially relevant in treating specific bacteria within a gut (Salmond and Fineran, 2015). Whilst specificity can have significant benefits, it also proves a potential challenge for the treatment of serious infection requiring fast and specific diagnostics in order to ensure a suitable phage or mixture is applied to eradicate the target pathogen. One exciting potential for phage technology is the ability to genetically engineer phage to alter their properties or cargoes. For example, changes to host specificity have been made and the introduction of enzymes that increase degradation of extracellular matrix components has increased efficacy in killing biofilms (Ryan et al., 2011). The development of phage therapy for systemic application has been beset by difficulties in delivery of the phage in a form that will survive and be translocated to the site of infection efficiently (Ryan et al., 2011). Systemic application in animal models has proved possible after both oral and parenteral application, although the route can greatly influence outcomes (Ryan et al., 2011).
Activity against biofilms
The good activity of phage against biofilms has been noted for a long time and makes phage a potentially valuable weapon in the treatment of biofilm infections. Biofilms are invaded by phage, which can move through the population of bacteria and significantly reduce viable numbers of cells. Phage have been used to treat respiratory infections in experimental mice where Pseudomonas or Burkholderia infections were either protected from killing or had significantly reduced pathology after inhalation or systemic phage application (Carmody et al., 2010; Debarbieux et al., 2010). Phage have also shown promise in preventing infection of catheters by Staphylococci or Pseudomonas (Donlan, 2009; Fu et al., 2010). Wound infection is a potentially favourable therapeutic niche for phage use as application is relatively easy, and the progress of treatment can be monitored simply. There have again been a number of studies showing efficacy in treating infection, notably of Pseudomonas in burn wound models (Sulakvelidze et al., 2001; McVay et al., 2007).
Phage resistance
One possible obstacle to the widespread adoption of phage therapy is the development of bacteria resistant to phage infection. Common mechanisms of resistance relate to changes in the membrane‐based targets including lipopolysaccharide, which prevent phage adsorption (Shen et al., 2016). It is possible that cocktails of phage with multiple targets can be developed to minimize this risk, although bacteria resistant to phage they encounter in their normal habitats are common.
Evidence for efficacy
Whilst there is a large body of in vitro and animal evidence supporting the efficacy of phage treatments for prevention or treatment of specific infections, there have been very few well‐designed clinical trials to investigate efficacy against a specific infection. Those that have been completed have shown safety but not proven efficacy to date, although in each case there have been potential confounding factors as patients also received other antimicrobial treatments as part of standards of care (Wright et al., 2009).
Safety
Whilst phage are often regarded as GRAS due to their ubiquitous nature and the lack of obvious toxicity from the anecdotal clinical data, which does exist, there are few data that specifically address or investigate cytotoxicity. These data would be required to support acceleration of a specific phage product into clinical trials under current regulations (Henein, 2013).
More work is needed to optimize delivery routes and formulations. The future development and licensing of phage treatments for specific conditions will require more clinical trials to provide a robust evidence base to support adoption of specific products for defined conditions into clinical practice (Pelfrene et al., 2016).
Photo inactivation
One novel approach to treat biofilms is the use of photo inactivation where light is used to either directly damage bacteria by exciting intracellular porphryins, which release reactive oxygen species, or to activate an inert photo‐sensitive dye that releases toxic reactive oxygen species (Sperandio et al., 2013). This approach promises to be relatively cheap and non‐toxic as a therapy such as blue light (wavelengths between 400 and 470 nm) is able to exert an antimicrobial effect (Dai et al., 2013b). Ultraviolet light has long been known to be antimicrobial, however, it is not suitable for application to humans due to mutagenic properties. One particular advantage of the use of light‐based antimicrobial therapies is equivalent activity against both drug resistant and sensitive pathogens, as the mechanisms of antibiotic resistance do not affect the efficacy of photo inactivation (Mulvey et al., 2000). Blue light in particular has been studied for activity against a range of bacteria and fungi and has been found to be efficacious (Enwemeka et al., 2009). There is in vitro evidence for activity against S. aureus , including methicillin resistant S. aureus (MRSA) strains, Clostridium difficile (both spores and vegetative cells), A. baumannii, E. coli , S. epidermidis , P. aeruginosa, K. pneumoniae , S. pyogenes and Mycobacterium spp. (Enwemeka et al., 2009; Maclean et al., 2009).
Activity against biofilms
A recent study specifically looked at the efficacy of blue light against biofilms and found blue‐light (405 nm) was effective at killing bacteria of a range of important nosocomial pathogens within biofilms in vitro (Halstead et al., 2016). The time taken to achieve a significant reduction in viable cells within the biofilm varied between species with illumination times between 15 and 60 min being required (Halstead et al., 2016). The clinical uses of blue light are still being developed, but effective action against biofilms and low toxicity suggest this may be a useful therapy to treat infected wounds or other sites, which can be irradiated. There is some clinical evidence to support this use, and blue light therapy has been shown to reduce the numbers of bacteria within wounds infected with P. aeruginosa, MRSA and A. baumannii in patients and mouse models (Dai et al., 2013a,b; Zhang et al., 2014).
Safety
Phototherapy using ultraviolet light has been well established as a therapeutic option for various skin conditions, although there are significant potential problems with damage to cells from the UV light (Moura et al., 2016). The use of blue light potentially reduces this concern, and the limited clinical evidence to date has not identified any side effects from application of blue light to humans (Keemss et al., 2016).
Resistance
Resistance to photo inactivation therapies has not been observed to date, although the production of bacterial pigments has been implicated as a resistance mechanism. Pigment production is linked to virulence in some bacterial species (Liu and Nizet, 2009). S. aureus are well known for producing triterpenoid carotenoids, which impart the golden colour to colonies. A correlation between pigment production and survival on surfaces exposed to natural light has been reported (Beard‐Pegler et al., 1988). One other possible mechanism by which pigments may protect against irradiation is by acting as antioxidants (Clauditz et al., 2006). It remains to be seen whether resistance would become a major issue if photodynamic therapy becomes widely adopted.
Discussion
Infections caused by biofilms remain a major challenge to human health, and current treatment regimens are not standardized or broadly effective. The problem is exacerbated by the lack of efficacy of treatments reliant on conventional antibiotics, which often do not effectively eradicate biofilms but are also being challenged by the development of resistance. There is therefore a need for approaches to treat and prevent biofilm‐related infections, which do not rely on antibiotics. We have described here the potential for use of weak organic acids, phage and irradiation as possible treatments to prevent or treat infections on indwelling devices or wounds. Each of these applications represents therapeutic niches where the delivery or application of a treatment may be relatively straightforward but are also areas of great clinical need. The three approaches have the potential to be at least as cost effective as current antimicrobial therapies; WOAs are cheap to manufacture, transport and apply and significantly cheaper than silver‐containing antimicrobial dressings (Halstead et al., 2015). Phage also offer a potentially cheap solution; technologies to prepare large scales of phage products have been developed, and these are commercially applied in food preparation (Kutter et al., 2015). Blue light therapy requires a small capital investment for the light source, but there are no further consumable costs, so this therapy again promises to be relatively cheap to apply in practice.
Each approach has some good in vitro and in some cases in vivo evidence for efficacy, safety and tolerability, and some impressive activity against biofilms formed by troublesome pathogens has been documented. For all, however, there is a lack of robust clinical evidence, which is required before specific treatments can be widely adopted. There are also large uncertainties about delivery, dosage and routes of application for all three approaches, which need further work to be defined. There may be some merit in combining aspects of these approaches together to achieve greatest efficacy, for example, a recent paper described a phage endolysin with anti Gram‐negative activity, which was potentiated by WOAs (Oliveira et al., 2014). Combining multiple active agents may also prevent the emergence of resistance to any novel therapy.
As numbers of device and wound infections increase in an ageing population, the need for novel ways to reduce the impact of infections becomes more urgent. Promising therapies to address this need are being developed and randomized clinical trials are planned or running. The results from these trials will hopefully be positive and offer novel ways to help minimize the burden of infection. This may pave the way for a new round of development of treatments and technologies to deal with infections that does not rely on the development of new antibiotics, which may not be forthcoming in the short term.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
Whilst no specific funding supported the preparation of this manuscript, work on the utility of organic acids in treating biofilm infections in MAW's laboratory has been supported by the NIHR funded Surgical Reconstruction and Microbiology Research Centre at Birmingham.
Hughes, G. , and Webber, M. A. (2017) Novel approaches to the treatment of bacterial biofilm infections. British Journal of Pharmacology, 174: 2237–2246. doi: 10.1111/bph.13706.
References
- Anderl JN, Franklin MJ, Stewart PS (2000). Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob Agents Chemother 44: 1818–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderl JN, Roe F, Stewart PS (2003). Role of nutrient limitation and stationary‐phase existence in. Society 47: 1251–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J, Kim Y‐T, Ryu S, Lee J‐H (2016). Biocontrol and rapid detection of food‐borne pathogens using bacteriophages and endolysins. Front Microbiol 7: 474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baik HS, Bearson S, Dunbar S, Foster JW (1996). The acid tolerance response of Salmonella typhimurium provides protection against organic acids. Microbiology 142 (Pt 1): 3195–3200. [DOI] [PubMed] [Google Scholar]
- Beard‐Pegler MA, Stubbs E, Vickery AM (1988). Observations on the resistance to drying of staphylococcal strains. J Med Microbiol 26: 251–255. [DOI] [PubMed] [Google Scholar]
- Bearson S, Bearson B, Foster JW (2006). Acid stress responses in enterobacteria. FEMS Microbiol Lett 147: 173–180. [DOI] [PubMed] [Google Scholar]
- Biase DD, Lund PA (2015). The Escherichia coli acid stress response and its significance for pathogenesis. Adv Appl Microbiol 92: 49–88. [DOI] [PubMed] [Google Scholar]
- Bjarnsholt T, Alhede M, Jensen PØ, Nielsen AK, Johansen HK, Homøe P et al. (2015). Antibiofilm properties of acetic acid. Adv Wound Care 4: 363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair JM, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJ (2015). Molecular mechanisms of antibiotic resistance. Nat Rev Microbiol 13: 42–51. [DOI] [PubMed] [Google Scholar]
- Brul S, Coote P (1999). Preservative agents in foods: mode of action and microbial resistance mechanisms. Int J Food Microbiol 50: 1–17. [DOI] [PubMed] [Google Scholar]
- Cano RJ, Borucki MK (1995). Revival and identification of bacterial spores in 25‐ to 40‐million‐year‐old Dominican amber. Science 268: 1060–1064. [DOI] [PubMed] [Google Scholar]
- Carmody LA, Gill JJ, Summer EJ, Sajjan US, Gonzalez CF, Young RF et al. (2010). Efficacy of bacteriophage therapy in a model of Burkholderia cenocepacia pulmonary infection. J Infect Dis 201: 264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauditz A, Resch A, Wieland K‐P, Peschel A, Götz F (2006). Staphyloxanthin plays a role in the fitness of Staphylococcus aureus and its ability to cope with oxidative stress. Infect Immun 74: 4950–4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon BP, Rowe SE, Lewis K (2015). Persister cells in biofilm associated infections. Adv Exp Med Biol 831: 1–9. [DOI] [PubMed] [Google Scholar]
- Dai T, Gupta A, Huang Y‐Y, Sherwood ME, Murray CK, Vrahas MS et al. (2013a). Blue light eliminates community‐acquired methicillin‐resistant Staphylococcus aureus in infected mouse skin abrasions. Photomed Laser Surg 31: 531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai T, Gupta A, Huang Y‐Y, Yin R, Murray CK, Vrahas MS et al. (2013b). Blue light rescues mice from potentially fatal Pseudomonas aeruginosa burn infection: efficacy, safety, and mechanism of action. Antimicrob Agents Chemother 57: 1238–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debarbieux L, Leduc D, Maura D, Morello E, Criscuolo A, Grossi O et al. (2010). Bacteriophages can treat and prevent Pseudomonas aeruginosa lung infections. J Infect Dis 201: 1096–1104. [DOI] [PubMed] [Google Scholar]
- Doles W, Wilkerson G, Morrison S, Richmond RG (2015). Glacial acetic acid adverse events: case reports and review of the literature. Hosp Pharm 50: 304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlan RM (2009). Preventing biofilms of clinically relevant organisms using bacteriophage. Trends Microbiol 17: 66–72. [DOI] [PubMed] [Google Scholar]
- Dunne WM, Mason EO, Kaplan SL (1993). Diffusion of rifampin and vancomycin through a Staphylococcus epidermidis biofilm. Antimicrob Agents Chemother 37: 2522–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enwemeka CS, Williams D, Enwemeka SK, Hollosi S, Yens D (2009). Blue 470‐nm light kills methicillin‐resistant Staphylococcus aureus (MRSA) in vitro. Photomed Laser Surg 27: 221–226. [DOI] [PubMed] [Google Scholar]
- Evans RC, Holmes CJ (1987). Effect of vancomycin hydrochloride on Staphylococcus epidermidis biofilm associated with silicone elastomer. Antimicrob Agents Chemother 31: 889–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JW, Hall HK (1990). Adaptive acidification tolerance response of Salmonella typhimurium . J Bacteriol 172: 771–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu W, Forster T, Mayer O, Curtin JJ, Lehman SM, Donlan RM (2010). Bacteriophage cocktail for the prevention of biofilm formation by Pseudomonas aeruginosa on catheters in an in vitro model system. Antimicrob Agents Chemother 54: 397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getliffe K (2012). The effect of acidic maintainance solutions on catheter longevity. Nurs Times 100: 32–34. [PubMed] [Google Scholar]
- Grudzinski A, Agarwal A, Bhatnagar N, Nesrallah G (2015). Benefits and harms of citrate locking solutions for hemodialysis catheters: a systematic review and meta‐analysis. Can J Kidney Health Dis 2: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O (2010). Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents 35: 322–332. [DOI] [PubMed] [Google Scholar]
- Hall‐Stoodley L, Costerton J, Stoodley P (2004). Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol 2: 95–108. [DOI] [PubMed] [Google Scholar]
- Halstead FD, Rauf M, Moiemen NS, Bamford A, Wearn CM, Fraise AP et al. (2015). The antibacterial activity of acetic acid against biofilm‐producing pathogens of relevance to burns patients. PLoS One 10: e0136190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead FD, Thwaite JE, Burt R, Laws TR, Raguse M, Moeller R et al. (2016). Antibacterial activity of blue light against nosocomial wound pathogens growing planktonically and as mature biofilms. Appl Environ Microbiol 82: 4006–4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henein A (2013). What are the limitations on the wider therapeutic use of phage? Bacteriophage 3: e24872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfield IN, Terzulli S, O'Byrne C (2003). Weak organic acids: a panoply of effects on bacteria. Sci Prog 86: 245–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolivet‐Gougeon A, Bonnaure‐Mallet M (2014). Biofilms as a mechanism of bacterial resistance. Drug Discov Today Technol 11: 49–56. [DOI] [PubMed] [Google Scholar]
- Keemss K, Pfaff S, Bornm M, Liebmann J, Merk H, Felbert VV (2016). Prospective, randomized study on the efficacy and safety of local UV‐free blue light treatment of eczema. Dermatology 232: 496–502. [DOI] [PubMed] [Google Scholar]
- Kutter EM, Kuhl SJ, Abedon ST (2015). Re‐establishing a place for phage therapy in western medicine. Future Microbiol 10: 685–688. [DOI] [PubMed] [Google Scholar]
- Liu GY, Nizet V (2009). Color me bad: microbial pigments as virulence factors. Trends Microbiol 17: 406–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclean M, MacGregor SJ, Anderson JG, Woolsey G (2009). Inactivation of bacterial pathogens following exposure to light from a 405‐nanometer light‐emitting diode array. Appl Environ Microbiol 75: 1932–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConoughey SJ, Howlin R, Granger JF, Manring MM, Calhoun JH, Shirtliff M et al. (2014). Biofilms in periprosthetic orthopedic infections. Future Microbiol 9: 987–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay CS, Velásquez M, Fralick JA (2007). Phage therapy of Pseudomonas aeruginosa infection in a mouse burn wound model. Antimicrob Agents Chemother 51: 1934–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihai MM, Holban AM, Giurcaneanu C, Popa LG, Oanea RM, Lazar V et al. (2015). Microbial biofilms: impact on the pathogenesis of periodontitis, cystic fibrosis, chronic wounds and medical device‐related infections. Curr Top Med Chem 15: 1552–1576. [DOI] [PubMed] [Google Scholar]
- Moura M, Coelho V, Apetato M (2016). The dark side of the light: Phototherapy adverse effects. Clin Dermatol 34: 556–562. [DOI] [PubMed] [Google Scholar]
- Mulvey M a, Schilling JD, Martinez JJ, Hultgren SJ (2000). From the cover: bad bugs and beleaguered bladders: interplay between uropathogenic Escherichia coli and innate host defenses. Proc Natl Acad Sci 97: 8829–8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagoba BS, Selkar SP, Wadher BJ, Gandhi RC (2013). Acetic acid treatment of pseudomonal wound infections – a review. J Infect Public Health 6: 410–415. [DOI] [PubMed] [Google Scholar]
- Nickel JC, Ruseska I, Wright JB, Costerton JW (1985). Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob Agents Chemother 27: 619–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira H, Thiagarajan V, Walmagh M, Sillankorva S, Lavigne R, Neves‐Petersen MT et al. (2014). A thermostable Salmonella phage endolysin, Lys68, with broad bactericidal properties against gram‐negative pathogens in presence of weak acids. PLoS One 9: e108376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelfrene E, Willebrand E, Cavaleiro Sanches A, Sebris Z, Cavaleri M (2016). Bacteriophage therapy: a regulatory perspective. J Antimicrob Chemother 71: 2071–2074. [DOI] [PubMed] [Google Scholar]
- Piddock LJV (2016). Reflecting on the final report of the O′Neill review on antimicrobial resistance. Lancet Infect Dis 16: 767–768. [DOI] [PubMed] [Google Scholar]
- Presterl E, Grisold AJ, Reichmann S, Hirschl AM, Georgopoulos A, Graninger W (2005). Viridans streptococci in endocarditis and neutropenic sepsis: biofilm formation and effects of antibiotics. J Antimicrob Chemother 55: 45–50. [DOI] [PubMed] [Google Scholar]
- Randall CP, Mariner KR, Chopra I, O'Neill AJ (2013). The target of daptomycin is absent from Escherichia coli and other gram‐negative pathogens. Antimicrob Agents Chemother 57: 637–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave LS, Sutton SB, Webber MA, Piddock LJV (2014). Fluoroquinolone resistance: mechanisms, impact on bacteria, and role in evolutionary success. Trends Microbiol 22: 438–445. [DOI] [PubMed] [Google Scholar]
- Resistance A, Usage A (2007). Maran 2007.
- Roe AJ, McLaggan D, Davidson I, O'Byrne C, Booth IR (1998). Perturbation of anion balance during inhibition of growth of Escherichia coli by weak acids. J Bacteriol 180: 767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan EM, Gorman SP, Donnelly RF, Gilmore BF (2011). Recent advances in bacteriophage therapy: how delivery routes, formulation, concentration and timing influence the success of phage therapy. J Pharm Pharmacol 63: 1253–1264. [DOI] [PubMed] [Google Scholar]
- Ryssel H, Kloeters O, Germann G, Schäfer T, Wiedemann G, Oehlbauer M (2009). The antimicrobial effect of acetic acid – an alternative to common local antiseptics? Burns 35: 695–700. [DOI] [PubMed] [Google Scholar]
- Salmond CV, Kroll RG, Booth IR (1984). The effect of food preservatives on pH homeostasis in Escherichia coIi . J Gen Microbiol 130: 2845–2850. [DOI] [PubMed] [Google Scholar]
- Salmond GPC, Fineran PC (2015). A century of the phage: past, present and future. Nat Rev Microbiol 13: 777–786. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan R, Wesley R, Somanathan T, Dhakad N, Shyamalakumary B, Amma NS et al. (1998). Visual inspection of the uterine cervix after the application of acetic acid in the detection of cervical carcinoma and its precursors. Cancer 83: 2150–2156. [PubMed] [Google Scholar]
- Shen X, Zhang J, Xu J, Du P, Pang B, Li J et al. (2016). The resistance of Vibrio cholerae O1 El Tor strains to the typing phage 919TP, a member of K139 phage family. Front Microbiol 7: 726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slonczewski JL, Fujisawa M, Dopson M, Krulwich TA (2009). Cytoplasmic pH measurement and homeostasis in bacteria and archaea. Adv Microb Physiol 55: 1–79 .317 [DOI] [PubMed] [Google Scholar]
- Sloss JM, Cumberland N, Milner SM (1993). Acetic acid used for the elimination of Pseudomonas aeruginosa from burn and soft tissue wounds. J R Army Med Corps 139: 49–51. [DOI] [PubMed] [Google Scholar]
- Solano C, Echeverz M, Lasa I (2014). Biofilm dispersion and quorum sensing. Curr Opin Microbiol 18: 96–104. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperandio FF, Huang Y‐Y, Hamblin MR (2013). Antimicrobial photodynamic therapy to kill Gram‐negative bacteria. Recent Pat Antiinfect Drug Discov 8: 108–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford M, Anslow PA (1998). Evidence that sorbic acid does not inhibit yeast as a classic ‘weak acid preservative’. Lett Appl Microbiol 27: 203–206. [DOI] [PubMed] [Google Scholar]
- Strominger JL, Tipper DJ (1965). Bacterial cell wall synthesis and structure in relation to the mechanism of action of penicillins and other antibacterial agents. Am J Med 39: 708–721. [DOI] [PubMed] [Google Scholar]
- Sulakvelidze A, Alavidze Z, Morris JG (2001). Bacteriophage therapy. Antimicrob Agents Chemother 45: 649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomanen E, Cozens R, Tosch W, Zak O, Tomasz A (1986). The rate of killing of Escherichia coli by ‐lactam antibiotics is strictly proportional to the rate of bacterial growth. Microbiology 132: 1297–1304. [DOI] [PubMed] [Google Scholar]
- Vakulenko SB, Mobashery S (2003). Versatility of aminoglycosides and prospects for their future versatility of aminoglycosides and prospects for their future. Clin Microbiol Rev 16: 430–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber MA, Piddock LJV (2003). The importance of efflux pumps in bacterial antibiotic resistance. J Antimicrob Chemother 51: 9–11. [DOI] [PubMed] [Google Scholar]
- Westley‐Horton E, Koestner JA (1991). Aztreonam: a review of the first monobactam. Am J Med Sci 302: 46–49. [DOI] [PubMed] [Google Scholar]
- WRAP (2013). Overview of waste in the UK hospitality and food service sector 52.
- Wright A, Hawkins CH, Anggård EE, Harper DR (2009). A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic‐resistant Pseudomonas aeruginosa; a preliminary report of efficacy. Clin Otolaryngol 34: 349–357. [DOI] [PubMed] [Google Scholar]
- Young KM, Foegeding PM (1993). Acetic, lactic and citric acids and pH inhibition of Listeria monocytogenes Scott A and the effect on intracellular pH. J Appl Bacteriol 74: 515–520. [PubMed] [Google Scholar]
- Zhang Y, Zhu Y, Gupta A, Huang Y, Murray CK, Vrahas MS et al. (2014). Antimicrobial blue light therapy for multidrug‐resistant Acinetobacter baumannii infection in a mouse burn model: implications for prophylaxis and treatment of combat‐related wound infections. J Infect Dis 209: 1963–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W‐H, Hu Z‐Q (2013). Epidemiology and genetics of CTX‐M extended‐spectrum β‐lactamases in Gram‐negative bacteria. Crit Rev Microbiol 39: 79–101. [DOI] [PMC free article] [PubMed] [Google Scholar]


