Abstract
Background
Agents currently used for the treatment and prevention of thrombosis have a number of side effects. We conducted this study to develop antithrombotic agents from herbs that are used in food.
Methods
The 80% (v/v) ethanol extracts of Phyllostachys pubescens leaf (PL) and Mume Fructus (MF) and their combinations—2:1 (PM21), 1:1 (PM11), and 1:2 (PM12)—were evaluated on rat platelet aggregation induced by adenosine diphosphate (ADP) in vitro and on arteriovenous shunt thrombosis after 3 days of oral treatment in rats in vivo.
Results
At 100 μg/mL, PM21 and PM11 inhibited in vitro ADP-induced aggregation by 44.0 ± 4.3% and 30.0 ± 3.2%, respectively, whereas PL, MF, and PM12 weakly or scarcely inhibited ADP-induced aggregation by 3.9 ± 3.2%, 13.0 ± 2.7%, and 5.2 ± 1.3%, respectively. The IC50 values of PM21 on ADP-, collagen-, and thrombin-induced platelet aggregations were 135.6 ± 7.4 μg/mL, 142.7 ± 5.8 μg/mL, and 186.5 ± 9.7 μg/mL, respectively. In an in vivo rat arteriovenous-shunt thrombosis model, thrombus weight was significantly decreased after the oral administration of 400 mg/kg PL (27.8 ± 3.0%, p < 0.01) or MF (35.2 ± 2.1%, p < 0.01), and with a good accord to the in vitro results, the combination of PL and MF in the ratio of 2:1, PM21 (60.9 ± 1.2%, p < 0.001), showed a superior antithrombotic effect to those of individual extracts. At dosages of 200 mg/kg, 100 mg/kg, and 50 mg/kg, PM21 dose-dependently decreased thrombosis weight (ED50, 314 mg/kg).
Conclusion
These results suggest that combination preparations of PL and MF, especially their 2:1 combination, can increase antiplatelet and antithromboticeffects more than PL and MF alone, offering evidence for a potential novel combination antithrombotic therapy.
Keywords: antiplatelet, antithrombosis, blood circulation, herbal combination, Mume Fructus, Phyllostachys pubescens
1. Introduction
Platelet aggregation is a physiological mechanism that seals off the damaged blood vessel wall to prevent blood loss. Various endogenous factors such as collagen, thrombin, adenosine diphosphate (ADP), serotonin, vasopressin, and epinephrine induce platelet activation followed by shape change and aggregation. Under pathological conditions, however, platelets also play a key role in the pathogenesis of coronary cardiovascular diseases, including artery disease and arthrosclerosis. Most acute coronary syndromes are caused by platelet adhesion, aggregation, and thrombus formation in areas of ruptured atheromatous plaques.1, 2 Since the discovery of acetylsalicylic acid, heparin, and warfarin, the use of antiplatelet and anticoagulation agents in the prevention and treatment of cardiac disease, especially during early treatment of acute myocardial infarction, has become widespread. The antiplatelet and antithrombotic agents of many plants such as Veratrum patulum,3 sesame seed,4 Magnolia obovata,5 Angelica sinensis,6 Ginkgo biloba,7 olive oil,8 Korean red ginseng,9 mulberries,10 Common thyme and rosemary,11 Lavandula hybrida,12 onion,13 Glycyrrhiza glabra,14 Gardenia jasminoides,15 Evodia rutaecarpa,16 and garlic,17 have been investigated.
Phyllostachys pubescens, which belongs to the Gramineae family, is an important bamboo species in Korea, Japan, and China. It has been commonly used throughout history in Chinese medicine for treating infections and for many forms of healing. Recently, it has been reported to contain agents of many kinds of biological activities, such as antioxidant activity,18 antiobesity activity,19 antitumor activity,20 and antimicrobial activity.21
Mume Fructus (MF) is produced by baking unripe fruits, which are gathered at the beginning of summer, until their skins turn black. It has been used in Chinese medicine to astringe the lungs and stop coughing, to restrain the intestines and stop diarrhea, as well as to promote the production of body fluids, and to expel roundworms. It has been reported that MF has antimicrobial activity,22 hypouricemic effect,23 antibacterial effect,24 antioxidant activity,25 antitumor activity,26 and immune-enhancing effect,27 etc.
The anticoagulant activity,28 antiplatelet aggregation, and antithrombotic activity29 of P. pubescens leaves have similarly been reported; however, their combined preparation has not been investigated until now. The aim of the present study was to investigate the ethanol extracts of Phyllostachys pubescens leaf (PL) and Mume Fructus (MF), and their combined preparations, on platelet aggregation in vitro and on rat arteriovenous (AV) shunt thrombosis in vivo.
2. Methods
2.1. Preparation of the ethanol extracts from the plants and their combinations
P. pubescens leaf was collected from Damyang, Korea, on July 27, 2012, and MF was purchased from the Bakjedang Herbal Medicine Shop in Daejeon, Korea. The voucher specimens were identified in the Basic Herbal Medicine Research Group at the Korea Institute of Oriental Medicine. Authenticated voucher specimens (BL-20120727; MF-20120725) were deposited in the Herbarium of the Korea Institute of Oriental Medicine.
The pulverized P. pubescens leaves (100 g) and MF (100 g) were each soaked in 1 L of 80% (v/v) ethanol in water for 5 hours under mantle reflux. The extracts were filtered and then were evaporated under reduced pressure in a rotary evaporator (N-1000 S; EYELA, Tokyo, Japan) to obtain P. pubescens leaf extract (PL, 8.7 g) and MF extract (18.9 g). Three combinations of these extracts were prepared as follows: PM21, PM11, and PM12 were formulated by mixing PL and MF at a ratio of 2:1, 1:1, and 1:2, respectively.
2.2. High-performance liquid chromatography analysis of marker compounds
High-performance liquid chromatography (HPLC)-grade reagents, acetonitrile, and water were obtained from J. T. Baker (Phillipsburg, NJ, USA). All other chemicals were of reagent grade.
The sample was analyzed by reverse-phase HPLC using Waters Alliance 2695 system (Waters Co., Milford, MA, USA), coupled with a 2996 photodiode array detector. Phenomenex Luna C18 column (250 × 4.6 mm; particle size 5 μm; Phenomenex, Torrance, CA, USA) was used as the stationary phase, and the mobile phase was composed of 0.1% (v/v) trifluoroacetic aqueous solution (A) and acetonitrile (B). The elution conditions were as follows: at t = 0 minute, the mobile phase consisted of 90% A/10% B and was held for 10 minutes. From 10 minutes to 30 minutes, a gradient was applied to 70% A/30% B, which was followed by a wash with 100% B for 5 minutes and a 15-minute equilibration period at 90% A/10% B. The separation temperature was kept at a constant 40 °C throughout the analysis, with a flow rate of 1.0 mL/min and injection volume of 20 μL.
Identification was based on retention time and UV spectra by comparison with commercial standards. For each compound, peak areas were determined at the wavelength providing maximal UV absorbance. Calibration curves of the standards ranging from 1.25 μg/mL to 40 μg/mL (6 levels) for 5-hydroxymethylfurfural and from 12.5 μg/mL to 400 μg/mL (6 levels) for isoorientin revealed good linearity, with R2 values exceeding 0.99 (peak areas vs. concentration).
Quantitation was performed on the basis of external standards with a mixture of standards of known concentration that were analyzed in duplicate prior to and after the batch of samples, and the peak areas were used to calculate the sample contents of the compounds.
2.3. Preparations of platelet-rich plasma and platelet aggregation assay
Samples were prepared according to standard practice30, 31, 32, 33: SD rat blood (10 mL) was obtained by cardiac puncture (by inserting a 23-gauge needle into the cardiac puncture), and samples were transferred to a 15-mL test tube containing 1 mL citrate phosphate dextrose solution (CPD: 90 mM Na3 C6H5O7·2H2O, 14 mM C6H8O7·H2O, 128.7 mM NaH2PO4·H2O,and 2.55 g/100 mL dextrose).
Platelet-rich plasma (PRP) was obtained after blood sample centrifugation at 170 × g for 5 minutes. The PRP samples were again centrifuged at 120 × g for 5 minutes to remove residual erythrocytes. In order to remove the CPD solution and to isolate the platelets, the PRP was centrifuged twice at 350 × g for 10 minutes, and the supernatant was then isolated as a platelet-poor plasma fraction, which was used as a reference solution in aggregation assays. The platelets of the precipitate were adjusted to the proper number (108/mL) for the aggregation assay in Tyrode buffer (137 mM NaCl, 12 mM NaHCO3, 5.5 mM glucose, 2 mM KCl, 1 mM MgCl2, 0.3 mM NaHPO4, and pH = 7.4). All platelet preparations were conducted at room temperature.
Samples and aspirin were dissolved in a 50% DMSO solution to obtain primary solutions of 0.2 g/L. These were then serially diluted with saline solution to obtain the required concentrations. The control was a saline solution with 0.5% DMSO solution. ADP, collagen, and thrombin (Sigma-Aldrich, St. Louis, MO, USA) were used to induce platelet aggregation. Aggregation was monitored by measuring light transmission via a platelet aggregometer (Chrono-Log corporation, Havertown, PA, USA). The washed platelets were preincubated at 37 °C for 5 minutes with either samples or vehicle, and then ADP (10 μM), collagen (5 μg/mL), or thrombin (0.5 unit/mL) was added. The aggregation curves were recorded for 6 minutes. Maximum aggregation was recorded for the control (CA) and for the tests (TA). The inhibition of aggregation (IA) was calculated as: IA = (CA – TA/CA) × 100.
2.4. In vivo experiments
2.4.1. Animals
Male Sprague–Dawley rats weighing 300–350 g were purchased from Japan SLC (Hamamatsu, Japan). They were kept in a temperature-controlled environment (20 °C) with a 12:12-hour light–dark cycle, and were fed with standard chow for at least 1 week prior to the initiation of any testing. Our study was approved by the committee for animal welfare at Daejeon University. Moreover, all animal procedures were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Korea Research Institute of Bioscience and Biotechnology (Daejeon, South Korea).
2.4.2. Treatments
Rats were orally administered (by gavage) with 400 mg/kg, 200 mg/kg, 100 mg/kg, and 50 mg/kg crude extract dissolved in 0.25% carboxymethylcellulose solution (Sigma) at the same time of the day for 3 consecutive days. The shunt thrombosis model was tested 2 hours after the last administration. Six rats were used for each test.
2.4.3. In vivo effect on a rat model of AV shunt thrombosis
The model tested was a rat AV shunt thrombosis model.34 After anesthesia with urethane (1.25 g/kg, i.p.; Sigma), an 8-cm polyethylene tube was inserted between the left jugular vein and the right carotid artery. The saline-filled shunt was assembled by connecting two cannulae with slightly curved 6-cm-long tygon tubing (internal diameter, 2 mm) containing a 5-cm-long cotton thread (diameter, 0.25 mm) that had been scraped with a scalpel blade to make it more thrombogenic. Extracorporeal circulation was maintained for 15 minutes, during which time a thrombus adhered to the cotton thread. The shunt was then removed and the thread, with its associated thrombus, was withdrawn and was immediately weighed. The thrombus wet weight was then determined by subtracting the weight of the dry 5 cm cotton thread.
2.5. Statistical analysis
Differences between treatment groups were examined using an unpaired Student t test. All data are presented as means ± standard deviation (SD). Differences between groups were considered significant when p < 0.05.
3. Results
3.1. Chromatographic separation of PM21
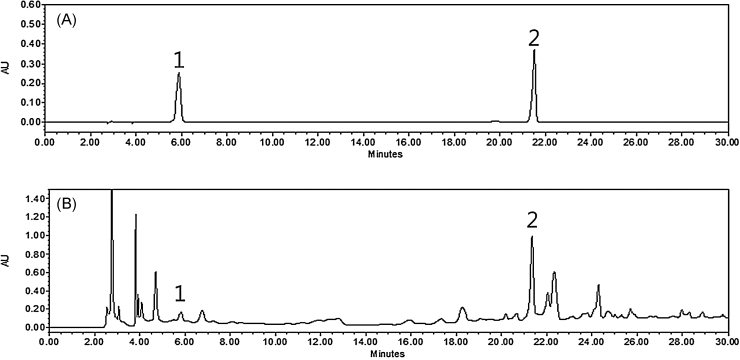
Two marker compounds were determined from major peaks by comparison of retention time and UV spectra on HPLC/PDA chromatogram with commercial standards. As shown in Fig. 1, PLC analysis of PM21 revealed two standards—5-hydroxymethylfurfural and isoorientin—at a retention time of approximately 5.8 minutes and 21.5 minutes. PM21 contained 0.08 ± 0.01 mg/g for 5-hydroxymethylfurfural and 4.8 ± 0.07 mg/g for isoorientin.
Fig. 1.
HPLC chromatogram of (A) two standards mixture and (B) the 80% (v/v) ethanol extracts combinational preparation PM21 at 280 nm. 5-Hydroxymethylfurfural (1) and isoorientin (2) appeared at a retention time of approximately 5.8 minutes and 21.5 minutes, respectively. PM21 contained 0.08 ± 0.01 mg/g for 5-hydroxymethylfurfural and 4.8 ± 0.07 mg/g for isoorientin. HPLC, high-performance liquid chromatography.
3.2. In vitro effect on agonist-induced rat platelet aggregation
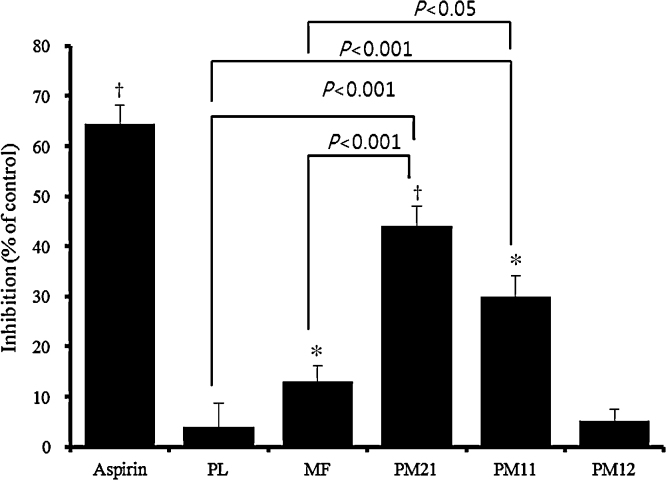
The 80% (v/v) ethanol extracts of PL and MF and their combinational preparations of 2:1 (PM21), 1:1 (PM11), and 1:2 (PM12) on ADP-induced platelet aggregation in vitro were evaluated using washed rat platelets. At the concentration of 100 μg/mL, PM21 and PM11 inhibited platelet aggregation by 44.0 ± 4.3% and 30.0 ± 3.2%, respectively, whereas PL, MF, and PM12 weakly or scarcely inhibited platelet aggregation by 3.9 ± 3.2%, 13.0 ± 2.7%, and 5.2 ± 1.3%, respectively, compared to control (Fig. 2). PM21 (p < 0.001, to both) and PM11 (p < 0.001 and p < 0.05, respectively) also showed a significant difference from PL or MF. We further determined the half-maximal inhibitory concentration (IC50) of PM21 on collagen- and thrombin-induced platelet aggregations as well as ADP-induced platelet aggregation. The IC50 values of PM21 on ADP-, collagen-, and thrombin-induced platelet aggregations were 135.6 ± 7.4 μg/mL, 142.7 ± 5.8 μg/mL, and 186.5 ± 9.7 μg/mL, respectively.
Fig. 2.
Effect of Phyllostachys pubescens leaf extract (PL), Mume Fructus extract (MF), and the 2:1, 1:1, and 1:1 combinations (PM21, PM11, and PM21, respectively) from these two extracts on adenosine diphosphate (ADP)-induced rat platelet aggregation in vitro. The washed platelets from SD rats were pre-incubated at 37 °C for 5 minutes with either samples (100 μg/mL) or vehicle, and then ADP (10 μM) was added. The aggregation curves were recorded for 6 minutes. Maximum aggregation was recorded for the control (CA) and for the tests (TA). The inhibition of aggregation (IA) was calculated as: IA = (CA – TA/CA) × 100. Data are presented as mean ± SD. *p < 0.05, †p < 0.01.
3.3. In vivo effect on a rat model of AV shunt thrombosis
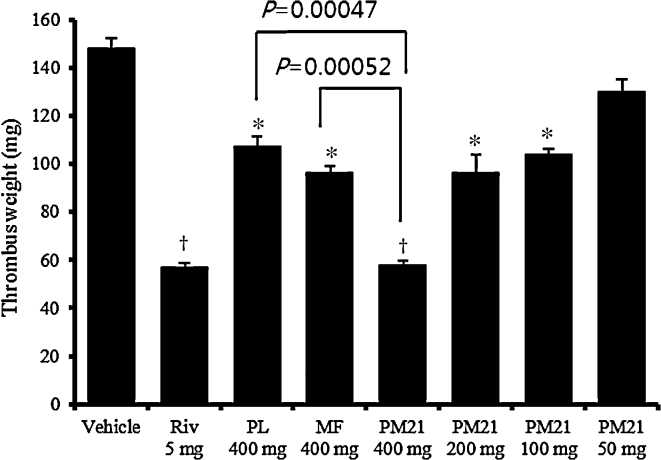
The AV shunt thrombosis model has been commonly used to assess antithrombotic effects.34 As shown in Fig. 3, after the oral administration of 400 mg/kg of PL or MF, thrombus weight was significantly decreased by 27.8 ± 3.0% (p < 0.01) and 35.2 ± 2.1%, (p < 0.01), respectively, and in good agreement with in vitro results, the combination of PL and MF in the ratio of 2:1 (PM21; 60.9 ± 1.2%, p < 0.001) showed a superior antithrombotic effect compared with that observed for each extract. PM21 also achieved statistical significance, with p = 0.00047 compared to PL and p = 0.00052 compared to MF. At the additional dosages of 200 mg/kg, 100 mg/kg, and 50 mg/kg, PM21 dose-dependently decreased the thrombosis weight by 35.2 ± 5.4% (p < 0.01), 29.8 ± 1.9% (p < 0.01), and 12.2 ± 3.6%, respectively, compared to the vehicle control (ED50, 314 mg/kg).
Fig. 3.
Effect of Phyllostachys pubescens leaf extract (PL), Mume Fructus extract (MF), and their 2:1 combination (PM21) of these two extracts on AV shunt thrombosis. Rivaroxaban (Riv) was used as a positive control. After anesthesia with urethane, an 8-cm polyethylene tube was inserted between the left jugular vein and the right carotid artery of SD rats. The saline-filled shunt was assembled by connecting two cannulae with slightly curved tubing containing a cotton thread that had been scraped with a scalpel blade to make it more thrombogenic. Extracorporeal circulation was maintained for 15 minutes, during which time a thrombus adhered to the cotton thread. The thrombus wet weight was then determined by subtracting the weight of the dry cotton thread. Data are shown as mean ± SD. *p < 0.01, †p < 0.001 versus vehicle control.
4. Discussion
Arterial thrombi are well known to be largely composed of aggregated platelets. Platelets play a vital role in both the initiation and growth of thrombi. Thus, the inhibition of platelet function represents a promising approach for the prevention of thrombotic disorder. In the present study, PL and MF, which are used in Oriental herbal medicine, and their combination preparations (PM21, PM11, and PM12) were evaluated in vitro for their antiplatelet aggregation activity induced by ADP in vitro. MF showed weak inhibition, and PL showed scarcely any inhibition at 100 μg/mL. By contrast, PM21 and PM11 showed significantly higher inhibition compared to PL or MF alone. Park et al29 reported that MF weakly inhibited platelet aggregation, even at a high concentration of 1 mg/mL. The antiplatelet aggregation effect of PL was not previously reported, but the anticoagulant activity, as an intrinsic pathway of activated partial thromboplastin time, had previously been shown to be weak, even at a high concentration of 1.2 mg/mL.28 This study suggests that the antiplatelet effect of PL or MF alone can be significantly increased by their combination.
Antithrombotic therapy has proven to be effective for the treatment of cardiovascular diseases. In the current study, PM21, which showed superior in vitro antiplatelet effects among the three combinations, was evaluated for its antithrombotic effect using the rat AV shunt thrombosis model in vivo, and in comparison with PL and MF used alone. PM21 showed a significantly (p < 0.001) higher antithrombotic effect compared to PL or MF alone. Considering the traditional applications of PL and MF in Oriental medicine, which have mostly been used to nourish the blood and improve blood circulation for the treatment of cardiovascular diseases, the antithrombotic effect of PM21 demonstrated in the present study might provide new evidence for the greater benefit of using this combined approach.
In conclusion, our results suggest that combined preparations of PL and MF, especially at a ratio of 2:1, can increase their antiplatelet and antithrombotic effects than when either agent is used alone, offering experimental evidence for a potential novel combination antithrombotic therapy. Further studies will be conducted to optimize the combination ratio of PL and MF and to investigate their synergistic mechanism.
Conflict of interest
All contributing authors declare no conflict of interest.
Acknowledgments
This work was supported by the Development of Medicinal Food for Blood Circulation Improvement from Oriental Herbal Medicine (project K13202), from the Ministry of Education, Science, and Technology of Korea.
References
- 1.Falk E. Plaque rupture with severe pre-existing stenosis precipitating coronary thrombosis. Characteristics of coronary atherosclerotic plaques underlying fatal occlusive thrombi. Br. Heart J. 1983;50:127–134. doi: 10.1136/hrt.50.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willerson J.T., Golino P., Eidt J., Campbell W.B., Buja L.M. Specific platelet mediators and unstable coronary artery lesions. Experimental evidence and potential clinical implications. Circulation. 1989;80:198–205. doi: 10.1161/01.cir.80.1.198. [DOI] [PubMed] [Google Scholar]
- 3.Song Q., Wang S., Zhao W. Total steroidal alkaloids from Veratrum patulum L. inhibit platelet aggregation, thrombi formation and decrease bleeding time in rats. J. Ethnopharmacol. 2012;141:183–186. doi: 10.1016/j.jep.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 4.Kinugasa C., Naemura A., Hyodo K., Nakai Y., Katsuta M., Yamamoto J. Experimental antithrombotic effects of sesame seed whole grains and extracts. Blood Coagul. Fibrinolysis. 2011;22:526–531. doi: 10.1097/MBC.0b013e328347b085. [DOI] [PubMed] [Google Scholar]
- 5.Park E.S., Lim Y., Lee S.H., Kwon B.M., Yoo H.S., Hong J.T. Antiplatelet activity of obovatol, a biphenolic component of Magnolia obovata, in rat arterial thrombosis and rabbit platelet aggregation. J Atheroscler Thromb. 2011;18:659–669. doi: 10.5551/jat.7427. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L., Du J.R., Wang J., Yu D.K., Chen Y.S., He Y. Z-ligustilide extracted from Radix Angelica sinensis decreased platelet aggregation induced by ADP ex vivo and arterio-venous shunt thrombosis in vivo in rats. Yakugaku Zasshi. 2009;129:855–859. doi: 10.1248/yakushi.129.855. [DOI] [PubMed] [Google Scholar]
- 7.Ryu K.H., Han H.Y., Lee S.Y., Jeon S.D., Im G.J., Lee B.Y. Ginkgo biloba extract enhances antiplatelet and antithrombotic effects of cilostazol without prolongation of bleeding time. Thromb. Res. 2009;124:328–334. doi: 10.1016/j.thromres.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Tsantila N., Karantonis H.C., Perrea D.N., Theocharis S.E., Iliopoulos D.G., Antonopoulou S. Antithrombotic and antiatherosclerotic properties of olive oil and olive pomace polar extracts in rabbits. Mediators Inflamm. 2007;2007:36204. doi: 10.1155/2007/36204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin Y.R., Yu J.Y., Lee J.J., You S.H., Chung J.H., Noh J.Y. Antithrombotic and antiplatelet activities of Korean red ginseng extract. Basic Clin Pharmacol Toxicol. 2007;100:170–175. doi: 10.1111/j.1742-7843.2006.00033.x. [DOI] [PubMed] [Google Scholar]
- 10.Yamatoto J., Naemura A., Ura M., Ijiri Y., Yamashita T., Kurioka A. Testing various fruits and anti-thrombotic effect: I. Mulberries. Plalelets. 2006;17:555–564. doi: 10.1080/09537100600759295. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto J., Yamada K., Naemura A., Yamashita T., Arai R. Testing various herbs for antithrombotic effect. Nutrition. 2005;21:580–587. doi: 10.1016/j.nut.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Ballabeni V., Tognolini M., Chiavarini M., Impicciatore M., Bruni R., Bianchi A. Novel antiplatelet and antithrombotic acvitities of essential oil from Lavandula hybrida reverchon “grosso”. Phytomedicine. 2004;11:596–601. doi: 10.1016/j.phymed.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Yamada K., Naemura A., Sawashita N., Noguchi Y., Yamamoto J. An onion variety has natural antithrombotic effect as assessed by thrombosis/thrombolysis models in rodents. Thromb. Res. 2004;114:213–220. doi: 10.1016/j.thromres.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Mendes-Silva W., Assafim M., Ruta B., Monteiro R.Q., Guimaraes J.A., Zingali R.B. Antithrombotic effect of Glycyrrhizin, a plant-derived thrombin inhibitor. Thromb. Res. 2003;112:93–98. doi: 10.1016/j.thromres.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki Y., Kondo K., Ikeda Y., Umemura K. Antithrombotic effect of geniposide and genipin in the mouse thrombosis model. Planta Med. 2001;67:807–810. doi: 10.1055/s-2001-18842. [DOI] [PubMed] [Google Scholar]
- 16.Sheu J.R., Hung W.C., Wu C.H., Lee Y.M., Yen M.H. Antithrombotic effect of rutaecarpine, an alkaloid isolated from Evodia rutaecrpa, on platelet plug formation in in vivo experiments. Br. J. Haematol. 2000;110:110–115. doi: 10.1046/j.1365-2141.2000.01953.x. [DOI] [PubMed] [Google Scholar]
- 17.el-Sabban F., Fahim M.A., Radwan G.M., Zaghloul S.S., Singh S. Garlic preserves patency and delays hyperthermia-induced thrombosis in pial microcirculation. Int. J. Hyperthermia. 1996;12:513–525. doi: 10.3109/02656739609023528. [DOI] [PubMed] [Google Scholar]
- 18.Wu D., Chen J., Lu B., Xiong L., He Y., Zhang Y. Application of near infrared spectroscopy for the rapid determination of antioxidant activity of bamboo leaf extract. Food Chem. 2012;135:2147–2156. doi: 10.1016/j.foodchem.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Koide C.L., Collier A.C., Berry M.J., Panee J. The effect of bamboo extract on hepatic biotransforming enzymes—findings from an obese–diabetic mouse model. J. Ethnopharmacol. 2011;133:37–45. doi: 10.1016/j.jep.2010.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuboyama N., Fujii A., Tamura T. antitumor activities of bamboo leaf extracts (BLE) and its lignin (BLL) Nihon Yakurigaku Zasshi. 1981;77:579–596. [In Japanese, English abstract] [PubMed] [Google Scholar]
- 21.Jin Y.C., Yuan K., Zhang J. Chemical composition, and antioxidant and antimicrobial activities of essential oil of Phyllostachys heterocycla cv. pubescens varieties from China. Moecules. 2011;16:4318–4327. doi: 10.3390/molecules16054318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seneviratne C.J., Wong R.W., Hagg U., Chen Y., Herath T.D., Samaranayake P.L. Prunus meme extract exhibits antimicrobial activity against pathogenic oral bacteria. Int. J. Paediatr. Dent. 2011;21(4):299–305. doi: 10.1111/j.1365-263X.2011.01123.x. [DOI] [PubMed] [Google Scholar]
- 23.Yi L.T., Li J., Su D.X., Dong J.F., Li C.F. Hypouricemic effect of the methanol extract from Prunus mume fruit in mice. Pharm Biol. 2012;50(11):1423–1427. doi: 10.3109/13880209.2012.683115. [DOI] [PubMed] [Google Scholar]
- 24.Kwon H.A., Kwon Y.J., Kwon D.Y., Lee J.H. Evaluation of antibacterial effects of a combination of Coptis Rhizoma, Meme Fructus, and Schizandrae Fructus against Salmonella. Int. J. Food Microbiol. 2008;127:180–183. doi: 10.1016/j.ijfoodmicro.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 25.Matsuda H., Morikawa T., Ishiwada T., Managi H., Kagawa M., Higashi Y. Medicinal flowers: VIII. Radical scavenging constituents from the flowers of Prunus mume: structure of prunose III. Chem. Pharm. Bull. 2003;51:440–443. doi: 10.1248/cpb.51.440. [DOI] [PubMed] [Google Scholar]
- 26.Shen H., Cheng T., Qiao C., Su Z., Li C. Antitumor effect in vitro and immuno-response in vivo of fructus Mume. Zhongguo Zhong Yao Za Zhi. 1995;20:365–368. [In Chinese, English abstract] [PubMed] [Google Scholar]
- 27.Jung B.G., Ko J.H., Cho S.J., Koh H.B., Yoon S.R., Han D.U. Immune-enhancing effect of fermented Maesil (Prunus mume) with probiotics against Bordetella bronchiseptica in mice. J. Vet. Med. Sci. 2010;72:1195–1202. doi: 10.1292/jvms.09-0555. [DOI] [PubMed] [Google Scholar]
- 28.Cho E., Kim S., Na I., Kim D.C. In MJ, Chae HJ. Antioxidant and anticoagulant activities of water and ethanol extracts of Phyllostachys pubescence leaf produced in Geoje. J Appl Chem. 2010;53:170–173. [Google Scholar]
- 29.Park S.H., Park K.J., Kim J.K. The effects of Mume Fructus extracts on blood flow improvement. Yakhak Hoeji. 2009;53:298–302. [In Korean, English abstract] [Google Scholar]
- 30.Bengmark S., Elmer O., Goransson G., Zoucas E. In vitro effect of ethanol on ADP and collagen-induced platelet aggregation. Thromb Haemast. 1981;46:673–675. [PubMed] [Google Scholar]
- 31.Komiya T., Higurashi K., Iizuka K., Mizuno Y. A novel free radical scavenger, nicaraven, inhibits human platelet aggregation in vitro. Clin. Neuropharmacol. 1999;22:11–14. doi: 10.1097/00002826-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Rand M.L., Packham M.A., Kinlough-Rathbone R.L., Fraser Mustard J. Effects of ethanol on pathways of platelet aggregation in vitro. Thromb. Haemost. 1988;59:383–387. [PubMed] [Google Scholar]
- 33.Xie M.L., Lu Q., Gu Z.L. Effect of quercetin on platelet aggregation induced by oxyradicals. Zhongguo Yao Li Xue Bao. 1996;17:334–336. [PubMed] [Google Scholar]
- 34.Perzborn E., Strassburger J., Wilmen A., Pohlmann J., Roehing S., Schlemmer K.H. In vitro and in vivo studies of the novel antithrombotic agent BAY 59-7939—an oral, direct factor Xa inhibitor. J Thromb Haemost. 2005;3:514–521. doi: 10.1111/j.1538-7836.2005.01166.x. [DOI] [PubMed] [Google Scholar]