Abstract
Most studies have considered the negative influence of obesity on fertility in both genders. In the present study, we assessed mitochondrial activity expressed as the mitochondrial potential index (MPI) in cumulus cells from obese women and women with a normal body mass index (BMI) during assisted reproductive therapy. The results revealed a significant reduction of MPI with increased body mass. The lower MPI levels in cumulus cells from obese women may reflect mitochondrial dysfunction caused by oxidative stress, which can affect the cumulus-oocyte complex and have an impact on oocyte development.
Keywords: Cumulus cells, Obesity, Adipokines, Mitochondria, Mitochondrial potential, IVF
Highlights
-
•
Reduction of MPI in cumulus cells with increase of body mass is revealed.
-
•
Correlation between cumulus cell MPI and serum level of adipokines was observed.
-
•
Decrease of MPI in cumulus from obese women reflects impaired mitochondrial activity.
1. Introduction
Obesity is considered as one of the worldwide pandemic diseases and has important consequences for fertility. Maternal obesity frequently results in negative outcomes both for mother and child [1], [2], [3], [4] thereby questioning how metabolic alterations affect oocyte and embryo development in overweight and obese patients. Fluctuations of free fatty acid levels in blood circulation and follicular fluid caused by triglyceride and lipid hydrolysis play an essential role as proinflammatory triggers and may contribute to the negative consequences of obesity on fertility [5], [22], [23]. Recent findings suggest that cumulus cells act as a barrier, protecting oocytes from lipotoxic effects [6], [7].
Mitochondria are key players in the regulation of cell energy metabolism and intracellular signaling, controlling cellular proliferation, differentiation and death, ultimately maintaining the homeostasis of the cell population [8]. Emerging evidence demonstrates the pivotal role of mitochondria in successful oocyte development and competence [9], [10], [11], [12], [13], [14]. Maternal mitochondria are the only source of the energy to support embryo development before they reach their own period of mitochondrial biogenesis corresponding to human embryonic genome activation (day 2–3 of embryonic development) [15], [16]. Mitochondrial transmembrane potential is essential for production of aerobic energy and a driving force of other synthetic processes in mitochondria [17]. It has been shown that low mitochondrial transmembrane potential in oocytes leads to abnormal embryos formation in mice [11], [18].
The relationship between mitochondrial dysfunction and infertility has been demonstrated in mice with obesity and/or diabetes [19], [20]. It was also shown that periconceptional obesity in mice is associated with the alteration of mitochondrial function in murine oocytes and zygotes [21]. Complex interactions between cumulus cells and oocytes influence oocytes development and competence through the processes of steroidogenesis, cellular signaling and metabolism [24]. Pyruvate production and free fatty acid oxidation in cumulus cells are the main sources of substrates for ATP production in the mitochondria of developing oocytes [25]. Cumulus cell mitochondrial membrane potential and ATP production positively correlate with the number of mature oocytes retrieved after ovarian stimulation during the in vitro fertilization (IVF) [26].
In this study we aim to assess mitochondrial activity in cumulus cells and its correlation with oocyte quality, the probability of fertilization and adequate early embryogenesis in obese and normal weight women that undergo ovarian stimulation using IVF protocols. A sufficient number of cumulus cells which are available during IVF/ICSI can be useful to improve the prediction of oocyte quality.
2. Materials and methods
2.1. Patients
IVF cycles were performed in the Federal State Budget Institution “Research Center for Obstetrics, Gynecology and Perinatology”, Ministry of Healthcare of the Russian Federation between January 2014 and April 2015 in accordance with the Ethics committee agreement, act No. 6 (May 16, 2013). Written informed consent was obtained from all women prior study.
Study participants were between the ages of 18 and 37 years, and each had a normal ovarian reserve and intact uterus. Ovarian reserve was assessed in accordance with the criteria of the European Society of Human Reproduction and Embryology. Primary diagnosis was tubal factor, ovulation disorders or male subfertile sperm. Exclusion criteria were previous ovarian surgery, signs of polycystic ovarian syndrome during clinical or ultrasound examinations, or other medical conditions that might have contraindications for pregnancy.
Prior to ovarian stimulation, following a standard GnRH antagonist protocol, the body mass index (BMI) was calculated from the weight and height recorded for each woman. BMI was defined as body mass in kilograms divided by height in metres squared. According to World Health Organization guidelines [27], all women in the present study were divided into two groups according to their BMI: five normal weight (NW) women with a BMI between 18.5 and 25.0 kg/m2 and eight obese women (OB) with BMIs of 30.0 kg/m2 and higher.
2.2. Stimulation protocol
All the patients underwent controlled multifollicular ovarian stimulation for assisted reproductive therapy with gonadotropins and GnRH antagonist (Cetrorelix, EMD Serono, USA). The gonadotropin injections were initiated on days 2–5 of the cycle, and Cetrorelix was injected when the follicles reach 12–14 mm in diameter. Ultrasound monitoring was performed during ovarian stimulation. The initial gonadotropin dose was determined in accordance with the ovarian reserve of each woman (based on antral follicles number, follicular stimulating hormone and anti-Mullerian hormone levels) and previous treatment experience. Human chorionic gonadotropin (hCG – Pregnyl®, Organon, Netherlands) was administered as the ovulation trigger when at least three follicles reached 18 mm in diameter. Transvaginal puncture was performed 36 h after hCG injection under ultrasound control.
The zygotes and blastocytes were cultured in cleavage medium (COOK Medical, Australia) until day 3 after fertilization, and in the blastocyst medium until day 5 after fertilization (COOK Medical, Australia), respectively. The embryos development was evaluated at the day 5 blastocyst stage. Day 5 embryos were graded by expansion of blastocyst cavity, number of cells and density of inner cell mass and number of trophectoderm cells using the Istanbul embryo grading criteria [28]. Embryo transfer (ET) was performed under ultrasound guidance on day 5 after fertilization. A serum β-hCG pregnancy test was carried out on the 14th day after ET.
2.3. Cumulus cells
The study comprised of 82 samples of cumulus cells derived from 13 randomly selected patients. Cumulus cells were mechanically separated from the cumulus oocyte complex within 2 h after oocyte aspiration and were cultured on Petri dishes adapted to Microscopy (Ibidi, Germany) in culture medium DMEM/F12 (PanEco, Russia) supplemented with penicillin/streptomycin (Life Technologies, USA) and 10% fetal bovine serum in a CO2 incubator (+ 37 °C, 5% CO2, 95% humidity). Cells remained to attach and analysed before day 5 after transvaginal puncture when ET was performed. Living adherent cells were stained with fluorescent dye MitoTracker Green FM (20 nM, Life Technologies, USA) to localize mitochondria regardless of mitochondrial membrane potential, Tetramethylrhodamine (TMRM, 20 nM, Life Technologies, USA) to identify active mitochondria, Hoechst 33,342 (5 μg/mL, Life Technologies, USA) to visualise the cell nuclei. Images were acquired immediately after staining using a Zeiss fluorescent microscope (Carl Zeiss, Germany) with 63 × oil objective lens; 8–10 view fields were randomly selected. The acquired images were processed and analysed using the ImageJ (NIH) open source software. For each image, the ratio of green and red fluorescence intensity per single cell was calculated and considered as the mitochondrial potential index (MPI). Approximately 100 ± 10 cumulus cells were analysed for each retrieved oocyte.
2.4. Glutathione and adipokines measurement
Fasting venous blood samples were collected in potassium-EDTA tubes (Sarstedt, Germany). Blood sampling was performed between 9 a.m. and 12 p.m. immediately before transvaginal puncture; therefore, in each individual case the precise time of blood sampling was dependent on the time of the procedure. Two aliquots of whole blood were collected and stored at − 80 °C for glutathione measurement (GSH). The remaining blood was centrifuged at 2880g for 10 min at + 4 °C (Eppendorf 5810R, Germany), and plasma was collected and stored at − 80 °C until subsequent analysis was performed.
Plasma concentrations of adipokines (leptin and adiponektin) were measured using special kits for the multiplex analyzer Luminex 200 (Luminex Corporation, USA). The measurement procedure was carried out in accordance with the manufacturer's instructions.
Glutathione was measured in whole blood samples by spectrometry. A GSH recycling method was implemented as described by Giustarini et al. [29]. The method is based on glutathione disulfide (GSSG) conversion to GSH by glutathione reductase (Roche, USA) in the presence of NADPH. GSH reacts with DTNB ((5,5′-dithiobis-(2-nitrobenzoic acid), Thermo Scientific™, USA) with chromophore formation, which can be detected at 412 nm. This method estimates the sample concentration of both forms of glutathione – total glutathione concentration (tGSH = GSH + GSSG). For each analysed sample, the total glutathione concentration was normalized to the haemoglobin level and expressed as μmol/1 mg of haemoglobin.
2.5. Statistical analysis
Statistical analysis was performed using SPSS Statistics 20.0 and Statistica 7.0. An independent t-test was used to compare the means between two unrelated groups (normal weight and obese women or mature and immature oocytes) on the same continuous dependent variable. ANOVA was used to compare three unrelated groups (embryo quality groups). For comparing three distribution-free samples (embryo quality group and MPI in each cumulus cells sample), a nonparametric Kruskal-Wallis test and Mann-Whitney U test were used for two groups. The dependent variables were evaluated using the correlation coefficient. Correlation analysis was performed using Pearson's parametric test for normal data distribution. To investigate whether distributions of categorical variables differ from one another between two or more groups, we used a chi-square (χ2) test by construction of contingency tables.
3. Results
The average age was 29.8 ± 3.0 years old in the normal weight (NW) group and 33.9 ± 3.6 years in the obese (OB) group (t-test, t = − 2.1, P = 0.06). The mean BMI in the normal weight group was 22.5 ± 1.7 kg/m2 and 33.0 ± 3.7 in the obese women group (P < 0.001).
After ovarian stimulation, 35 oocytes were retrieved in the NW group and 52 oocytes in the OB group. There was no significant difference in the number of retrieved immature and mature oocytes between the two studied groups (P = 0.537). From the oocytes which were selected for fertilization and following incubation for 5 days before ET, 25 embryos were obtained in the NW women and 34 in the OB group (Table 1). It should be mentioned that the number of good quality embryos obtained was significantly higher in the NW than in OB group (P = 0.013, Table 1.).
Table 1.
Embryo quality on day 5 after fertilization.
| Embryo quality | Number of embryos |
|
|---|---|---|
| NW group | OB group | |
| Degenerated | 8 | 18 |
| Good | 14⁎ | 6⁎ |
| Fair | 3 | 4 |
| Poor | 0 | 6 |
| Embryo number | 25 | 34 |
P < 0.05.
82 samples of cumulus cells were available for MPI analysis (32 samples from NW and 50 from OB women, several oocytes for each woman). Cumulus cells for mature oocytes only (minimum 3 and maximum 11 oocytes per patient) have been collected and analysed. The quality and number of oocytes retrieved after hormonal stimulation varies from patient to patient, and within the patient. MPI levels in cumulus cells were determined based on fluorescent images analysis. A representative image of double-stained mitochondria in cumulus cells is shown in Fig. 1. We did not observe significant difference of MPI among cumulus cells from oocytes in different maturation states (P = 0.511) as well as for blastocyst embryos of different quality (P = 0.542). Taking into account the tendency towards an elder average age in the OB group, correlation analysis was used to eliminate the influence of this aspect on MPI. No significant correlation was revealed between age and MPI in cumulus cells within the studied groups (ρ = − 0.142, P = 0.214).
Fig. 1.
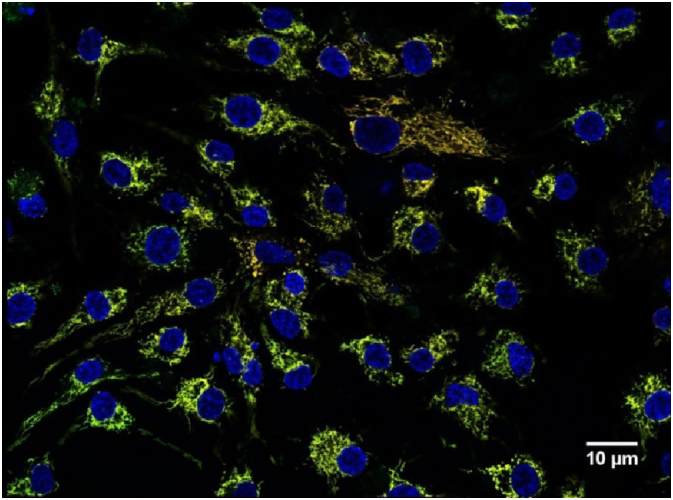
Representative fluorescent image of patient cumulus cells. Cells were stained to localize mitochondria regardless of mitochondrial membrane potential (MitoTracker, green), active mitochondria (TMRM, red) and nuclei (Hoechst, blue). Scale bar = 10 μm.
The MPI values were significantly higher in cumulus cells obtained from NW women in comparison with those from OB women (mean value 0.87 ± 0.16 AU and 0.78 ± 0.15 AU correspondingly, P = 0.017, Fig. 2). Our study shown a significant negative correlation between BMI value and mitochondrial transmembrane potential index in cumulus cells (r = − 0.30, P = 0.005; Fig. 3).
Fig. 2.
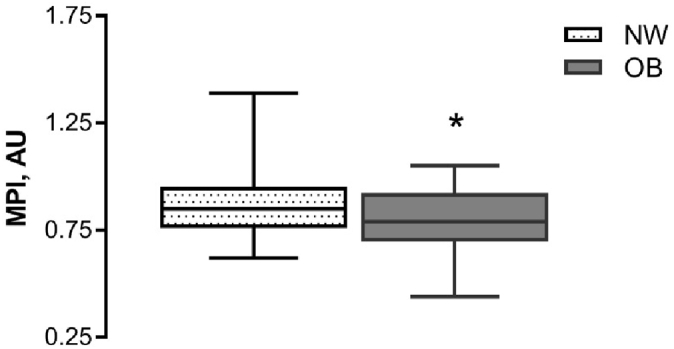
Mitochondrial potential index (MPI) in cumulus cells retrieved from women with normal body weight (NW, n = 32) and obese women (OB, n = 50). Cumulus cells for mature oocytes only have been collected and analysed. Cells were double-stained to localize all the mitochondria regardless of membrane potential and active mitochondria. The MPI was calculated based on fluorescence signal ratio. Boxes represent the 75th percentile, median (0.85 AU in NW, 0.79 AU in OB), 25th percentile, and whiskers represent maximum and minimum values; * P < 0.05.
Fig. 3.
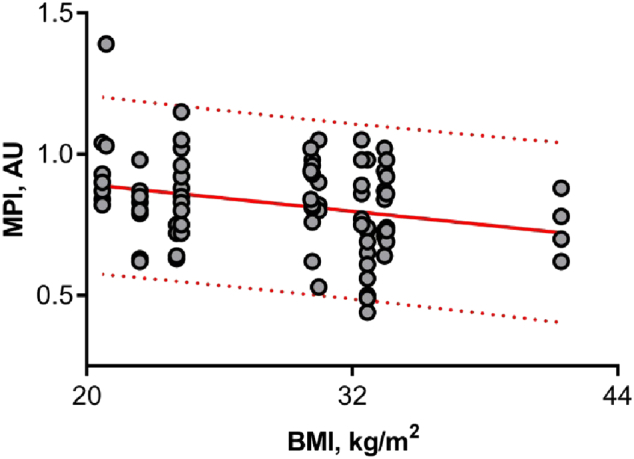
Regression of mitochondrial potential index (MPI) in cumulus cells with body mass index (BMI). Cumulus cells for mature oocytes only have been collected and analysed. Cells were double-stained to localize all the mitochondria regardless of membrane potential and active mitochondria. The MPI was calculated based on fluorescence signal ratio, n = 82. The MPI value in cumulus cells was negatively correlated with the BMI (r = − 0.30, P = 0.005); 95% prediction bands are shown in the red dotted line.
Embryo transfer was performed for all the participants (5 normal weight women and 8 obese women) on day 5 after fertilization. In NW group 9 embryos were transferred and 4 gestational sacs were detected on ultrasound 3 weeks after ET (implantation rate is 44.4%). In OB group 14 embryos were transferred and 3 gestational sacs were detected on ultrasound (implantation rate is 21.4%). Unfortunately, we had no possibility to link each embryo used for ET with corresponding cumulus cells to correlate MPI with pregnancy outcome because of two embryos transfer routinely used in our department and small number of the participants. The best quality embryos for ET were chosen only by morphological criteria.
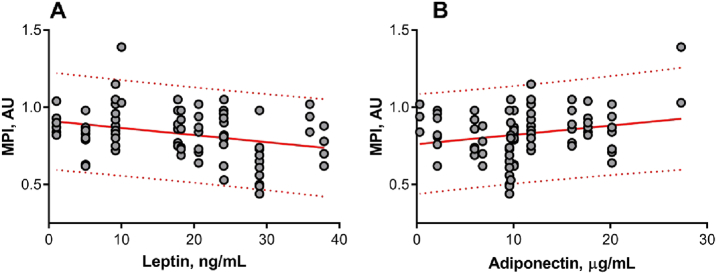
The serum concentrations of leptin, adiponectin and level of nonenzymatic antioxidant tGSH were measured. The data are summarized in Table 2. Since blood samples were available only from four patients in the NW group we were not able to compare two groups. However, our data allowed analysing whether there was any association between mitochondrial potential index in cumulus cells and adipokines serum levels on the day of oocyte retrieval for all participants. Correlation analysis revealed a negative association between cumulus cell MPI and leptin serum levels (r = − 0.36, P = 0.001; Fig. 4A). Also, positive correlations between mitochondrial index and adiponectin serum levels have been observed (Pearson test, r = 0.27, P = 0.014; Fig. 4B). A positive correlation was also observed between MPI and glutathione (Pearson test, r = 0.29, P = 0.008; Fig. 5).
Table 2.
Serum levels of adipokines and glutathione.
| NW (n = 4) |
OB (n = 8) |
|
|---|---|---|
| Leptin, ng/mL | 6.3 ± 2.1 | 26.0 ± 2.7 |
| Adiponectin, μg/mL | 16.7 ± 3.9 | 8.8 ± 2.4 |
| tGSH, μmol/L | 835 ± 59 | 594 ± 122 |
Fig. 4.
Regression of mitochondrial potential index (MPI) in cumulus cells with serum levels of (A) leptin and (B) adiponectin. Cumulus cells for mature oocytes only have been collected and analysed. Cells were double-stained to localize all the mitochondria regardless of membrane potential and active mitochondria. The MPI was calculated based on fluorescence signal ratio, n = 82. The MPI in cumulus cells negatively correlated with leptin levels (r = − 0.36, P = 0.001) and positively correlated with adiponectin levels (r = 0.27, P = 0.014); 95% prediction bands are shown in the red dotted line.
Fig. 5.
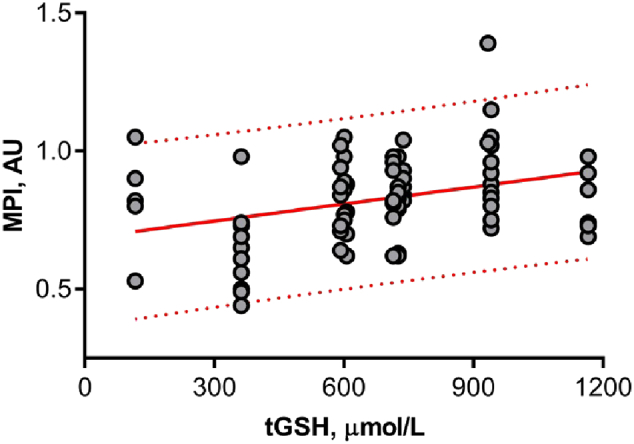
Regression of mitochondrial potential index (MPI) in cumulus cells with serum levels of glutathione (tGSH). Cumulus cells for mature oocytes only have been collected and analysed. Cells were double-stained to localize all mitochondria regardless of membrane potential and active mitochondria. The MPI was calculated based on fluorescence signal ratio, n = 82. 95% prediction bands are shown in the red dotted line. The MPI showed a positive correlation with glutathione levels (r = 0.29, P = 0.008).
4. Discussion
It is widely accepted that bi-directional communication exists between the cumulus cells and human oocyte. The developing oocyte is surrounded by cumulus cells that are connected to the oocyte by gap junctions, and this complex is indispensable for competent oocyte development. Cumulus cells provide growing oocytes with nutrients for ATP synthesis and the oocytes control metabolism of cumulus cells in accordance with its changing energy demands [30], [31]. Therefore, highly metabolically active mitochondria in cumulus cells are an essential part of this process, and any mitochondrial dysfunction may have significant consequences for oocyte growth, fertility competence and chromosomal segregation [26]. Numerous studies have reported that obesity has a negative impact on folliculo-, oo- and early embryogenesis, endometrial receptivity and pregnancy rate [32], [33]. Given the exclusively maternal origin of mitochondria, a deleterious influence of maternal BMI on mitochondria in the cumulus-oocyte complex may strongly affect embryonic metabolism [21]. We hypothesized that mitochondrial reticulum dysfunction in obesity could affect the spindle cleavage and chromosome segregation, resulting in higher aneuploidy rate and impairment of embryo developmental potential.
In the present study, MPI was analysed as an indicator of mitochondrial activity in cumulus cells retrieved from obese women and those with normal BMIs who were undergoing assisted reproductive therapy. Our results revealed a significant reduction of MPI with increase of body mass allowing to assume, that a lower MPI value in cumulus cells from obese women may be an indication of mitochondrial dysfunction caused by oxidative stress.
Excessive lipid intake with the accumulation of triglyceride droplets and intracellular free fatty acids in non-adipose tissues lead to endoplasmic reticulum stress and mitochondrial dysfunction called lipotoxicity. Possible mechanisms of tissue damage in this case include an increase of free fatty acid β-oxidation and oxidative stress, direct free fatty acid toxicity, alteration of cell membrane composition by phospholipid and triglyceride peroxidation, mitochondrial permeabilisation resulting in the activation of apoptosis cascades [34]. Furthermore, it is known that in addition to participating in β-oxidation, free fatty acids directly uncouple mitochondrial respiration reducing their membrane potential and ATP synthesis level [35]. Loss of membrane potential is reported as mitochondrial damage manifestation, which is related to oxidative phosphorylation uncoupling and increased oxygen radical generation, release of cytochrome C, activation of caspases and initiation of DNA fragmentation [35]. Thus, the decrease in mitochondrial membrane potential value in cumulus cells obtained from the obese women in this study may reflect a response to permanent cellular stress caused by lipotoxicity and a reflection of impaired mitochondrial activity.
Data about characterization of mitochondrial function assessed by determining the membrane potential are not numerous. Reduced mitochondrial membrane potential was reported in both immature and mature oocytes obtained from mice exposed to a high-fat diet and in cumulus cells from diabetic mice [36], [37]. It was also shown that mitochondrial potential resistance to stress positively correlates with the number of total and mature oocytes retrieved in an IVF programme after ovarian stimulation [26]. In vitro model experiments with cow and mice oocytes demonstrated that cumulus cells actively protect oocytes from lipotoxicity by the accumulation of free fatty acids in lipid droplets [7]. Also, significant deterioration of the cumulus cell layer and increased apoptosis was observed in the cumulus-oocyte complex exposed to increased levels of free fatty acids [7], [38].
We also analysed the association between MPI in cumulus cells and adipokine serum levels on the day of oocyte retrieval. Leptin and adiponectin were selected adipokines in this study due to their important impact on fertility at various levels including the ovary, endometrium and processes of fetus implantation in obese individuals. We observed a negative correlation between cumulus cell MPI and serum levels of leptin and a positive correlation between MPI and adiponectin concentration. It is known that obese individuals have elevated levels of circulating leptin, which is directly proportional to their BMI. Glucose and lipid metabolism are highly regulated by leptin. Chronic elevation of leptin stimulates fatty acid oxidation (FAO) and triglyceride hydrolysis thereby reducing total fatty acid uptake [39]. At the same time, an increase of FAO may cause uncoupling of the mitochondrial respiratory chain and oxidative phosphorylation, free radical generation and calcium homeostasis disorders resulting in cell damage and functional incompetence. Adiponectin stimulates mitochondrial biogenesis and reduces lipid content in human and animal adipocytes. The overexpression of adiponectin increases mitochondrial density and mitochondrial DNA content enhancing mitochondrial ATP synthesis [40]. Unlike other adipokines, the concentration of plasma adiponectin decreases in abdominal obesity. Jansson et al. reported that obese women have lower adiponectin levels throughout pregnancy compared with those of lean controls [41]. It was shown in human and mice that during pregnancy maternal adiponectin is a powerful regulator of nutrient (glucose, fatty acids and amino acids) transport in the placenta and fetal growth [42]. One of the mechanisms of adiponectin protective action is the upregulation of uncoupling protein 2 in the mitochondria, which restores cellular redox homeostasis by promoting mitochondrial respiration, attenuates fatty acid accumulation in mitochondria and reactive oxygen species production [43]. To characterize the antioxidant capacity, we measured blood levels of GSH because it is one of the most abundant non-enzymatic antioxidants, and the elevation of GSH content may provide protection from pathological conditions associated with elevated oxidative damage. Moreover, it was shown that perturbations in the system of GSH and GSH-dependent enzymes are involved in obesity and are associated with the development of obesity comorbidities [44]. Also, GSH has been shown to be important in successful recovery and developmental potential in mouse oocytes after cryopreservation and vitrification. Supplementation with GSH donor significantly improves oocyte culture conditions and decreases the risk of chromosome segregation errors, alterations in mRNA expression patterns, levels of reactive oxygen species (ROS) and increases redox capacity of oocyte mitochondria [45]. The activity of antioxidant enzymes is very high in follicular fluid including GSH-dependent enzymes. In addition, GSH is synthesized by cumulus cells and can be transferred to the oocyte if needed to ensure the effective scavenging of ROS and to protect the oocyte during its resumed metabolic and meiotic activity [46], [45]. Based on published data, we assume that the positive correlations between MPI and the levels of GSH and adiponectin observed in this study may reflect a positive impact of these regulators due to close regulatory interactions in the cumulus-oocyte complex during follicle maturation.
5. Conclusion
Results of our study support a concept that obesity leads to mitochondrial dysfunction. In cumulus cells, mitochondrial dysfunction can reflect or even significantly affect the oocyte energy supply and contribute to the reduced oocyte quality and pregnancy rates for women with obesity. A better understanding of the energetic relationships in cumulus-oocyte complex can provide a new approach for better quality oocyte selection and assist the optimization of IVF implementation.
6. Limitations of the study
Our study data should be interpreted with caution because of some limitations. First, small number of patients consent to participate in the present study. The quality and number of oocytes retrieved after hormonal stimulation varies from patient to patient, and within the patient. Second, cumulus cell cultivation can influence MPI. Our experimental design required attachment of cells to the culture dish to allow further analysis by microscopy of alive, non-fixed cells. But nevertheless cumulus cells retrieved from women with normal body weight and from obese women were processed in the same way so the results may be compared. Third, trend in elder age within the obese group even if no significant correlation was revealed between age and MPI in cumulus cells within the studied groups.
Competing interests
The authors declare that there are no competing interests. This research is not supported by grants from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Johansson S., Villamor E., Altman M., Bonamy A.K., Granath F., Cnattingius S. Maternal overweight and obesity in early pregnancy and risk of infant mortality: a population based cohort study in Sweden. BMJ. 2014;349:g6572. doi: 10.1136/bmj.g6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crane J.M., White J., Murphy P., Burrage L., Hutchens D. The effect of gestational weight gain by body mass index on maternal and neonatal outcomes. J. Obstet. Gynaecol. Can. 2009;31(1):28–35. doi: 10.1016/s1701-2163(16)34050-6. [DOI] [PubMed] [Google Scholar]
- 3.Tenenbaum-Gavish K., Hod M. Impact of maternal obesity on fetal health. Fetal Diagn. Ther. 2013;34(1):1–7. doi: 10.1159/000350170. [DOI] [PubMed] [Google Scholar]
- 4.Knight-Agarwal C.R., Williams L.T., Davis D., Davey R., Cochrane T., Zhang H., Rickwood P. Association of BMI and interpregnancy BMI change with birth outcomes in an Australian obstetric population: a retrospective cohort study. BMJ Open. 2016;6(5):e010667. doi: 10.1136/bmjopen-2015-010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gregor M.F., Hotamisligil G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 6.Aardema H., Lolicato F., van de Lest C.H., Brouwers J.F., Vaandrager A.B., van Tol H.T., Roelen B.A., Vos P.L., Helms J.B., Gadella B.M. Bovine cumulus cells protect maturing oocytes from increased fatty acid levels by massive intracellular lipid storage. Biol. Reprod. 2013;88(6):164. doi: 10.1095/biolreprod.112.106062. [DOI] [PubMed] [Google Scholar]
- 7.Lolicato F., Brouwers J.F., de Lest C.H., Wubbolts R., Aardema H., Priore P., Roelen B.A., Helms J.B., Gadella B.M. The cumulus cell layer protects the bovine maturing oocyte against fatty acid-induced lipotoxicity. Biol. Reprod. 2015;92(1):16. doi: 10.1095/biolreprod.114.120634. [DOI] [PubMed] [Google Scholar]
- 8.Skulachev V., Bogachev A., Kasparinskiy F. Moscow State University Press; Moscow: 2010. Membrane Bioenergetics; p. 367. [Google Scholar]
- 9.Van Blerkom J. Mitochondria in human oogenesis and preimplantation embryogenesis: engines of metabolism, ionic regulation and developmental competence. Reproduction. 2004;128(3):269–280. doi: 10.1530/rep.1.00240. [DOI] [PubMed] [Google Scholar]
- 10.Van Blerkom J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion. 2011;11(5):797–813. doi: 10.1016/j.mito.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Grindler N.M., Moley K.H. Maternal obesity, infertility and mitochondrial dysfunction: potential mechanisms emerging from mouse model systems. Mol. Hum. Reprod. 2013;19(8):486–494. doi: 10.1093/molehr/gat026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chappel S. The role of mitochondria from mature oocyte to viable blastocyst. Obstet. Gynecol. Int. 2013;2013:183024. doi: 10.1155/2013/183024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schatten H., Sun Q.Y., Prather R. The impact of mitochondrial function/dysfunction on IVF and new treatment possibilities for infertility. Reprod. Biol. Endocrinol. 2014;12:111. doi: 10.1186/1477-7827-12-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fragouli E., Wells D. Mitochondrial DNA assessment to determine oocyte and embryo viability. Semin. Reprod. Med. 2015;33(6):401–409. doi: 10.1055/s-0035-1567821. [DOI] [PubMed] [Google Scholar]
- 15.Hartshorne G. The embryo. Hum. Reprod. 2000;15(Suppl. 4):31–41. doi: 10.1093/humrep/15.suppl_4.31. [DOI] [PubMed] [Google Scholar]
- 16.Ao A., Erickson R.P., Winston R.M., Handyside A.H. Transcription of paternal Y-linked genes in the human zygote as early as the pronucleate stage. Zygote. 1994;2(4):281–287. doi: 10.1017/s0967199400002100. [DOI] [PubMed] [Google Scholar]
- 17.Nicholls D.G. Mitochondrial membrane potential and aging. Aging Cell. 2004;3(1):35–40. doi: 10.1111/j.1474-9728.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- 18.Wilding M., Dale B., Marino M., di Matteo L., Alviggi C., Pisaturo M.L., Lombardi L., De Placido G. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum. Reprod. 2001;16(5):909–917. doi: 10.1093/humrep/16.5.909. [DOI] [PubMed] [Google Scholar]
- 19.Ou X.H., Li S., Wang Z.B., Li M., Quan S., Xing F., Guo L., Chao S.B., Chen Z., Liang X.W., Hou Y., Schatten H., Sun Q.Y. Maternal insulin resistance causes oxidative stress and mitochondrial dysfunction in mouse oocytes. Hum. Reprod. 2012;27(7):2130–2145. doi: 10.1093/humrep/des137. [DOI] [PubMed] [Google Scholar]
- 20.Zhang C.H., Qian W.P., Qi S.T., Ge Z.J., Min L.J., Zhu X.L., Huang X., Liu J.P., Ouyang Y.C., Hou Y., Schatten H., Sun Q.Y. Maternal diabetes causes abnormal dynamic changes of endoplasmic reticulum during mouse oocyte maturation and early embryo development. Reprod. Biol. Endocrinol. 2013;11:31. doi: 10.1186/1477-7827-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Igosheva N., Abramov A.Y., Poston L., Eckert J.J., Fleming T.P., Duchen M.R., McConnell J. Maternal diet induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS One. 2010;5(4):e10074. doi: 10.1371/journal.pone.0010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jungheim E.S., Macones G.A., Odem R.R., Patterson B.W., Lanzendorf S.E., Ratts V.S., Moley K.H. Associations between free fatty acids, cumulus oocyte complex morphology and ovarian function during in vitro fertilization. Fertil. Steril. 2011;95(6):1970–1974. doi: 10.1016/j.fertnstert.2011.01.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mori M.A., Liu M., Bezy O., Almind K., Shapiro H., Kasif S., Kahn C.R. A systems biology approach identifies inflammatory abnormalities between mouse strains prior to development of metabolic disease. Diabetes. 2010;59(11):2960–2971. doi: 10.2337/db10-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dumesic D.A., Meldrum D.R., Katz-Jaffe M.G., Krisher R.L., Schoolcraft W.B. Oocyte environment: follicular fluid and cumulus cells are critical for oocyte health. Fertil. Steril. 2015;103(2):303–316. doi: 10.1016/j.fertnstert.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 25.Simerman A.A., Hill D.L., Grogan T.R., Elashoff D., Clarke N.J., Goldstein E.H., Manrriquez A.N., Chazenbalk G.D., Dumesic D.A. Intrafollicular cortisol levels inversely correlate with cumulus cell lipid content as a possible energy source during oocyte meiotic resumption in women undergoing ovarian stimulation for in vitro fertilization. Fertil. Steril. 2015;103(1):249–257. doi: 10.1016/j.fertnstert.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dumesic D.A., Guedikian A.A., Madrigal V.K., Phan J.D., Hill D.L., Alvarez J.P., Chazenbalk G.D. Cumulus cell mitochondrial resistance to stress in vitro predicts oocyte development during assisted reproduction. J. Clin. Endocrinol. Metab. 2016;101(5):2235–2245. doi: 10.1210/jc.2016-1464. [DOI] [PubMed] [Google Scholar]
- 27.WHO Media center fact sheet: obesity and overweight [digital source] 2015. http://www.who.int/mediacentre/factsheets/fs311/en/ – fact sheet №311. – access mode:
- 28.The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of EmbryologyHum. Reprod. 2011;26(6):1270–1283. doi: 10.1093/humrep/der037. [DOI] [PubMed] [Google Scholar]
- 29.Giustarini D., Dalle-Donne I., Milzani A., Fanti P., Rossi R. Analysis of GSH and GSSG after derivatization with N-ethylmaleimide. Nat. Protoc. 2013;8(9):1660–1669. doi: 10.1038/nprot.2013.095. [DOI] [PubMed] [Google Scholar]
- 30.Gilchrist R.B., Lane M., Thompson J.G. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum. Reprod. Update. 2008;14(2):159–177. doi: 10.1093/humupd/dmm040. [DOI] [PubMed] [Google Scholar]
- 31.Su Y.Q., Sugiura K., Eppig J.J. Mouse oocyte control of granulosa cell development and function: paracrine regulation of cumulus cell metabolism. Semin. Reprod. Med. 2009;27(1):32–42. doi: 10.1055/s-0028-1108008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rittenberg V., Sobaleva S., Ahmad A., Oteng-Ntim E., Bolton V., Khalaf Y., Braude P., El-Toukhy T. Influence of BMI on risk of miscarriage after single blastocyst transfer. Hum. Reprod. 2011;26(10):2642–2650. doi: 10.1093/humrep/der254. [DOI] [PubMed] [Google Scholar]
- 33.Moragianni V.A., Jones S.M., Ryley D.A. The effect of body mass index on the outcomes of first assisted reproductive technology cycles. Fertil. Steril. 2012;98(1):102–108. doi: 10.1016/j.fertnstert.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Hauck A.K., Bernlohr D.A. Oxidative stress and lipotoxicity. J. Lipid Res. 2016;57(11):1976–1986. doi: 10.1194/jlr.R066597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maassen J.A., Romijn J.A., Heine R.J. Fatty acid-induced mitochondrial uncoupling in adipocytes as a key protective factor against insulin resistance and beta cell dysfunction: a new concept in the pathogenesis of obesity-associated type 2 diabetes mellitus. Diabetologia. 2007;50(10):2036–2041. doi: 10.1007/s00125-007-0776-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu L.L., Dunning K.R., Yang X., Russell D.L., Lane M., Norman R.J., Robker R.L. High-fat diet causes lipotoxicity responses in cumulus-oocyte complexes and decreased fertilization rates. Endocrinology. 2010;151(11):5438–5445. doi: 10.1210/en.2010-0551. [DOI] [PubMed] [Google Scholar]
- 37.Wang Q., Frolova A.I., Purcell S., Adastra K., Schoeller E., Chi M.M., Schedl T., Moley K.H. Mitochondrial dysfunction and apoptosis in cumulus cells of type I diabetic mice. PLoS One. 2010;5(12):e15901. doi: 10.1371/journal.pone.0015901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang X., Wu L.L., Chura L.R., Liang X., Lane M., Norman R.J., Robker R.L. Exposure to lipid-rich follicular fluid is associated with endoplasmic reticulum stress and impaired oocyte maturation in cumulus-oocyte complexes. Fertil. Steril. 2012;97(6):1438–1443. doi: 10.1016/j.fertnstert.2012.02.034. [DOI] [PubMed] [Google Scholar]
- 39.Gan L., Liu Z., Cao W., Zhang Z., Sun C. FABP4 reversed the regulation of leptin on mitochondrial fatty acid oxidation in mice adipocytes. Sci. Rep. 2015;5:13588. doi: 10.1038/srep13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gan L., Yan J., Liu Z., Feng M., Sun C. Adiponectin prevents reduction of lipid-induced mitochondrial biogenesis via AMPK/ACC2 pathway in chicken adipocyte. J. Cell. Biochem. 2015;116(6):1090–1100. doi: 10.1002/jcb.25064. [DOI] [PubMed] [Google Scholar]
- 41.Jansson N., Nilsfelt A., Gellerstedt M., Wennergren M., Rossander-Hulthén L., Powell T.L., Jansson T. Maternal hormones linking maternal body mass index and dietary intake to birth weight. Am. J. Clin. Nutr. 2008;87(6):1743–1749. doi: 10.1093/ajcn/87.6.1743. [DOI] [PubMed] [Google Scholar]
- 42.Howell K.R., Powell T.L. Effects of maternal obesity on placental function and fetal development. Reproduction. 2017;153(3):R97–R108. doi: 10.1530/REP-16-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou M., Xu A., Tam P.K., Lam K.S., Huang B., Liang Y., Lee I.K., Wu D., Wang Y. Upregulation of UCP2 by adiponectin: the involvement of mitochondrial superoxide and hnRNP K. PLoS One. 2012;7(2):e32349. doi: 10.1371/journal.pone.0032349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Picklo M.J., Long E.K., Vomhof-DeKrey E.E. Glutathionyl systems and metabolic dysfunction in obesity. Nutr. Rev. 2015;73(12):858–868. doi: 10.1093/nutrit/nuv042. [DOI] [PubMed] [Google Scholar]
- 45.Trapphoff T., Heiligentag M., Simon J., Staubach N., Seidel T., Otte K., Fröhlich T., Arnold G.J., Eichenlaub-Ritter U. Improved cryotolerance and developmental potential of in vitro and in vivo matured mouse oocytes by supplementing with a glutathione donor prior to vitrification. Mol. Hum. Reprod. 2016;22(12):867–881. doi: 10.1093/molehr/gaw059. [DOI] [PubMed] [Google Scholar]
- 46.Carbone M.C., Tatone C., Delle Monache S., Marci R., Caserta D., Colonna R., Amicarelli F. Antioxidant enzymatic defences in human follicular fluid: characterization and age-dependent changes. Mol. Hum. Reprod. 2003;9(11):639–643. doi: 10.1093/molehr/gag090. [DOI] [PubMed] [Google Scholar]