Abstract
Background
Ashwagandharishtha is a liquid polyherbal formulation traditionally prepared by fermentation process using the flowers of Woodfordia fruticosa. It contains roots of Withania somnifera as a major crude drug. Alcohol generated during the fermentation causes the extraction of water insoluble phytoconstituents. Yeasts present on the flowers are responsible for this fermentation.
Methods
Total nine formulations of ashwagandharishtha were prepared by fermentation process using traditional Woodfordia fruticosa flowers (ASG-WFS) and using yeasts isolated from the same flowers. During fermentation, kinetic of alcohol generation, sugar consumption, changes in pH and withanolides extraction were studied. All the formulations were tested for in vitro antioxidant potential by 1,1-diphenyl-2-picryl-hydrazyl (DPPH) free radical scavenging, hydrogen peroxide scavenging and total reducing power assay. The results were compared with standard ascorbic acid.
Results
Traditional formulation (ASG-WFS) showed the highest activity (p < 0.001) relative to other formulations and standard ascorbic acid. ASG-WFS showed significant (DPPH) free radical scavenging (78.75%) and hydrogen peroxide scavenging (69.62%) at the concentration of 1000 μg/mL and 100 μg/mL, respectively.
Conclusion
Traditional process is the best process for preparing ashwagandharishtha to obtain significant antioxidant activity.
Keywords: Alcohol, withaferine A, Withanolide A, Antioxidant potential
1. Introduction
Herbal formulations belonging to the category of asavas and arishthas are prepared by traditional fermentation process using the flowers of Woodfordia fruticosa. These flowers are added as inoculums for the induction and maintenance of fermentation process.1 Alcohol generated during the process causes the extraction of water insoluble active principles of crude drugs. This process uses the native yeast Saccharomyces cerevisiae present on the flowers.2 Ashwagandharishtha is one of the herbal formulation which is prepared by traditional fermentation process containing 8-12%v/v of self generated alcohol. It contains roots of Withania somnifera (ashwagandha) as a major crude drug along with 26 other drugs in minor quantities.1 Roots exhibit various pharmacological effects like impotency, antiaging, cardio protective and antioxidant effect.3, 4, 5 These activities are attributed towards the presence of withanolides like withaferine A and withanolide A.6, 7 These withanolides contains steroidal nucleus and are soluble in non-aqueous solvents like ethanol, methanol and in some organic solvents but insoluble in water. Due to their solubility nature, they get extracted from the roots to formulation by generated alcohol.
Traditional method has certain drawbacks like longer period (30-45 day) for the completion of fermentation process, and production of toxic non-ethanol volatile compounds due to presence of other microorganisms on the flowers.8 Thus, there is a definite need of suitable process for preparing of ashwagandharishtha that overcomes above problems. In this work, various formulations of ashwagandharishtha were prepared by traditional fermentation process using the flowers of W. fruticosa and by non-traditional fermentation process using the yeasts isolated from the same flowers. During the fermentation process, kinetic of alcohol generation, consumption of sugar, changes in pH and withanolides extraction were studied. Antioxidant molecules are capable of reducing oxidative stress of free radicals by inhibiting the oxidation of other molecules.9 Antioxidant activity of ashwagandharishtha is well documented.10 Assessment of antioxidant activity of formulations will explain at least in part how preparation method affects antioxidant activity. Its results will also be useful to correlate potency with method of preparation. This study will provide a means to standardize and optimize the herbal formulations like ashwagandharishtha. Data of standardization formulation in terms of active phytoconstituents and non-ethanol impurities will provide useful information about the quality of formulations prepared by both fermentation processes. Minor ingredients of the formulations like arjuna, musali and manjistha also contribute the antioxidant activity.11, 12, 13 Therefore, to avoid there interference in the study, all minor ingredients of formulation were omitted. All the formulations were tested for in vitro antioxidant potential. Jaggery sugar was used as a substrate for fermentation.
2. Methods
2.1. Material
Plant material of W. Somnifera was collected from Green Pharmacy, Pune, and dried flowers of the W. fruticosa were collected from the different regions of Solapur district (Maharashtra). Both the plant materials were authenticated from Agharkar Research Institute, Pune. Standard culture of alcohol producing yeast Saccharomyces cerevisiae SC1011 was collected from Vasantdada Sugar Institute, Pune. Pure drug sample of withaferine A and withanolide A were procured from Natural Remedies Pvt Ltd., Bangalore, India. Standards of l- ascorbic acid (catalogue no. 605999), stable free radical 1,1-diphenyl-2-picryl-hydrazyl (catalogue no. D9132), and trichloroacetic acid (catalogue no. T6399) were obtained from Sigma Aldrich, St. Louis, USA. All other chemicals used were analytical grade and obtained from Merck, US. The instruments used in work were autoclave (Lab Hosp, India), BOD Incubator (REMI instruments, Mumbai, India), Shaker incubator (model-HRIS, Steelmet Novatech, India), Centrifuge machine (REMI laboratories, Mumbai, India), Rotary vacuum evaporator (Model Laborota 4000, Heidolph, Washington, USA) and UV-Vis spectrophotometer (Shimadzu UV-1601, Hong Kong, China).
2.2. Source of strains
Four yeasts strains i.e. S. cerevisiae Jm.20, Saccharomycopsis fibuligera Jm.8, S. fibuligera Jm.10 and S. fibuligera Jm.16 were isolated from the flowers of W. fruticosa in our previous work. All the strains were maintained on MGYP agar (3 g malt extract, 10 g glucose, 3 g yeast extract, 5 g peptone and 20 g agar per liter of water, pH 4.5) slants and preserved for routine use at 4 °C.
2.3. Preparation of inoculums
The inoculums for all the strains including standard strain were prepared separately by transferring one loopful of yeast culture into 100 mL of sterile 12°brix jaggery medium containing 0.01%w/v of diamino phosphate and 0.01%w/v of urea. For mixed inoculums, 1 loopful of each of four isolated strains was used. The inoculums of S. cerevisiae Jm.20 and S. cerevisiae SC1011 were incubated at 32.5 °C for 24 h, whereas those of three strains of S. fibuligera were incubated at 32.5 °C for 48 h in shaker incubator at 180 rpm. After incubation, cell contents (cell/ml) of S. cerevisae jm.20 and S. cerevisae SC1011 were measured using neubauer counting chamber as these cells were free and countable.
2.4. Preparation of formulations by traditional process
About 50 g of powder of dried roots of W. somnifera was taken in 2L distilled water in a 5L capacity stainless steel pot. The material was allowed to soak overnight. The decoction was concentrated to 250 mL by boiling the contents for 1-2 h. While hot, about 250 g of jaggery (sugar) was added slowly with stirring. After the dissolution of jaggery, the contents were taken to 500 mL measuring cylinder. Final volume of media was made up to the mark with distilled water. The contents were then transferred to 1L conical flask, plugged with cotton and sterilized by autoclaving at 121 °C for 20 min at 15 lbs pressure. After sterilization, flask was cooled to room temperature and then inoculated aseptically with the 16 g of W. fruticosa flowers. The flask was then incubated for fermentation in BOD incubator at 32.5 °C till the fermentation process is stopped. This traditional formulation was named as ASG-WFS.
2.5. Preparation of blank formulations by traditional process
Traditional blank formulation was prepared by dissolving 250 g of jaggery in hot water. After dissolution, the media was transferred to 500 mL measuring cylinder and final volume was made up to the mark with distilled water. The contents were then transferred to 1 L conical flask, plugged with cotton and then sterilized by autoclaving at 121 °C for 20 min at 15 lbs pressure. After sterilization, flask was cooled to room temperature and inoculated aseptically with the flowers of W. fruticosa (16 g). The flask was incubated for fermentation at 32.5 °C in BOD incubator till the fermentation process is stopped. The formulation was named as ASG-WFB.
2.6. Preparation of formulations by non-traditional process
The decoctions of roots of W. sonifera were prepared in the similar manner as described above. After dissolution of jaggery, the contents were then taken to 500 mL measuring cylinder, and final volume of media was made up to 450 mL with distilled water. The contents were transferred to 1 L conical flask, plugged, and then sterilized by autoclaving at 121 °C for 20 min at 15 lbs pressure. After sterilization, flask was cooled to room temperature and then inoculated with 50 mL of yeasts inoculums. Total six formulations were prepared in the similar manner in different 1L conical flasks with different inoculums. These formulations were made using the inoculums of (i) S. cerevisiae Jm.20 {cell content- 4.2 x 108cells/mL}, (ii) S. fibuligera Jm.8, (iii) S. fibuligera Jm.10, (iv) S. fibuligera Jm.16,(v) mixture of isolated strains ‘i’ to ‘iv’, and (vi) standard strain S. cerevisiae SC1011{cell content- 5.1 x 108 cells/mL}.
Formulations made using the strain S. cerevisiae Jm.20, S. fibuligera Jm.8, S. fibuligera Jm.10, S. fibuligera Jm.16 and mixture of isolated were named as ASG-20, ASG-8, ASG-10, ASG-16 and ASG-Mix, respectively; whereas, formulations made using the standard strain S. cerevisiae SC1011 was named as ASG-SC1011. All the formulations except ASG-SC1011 were incubated for fermentation in BOD incubator at 32.5 °C till the fermentation process is stopped. To study the effect of time period on the extraction of withanolides, formulation ASG-SC1011 was kept for 720 h (1 month).
2.7. Preparation of formulations by non-traditional process at optimum conditions
One formulation was prepared using S. cerevisiae Jm.20 at optimum fermentation conditions. The conditions selected were jaggery (40%w/v), and inoculums 8%v/v and temperature 30 °C.14 The formulation was named as ASG-20z.
2.8. Analysis of formulations
At the regular intervals, 15 mL of fermentation broths was withdrawn from each formulation, centrifuged and the supernatant liquid was used for the analysis. Analysis of ASG-WFS, ASG-WFB, ASG-8, ASG-10 and ASG-16 were carried out at the interval of 72 h whereas, analysis of remaining formulations were carried out at the interval 24 h. Kinetics of fermentation was studied by determining the relationship between alcohol generated, residual sugar consumed and amount of withanolides (withaferine A, withanolide A) extracted. Amount of withanolides were determined by HPLC method.15 Amount of alcohol generated was determined by dichromate oxidation method and that of residual sugar was determined by titration method.15, 16, 17 pH was measured by digital pH meter. Non-ethanol volatile (NEV) compounds in preparations were determined by Gas chromatography method.18 Amount of NEV compounds in finished were compared with those of marketed formulation.
2.9. Evaluation of in vitro antioxidant potential
After the completion of fermentation process, all the formulations were centrifuged at 3000 rpm for 20 min. Upper supernatant layers were used for evaluation of in vitro antioxidant potential by following assay methods. 1-ascorbic acid (100 μg/ml) was used as standard antioxidant for the comparing the results.
-
(i)
1, 1-diphenyl-2-picryl-hydrazyl (DPPH) scavenging assay
DPPH scavenging ability of formulations (samples) were determined by the slightly modifying the procedure given by Chidambara.19 1 mL of different concentration of sample (100, 200, 400, 600 and 1000 μL/mL) were taken in different test tubes. To each tube, 5 mL of DPPH solution was added. Tubes were shaken for 2 min and immediately kept in dark at 27 °C for 20 min. After incubation, the tubes were centrifuged at 6000 rpm for 5 minutes and changes in absorbance were measured using supernatant liquid at 517 nm. Control solution was prepared and zero was set using methanol. % DPPH scavenging effects of samples were calculated from following formula:Where, Ac is the absorbance of control solution, and As is the absorbance of sample solution.
-
(ii)
Hydrogen peroxide scavenging assay
Ability of sample to scavenge hydrogen peroxide was determined using the procedure given by Ruch.20 A solution of hydrogen peroxide (60 mM) was prepared in phosphate buffer (pH 7.4). In test tubes, different concentrations of sample (10, 20, 30, 40, 60 and 100 μL/mL) in water were added to a hydrogen peroxide solution (60 mM). The tubes were incubated at room temperature for 10 min. Absorbance of hydrogen peroxide was measured at 230 nm against blank solution containing phosphate buffer solution without hydrogen peroxide. The percent hydrogen peroxide scavenging activity was calculated from the following formula:Where, Ac is the absorbance of control solution, and As is the absorbance of sample solution.
-
(iii)
Total reducing power assay
The reducing capability of various samples were measured by the transformation of Fe3+ to Fe2+ as per the procedure reported by Oyaizu.21 The different concentration of sample (10, 20, 30, 40, 60 and 100 μL/mL) were mixed with phosphate buffer (2.5 mL, 0.2 M, pH 6.6) and potassium ferricyanide (2.5 mL, 1%). The mixture was incubated at 50 °C for 20 min by adding 10% trichloroacetic acid (2.5 mL). The mixture was then centrifuged and upper supernatant layer of solution (2.5 mL) was mixed with distilled water (2.5 mL) and FeCl3 (0.5 mL, 0.1%), and absorbance was measured at 700 nm. The increased absorbance of the reaction mixture indicated increased reducing power.
3. Results
3.1. Statistical analysis of data
The data of results are expressed as mean ± standard error of the mean (SEM). The statistical analysis of results was carried out using software Graph pad (Instat and Prism 5.0 Demo version). One way ANOVA followed by Dunnett's multiple comparison tests was done for in vitro antioxidant tests. p < 0.05 was considered to be significant.
3.2. Preparation of formulations
Total nine formulations of ashwagandharishtha were prepared by traditional and non-traditional fermentation process. Traditional process involved the use of W. fruticosa flowers whereas, non-traditional formulation involved the use of yeasts isolated from the same flowers. One formulation was prepared by using inoculums of standard strain yeast S. cerevisiae SC1011.
3.3. Kinetic of fermentation process
All the formulations were yellowish in colour and turbid throughout the fermentation process. Kinetic study of fermentation showed that alcohol generation in all formulations was proportionally increased with the consumption of residual sugar (substrate). Stopping of fermentation was indicated stopping of alcohol generation and no further consumption of residual sugar. Formulations i.e. ASG-20, ASG-Mix, ASG-20z, ASG-SC1011, ASG-WFS and ASG-WFB which were prepared using the strain of S. cerevisiae, mixture of yeasts, and using flowers of W. fruticosa have produced the alcohol in the recommended range 8-12%v/v (63-95 g/L) (Table 1). Thus, the strain S. cerevisiae is essential for the alcohol production in the required range. Whereas, all those formulations which were prepared using the strains of S. fibuligera did not produced alcohol in the recommended range.
Table 1.
Results of kinetic study with respect to hours.
| Preparation | Alcohol production (g/L) | Substrate consumption (%w/v) | Withaferine A (ng/μL) | Withanolide A (ng/μL) | ||||
|---|---|---|---|---|---|---|---|---|
| Str. Line equation | R2 | Str. Line equation | R2 | Str. Line equation | R2 | Str. Line equation | R2 | |
| ASG-WFS | y = 0.118x + 8.288 | 0.976 | y = -0.031x + 36.19 | 0.974 | y = 0.038x + 5.518 | 0.987 | y = 0.027x + 4.742 | 0.978 |
| ASG-8 | y = 0.074x + 3.136 | 0.913 | y = -0.019x + 33.08 | 0.941 | y = 0.024x + 5.474 | 0.968 | y = 0.022x + 5.134 | 0.982 |
| ASG-10 | y = 0.076x + 0.645 | 0.924 | y = -0.021x + 26.28 | 0.782 | y = 0.015x + 6.459 | 0.964 | y = 0.015x + 6.320 | 0.968 |
| ASG-16 | y = 0.098x + 0.088 | 0.992 | y = -0.031x + 2.67 | 0.997 | y = 0.016x + 6.582 | 0.966 | y = 0.017x + 5.467 | 0.947 |
| ASG-20 | y = 0.764x + 6.32 | 0.955 | y = -0.267x + 26.80 | 0.956 | y = 0.079x + 5.924 | 0.983 | y = 0.091x + 5.037 | 0.973 |
| ASG-Mix | y = 0.803x + 8.174 | 0.945 | y = -0.484x + 24.90 | 0.925 | y = 0.048x + 7.004 | 0.981 | y = 0.060x + 5.606 | 0.990 |
| ASG-SC1011 | y = 1.810x + 3.583 | 0.980 | y = -0.469x + 25.74 | 0.972 | y = 0.080x + 5.643 | 0.979 | y = 0.083x + 5.261 | 0.981 |
| ASG-20z | y = 0.942x + 2.341 | 0.992 | y = -0.281x + 27.73 | 0.960 | y = 0.074x + 6.545 | 0.981 | y = 0.089x + 5.757 | 0.973 |
| ASG-WFB* | y = 0.129x + 4.164 | 0.992 | y = -0.034x + 33.00 | 0.990 | - | - | - | - |
*Formulation without roots of W. somnifera
3.4. Withanolides extraction kinetic
From the results, it was observed that there no changes in extraction of withanolides were observed after increased in the alcohol concentration. In all, extraction of withanolides was continued even after the stopping of alcohol generation. Withanolides contents and extraction rate was low in those formulations which were prepared using strains of S. fibuligera. But, in other formulations including traditional, withanolides contents and extraction rate was higher (Table 1). Results of formulation ASG-SC1011 which was kept for a month clearly indicated that for the complete extraction of withanolides at least one month of period is required. Contents of withanolides after 0 h indicate that about 20-25% withanolides gets extracted during the decoction. This result tells the need of decoction of roots before the fermentation process.
3.5. Analysis of non-ethanol volatile (NEV) compounds
Total 11 different NEV compounds i.e. acetaldehyde, ethyl acetate, methanol, diacetyl, 1-propanol, 2,3-pentadione, isobutanol, 1-butanol, isoamyl acetate, isoamyl alcohol and furfural were produced in different formulations. The amount of NEV compounds in prepared formlations was closer to the amount present in marketed formulation. The quantity of all the compounds was within the ICH limit.22 This indicates the safety of formulations for the human being or patient. Compounds acetaldehyde, methanol and isoamyl alcohol were common in all formulations. Among them, isoamyl alcohol was present in higher amount. Traditional formulation (ASG-WFS) showed highest amount of methanol. This may be due to the contamination of flowers with other microorganisms.8
4. Discussion
4.1. Evaluation of antioxidant potential
4.1.1. DPPH scavenging assay
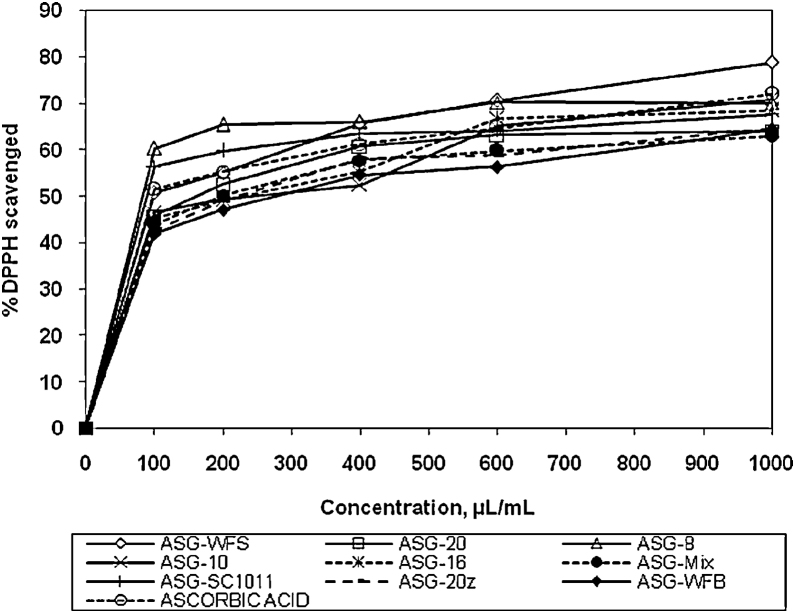
All the formulations and ascorbic acid were found to decreases the concentration of DPPH significantly (p < 0.05) from the concentration of 100 μL/mL to 1000 μL/mL. The DPPH scavenging potential was observed in order of ASG-WFS > ASG-WFB > ASG-8 > ASG-10 > ASG-16 > ascorbic acid > ASG-20 > ASG-Mix > ASG-SC1011. The IC50 values of the preparations ASG-WFS, ASG-WFB, ASG-8, ASG-10, ASG-16, ascorbic acid, ASG-20, ASG-Mix, ASG-SC1011 and ASG-20z were 13.11, 13.94, 14.69, 18.44, 18.57, 19.82, 21.57, 22.38, 22.90 and 31.65, respectively. The free radical scavenging potential was decreased with decrease in concentration. The results obtained were statistically significant (p < 0.05) compared to control (Fig. 1).
Fig. 1.
DPPH scavenging potential of various formulations.
ASG-8, Ashwagandharishtha prepared using strain S. fibuligera Jm.8; ASG-Mix, Ashwagandharishtha prepared using mixture of strains S. fibuligera Jm.8, S. fibuligera Jm.10, S. fibuligera Jm.16 and S. cerevisiae Jm.20; ASG-SC1011, Ashwagandharishtha prepared using standard strain of S. cerevisiae SC1011; ASG-16, Ashwagandharishtha prepared using strain S. fibuligera Jm.16; ASG-10, Ashwagandharishtha prepared using strain S. fibuligera Jm.10; ASG-20, Ashwagandharishtha prepared using strain S. cerevisiae Jm.20; ASG-20z, Ashwagandharishtha prepared using strain S. cerevisiae Jm.20 at optimum fermentation conditions; ASG-WFS, Ashwagandharishtha prepared using W. fruticosa flowers.
4.1.2. Hydrogen peroxide scavenging assay
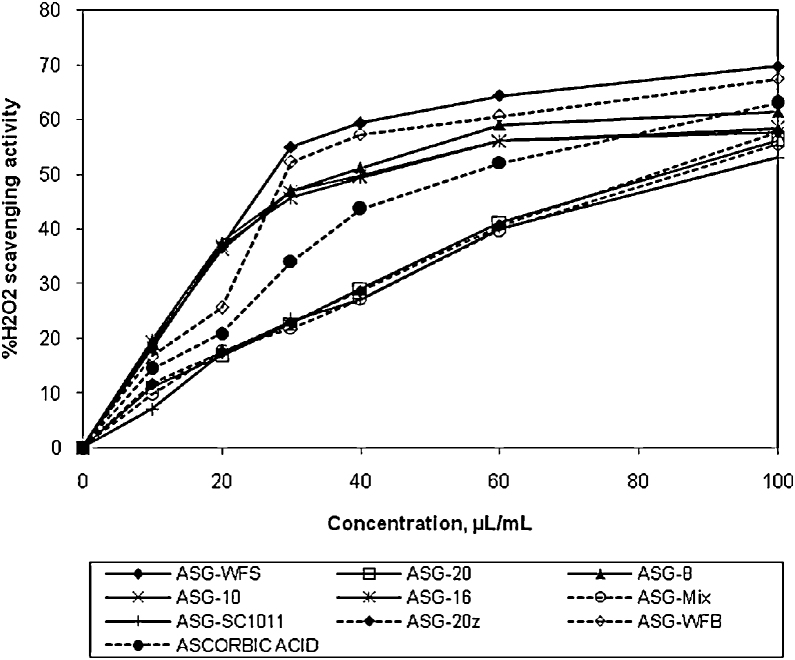
Traditional formulation (ASG-WFS) up to the concentration 100 μL/mL showed higher hydrogen peroxide scavenging potential than that of ascorbic acid and other preparations Preparation ASG-8, ASG-10 and ASG-16 showed lesser and almost equal activity. Formulation ASG-20, ASG-Mix and ASG-SC1011 have also exhibited intermediate and almost equal activity. Hydrogen peroxide scavenging effect was observed in order of ASG-WFS > ASG-8 > ASG-10 > ASG-16 > ASG-WFB > ascorbic acid > ASG-20 > ASG-20z > ASG-Mix > ASG-SC1011. IC50 values of the preparations ASG-WFS, ASG-8, ASG-10, ASG-16, ASG-WFB, ascorbic acid, ASG-20, ASG-Mix and ASG-SC1011 were 30.06, 30.60, 30.85, 31.51, 33.35, 39.72, 44.50, 44.62, 44.96 and 45.14, respectively. The results obtained were statistically significant (p < 0.05) compared to control (Fig. 2).
Fig. 2.
Hydrogen peroxide scavenging potential of various formulations.
ASG-8, Ashwagandharishtha prepared using strain S. fibuligera Jm.8; ASG-Mix, Ashwagandharishtha prepared using mixture of strains S. fibuligera Jm.8, S. fibuligera Jm.10, S. fibuligera Jm.16 and S. cerevisiae Jm.20; ASG-SC1011, Ashwagandharishtha prepared using standard strain of S. cerevisiae SC1011; ASG-16, Ashwagandharishtha prepared using strain S. fibuligera Jm.16; ASG-10, Ashwagandharishtha prepared using strain S. fibuligera Jm.10; ASG-20, Ashwagandharishtha prepared using strain S. cerevisiae Jm.20; ASG-20z, Ashwagandharishtha prepared using strain S. cerevisiae Jm.20 at optimum fermentation conditions; ASG-WFS, Ashwagandharishtha prepared using W. fruticosa flowers.
4.1.3. Total reduction capability assay
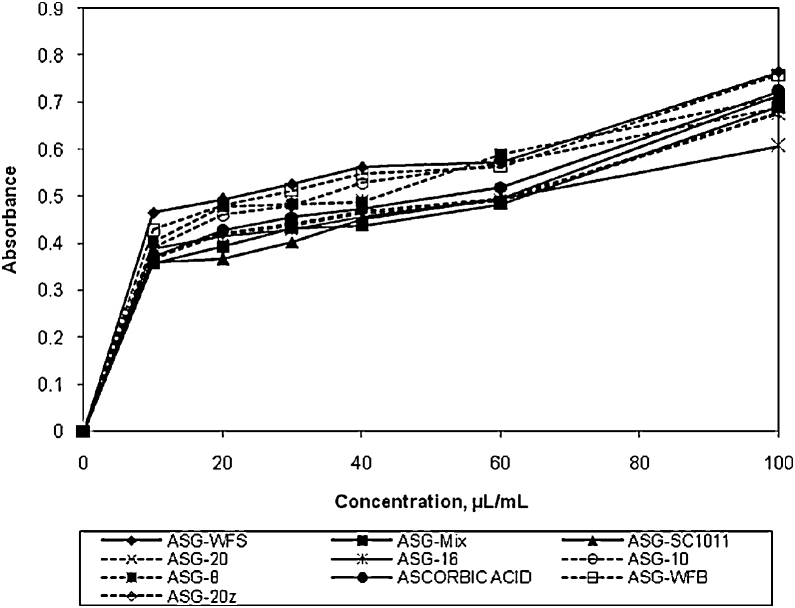
Total reducing power of all the formulations was increased with increasing concentration. Order of reducing power was ASG-WFS > ASG-WFB > (ASG-8 = ASG-10) > ASG-16 > (ASG-20 = ASG-20z = ascorbicacid)>ASG-Mix>ASG-SC1011. All the formulation and ascorbic acid showed almost same IC50 values. The IC50 values of the preparations ASG-WFS, ASG-8, ASG-10, ASG-16, ascorbic acid, ASG-20, ASG-Mix and ASG-SC1011 were 49.72, 49.74, 49.74, 49.76, 49.77, 49.77, 49.79 and 49.80, respectively. The reducing power exhibited by the preparations were statistically significant (p < 0.05) (Fig. 3).
Fig. 3.
Total reducing potential of various formulations.
ASG-8, Ashwagandharishtha prepared using strain S. fibuligera Jm.8; ASG-Mix, Ashwagandharishtha prepared using mixture of strains S. fibuligera Jm.8, S. fibuligera Jm.10, S. fibuligera Jm.16 and S. cerevisiae Jm.20; ASG-SC1011, Ashwagandharishtha prepared using standard strain of S. cerevisiae SC1011; ASG-16, Ashwagandharishtha prepared using strain S. fibuligera Jm.16; ASG-10, Ashwagandharishtha prepared using strain S. fibuligera Jm.10; ASG-20, Ashwagandharishtha prepared using strain S. cerevisiae Jm.20; ASG-20z, Ashwagandharishtha prepared using strain S. cerevisiae Jm.20 at optimum fermentation conditions; ASG-WFS, Ashwagandharishtha prepared using W. fruticosa flowers.
Despite the variation in withanolides concentration, all the formulations showed significant in vitro antioxidant activity by all methods relative to standard ascorbic acid. This might be due to the presence of higher amount of jaggery which itself also act as strong antioxidant substance.23 Among all formulations, traditional ASG-WFS showed slightly more activity by all the model systems. This increased in activity may be due to the presence of phenolic compounds in the flowers.24, 25 Traditional blank formulation (ASG-WFB) confirms that flowers are responsible for this increased in activity. Data of antioxidant study also explains at least in a part how preparation process affects the biological activity. This study also act as supporting evidence to the traditional process reported in Ayurvedic Pharmacopoeia of India which recommend the need of at least 1 month of period for completing fermentation process for preparing Ashwagandharishtha.
From the results, it can be concluded that the traditional method is the best method for preparing ashwagandharishtha for a significant antioxidant activity. The results also supports that the W. fruticosa flowers are not only important for the regulation of fermentation processes for preparation of formulation but are also essential for significant antioxidant activity.
Conflicts of interest
All authors have none to declare.
References
- 1.Ayurvedic Formulary of India, Ministry of Health and Family Planning, Government of India. The Controller of Publications: New Delhi;2001.
- 2.Atal C.K., Bhatia A.K., Singh R.P. Role of Woodfordia fruticosa Kurz (Dhataki) in the preparation of Asavas and Aristhas. J Res Ayurved Sidd. 1982;3:193–199. [Google Scholar]
- 3.Bone K. Phytotherapy Press; Australia: 1996. Clinical applications of Ayurvedic and Chinese Herbs. Monographs for Western Herbal Practitioner. [Google Scholar]
- 4.Andallu B., Radhika B., Hypoglycemic diuretic and hypocholesterolemic effect of winter cherry (Withania somnifera) root. Indian J Exp Biol. 2000;38:607–609. [PubMed] [Google Scholar]
- 5.Bhattacharya S.K., Bhattacharya A., Sairam K., Ghoshal S. Anxiolytic-antidepressant activity of Withania somnifera glycowithanolides, an experimental study. Phytomedicine. 2000;7:463–469. doi: 10.1016/S0944-7113(00)80030-6. [DOI] [PubMed] [Google Scholar]
- 6.Misra L., Mishra P., Pandey A., Sangwan R.S., Sangwan N.S., Tuli R. Withanolides from Withania somnifera roots. Phytochemistry. 2008;69:1000–1004. doi: 10.1016/j.phytochem.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 7.Ichikawa H., Takada Y., Shishodia S., Jayaprakasam B., Nair M.G., Agarwal B.B. Withanolides potentiate apoptosis, inhibit invasion and abolish osteoclastogenesis through suppression of nuclear factor-kappa B (NF-kappa B) activation and NF-kappa B regulated gene expression. Mol Cancer Ther. 2006;5:1434–1445. doi: 10.1158/1535-7163.MCT-06-0096. [DOI] [PubMed] [Google Scholar]
- 8.Maheshwari D.K., Lal R. Role of microflora associated with Dhataki flowers (Woodfordia fruticosa Kurz) in the production of Ayurvedic tonic Amritarishta. J Ind Bot Soc. 1999;78:91–94. [Google Scholar]
- 9.Ali K.M., Chatterjee K., De D., Maiti S., Pathak T.K. Evaluation of antioxidant activity of seed of Holarrhena antidysenterica: An approach through different in vitro models. J Nat Pharm. 2011;2:115–118. [Google Scholar]
- 10.Tiwari Preeti, Patel R.K. Comparison of anti-hyperlipidemic activity in ashwagandharishta prepared by traditional and modern methods. Asian J Research Chem. 2010;3(3):574. [Google Scholar]
- 11.Bhattacharya P.N., Jha D.K. Antidermatophytic and Antioxidant Activity of Terminalia arjuna (roxb.) Wight & Arn. Bark. Int J Res Pharm Biol Arch. 2011;2:973–979. [Google Scholar]
- 12.Narasimhan S., Govindarajan R., Vijayakumar M., Mehrotra S. Free radical scavenging potential of Chlorophytum tuberosum baker. J Ethnopharmacol. 2006;104:423–425. doi: 10.1016/j.jep.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 13.Joharapurkar A.A., Zambad S.P., Wanjari M.M., Umathe S.N. In vivo evaluation of antioxidant activity of alcoholic extract ofRubia cordifolia Linn. and its influence on ethanol-induced immunosuppression. Ind J Pharmacol. 2003;35:232–236. [Google Scholar]
- 14.Manwar J., Mahadik K., Paradkar A. Plackett-Burman Design: A statistical method for the optimization of fermentation process for the yeast saccharomyces cerevisiae isolated from the flowers of woodfordia fruticosa. Ferment Technol. 2013;2(1):1000109. [Google Scholar]
- 15.Manwar J.V., Mahadik K.R., Paradkar A.R., Takle S.P., Sathiyanarayanan L., Patil S.V. Determination of withanolides from the roots and herbal formulation of Withania somnifera by HPLC using DAD and ELSD detector. Der Pharmacia Sinica. 2012;3:41–46. [Google Scholar]
- 16.Crowell E.A., Ough C.S. A modified procedure for alcohol determination by dichromate oxidation. Am J Enol Vitic. 1979;30:61–63. [Google Scholar]
- 17.Lane J.H., Eynon L. Determination of reducing sugars by means of Fehling's solution with methylene blue as internal indicator. J Soc Chem Ind Trans. 1923:32T–36T. [Google Scholar]
- 18.Manwar J., Mahadik K., Paradkar A., Patil S., Sathiyanarayanan L., Manmode R. Gas chromatography method for the determination of non-ethanol volatile compounds in herbal formulation. Int J Ana Bioanal Chem. 2013;1(3):12–17. [Google Scholar]
- 19.Chidambra K.N., Singh R.P., Jayaprakasha G.K.J. Antioxidant activities of grape (Vitis vinifera) pomace extracts. J Agric Food Chem. 2002;50:5909–5914. doi: 10.1021/jf0257042. [DOI] [PubMed] [Google Scholar]
- 20.Ruch R.J., Cheng S.J., Klaunig J.E. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10:1003–1008. doi: 10.1093/carcin/10.6.1003. [DOI] [PubMed] [Google Scholar]
- 21.Oyaizu M. Studies on products of the browning reaction Antioxidative activities of browning reaction products prepared from glucosamine. Jpn J Nutr. 1986;44:307–315. [Google Scholar]
- 22.International Conference on Harmonization, ICH Topic Q3C(R4) Impurities: Guidelines for residual solvents. Feb. 2009, available at http://www.ich.org.
- 23.Harish Nayaka M.A., Sathisha U.V., Manohar M.P., Chandrashekar K.B., Dharmesh S.M. Cytoprotective and antioxidant activity studies of jaggery sugar. Food Chem. 2009;115:113–118. [Google Scholar]
- 24.Chandan B.K., Saxena A.K., Shukla S., Sharma N., Gupta D.K. Hepatoprotective activity of Woodfordia fruticosa Kurz flowers against carbon tetrachloride induced hepatotoxicity. J Ethnopharmacol. 2008;119:218–224. doi: 10.1016/j.jep.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 25.Shome U., Mehrotra S., Sharma H.P. Pharmacognostic studies on the flower of Woodfordia fruticosa Kurz. Proc Ind Acad Sci. 1981;90:335–351. [Google Scholar]