Abstract
Medicinal tablets have been used for a long time to treat cardiovascular disease. However, mortality rate is steadily increasing partly because of the patients’ sedentary lifestyle and unhealthy diet. By contrast, exercise has been systematically shown to have multiple benefits. Regular exercise training can prevent various diseases in healthy individuals. Combined exercise and cardiac medications may lead to the improvement of heart disease. Numerous exercise training pathways still need further investigations. How exercise can prevent, treat, or attenuate diseases remains somewhat elusive. Thus, this review will discuss cardiac medications in parallel with the mechanism of action of exercise.
Keywords: cardiac medication, exercise, heart disease
1. Introduction
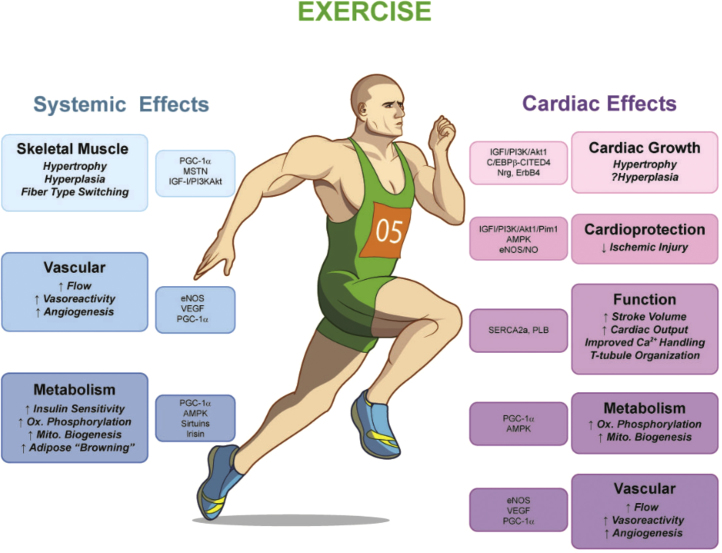
Exercise training has been recognized to prevent and ameliorate cardiovascular disease. Although exercise can reduce cardiovascular disease, it is also suggested that it can be instrumental in preventing chronic disease such as hypertension, dyslipidemia, and diabetes mellitus. A recent study by Mann and Rosenzweig1 wonderfully highlighted the beneficial effects of exercise including cardiac effects and systemic factors on skeletal muscle, metabolism, and vascular dysfunction (Fig. 1).
Fig. 1.
Overview of the systemic and cardiac-specific effects of exercise. Endurance exercise has multiple systemic effects, ranging from increased skeletal muscle growth to vascular remodeling and improved energetics. Exercise also exerts direct effects on the heart itself, including increased cardiac growth, protection against ischemic damage, and modulation of cardiac function, metabolism, and vascular supply. AMPK, AMP-activated kinase; C/EBP β, CCAAT/enhancer binding protein β; CITED4, cbp/p300-interacting trans-activator with Glu/Asprich carboxy-terminal domain 4; eNOS, endothelial nitric oxide synthase; IGF-1, insulin-like growth factor-1; MSTN, myostatin; Nrg1, neuregulin1; PGC-1α, peroxisome proliferator activated receptor gamma co-activator 1 α; PI3K, phosphoinositide kinase-3; Pim1, proto-oncogene serine/threonine-protein kinase-1; PLB, phospholamban; SERCA2a, sarco/endoplasmic reticulum Ca2+-ATPase, 2a; and VEGF, vascular endothelial growth factor. Reprinted with permission form Mann et al.1
The key role of the cardiovascular system, which is composed of the heart and the intricate network of veins and arteries throughout the body, is the transportation of vital substances. Modern-day technological advancements brought us ease and convenience, allowing us to accomplish more at a faster rate, but they also translated to less physical activity. As a consequence, diseases have also evolved and some of them involve the cardiovascular system; for example, ischemia is the leading cause of death as reported by the World Health Organization based on the 2008 statistics.2 Increase in correlation between cardiac disorders and diabetes is also prevalent due to a similar contributing factor—sedentary lifestyle.3 Medications for the treatment and rehabilitation of cardiac diseases are continually being developed. Despite the effectiveness of medicines, a chemical ingested may still bring harm to vital parts of the body as specified by their respective adverse effects. Accordingly, a natural activity such as exercise is advised and advocated by sports trainers and physicians. Exercise is beneficial for the heart in a number of ways, although the exact process as to how exercise intervenes in the cardiovascular system is unknown. As advised, walking by stairs is more beneficial than taking the elevator. This review will focus on current heart medication categories commonly used to treat diseases. Particular exercise pathways similar or different from medications work of action will also be discussed.
2. Anticoagulant
Thrombogenesis is the formation of blood clots to prevent excessive hemorrhage. Coagulation is a homeostatic process that primarily involves the platelets followed by protein clotting factors. To eliminate the formed thrombus, natural anticoagulants exist such as protein C, which stops the clotting process, followed by fibrinolysis, in which the thrombus is dissolved thus preventing dislodgement. Anticoagulant drugs are medicines used for cardiovascular diseases (CVDs) such as acute coronary syndromes, and they work by inhibiting one of the clotting factors or mimicking natural anticoagulants.4
Exercise simultaneously increases coagulation and fibrinolysis without altering the balance between them. It is suggested to increase coagulation in the blood by an elevation in factor VIII, an important blood clotting protein, and to balance this equation by increasing fibrinolysis through the release of more tissue type plasminogen activator (t-PA) and limiting plasminogen activator inhibitor.5 In another endurance exercise study, changes in conditions with treadmill running less than 2 hours below the individual anaerobic threshold resulted in improved fibrinolysis with reduced coagulation in healthy men.6 Hypoxic training induced increased thrombin activity with opposing effect from normoxic and relative hypoxic trained individuals.7 Interestingly, this difference in result, an inverse proportion of fibrinolysis with thrombin formation, although both favoring exercise, relates to the flexibility of exercise outcome with changes in its components. Short-term exercise in healthy individuals balanced the increase in coagulation by increasing fibrinolysis, whereas in individuals with CVD it triggered an ischemic condition with the increase in plasminogen activator inhibitor.7 Chronic aerobic exercise, by contrast, constitutively decreased coagulation and increased fibrinolysis in patients with CVDs and normal individuals.8
In CVDs, although acute exercise can negatively affect the case, benefit of endurance exercise are manifested. Change in exercise intensity caused a change in outcome (e.g., moderate exercise increased fibrinolysis) whereas strenuous exercise greatly affected coagulation, both without interruption in homeostasis in healthy individuals.36 The intensity and time of exercise in a normal individual do not alter the sustenance of coagulation and fibrinolysis balance. The combination of thrombin inhibitors and exercise augments fibrinolysis caused by exercise without counteracting exercise-induced platelet or leukocyte activation.9 This shows an effective way to boost the drug's potential. Although exercise promises a positive effect, risk is present with individuals undergoing rehabilitation because of the probability of emboli. Studies regarding the most effective exercise method and factors are still to be uncovered.
3. Antiplatelet
Platelet activity is involved in the coagulation process as the first step in preventing hemorrhage. Antiplatelet drugs lessen the interaction of platelets to aggregate, thus preventing thrombus. The effect of exercise in platelets remains controversial until today.10 Less disagreements arise from patients with coronary heart disease where exercise boosted up platelet number, aggregation, and function.10, 11, 12 Platelet function was also increased with a single bout of aerobic exercise, 13 whereas reduced platelet count associated with exercise was found as one of the factors to decrease cardiovascular risk.14, 15, 16 In sickle cell anemia, exercise decreases platelet aggregation, giving a late, positive benefit.17 These reports indicate the need to evaluate platelet function focusing on the age of the population as well as the disease condition and exercise parameters. Differences in methodology make it difficult to derive a definite conclusion on the direct or indirect effect of exercise to platelet. Also, the duration of increased platelet after exercise is unknown. Further investigation is also warranted to differentiate the impact of exercise on platelet between genders. Aspirin, which is one of the frequently used antiplatelet agents, does not attenuate the exercise-induced increase in platelet aggregation.10, 11, 12
4. Angiotensin II receptor blockers
The regulation of internal circulation of blood and fluids is handled by the renin–angiotensin system (RAS), governed by hormones controlling the decrease in water balance and blood pressure. This systemic process involves the kidneys that secrete the renin after the detection of low blood pressure. Renin stimulates the transformation of angiotensinogen in the liver into angiotensin I (Ang I) that is further transformed into angiotensin II (Ang II) by the angiotensin-converting enzyme (ACE) from the lungs. This hormone, in turn, prompts aldosterone release from the adrenal cortex, relaying to the kidneys information to compensate for the loss. Involvement of this process is observed in pathological left ventricular hypertrophy, which occurs in certain conditions such as myocardial infarct (MI) and heart failure (HF). The action of angiotensin receptor blockers is the inhibition of Ang II (the main hormone during the process acting as a vasoconstrictor).18 The prevention of veins from narrowing decreases the potential of increase in blood pressure. Exercise is reported to influence the RAS.19, 20
Acute exercise was associated with elevations in plasma aldosterone, renin activity, potassium, and vasopressin.21, 22 Generally, renin plasma level is increased after a period of exercise, activating more Ang I and Ang II overtaking renin levels due to lactic acidosis, which inhibits angiotensinases.19, 23 Angiotensin receptor 1 (ATr1) decreased by Ang II leads to unfavorable cardiac remodeling.24 In the normal heart, Ang II was decreased after aerobic exercise accompanied by increased angiotensin receptor 2 (ATr2); similarly, ATr2 elevation in exercised HF condition is associated with vasodilation, stimulating nitric oxide and bradykinin.20, 22 In contrast to this result, ATr1 protein as well as ACE binding decreased in response to exercise in MI rats.25 Decline in ATr1 was constituted with inhibition of Ang II binding, thereby resulting in favorable reduced fibrosis formation. Aerobic exercise also increased ACE2 and decreased cardiac AngII and ACE in obese rats.26
5. ACE inhibitors
The target of the ACE inhibitors is the ACE, which mediates in the conversion of Ang I into Ang II. ACE inhibition leads to a decrease in Ang II, finally lowering blood pressure. In the serum, ACE increases in response to acute exercise independent of ACE genotype, which is correlated with elevation in cardiac ACE thus elevated Ang II.27, 28, 29 A study revealed that ACE majorly contributes to Ang II formation in the heart.27 ACE's importance is portrayed with its gene insertion and deletion polymorphism that is associated with MI, cardiomyopathy, left ventricular hypertrophy, coronary artery disease, and HF.28, 30 ACE was observed to elevate in the early development of myocardial dysfunction with a potential contribution in ventricular remodeling.31 Post-MI exercise treatment can decrease ACE, attenuating the pathological remodeling.25 ACE2 is an ACE homolog that is unresponsive to ACE inhibitors and reported to have a cardioprotective role. Adding up to the RAS pathway, ACE2 hydrolyzes Ang I, resulting to Ang-(1–9), which is in turn transformed into Ang-(1–7) by a neutral endopeptidase and ACE.32, 33 ACE2 is a vasodilator activating Ang (1–7) releasing vasofactors, a contradictory mechanism from ACE balancing the latter. ACE levels do not directly contribute to left ventricular hypertrophy; however, increase in ACE2—as reflected in exercise—attenuates left ventricular hypertrophy by decreasing angiotensin II and increasing expression of angiotensin (1–7).20 Chronic inhibition of ACE2 in transgenic hypertensive rats elevates angiotensin II in the heart that resulted in fibrosis and pathological hypertrophy.23
In relation to neurophysiology, animal models from young to old age exhibited improvement in memory with exercise. In humans, increased physical activity was reported to decrease the chance of acquiring dementia.34 ACE inhibitors are known to have positive impact on the cognition of animals and people with HF, which may be associated with the blockage of AngII.35
6. Beta blockers
Tissues that respond to sympathetic stimulation contain beta adrenoceptors, which are the binding sites of catecholamine. Predominantly in the heart, the most abundant among the three distinct types of adrenoceptors with highest functionality are the β1 adrenoceptors.36 In the heart, β1 are localized on large conduit coronary arteries, whereas β2 are dispersed in the small arteries and arterioles. On a cellular basis, β1 reside in the cell membrane, whereas β2 are secluded in the caveolae. Beta-blockers prevent the normal ligand from binding to the beta-adrenoceptor by competing for the binding site. The inhibition of epinephrine and norepinephrine results in lower blood pressure with slower heart rate and less cardiac output. The sympathetic activity produced by these receptors depends on their expression, distribution, and responsiveness to catecholamine.
Exercise affects catecholamine regulation as well as the distribution of adrenoceptors. Generally, exercise stimulates epinephrine and norepinephrine increase in the serum.36 Coronary vessels respond with significant elevation in left ventricular norepinephrine secretion and epinephrine usage. This sympathetic response of both receptors increases heart rate, contractility, and a β-adrenoceptor-mediated coronary vasodilation.36 The norepinephrine binding with β1 is mostly responsible for the coronary vasodilation and partially by β2 and epinephrine binding.36
A disproportion in this constituent leads to predictable responses. The initial course of HF is accompanied by compensatory expression catecholamine. Chronically, it progresses to a higher β2/β1 ratio, which finally results to improper calcium handling contributing to arrhythmias.37, 38 Exercise reverses this outcome by increasing the responsiveness to β1 adrenoreceptor and decreasing sympathetic activation.38 Also, a recovery of beta adrenergic signaling pathway was achieved via equilibrating the β2/β1 ratio after exercise in dogs susceptible for ventricular fibrillation.39 Acute voluntary wheel models maintained β1 expression with a decline in β2 regulation and function rehabilitating HF models.38
Diabetic heart comes along with bradycardia and cardiomyopathy because of decreased responsiveness with beta adrenoceptors responsible for the sympathetic activities.40 Different models of exercise contribute to multiple alterations in adrenoceptor response. Intensive exercises effect on normal rats reflects diabetic adrenoceptor effects such as β1 down-regulation decreasing positive inotropy and β3 up-regulation improving negative inotropy. In addition, intense exercise accompanies decreased β2 adrenoceptor, inhibition of β1 loss without restoration, and normalized β3 as opposed to β1 restoration with β2 normalization and β3 upregulation in moderate exercise. Intensive exercise in diabetic rats accentuates bradycardia by rendering less sympathetic response, although it remains unknown whether it provides cardioprotection against arrhythmias.41 β3's impact in the heart still needs to be further studied with its chronotropic effect remaining as a controversial topic.
Exercise alters the distribution of receptors as well as regulating the catecholamine that bind with them. Therefore, the benefits surpass the normal mechanism as it greatly contributes to cardiometabolic disorders.
7. Calcium channel blockers
Calcium ions in the heart are factors responsible for smooth muscle contraction. Systole depends on the accumulation of calcium in the cytoplasm and diastole proceeds upon the extrusion of calcium. The amount and pooling duration of cytoplasmic calcium determines the force of contractility of the myocytes. Active transport of calcium extracellularly to the cell is mediated by voltage-gated calcium channels. Calcium channel blockers (CCBs) interrupt calcium transposition by binding to the channels such as the l-type calcium channels in the heart and in smooth muscle of the peripheral vasculature promoting vasodilation of peripheral vasculature and coronary arteries excluding veins, thus a decrease in cardiac contractility and output. However, there are irregularities with the relationship of CCBs and mortality rate. Women with hypertension without CVD history were reported to have a higher mortality rate than those who took beta blockers with diuretics,56 whereas patients at risk for CVD undergoing hemodialysis and hypertensive end-stage renal disease patients showed a lower mortality rate with CCB treatment.42, 43
Exercise has systemic outcome to calcium balance in multiple organs in the body. It is well known how exercise strengthens bones and prevents calcium loss especially in lactating women.44 Aside from voltage-gated channels, calcium homeostasis in cells is mediated by protein pathways. A review highlighted multiple proteins involved in myocyte calcium handling specifically related to cardiac hypertrophy and exercise effect.50 In the myocytes, proteins mainly responsible for calcium handling regulated by exercise are cardiac sarcoplasmic reticulum calcium-ATPase (SERCA2a), cardiac sodium/calcium exchanger (NCX1), and ryanodine receptor 2 (RyR2). All three proteins take part in systole and diastole of the heart with SERCAa2 contributing the most with relaxation. Disturbance in the balance of these proteins was seen with failing hearts, where SERCA2a was downregulated and NCX was increased.45, 46 Long-term aerobic exercise in animal models with HF resulted in restored abnormality in calcium handling proteins.45, 46, 47, 48
8. Statins
Statins are inhibitors of HMG-CoA reductase that are enzymes involved in cholesterol formation in the liver. High levels of cholesterol, specifically low-density lipoproteins (LDLs), are elevated in most CVDs leading to viscous blood and plaque buildup potentiating atherosclerosis. Therefore, limiting this level will prevent damage to the heart.
Exercise can lower cholesterol by burning and using it as an energy source. Exercise is related with both improved muscle function and heart strength, whereas the opposite was observed with sedentary living.49 Obese and overweight men who have increased lipids and LDLs were found to have better prognosis against diseases when treated with aerobic muscular conditioning.50 Also, a new protein, irisin, which is induced by exercise, was found to turn white adipose tissue to brown, such as adipose tissue, and has decreased expression in sedentary diabetic and obese individuals.51
Statin therapy is safe for reducing cholesterol levels with few cases of adverse effects.52 However, precaution is needed regarding the use of statins and exercise together. Reports about statin affecting muscle strength are evident, although a deeper investigation is needed.53, 54 However, in patients with coronary arterial diseases, statin combined with exercise appeared to be beneficial in attenuating arterial wall stiffness and increasing high-density lipoproteins.55, 56
9. Concluding remarks and future perspectives
Man's increasing propensity toward obesity is a threat that poses high risk because it could lead to heart problems. Awareness of the outcome of a sedentary lifestyle offers a better understanding of the ways that we can combat this problem. Promotion of programs providing more information about the merits of exercise therapy to high risk populations should be adopted. Medications are continually being developed, with the aim of preserving and serving as cardioprotective agents. However, a pill for exercise remains unavailable to date—exercise coupled with proper nutrition still serves as the best option to prevent diseases as well as to have a healthy heart able to resist future threats.
Multiple findings showcase how beneficial exercise is to healthy individuals and those with various diseases. Moderate exercise done regularly produces cardioprotection.57 Although 30 minutes was said to suffice as weekly exercise to improve health, conflicts about its intensity, duration, and timing still need be resolved.58 Exercise serves as cardiac medication that works at the cellular level to balance proper signaling molecules. Although interaction between exercise and medications has been reported, further investigations are needed to explain how acute and chronic exercises exert their beneficial effects. Molecular studies in exercise might lead to the development of drug(s) that can alter some pathways and thus prevent diseases.
This review highlighted the ways in which exercise provides positive contributions to various “conditions”; however, it is also apparent that much information is still needed before we can “see” the whole picture. Exercise definitely provides cardioprotection, although the main mechanism it targets is yet to be unveiled. Further research is needed to establish the proper exercise settings needed for particular diseases.
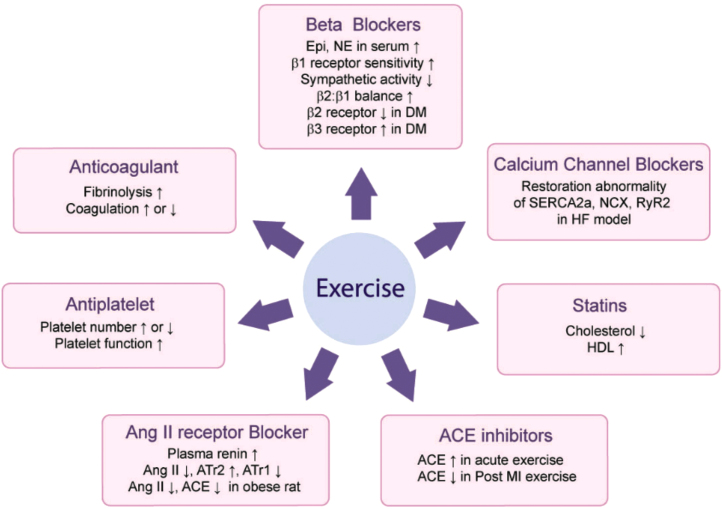
The integration of medicine and exercise is beneficial in some cases and can be detrimental in other situations (Fig. 2). The resulting adverse effects should be further studied in depth.
Fig. 2.
Exercise effect on cardiac medication. Epi, epinephrine; NE, norephinephrine; DM, diabetes mellitus; AngII, angiotensin 2; ATr1, angiotensin receptor 1; ATr2, angiotensin receptor 2; ACE, angiotensin converting enzyme; MI, myocardial infarction; HDL, high density lipoprotein; NCX, Na+/Ca+ exchanger; RyR2, ryanodine recepter2; HF, heart failure; SERCA2a, cardiac sarcoplasmic reticulum calcium-ATPase.
Conflicts of interest
All contributing authors declare no conflict of interest.
Acknowledgements
This study was supported by Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (2010–0020224).
References
- 1.Mann N., Rosenzweig A. Can exercise teach us how to treat heart disease? Circulation. 2012;126:2625–2635. doi: 10.1161/CIRCULATIONAHA.111.060376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. The top 10 causes of death. World Health Organization. Available from: http://www.who.int/mediacentre/factsheets/fs310/en/index2.html. Published 2013. Accessed March 20, 2013.
- 3.Grundy S.M., Benjamin I.J., Burke G.L., Chait A., Eckel R.H., Howard B.V. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100:1134–1146. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- 4.De Caterina R., Husted S., Wallentin L., Andreotti F., Arnesen H., Bachmann F. General mechanisms of coagulation and targets of anticoagulants (Section I). Position Paper of the ESC Working Group on Thrombosis-Task Force on Anticoagulants in Heart Disease. Thromb Haemost. 2013;109:569–579. doi: 10.1160/TH12-10-0772. [DOI] [PubMed] [Google Scholar]
- 5.El-sayed M.S. Effects of exercise on blood coagulation, fibrinolysis and platelet aggregation. Sports Med. 1996;22:282–298. doi: 10.2165/00007256-199622050-00002. [DOI] [PubMed] [Google Scholar]
- 6.Hilberg T., Gläser D., Reckhart C., Prasa D., Stürzebecher J., Gabriel H.H. Blood coagulation and fibrinolysis after long-duration treadmill exercise controlled by individual anaerobic threshold. Eur. J. Appl. Physiol. 2003;90:639–642. doi: 10.1007/s00421-003-0907-2. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y.W., Chen Y.C., Wang J.S. Absolute hypoxic exercising training enhances in vitro thrombin generation by increasing procoagulant platelet-derived microparticles under high shear stress in sedentary men. Clin Sci (Lond) 2013;124:639–649. doi: 10.1042/CS20120540. [DOI] [PubMed] [Google Scholar]
- 8.Womack C.J., Nagelkirk P.R., Coughlin A.M. Exercise-induced changes in coagulation and fibrinolysis in healthy populations and patients with cardiovascular disease. Sports Med. 2003;33:795–807. doi: 10.2165/00007256-200333110-00002. [DOI] [PubMed] [Google Scholar]
- 9.Li N., He S., Blombäck M., Hjemdahl P. Platelet activity, coagulation, and fibrinolysis during exercise in healthy males: effects of thrombin inhibition by argatroban and enoxaparin. Arterioscler. Thromb. Vasc. Biol. 2007;27:407–413. doi: 10.1161/01.ATV.0000253906.19648.ac. [DOI] [PubMed] [Google Scholar]
- 10.El-Sayed M.S., Ali N., El-Sayed Ali Z. Aggregation and activation of blood platelets in exercise and training. Sports Med. 2005;35:11–22. doi: 10.2165/00007256-200535010-00002. [DOI] [PubMed] [Google Scholar]
- 11.Wallén N.H., Held C., Rehnqvist N., Hjemdahl P. Effects of mental and physical stress on platelet function in patients with angina pectoris and healthy controls. Eur. Heart J. 1997;18:807–815. doi: 10.1093/oxfordjournals.eurheartj.a015346. [DOI] [PubMed] [Google Scholar]
- 12.El-Sayed M.S., El-Sayed Ali Z., Ahmadizad S. Exercise and training effects on blood haemostasis in health and disease: an update. Sports Med. 2004;34:181–200. doi: 10.2165/00007256-200434030-00004. [DOI] [PubMed] [Google Scholar]
- 13.Whittaker J.P., Linden M.D., Coffey V.G. Effect of aerobic interval training and caffeine on blood platelet function. Med. Sci. Sports Exerc. 2013;45:342–350. doi: 10.1249/MSS.0b013e31827039db. [DOI] [PubMed] [Google Scholar]
- 14.Adams R.A., Higgins T., Potter S., Evans S.A. The effect of physical activity on haematological predictors of cardiovascular risk: evidence of a dose response. Clin. Hemorheol. Microcirc. 2012;52:57–65. doi: 10.3233/CH-2012-1566. [DOI] [PubMed] [Google Scholar]
- 15.Hilberg T. Physical activity in the prevention of cardiovascular diseases. Epidemiology and mechanisms. Hamostaseologie. 2008;28:9–15. [PubMed] [Google Scholar]
- 16.Pagar A.B., Raut S.E., Hawaldar V.B. The effect of exercise on platelet aggregability and other cardiovascular parameters. IJBMS. 2012;2:273–277. [Google Scholar]
- 17.Waltz X., Hedreville M., Sinnapah S., Lamarre Y., Soter V., Lemonne N. Delayed beneficial effect of acute exercise on red blood cell aggregate strength in patients with sickle cell anemia. Clin. Hemorheol. Microcirc. 2012;52:15–26. doi: 10.3233/CH-2012-1540. [DOI] [PubMed] [Google Scholar]
- 18.Benigni A., Cassis P., Remuzzi G., Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med. 2010;2:247–257. doi: 10.1002/emmm.201000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aldigier J.C., Huang H., Dalmay F., Lartigue M., Baussant T., Chassain A.P. Angiotensin-converting enzyme inhibition does not suppress plasma angiotensin II increase during exercise in humans. J. Cardiovasc. Pharmacol. 1993;21:289–295. doi: 10.1097/00005344-199302000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Fernandes T., Hashimoto N.Y., Magalhães F.C., Fernandes F.B., Casarini D.E., Carmona A.K. Aerobic exercise training-induced left ventricular hypertrophy involves regulatory microRNAs, decreased angiotensin-converting enzyme-angiotensin II, and synergistic regulation of angiotensin-converting enzyme 2-angiotensin (1-7) Hypertension. 2011;58:182–189. doi: 10.1161/HYPERTENSIONAHA.110.168252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luger A., Deuster P.A., Debolt J.E., Loriaux D.L., Chrousos G.P. Acute exercise stimulates the renin–angiotensin–aldosterone axis: adaptive changes in runners. Horm. Res. 1988;30:5–9. doi: 10.1159/000181017. [DOI] [PubMed] [Google Scholar]
- 22.Negrao C.E., Middlekauff H.R. Exercise training in heart failure: reduction in angiotensin II, sympathetic nerve activity, and baroreflex control. J. Appl. Physiol. 2008;104:577–578. doi: 10.1152/japplphysiol.01368.2007. [DOI] [PubMed] [Google Scholar]
- 23.Fallo F. Renin–angiotensin–aldosterone system and physical exercise. J. Sports Med. Phys. Fitness. 1993;33:306–312. [PubMed] [Google Scholar]
- 24.Santos R.A., Ferreira A.J., Simões E., Silva A.C. Recent advances in the angiotensin-converting enzyme 2-angiotensin(1-7)-Mas axis. Exp. Physiol. 2008;93:519–527. doi: 10.1113/expphysiol.2008.042002. [DOI] [PubMed] [Google Scholar]
- 25.Xu X., Wan W., Ji L., Lao S., Powers A.S., Zhao W. Exercise training combined with angiotensin II receptor blockade limits post-infarct ventricular remodelling in rats. Cardiovasc. Res. 2008;78:523–532. doi: 10.1093/cvr/cvn028. [DOI] [PubMed] [Google Scholar]
- 26.Barretti D.L., Magalhães Fde C., Fernandes T., do Carmo E.C., Rosa K.T., Irigoyen M.C. Effects of aerobic exercise training on cardiac renin–angiotensin system in an obese Zucker rat strain. PLOS One. 2012;7:461–514. doi: 10.1371/journal.pone.0046114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yaul A., Moss A.D., James L.J., Hynes J., Ashworth J.J., Evans G.H. Serum angiotensin I-converting enzyme response to exercise; no differential effect of genotype. Br J Sports Med. 2011;45:A4. [Google Scholar]
- 28.Danser J.A.H., Schalekamp M.A.D.H., Bax W.A., van den Brink A.M., Saxena P.R., Riegger G.A.J. Angiotensin-converting enzyme in the human heart. Effect of the deletion/insertion polymorphism. Circulation. 1995;92:1387–1388. doi: 10.1161/01.cir.92.6.1387. [DOI] [PubMed] [Google Scholar]
- 29.Zisman L.S., Abraham W.T., Meixell G.E., Vamvakias B.N., Quaife R.A., Lowes B.D. Angiotensin II formation in the intact human heart. Predominance of the angiotensin-converting enzyme pathway. J Clin Invest. 1995;96:1490–1498. doi: 10.1172/JCI118186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schut A.F., Bleumink G.S., Stricker B.H., Hofman A., Witteman J.C., Pols H.A. Angiotensin converting enzyme insertion/deletion polymorphism and the risk of heart failure in hypertensive subjects. Eur. Heart J. 2004;25:2143–2148. doi: 10.1016/j.ehj.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 31.Davis G.K., Millner R.W., Roberts D.H. Angiotensin converting enzyme ACE gene expression in the human left ventricle: effect of ACE gene insertion/deletion polymorphism and left ventricular function. Eur. J. Heart Fail. 2000;2:253–256. doi: 10.1016/s1388-9842(00)00070-2. [DOI] [PubMed] [Google Scholar]
- 32.Raizada M.K., Ferreira A.J. ACE2: a new target for cardiovascular disease therapeutics. J. Cardiovasc. Pharmacol. 2007;50:112–119. doi: 10.1097/FJC.0b013e3180986219. [DOI] [PubMed] [Google Scholar]
- 33.Iwai M., Horiuchi M. Devil and angel in the renin–angiotensin system: ACE–angiotensin II–AT1 receptor axis vs. ACE2–angiotensin-(1-7)–Mas receptor axis. Hypertens Res. 2009;32:533–536. doi: 10.1038/hr.2009.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gregory S.M., Parker B., Thompson P.D. Physical activity, cognitive function, and brain health: what is the role of exercise training in the prevention of dementia? Brain Sci. 2012;2:684–708. doi: 10.3390/brainsci2040684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Menzel K., Hilberg T. Blood coagulation and fibrinolysis in healthy, untrained subjects: effects of different exercise intensities controlled by individual anaerobic threshold. Eur. J. Appl. Physiol. 2011;111:253–260. doi: 10.1007/s00421-010-1640-2. [DOI] [PubMed] [Google Scholar]
- 36.Gao F., de Beer V.J., Hoekstra M., Xiao C., Duncker D.J., Merkus D. Both β1- and β2-adrenoceptors contribute to feedforward coronary resistance vessel dilation during exercise. Am J Physiol Heart Circ Physiol. 2010;298:921–929. doi: 10.1152/ajpheart.00135.2009. [DOI] [PubMed] [Google Scholar]
- 37.Stones R., Natali A., Billeter R., Harrison S., White E. Voluntary exercise-induced changes in beta2-adrenoceptor signalling in rat ventricular myocytes. Exp. Physiol. 2008;93:1065–1075. doi: 10.1113/expphysiol.2008.042598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duncker D.J., Merkus D. Exercise hyperaemia in the heart: the search for the dilator mechanism. J Physiol. 2007;583:847–854. doi: 10.1113/jphysiol.2007.135525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holycross B.J., Kukielka M., Nishijima Y., Altschuld R.A., Carnes C.A., Billman G.E. Exercise training normalizes beta-adrenoceptor expression in dogs susceptible to ventricular fibrillation. Am J Physiol Heart Circ Physiol. 2007;293:2702–2709. doi: 10.1152/ajpheart.00763.2007. [DOI] [PubMed] [Google Scholar]
- 40.Wang HYS. Cardiac function and β-adrenergic receptor responsiveness in the isolated human diabetic myocardium. Thesis, Bachelor of Biomedical Sciences with Honours, University of Otago. Available from: http://hdl.handle.net/10523/2637. Published 2012. Accessed Mar 7, 2013.
- 41.Lahaye Sle D., Gratas-Delamarche A., Malardé L., Vincent S., Zguira M.S., Morel S.L. Intense exercise training induces adaptation in expression and responsiveness of cardiac beta-adrenoceptors in diabetic rats. Cardiovasc Diabetol. 2010;9:72. doi: 10.1186/1475-2840-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tepel M., Giet M.V., Park A., Zidek W. Association of calcium channel blockers and mortality in haemodialysis patients. Clin. Sci. (Lond.) 2002;103:511–515. doi: 10.1042/cs1030511. [DOI] [PubMed] [Google Scholar]
- 43.Kestenbaum B., Gillen D.L., Sherrard D.J., Seliger S., Ball A., Stehman-Breen C. Calcium channel blocker use and mortality among patients with end-stage renal disease. Kidney Int. 2002;61:2157–2164. doi: 10.1046/j.1523-1755.2002.00355.x. [DOI] [PubMed] [Google Scholar]
- 44.Lovelady C.A., Bopp M.J., Colleran H.L., Mackie H.K., Wideman L. Effect of exercise training on loss of bone mineral density during lactation. Med. Sci. Sports Exerc. 2009;41:1902–1907. doi: 10.1249/MSS.0b013e3181a5a68b. [DOI] [PubMed] [Google Scholar]
- 45.Lu L., Mei D.F., Gu A.G., Wang S., Lentzner B., Gutstein D.E. Exercise training normalizes altered calcium-handling proteins during development of heart failure. J. Appl. Physiol. 2002;92:1524–1530. doi: 10.1152/japplphysiol.00405.2001. [DOI] [PubMed] [Google Scholar]
- 46.Medeiros A., Rolim N.P., Oliveira R.S., Rosa K.T., Mattos K.C., Casarini D.E. Exercise training delays cardiac dysfunction and prevents calcium handling abnormalities in sympathetic hyperactivity-induced heart failure mice. J. Appl. Physiol. 2008;104:103–109. doi: 10.1152/japplphysiol.00493.2007. [DOI] [PubMed] [Google Scholar]
- 47.Locatelli J., de Assis L.V., Isoldi MC Calcium handling proteins: structure, function, and modulation by exercise. Heart Fail Rev. 2013 doi: 10.1007/s10741-013-9373-z. Accessed Feb 24, 2013. [DOI] [PubMed] [Google Scholar]
- 48.Rolim N.P., Medeiros A., Rosa K.T., Mattos K.C., Irigoyen M.C., Krieger E.M. Exercise training improves the net balance of cardiac Ca2+ handling protein expression in heart failure. Physiol. Genomics. 2007;29:246–252. doi: 10.1152/physiolgenomics.00188.2006. [DOI] [PubMed] [Google Scholar]
- 49.Kosola J., Ahotupa M., Kyröläinen H., Santtila M., Vasankari T. Both poor cardiorespiratory and weak muscle fitness are related to a high concentration of oxidized low-density lipoprotein lipids. Scand. J. Med. Sci. Sports. 2012;22:746–755. doi: 10.1111/j.1600-0838.2011.01326.x. [DOI] [PubMed] [Google Scholar]
- 50.Kosola J., Ahotupa M., Kyrolainen H., Santtila M., Vasankari T. Good aerobic or muscular fitness protects overweight men from elevated oxidized LDL. Med. Sci. Sports Exerc. 2012;44:563–568. doi: 10.1249/MSS.0b013e31823822cc. [DOI] [PubMed] [Google Scholar]
- 51.Moreno-Navarrete J.M., Ortega F., Serrano M., Guerra E., Pardo G., Tinahones F. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2013;98:E769–E778. doi: 10.1210/jc.2012-2749. [DOI] [PubMed] [Google Scholar]
- 52.McKenney J.M. Pharmacologic options for aggressive low-density lipoprotein cholesterol lowering: benefits versus risks. Am. J. Cardiol. 2005;96:60–66. doi: 10.1016/j.amjcard.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 53.Wassertheil-Smoller S., Psaty B., Greenland P., Oberman A., Kotchen T., Mouton C. Association between cardiovascular outcomes and antihypertensive drug treatment in older women. JAMA. 2004;292:2849–2859. doi: 10.1001/jama.292.23.2849. [DOI] [PubMed] [Google Scholar]
- 54.Parker B.A., Thompson P.D. Effect of statins on skeletal muscle: exercise, myopathy, and muscle outcomes. Exerc. Sport Sci. Rev. 2012;40:188–194. doi: 10.1097/JES.0b013e31826c169e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Toyama K., Sugiyama S., Oka H., Iwasaki Y., Sumida H., Tanaka T. Combination treatment of rosuvastatin or atorvastatin, with regular exercise improves arterial wall stiffness in patients with coronary artery disease. PLOS One. 2012;7:e41369. doi: 10.1371/journal.pone.0041369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Toyama K., Sugiyama S., Oka H., Iwasaki Y., Sumida H., Tanaka T. Rosuvastatin combined with regular exercise preserves coenzyme Q10 levels associated with a significant increase in high-density lipoprotein cholesterol in patients with coronary artery disease. Atherosclerosis. 2011;217:158–164. doi: 10.1016/j.atherosclerosis.2011.02.050. [DOI] [PubMed] [Google Scholar]
- 57.Agarwal S.K. Cardiovascular benefits of exercise. Int J Gen Med. 2012;5:541–545. doi: 10.2147/IJGM.S30113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paschalis V., Nikolaidis M.G., Theodorou A.A., Panayiotou G., Fatouros I.G., Koutedakis Y. A weekly bout of eccentric exercise is sufficient to induce health-promoting effects. Med. Sci. Sports Exerc. 2011;43:64–73. doi: 10.1249/MSS.0b013e3181e91d90. [DOI] [PubMed] [Google Scholar]