Abstract
Background
The purpose of this study is to assess the combined exercise programs (12 weeks' physical exercise training, resistance and aerobic) and 6 weeks' detraining on the correlation of metabolic syndrome (MS) markers and plasma adiponectin level in two groups.
Methods
Participants were divided into two groups [physical exercise training group (EG, n = 8) and control group (CG, n = 7)]. The EG performed a 12-week training program (aerobic and resistance training twice/wk, more than 40 min/d). After 12 weeks' exercise training and 6 weeks' detraining, we also evaluated MS markers and plasma adiponectin at three time periods (baseline, EBP; 12 weeks' exercise program, 12 EP; 12 weeks' and 6 weeks' detraining, 12 + 6 EDP) in overweight and obese children.
Results
Compared with the CG, After the 12 weeks' exercise treatment, weight, body mass index (BMI), waist girth, percent body fat, lean body mass (LBM), percentage lean body, systolic blood pressure, and insulin and homeostatic model assessment (HOMA) indices were lowered in the EG, and plasma adiponectin levels were not altered in the EG. After 6 weeks' detraining, insulin, insulin resistance, and plasma adiponectin levels were significantly increased in the EG. In the adiponectin level, there were positive correlations with LBM and percent lean body and negative correlations with percent body fat, insulin, and insulin resistance after 12 weeks' physical exercise intervention and 6 weeks' detraining.
Conclusion
These findings suggest that combined physical training is a useful tool in the management of MS markers in the training periods. Moreover, there was an additive effect even after the 6-weeks detraining period.
Keywords: adiponectin, detraining, overweight and obese children, physical exercise training
1. Introduction
Metabolic syndrome (MS) is a combined medical disorder that affects the risk of developing type 2 diabetes mellitus (T2DM) and cardiovascular diseases. It has been revealed that the estimated prevalence is up to 25% of the US population.1
It is well known that MS is the clustering of dyslipidemia, impaired insulin sensitivity, hypertension, obesity, T2DM, and cardiovascular disease.2, 3
It is well reported that regular physical exercise can enhance MS markers and decrease cardiovascular diseases and premature mortality.4 Also, various kinds of physical activity programs based either on aerobic exercise, resistance training, or a combined type of physical activity may promote insulin sensitivity and weaken or suppress MS in children.
Several sets of criteria are in use for MS diagnosis: atherogenic dyslipidemia, hypertension, elevated blood glucose, obesity, insulin sensitivity, and T2DM.5 Adiponectin level is also an important factor in estimating MS.
Adiponectin is known as an endogeneous insulin sensitizer, which plays an important role as a modulator of peroxisome proliferator activated receptor gamma action. It has a reverse correlation with obesity and serum levels.6
It has been found that plasma adiponectin plays a key role in regulating obesity, carbohydrate metabolism, and insulin resistance,7, 8 and it has been revealed that it decreases in obese individuals and T2DM patients.9, 10, 11
Plasma adiponectin also increases insulin sensitivity through enhancing fatty acid oxidation in skeletal muscle, liver, and blood vessels.12, 13 It has also been reported that exercise training improves insulin sensitivity activating 5′ adenosine monophosphate-activated protein kinase (AMPK) in skeletal muscle.14 Most of the previous researches have revealed the negative relationship between insulin level and plasma adiponectin level.11, 15 Several papers revealed that the adiponectin level increased meaningfully with the enhancement of insulin resistance.15, 16, 17, 18, 19, 20, 21 However, some research papers report that there is no connection between insulin sensitivity and adiponectin level. Other studies reported no change in plasma adiponectin level or even decreased rather than increased levels after exercise intervention, despite the enhanced level of insulin.18, 19, 22, 23, 24
Therefore, we can conclude that regular exercise training enhances MS with insulin resistance. Generally, there are two possible mechanisms: the first is adiponectin dependent, and the second is adiponectin independent. Physical exercise increases insulin sensitivity through AMP kinase pathway activation. It has recently been revealed that adiponectin also improves muscular insulin sensitivity through the same process. However, it is generally understood that several weeks of exercise training increases adiponectin levels with insulin sensitivity.
Further research is needed on the correlation between plasma exercise training and adiponectin level. However, little research has been carried out on the mechanisms and correlation of exercise and adiponectin responses. Therefore, in our study, we analyzed the relationships between plasma adiponectin level and insulin sensitivity following 12 weeks' exercise training and 6 weeks' detraining.
2. Methods
2.1. Participants
Fifteen young overweight [known body mass index (BMI) percentile 85•94] and obese children (known BMI percentile > 95) participated in the present study.25 The criteria for participating in the current research included no experience of cardiovascular disease,1, 2 no use of medical substances,3 no experiences of regular exercise training, and no smoking.
The 15 participants were unintentionally divided into two groups [exercise training group (EG, n = 8) and control group (CG, n = 7)] and three periods (baseline, EBP; 12 weeks' exercise program, 12 EP; 12 weeks' and 6 weeks' detraining, 12 + 6 EDP).
Informed consent was taken from the participants and their parents after they were given a detailed explanation about the study's purpose and methods. This experiment was approved by the Dong-Eui University and Pusan National University Ethics Committee.
2.2. Exercise protocol
The exercise program was chosen from a randomized and controlled clinical trial designed to study the combined effects of physical exercise programs. The combined exercise training program was composed of walking exercises and band exercises, which were supervised by many exercise specialists.
Walking exercise was practiced twice a week at 55•64% heart rate max (HRmax) and 65•75% HRmax, based on the Karvonen formula, for Weeks 1•6 and Weeks 7•12, respectively, as an aerobic exercise (30 min for 1•6 wk and 35 min for 7•12 wk).
Rubber band exercises were practiced twice a week as resistance training (50 min/d). Rubber band exercises were composed of nine exercises: squat, sit-up, seated row, knee extension, knee curl, seated leg press, overhead press, elbow curl, and bench press.
Each movement was performed at 70% of the maximal single repetition. Resistance exercise was increased slowly when the individuals could perform 20 repetitions without recess.
The participants increased the thickness of the rubber to increase the exercise intensity. This was accomplished by utilizing the different thicknesses of the band products.
All physiological variables were assessed at three periods (EBP, 12 EP, and 12 + 6 EDP).
2.3. Baseline, 12 weeks' exercise training, and 6 weeks' detraining measurements
Body weight and height were gauged using a digital scale while wearing pants but no shoes. Waist girth was also gauged using a tapeline between the iliac crest at the bottom of the rip.
Blood pressure was measured using an automatic device (DINMAP; Critikon, Inc., Lockbourne, OH, USA) and measured twice on arms after a 10-minute rest on the bench with an appropriate-sized cuff, and the mean value was used in the result.
Body composition was determined using dual-energy X-ray absorptiometry (DEXA, Lunar Prodigy; GE Medical Systems, Waukesha, WI, USA), a sensitive test for quantifying alterations in fat and lean mass in vivo26 and for evaluating regional fat distribution.27
For all individuals, blood samples were taken in the morning from the antecubital vein following a 12-hour overnight fast; fasting glucose, serum insulin, total cholesterol, triglyceride, and adiponectin levels were determined. Blood samples were taken at baseline, 12 weeks' training, and 6 weeks' detraining.
Blood samples were drawn into chilled tubes containing Na2EDTA (1 g/L) and aprotinin (500 U/mL). Extracted plasma was immediately isolated by centrifugation at 4 °C and kept at •80 °C until analyzed. Glucose levels were also gauged enzymatically (glucose oxidase) within 4 hours of collection, utilizing a Synchron LX 20 (Beckman Coulter Inc., Fullerton, CA, USA). Insulin levels were assayed by radioimmunoassay (RIA, Coat-A-Count; Diagnostic Products Corporation, Los Angeles, CA, USA), using antibody-coated tubes. The mean coefficients of variation (CVs) were 4.2% and 6.3% separately. The homeostatic model assessment (HOMA) index was also measured by using the following formula: fasting plasma glucose (mmol/L) í fasting plasma insulin (mU/L)/22.5.28
Plasma adiponectin was measured using an enzyme-linked immunosorbent assay (ELISA) kit (B-Bridge international, Inc., Sunnyvale, CA, USA). The mean CVs were 4.1% and 7.4% for adiponectin. High density lipoprotein cholesterol (HDL) and triglyceride levels were calculated utilizing a Synchron LX20 analyzer (Beckman Coulter, Fullerton, CA, USA).
2.4. Data analysis
In this study, we analyzed the relationships between plasma adiponectin level and insulin sensitivity following 12 weeks' physical exercise training and 6 weeks' detraining. The results were expressed as mean ± standard deviation (SD).
The Kolmogorov-Smirnov test (K-S test) was performed in advance to confirm normal distribution of each variable. We analyzed the data utilizing two-way repeated measures of analysis of variance (ANOVA) in the comparison study to analyze the effects of groups and periods. The correlation between plasma adiponectin and other variables was estimated by using Pearson's correlation coefficients. A p < 0.05 was considered significant.
All data analyses were performed using SPSS version 15.0 statistics program (SPSS Inc., Chicago, IL, USA).
3. Results
The measurements of MS markers and adiponectin levels after 12 weeks' exercise intervention and 6 weeks' detraining are shown in Table 1.
Table 1.
The changes in metabolic syndrome (MS) parameters and adiponectin after 12 weeks' exercise and 6 weeks' detraining.
| EG (n = 8) |
CG (n = 7) |
F* | F§ | |||||
|---|---|---|---|---|---|---|---|---|
| Pre (0 week) | Post (12 weeks) | Detraining (6 weeks) | Pre (0 week) | Post (12 weeks) | Detraining (16 weeks) | |||
| Weight (kg) | 54.44 ± 8.2 | 50.75 ± 7.31 | 52.10 ± 8.16 | 59.36 ± 5.88 | 60.83 ± 6.29 | 61.76 ± 6.35 | 57.57‡ | 36.03** |
| BMI (kg/m2) | 24.68 ± 2.68 | 22.41 ± 2.22 | 22.90 ± 2.42 | 25.32 ± 1.60 | 25.45 ± 1.72 | 25.71 ± 1.68 | 54.21‡ | 31.56** |
| Waist girth (cm) | 83.45 ± 3.75 | 82.40 ± 3.14 | 82.94 ± 2.87 | 83.86 ± 2.92 | 84.29 ± 2.53 | 84.14 ± 2.72 | 8.40† | 0.85 |
| BFM (kg) | 18.83 ± 3.62 | 14.92 ± 3.24 | 15.82 ± 3.56 | 21.23 ± 2.11 | 21.35 ± 2.63 | 21.85 ± 2.69 | 39.29‡ | 25.56** |
| PBF (%) | 34.49 ± 3.04 | 29.32 ± 4.25 | 30.20 ± 4.16 | 35.81 ± 2.11 | 35.11 ± 2.45 | 35.39 ± 2.47 | 26.09‡ | 16.84¶ |
| LBM (kg) | 34.01 ± 5.08 | 34.23 ± 5.25 | 34.64 ± 5.41 | 36.42 ± 4.17 | 37.70 ± 4.28 | 38.09 ± 4.09 | 4.75† | 5.43|| |
| PLB (%) | 62.59 ± 2.91 | 67.53 ± 4.12 | 66.66 ± 4.01 | 61.27 ± 2.06 | 61.94 ± 2.39 | 61.64 ± 2.24 | 22.99‡ | 15.22¶ |
| SBP (mmHg) | 100.38 ± 8.35 | 98.36 ± 6.01 | 101.75 ± 6.14 | 115.00 ± 101.29 | 107.71 ± 10.01 | 109.14 ± 9.84 | 6.41† | 3.69 |
| DBP (mmHg) | 60.88 ± 7.20 | 75.63 ± 8.62 | 71.25 ± 8.41 | 64.71 ± 5.22 | 86.29 ± 8.30 | 83.71 ± 8.58 | .98 | 2.87 |
| TG (mg/dL) | 110.63 ± 72.56 | 33.00 ± 12.07 | 74.13 ± 32.29 | 102.71 ± 74.61 | 74.14 ± 66.88 | 87.14 ± 66.44 | 3.40 | 0.57 |
| HDL-C (mg/dL) | 44.00 ± 12.02 | 56.37 ± 11.88 | 53.75 ± 7.59 | 41.57 ± 6.95 | 50.57 ± 12.58 | 52.86 ± 12.94 | 0.30 | 0.60 |
| Glucose (mg/dL) | 70.63 ± 8.26 | 90.38 ± 7.01 | 91.13 ± 4.38 | 73.00 ± 7.94 | 94.43 ± 7.14 | 95.43 ± 5.32 | 0.12 | 0.19 |
| Insulin (α/4IU/mL) | 15.68 ± 12.64 | 5.14 ± 1.01 | 6.86 ± 3.90 | 12.44 ± 6.13 | 13.87 ± 6.86 | 14.43 ± 14.20 | 5.58† | 2.53 |
| HOMA-IR index | 2.65 ± 2.04 | 0.90 ± 0.22 | 1.21 ± 0.71 | 2.22 ± 1.05 | 2.54 ± 1.37 | 2.71 ± 2.94 | 5.78† | 2.37 |
| Adiponectin (α/4g/mL) | 12.11 ± 4.95 | 12.16 ± 4.10 | 17.17 ± 5.91 | 14.06 ± 5.61 | 14.42 ± 4.27 | 13.6 ± 4.85 | 0.10 | 14.71¶ |
* Significant difference of interaction effect between group and time in two-way repeated ANOVA after 12 weeks' exercise treatment based on baseline, †p < 0.05, ‡p < 0.001.
§ Significant difference of interaction effect between group and time in two-way repeated ANOVA after 18 weeks' detraining based on baseline, ||p < 0.05, ¶p < 0.01, **p < 0.001.
ANOVA, analysis of variance; BMI, body mass index; BFM, body fat mass; CG, control group; DBP, diastolic blood pressure; EG, exercise group; HDL-C, high density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment-insulin resistance; LBM, lean body mass; PBF, percentage of body fat; PLB, percentage of lean body; SBP, systolic blood pressure; TG, triglyceride.
In the weight (p < 0.001), BMI (p < 0.001), waist girth (p < 0.05), fat mass (p < 0.001), % fat (p < 0.001), lean body mass (LBM, p < 0.05), % LBM (p < 0.001), systolic blood pressure (SBP, p < 0.05), insulin (p < 0.05), and HOMA-IR (p < 0.05) levels, there were significant differences between EG and CG following 12 weeks' exercise training. However, there were no significant differences between time and group in DBP, TG, HDL-C, blood glucose, and adiponectin.
After 6 weeks' detraining, group analyses showed significant differences in time í group interaction in several MS markers. They were significantly much improved in weight (p < 0.001), BMI (p < 0.001), fat mass (p < 0.001), % fat (p < 0.01), LBM (p < 0.05), % LBM (p < 0.01), and adiponectin (p < 0.01) compared to CG. However, there were no significant differences in time í group in waist girth, SBP, DBP, TG, HDL-C, blood glucose, insulin, and HOMA-IR.
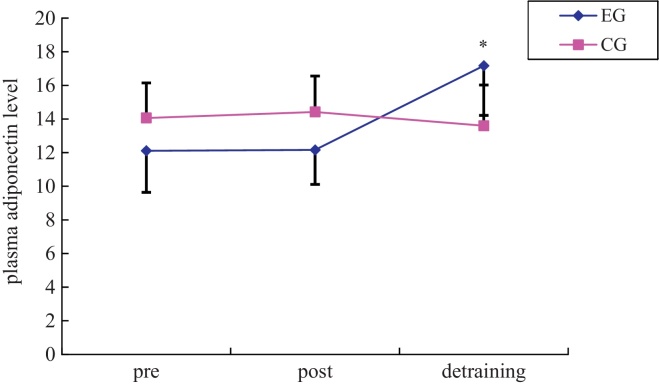
The correlations between adiponectin and MS markers are shown in Table 2. There was no correlation between adiponectin and MS markers after 12 weeks' physical exercise training and 6 weeks' detraining (12 weeks' exercise training + 6 weeks' detraining). However, there was a positive correlation in the 6-week detraining period (Fig. 1).
Table 2.
The correlations between plasma adiponectin concentration and metabolic syndrome (MS) markers.
| 0•12 weeks |
0•18 weeks |
12•18 weeks |
||||
|---|---|---|---|---|---|---|
| EG (n = 8) | CG (n = 7) | EG (n = 8) | CG (n = 7) | EG (n = 8) | CG (n = 7) | |
| Weight (kg) | •0.322 | •0.105 | 0.148 | •0.433 | 0.179 | •0.198 |
| BMI (kg/m2) | •0.196 | •0.023 | 0.099 | •0.362 | 0.218 | •0.394 |
| Waist circumference (cm) | 0.189 | 0.035 | 0.154 | •0.398 | 0.262 | 0.110 |
| SBP (mmHg) | 0.560 | 0.048 | •0.074 | •0.012 | •0.167 | •0.159 |
| DBP (mmHg) | 0.376 | 0.067 | •0.142 | •0.614 | 0.335 | 0.745 |
| BFM (kg) | •0.415 | •0.595 | 0.128 | •0.561 | •0.489 | •0.323 |
| PBF (%) | •0.330 | •0.732 | 0.252 | •0.462 | •0.717* | •0.327 |
| LBM (kg) | 0.157 | 0.534 | 0.005 | 0.088 | 0.792* | 0.167 |
| Percentage of lean body (%) | 0.315 | 0.733 | •0.270 | 0.476 | 0.732* | 0.315 |
| TG (mg/dL) | 0.591 | •0.264 | 0.628 | 0.013 | •0.531 | •0.594 |
| HDL-C (mg/dL) | •0.064 | •0.247 | •0.252 | •0.406 | 0.281 | •0.117 |
| Blood glucose (mg/dL) | •0.376 | •0.528 | •0.183 | •0.717 | •0.083 | •0.710 |
| Insulin (α/4IU/mL) | •0.075 | 0.313 | 0.295 | 0.763 | •0.750* | 0.425 |
| HOMA-IR index | •0.109 | 0.298 | 0.319 | 0.744 | •0.728* | 0.432 |
, baseline data minus data at 12 weeks and baseline data minus data at detraining (18 weeks) and 12 weeks data minus data at detraining (18 weeks).
* p < 0.05.
BFM, body fat mass; CG, control group; DBP, diastolic blood pressure; EG, exercise group; HDL-C, high density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment-insulin resistance; LBM, lean body mass; PBF, percentage of body fat; SBP, systolic blood pressure; TG, triglyceride.
Fig. 1.
Changes in adiponectin.
* Significant difference of interaction effect between groups and times in two-way repeated ANOVA after 12 weeks' exercise training and 6 weeks' detraining based on baseline, p < 0.01.
ANOVA, analysis of variance; CG, control group; EG, exercise group.
There were positive correlations between adiponectin and LBM (r = 0.792, p < 0.05) and percent of lean body (r = •0.732, p < 0.05); there were negative correlations with percent body fat (r = •0.717, p < 0.05), insulin (r = •0.750, p < 0.05), and insulin resistance (r = •0.728, p < 0.05) after 6 weeks' detraining.
4. Discussion
Adiponectin is an adipose-specific secretory protein that shows great potential as a novel mediator, having an anti-atherogenic properties. It enhances the peripheral insulin sensitivity and affects beta-cell function.29, 30
It is well published that adiponectin is significantly decreased in rodents,31 monkeys,32 and human patients with obesity and T2DM patients,10, 11, 33 and also in patients with coronary artery disease.34 There is a reverse correlation between adiponectin level and obesity-related factors such as fasting plasma insulin, abnormal oral glucose tolerance, fat distribution, and percentage body fat, and a positive correlation with glucose disposal during a euglycemic insulin clamp.10, 11, 31, 33
It is generally understood that plasma adiponectin levels increased after weight loss caused by dietary therapy. Also, levels were reduced in patients with T2DM,11 obesity,9, 35 and coronary artery disease.36
According to some research papers, it is reported that plasma adiponectin level is increased after flexibility exercise and aerobic exercise intervention.19, 37
On the contrary, Boudou et al22 reported that plasma adiponectin level was not changed despite reduced abdominal fat and increased insulin sensitivity without a significant alteration in body weight after 8 weeks' exercise intervention in 16 middle-aged men with T2DM.
Although regular exercise training is used as a useful tool to ameliorate obesity, insulin resistance, and weight control, only a few research studies have been executed on the relationship between adiponectin level and physical exercise training.38
In these experiments, adiponectin levels increased or remained constant following exercise training. Therefore, we need to further investigate the mechanism behind the correlation between plasma adiponectin level and physical exercise training.
Moreover, in this paper, we evaluated the adiponectin and metabolic markers in two periods: 12 weeks' exercise training and 6 weeks' detraining. The main finding of this investigation was that 12 weeks' combined training improved waist circumference, body weight, body fat, BMI, percent body fat, insulin, and HOMA-IR without adiponectin. A previous study showed that exercise training improved MS markers for insulin sensitivity.39
Adiponectin levels did not vary after 12 weeks of physical training in the present research. However, insulin sensitivity improved in obese children. Similar results have been revealed in other papers.40 The beneficial effects of 6 weeks' detraining on MS markers and adiponectin were observed. After 6 weeks' detraining, there was an additive effect of weight, BMI, body fat, and percent body fat.
An amusing finding of the present investigation is that adiponectin was much improved following 6 weeks' detraining not following the 12 weeks' exercise training. These results are also in accordance with those of previous experiments on other overweight and obese children that exercise training induced enhancements in body fat, waist circumference, body weight, BMI, and insulin sensitivity without adiponectin.41
Detraining has been redefined as the complete or partial loss of training-induced psychological, anatomical, and physiological performance adaptation, as an outcome of training reduction or training cessation.42 This is consistent with another research paper where detraining increased the level of adiponectin through increased insulin sensitivity and glucose transporters.43 In another study, body fat mass was significantly improved after 8 weeks of physical exercise training in young obese men.
BMI, weight, percent body fat, and body fat mass were reported to be significantly improved after 5 months of combined exercise (aerobic exercise and resistance exercise) training, whereas there was no alteration in insulin sensitivity and adiponectin in both groups.44 Generally, plasma adiponectin levels have been shown to reduce simultaneously with the development of insulin resistance.32
According to this study, after 12 weeks' combined physical exercise intervention, some MS markers and insulin resistance were improved significantly but plasma adiponectin levels were not altered statistically. However, after 6 weeks' detraining, plasma adiponectin levels significantly increased. We find that adiponectin level is altered after 12 weeks' exercise and 6 weeks' detraining. With such results showing similarity in the negative relationship between insulin resistance and adiponectin (and its process of metabolism), it is clear that exercise enhances muscle and blood functions and also improves insulin sensitivity through activation of AMPK in skeletal muscle.22
After 6 weeks' detraining, there was a positive correlation between lean body mass and percent lean body with adiponectin, whereas there was a negative correlation between adiponectin and percent body fat, insulin, and insulin resistance. With such results, we find that decreased insulin promotes transportation of glucose, and then it influences the increase in plasma adiponectin levels.45 Also, plasma adiponectin level has been correlated with percent body fat and BMI.9, 46
These results suggest that combined physical training is not only an effective tool in the management of syndrome markers in the training periods but also these changes were retained or even accumulated after 6 weeks' detraining. Therefore, we can conclude that there is an enhanced adiponectin effect in the detraining period.
Conflict of Interest
All contributing authors declare no conflicts of interest.
References
- 1.Ford E.S., Giles W.H., Dietz W.H. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 2.Lakka H.M., Laaksonen D.E., Lakka T.A., Niskanen L.K., Kumpusalo E., Tuomilehto J. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 3.Wilson P.W., D'Agostino R.B., Parise H., Sullivan L., Meigs J.B. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–3072. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 4.Stensvold D., Tjonna A.E., Skaug E.A., Aspenes S., Stølen T., Wisloff U. Strength training versus aerobic interval training to modify risk factors of the metabolic syndrome. J Appl Physiol. 2010;108:804–810. doi: 10.1152/japplphysiol.00996.2009. [DOI] [PubMed] [Google Scholar]
- 5.Nam J.S., Park J.S., Cho M.H., Jee S.H., Lee H.S., Ahn C.W. The association between pulse wave velocity and metabolic syndrome and adiponectin in patients with impaired fasting glucose: cardiovascular risks and adiponectin in IFG. Diabetes Res Clin Pract. 2009;84:145–151. doi: 10.1016/j.diabres.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Ziemke F., Mantzoros C.S. Adiponectin in insulin resistance: lessons from translational research. Am J Clin Nutr. 2010;91:258S–261S. doi: 10.3945/ajcn.2009.28449C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koerner A., Kratzsch J., Kiess W. Adipocytokines: leptin the classical, resistin the controversical, adiponectin the promising, and more to come. Best Pract Res Clin Endocrinol Metab. 2005;19:525–546. doi: 10.1016/j.beem.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Yamauchi T., Kamon J., Waki H., Terauchi Y., Kubota N., Hara K. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 9.Arita Y., Kihara S., Ouchi N., Takahashi M., Maeda K., Miyagawa J. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 10.Hotta K., Funahashi T., Bodkin N.L., Ortmeyer H.K., Arita Y., Hansen B.C. Circulating concentrations of the adiposite protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression too type 2 diabetes in rhesus monkeys. Diabetes. 2001;50:1126–1133. doi: 10.2337/diabetes.50.5.1126. [DOI] [PubMed] [Google Scholar]
- 11.Weyer C., Funahashi T., Tanaka S., Hotta K., Matsuzawa Y., Pratley R.E. Hypo-adiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyper-insulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 12.Schulze M.B., Rimm E.B., Shai I., Rifai N., Hu F.B. Relationship between adiponectin and glycemic control, blood lipids, and inflammatory markers in men with type 2 diabetes. Diabetes Care. 2004;27:1680–1687. doi: 10.2337/diacare.27.7.1680. [DOI] [PubMed] [Google Scholar]
- 13.Wang H., Zhang H., Jia Y., Zhang Z., Craig R., Wang X. Adiponectin receptor 1 gene (ADIPOR1) as a candidate for type 2 diabetes and insulin resistance. Diabetes. 2004;53:2132–2136. doi: 10.2337/diabetes.53.8.2132. [DOI] [PubMed] [Google Scholar]
- 14.Musi N., Fujii N., Hirshman M.F., Ekberg I., Fröberg S., Ljungqvist O. AMP-activated protein kinase (AMPK) is activated in muscle of subjects with type 2 diabetes during exercise. Diabetes. 2001;50:921–927. doi: 10.2337/diabetes.50.5.921. [DOI] [PubMed] [Google Scholar]
- 15.Kriketos A.D., Gan S.K., Poynten A.M., Furler S.M., Chisholm D.J., Campbell L.V. Exercise increases adiponectin levels and insulin sensitivity in humans. Diabetes Care. 2004;27:629–630. doi: 10.2337/diacare.27.2.629. [DOI] [PubMed] [Google Scholar]
- 16.Kondo T., Kobayashi I., Murakami M. Effect of exercise on circulating adipokine levels in obese young women. Endocr J. 2006;53:189–195. doi: 10.1507/endocrj.53.189. [DOI] [PubMed] [Google Scholar]
- 17.Blñ/4her M., Bullen J.W., Jr., Lee J.H., Kralisch S., Fasshauer M., Klöting N. Circulating adiponectin and expression of adiponectin receptors in human skeletal muscle: associations with metabolic parameters and insulin resistance and regulation by physical training. J Clin Endocrinol Metab. 2006;91:2310–2316. doi: 10.1210/jc.2005-2556. [DOI] [PubMed] [Google Scholar]
- 18.Reinehr T., Roth C., Menke T., Andler W. Adiponectin before and after weight loss in obese children. J Clin Endocrinol Metab. 2004;89:3790–3794. doi: 10.1210/jc.2003-031925. [DOI] [PubMed] [Google Scholar]
- 19.Balagopal P., George D., Yarandi H., Funanage V., Bayne E. Reversal of obesity-related hypoadiponectinemia by lifestyle intervention: a controlled, randomized study in obese adolescents. J Clin Endocrinol Metab. 2005;90:6192–6197. doi: 10.1210/jc.2004-2427. [DOI] [PubMed] [Google Scholar]
- 20.Esposito K., Pontillo A., Di Palo C., Giugliano G., Masella M., Marfella R. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003;289:1799–1804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 21.Carrel A.L., McVean J.J., Clark R.R., Peterson S.E., Eickhoff J.C., Allen D.B. School-based exercise improves fitness, body composition, insulin sensitivity, and markers of inflammation in non-obese children. J Pediatr Endocrinol Metab. 2009;22:409–415. doi: 10.1515/jpem.2009.22.5.409. [DOI] [PubMed] [Google Scholar]
- 22.Boudou P., Sobngwi E., Mauvais-Jarvis F., Vexiau P., Gautier J.F. Absence of exercise-induced variations in adiponectin levels despite decreased abdominal adiposity and improved insulin sensitivity in type 2 diabetic men. Eur J Endocrinol. 2003;149:421–424. doi: 10.1530/eje.0.1490421. [DOI] [PubMed] [Google Scholar]
- 23.Hulver M.W., Zheng D., Tanner C.J., Houmard J.A., Kraus W.E., Slentz C.A. Adiponectin is not altered with exercise training despite enhanced insulin action. Am J Physiol Endocrinol Metab. 2002;283:E861–E865. doi: 10.1152/ajpendo.00150.2002. [DOI] [PubMed] [Google Scholar]
- 24.Panagopoulou P., Galli-Tsinopoulou A., Fleva A., Pavlitou-Tsiontsi E., Vavatsi-Christaki N., Nousia-Arvanitakis S. Adiponectin and insulin resistance in childhood obesity. J Pediatr Gastroenterol Nutr. 2008;47:356–362. doi: 10.1097/MPG.0b013e31817fcb67. [DOI] [PubMed] [Google Scholar]
- 25.Hong Y.M., Moon K.R., Seo J.W., Sim J.G., Yoo K.W., Jeong B.J. Guideline of diagnosis and treatment in childhood obesity. J Kor Pediat Soc. 1999;42:1338–1345. [In Korean, English Abstract] [Google Scholar]
- 26.Going S.B., Massett M.P., Hall M.C., Bare L.A., Root P.A., Williams D.P. Detection of small changes in body composition by dual-energy X-ray absorptiometry. Am J Clin Nutr. 1993;57:845–850. doi: 10.1093/ajcn/57.6.845. [DOI] [PubMed] [Google Scholar]
- 27.Taylor R.W., Keil D., Gold E.J., Williams S.M., Goulding A. Bodymass index, waist girth, and waist-to-hip ratio as indexes of total and regional adiposity in women: evaluation using receiver operating characteristic curves. Am J Clin Nutr. 1998;67:44–49. doi: 10.1093/ajcn/67.1.44. [DOI] [PubMed] [Google Scholar]
- 28.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and beta cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 29.Moller D.E. Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol Metab. 2000;11:212–217. doi: 10.1016/s1043-2760(00)00272-1. [DOI] [PubMed] [Google Scholar]
- 30.Shulman G.I. Cellular mechanisms of insulin resistance in humans. Am J Cardiol. 1999;84(Suppl 1):3–10. doi: 10.1016/s0002-9149(99)00350-1. [DOI] [PubMed] [Google Scholar]
- 31.Hu E., Liang P., Spiegelman B.M. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 32.Hotta K., Funahashi T., Arita Y., Takahashi M., Matsuda M., Okamoto Y. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 33.Statnick M.A., Beavers L.S., Conner L.J., Corominola H., Johnson D., Hammond C.D. Decreased expression of apM1 in abdominal and subcutaneous adipose tissue of humans with type 2 diabetes. Int J Exp Diabetes Res. 2000;1:81–88. doi: 10.1155/EDR.2000.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ouchi N., Kihara S., Arita Y., Maeda K., Kuriyama H., Okamoto Y. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 35.Kern P.A., Di Gregorio G.B., Lu T., Rassouli N., Ranganathan G. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes. 2003;52:1779–1785. doi: 10.2337/diabetes.52.7.1779. [DOI] [PubMed] [Google Scholar]
- 36.Kumada M., Kihara S., Sumitsuji S., Kawamoto T., Matsumoto S., Ouchi N. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–89. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 37.Expósito J., Hernández J., Fernández Feijóo A., Nieto T., Briones E. New chemotherapy treatments in advanced cancer patients: an easily applicable evaluation of clinical efficacy and cost-effectiveness. Acta Oncol. 2003;42:895–902. doi: 10.1080/02841860310018981. [DOI] [PubMed] [Google Scholar]
- 38.Nemet D., Wang P., Funahashi T., Matsuzawa Y., Tanaka S., Engelman L. Adipocytokines, body composition, and fitness in children. Pediatr Res. 2003;53:148–152. doi: 10.1203/00006450-200301000-00025. [DOI] [PubMed] [Google Scholar]
- 39.Rochlitz H., Akpulat S., Bobbert T., Mai K., Möhlig M., Osterhoff M. Significance of biomarkers for metabolic syndrome during weight reduction. Dtsch Med Wochenschr. 2005;130:1061–1066. doi: 10.1055/s-2005-866789. [DOI] [PubMed] [Google Scholar]
- 40.Xydakis A.M., Case C.C., Jones P.H., Hoogeveen R.C., Liu M.Y., Smith E.O. Adiponectin, inflammation, and the expression of the metabolic syndrome in obese individuals: the impact of rapid weight loss through caloric restriction. J Clin Endocrinol Metab. 2004;89:2697–2703. doi: 10.1210/jc.2003-031826. [DOI] [PubMed] [Google Scholar]
- 41.Nassis G.P., Papantakou K., Skenderi K., Triandafillopoulou M., Kavouras S.A., Yannakoulia M. Aerobic exercise training improves insulin sensitivity without changes in body weight, body fat, adiponectin, and inflammatory markers in overweight and obese girls. Metabolism. 2005;54:1472–1479. doi: 10.1016/j.metabol.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 42.Mujika I., Padilla S. Detraining: loss of training-induced physiological and performance adaptations. Part I: short term insufficient training stimulus. Sports Med. 2000;30:79–87. doi: 10.2165/00007256-200030020-00002. [DOI] [PubMed] [Google Scholar]
- 43.Mostarda C., Rogow A., Silva I.C., De La Fuente R.N., Jorge L., Rodrigues B. Benefits of exercise training in diabetic rats persist after three weeks of detraining. Auton Neurosci. 2009;145(1•2):11–16. doi: 10.1016/j.autneu.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 44.Hara T., Fujiwara H., Nakao H., Mimura T., Yoshikawa T., Fujimoto S. Body composition is related to increase in plasma adiponectin levels rather than training in young obese men. Eur J Appl Physiol. 2005;94(5•6):520–526. doi: 10.1007/s00421-005-1374-8. [DOI] [PubMed] [Google Scholar]
- 45.Motoshima H., Wu X., Sinha M.K., Hardy V.E., Rosato E.L., Barbot D.J. Differential regulation of adiponectin secretion from cultured human abdomenal and subcutaneous adipocytes: effects of insulin and rosiglitazone. J Clin Endocrinol Metab. 2002;87:5662–5667. doi: 10.1210/jc.2002-020635. [DOI] [PubMed] [Google Scholar]
- 46.Hara T., Fujiwara H., Shoji T., Mimura T., Nakao H., Fujimoto S. Decreased plasma adiponectin levels in young obese males. J Atheroscler Thromb. 2003;10:234–238. doi: 10.5551/jat.10.234. [DOI] [PubMed] [Google Scholar]