Abstract
Background
Orodispersible tablets or fast dissolving tablets dissolve or disintegrate immediately on the patients’ tongue or buccal mucosa. This drug delivery system is suitable for drugs undergoing high first pass metabolism. It improves bioavailability, reduces dosing frequency, and thereby minimizes the side effects and also makes the dosage form more cost-effective. In this study, polysaccharide isolated from the seeds of Cassia tora was investigated as a superdisintegrant in the orodispersible tablets. The model drug chosen was valsartan, an antihypertensive drug.
Methods
Valsartan tablets were prepared separately using different concentrations (1%, 2.5%, 5%, and 7.5% w/w) of isolated C. tora seed polysaccharide (natural) and sodium starch glycolate (synthetic) as superdisintegrant by the direct compression method. Evaluation of tablets was done for various pre- and postcompression parameters. The stability studies were performed on optimized formulation F4. The disintegration time and in vitro drug release of the formulation F4 were compared with marketed formulations (conventional tablets).
Results
The drug excipient interactions were characterized by Fourier transform infrared studies. The formulation F4 containing 7.5% polysaccharide showed good wetting time and disintegration time as compared to a formulation prepared using a synthetic superdisintegrant at the same concentration level. Hence, batch F4 was considered optimized formulation.
Conclusion
The present work revealed that C. tora seed polysaccharide has a good potential as a disintegrant in the formulation of orodispersible tablets. Because C. tora polysaccharide is inexpensive as compared to synthetic superdisintegrants, nontoxic, compatible, and easy to manufacture, it can be used in place of currently marketed superdisintegrants.
Keywords: Cassia tora, direct compression, orodispersible tablets, patient compliance, sodium starch glycolate
1. Introduction
Tablets and capsules are the most extensively used and widely acceptable dosage forms. However, they face a drawback for some patients because of the difficulty to swallow. Tablets that dissolve rapidly in the oral cavity have become very popular in recent times. Orodispersible tablets (ODTs) represent a rapidly emerging drug delivery system with better patient compliance. ODTs are used for people who have swallowing difficulties as well as for active people.1, 2, 3
ODTs disintegrate in the patient's mouth within a few seconds and are ideal for patients having dysphasia.4, 5 Some drugs are absorbed from the mouth, pharynx, and esophagus as the saliva passes down the stomach, which leads to an increase in bioavailability. The advantages of mouth dissolving dosage form are increasingly being acknowledged in both industry and academia.6 ODTs are commonly known as orally disintegrating tablets, mouth dissolving tablets, fast dissolving tablets, or rapid melt tablets.
The additives used to convert active pharmaceutical ingredients into pharmaceutical dosage form are known as excipients.7 Excipients from natural sources have an advantage over synthetic excipients in that they are locally accessible, nonpolluting, biocompatible, and cheap as compared to imported synthetic products. Herbs are renewable resources for maintainable supplies of cheaper pharmaceutical products.8, 9, 10, 11, 12, 13, 14, 15
Fast disintegrating tablets are made by a direct compression method using superdisintegrants as an important component. Disintegrants are the substances or mixture of substances added to the drug formulation that helps in the disintegration of the tablet content into smaller particles that dissolve more rapidly than tablets without disintegrants. Examples of superdisintegrants are croscarmelose, crospovidone, and sodium starch glycolate (SSG), which symbolize the example of crosslinked cellulose, crosslinked polymer, and a crosslinked starch, respectively.16, 17 These are the commonly used synthetic origin superdisintegrants. Various natural origin substances such as karaya, modified starch, and agar have been used in the formulations of ODTs. The natural origin substances are comparatively cheaper with certain desired properties such as abundantly available, nonirritating, and nontoxic in nature.19 They have several advantages over synthetic superdisintegrants, such as ease of isolation, local availability, and biocompatibility.
Cassia tora (Family: Leguminosae) is an annual under shrub commonly found in India and other tropical countries.20 Seeds of C. tora contain several anthraquinones and sennosoids.21, 22, 23 It has been used for treatment of asthma and to improve visual activity.24 It has anti-inflammatory and hepatoprotective activities.25 Gum obtained from the seeds of C. tora is known as “Panwar gum”. Chemically, it is a neutral heteropolysaccharide of galactose and mannose (i.e., galactomannans). The pH of the Panwar gum mucilage is approximately 7.20 A literature survey revealed that the C. tora polysaccharide has not been used to date as a disintegrant, and hence this polysaccharide was selected for the present investigation. The present study deals with the investigation of a polysaccharide isolated from the seeds of C. tora as a superdisintegrant in ODTs and compares its disintegrating potential with synthetic superdisintegrants.
The model drug used for the study was valsartan, which is an antihypertensive drug belonging to the category of angiotensin II receptor antagonist. The molecular weight of valsartan is 435.5, and its half-life is 4–6 hours; it has poor oral bioavailability (ranging from 10 to 35), because of poor solubility, dissolution, 95% of the drug undergoes protein binding, and most importantly extensive first pass hepatic metabolism.26, 27 The present research work was aimed at the development and characterization of ODTs of valsartan using a novel superdisintegrant to produce a rapid onset of action and patient compliance.
2. Methods
2.1. Materials
Valsartan was obtained from Unichem Laboratories Ltd., Mumbai, India as a gift sample. Microcrystalline cellulose (MCC PH 102), SSG, magnesium stearate, talc, aspartame, and vanilla flavor were obtained from Colorcon Asia Pvt. Ltd. (Mumbai, India). C. tora seeds were collected from the Maharashtra region (India) in the month of October. The plant material was authenticated at the Blatter herbarium. The specimen sample of the plant is preserved with the Department of Quality Assurance, Dr. L.H. Hiranandani College of Pharmacy, Ulhasnagar, India. All chemicals and reagents used in this study were of analytical grade.
2.2. Isolation of polysaccharide from C. tora seeds
The seeds of C. tora were dry milled in a mixer to separate the endosperm from the seed coat. The endosperm of C. tora seeds (100 g) was soaked in distilled water and shaken for 4–5 hours. The viscous solution obtained was filtered through a muslin cloth. Precipitation of the mucilage (polysaccharide) was carried out with the addition of 95% ethanol (1:1 ratio) by continuous stirring. The precipitated polysaccharide was transferred to an evaporating dish and treated consecutively with ethanol. The polysaccharide obtained was dried in oven at 40–45 °C. It was then powdered and passed through sieve number 60 and stored in an airtight container. The isolated polysaccharide was characterized for various physicochemical properties such as solubility, pH (1% w/w in water), swelling index, loss on drying, ash value, bulk and tapped density, compressibility index, Hausner's ratio, and angle of repose as per the reported method.28, 29
2.3. Formulation of ODTs
ODTs of valsartan were prepared with the direct compression method using isolated polysaccharide and synthetic superdisintegrant at concentrations of 1%, 2.5%, 5%, and 7.5% w/w. All the ingredients were passed through a 60 mesh sieve. A weighed quantity of each ingredient was taken, and the blend (powder mix) was uniformly mixed and compressed into tablets of 200 mg using 9-mm round flat punches on a single station rotary tablet machine (Royal artist, Mumbai, India). The composition of each formulation is given in Table 1.
Table 1.
Composition of orodispersible tablet
| Components mg/tablet | Formulation code |
|||||||
|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | |
| Drug | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 |
| Microcrystalline cellulose PH102 | 151.2 | 148.2 | 143.2 | 138.2 | 151.2 | 148.2 | 143.2 | 138.2 |
| Cassia tora polysaccharide | 2 | 5 | 10 | 15 | – | – | – | – |
| Sodium starch glycolate | – | – | – | – | 2 | 5 | 10 | 15 |
| Aspartame | 2.8 | 2.8 | 2.8 | 2.8 | 2.8 | 2.8 | 2.8 | 2.8 |
| Vanilla | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Talc | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Magnesium stearate | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Total weight | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 |
2.4. Evaluation of powder blend (precompression parameters)
The powder mix was evaluated for various flow properties such as angle of repose, bulk and tapped density, Hausner's ratio, and Carr's index.
2.4.1. Angle of repose
The angle of repose of powder was carried out using the fixed funnel method. The accurately weighed quantity of powder mix was taken in a funnel. The height of the funnel was maintained in such a way that the tip of the funnel just touched the apex of the heap of the powder. The powder was allowed to flow through the funnel without any resistance on to the surface. The diameter and height of the powder cone was measured. The angle of repose was determined using the following equation:
where h and r are the height and radius of the powder cone, respectively.
2.4.2. Bulk density and tapped density
Powder weighing 5 g from each formula was introduced into a 25-mL measuring cylinder. It was initially shaken lightly to break any agglomerates that may have formed. The initial volume was noted, and the cylinder was allowed to fall under its own weight onto a hard surface from the height of 2.5 cm at 2-second intervals. The tapping was continued until a constant volume was observed.30 LBD (Loose bulk density) and TBD (Tapped bulk density) were calculated using the following formulas:
2.4.3. Compressibility index and Hausner's ratio
The following formula was used to determine the compressibility index of granules:
Hausner's ratio was calculated by the following formula:
2.5. Evaluation of tablets (postcompression parameters)
2.5.1. Tablet hardness
Hardness is a vital parameter that prevents breakage of tablets during transportation, handling, and storage. The hardness of tablet was measured with the Monsanto hardness tester and was expressed in terms of kg/cm2.
2.5.2. Tablet thickness
The tablet was placed between the two arms of the Vernier caliper, and thickness was determined. Five measurements were taken.
2.5.3. Weight variation
Twenty tablets were selected arbitrarily from each formulation and weighed individually using a digital balance (Shimadzu Corporation, Japan; Model No. BL 220H). The individual weights were noted and compared with the average weight for the weight variation.31
2.5.4. Friability
Twenty tablets were weighed and then placed in a plastic chambered friabilator USP type Roche friabilator (Pharmalab, Ahmedabad, India) attached to a motor revolving at a speed of 25 rpm for 4 minutes. The tablets were reweighed, and the percentage weight loss (friability) was calculated using the following formula:
2.5.5. Drug content
Ten tablets were weighed and crushed to a fine powder, and a quantity of powder equivalent to 40 mg of valsartan was introduced into a 100-mL volumetric flask and extracted using pH 6.8 phosphate buffer. The solution obtained was filtered, and the filtrate was suitably diluted with pH 6.8 phosphate buffer. The valsartan content was determined by measuring the absorbance at 250 nm using a UV–Visible Spectrophotometer (UV-1800 with UV–Probe Version 2.34 software, Shimadzu Corporation, Japan). The drug content was determined using the standard calibration curve.32 The mean percent drug content was calculated as an average of three determinations.
2.5.6. Wetting time and water absorption ratio (R)
A tissue paper was taken and folded twice and placed in a Petri dish (with an internal diameter of 5 cm) containing 6 mL of water. A tablet was cautiously placed on the top of the tissue paper in the Petri dish. Wetting time was noted as the time required for water to reach the upper surface of the tablet and to completely wet it.33 Water absorption ratio (R) was then determined according to the following equation:
where wb and wa denote the tablet weights before and after water absorption, respectively.
2.5.7. In vitro disintegration time
The tablet disintegration test apparatus was used to determine the disintegration time for all formulations. Six tablets were placed individually in each tube of disintegration test apparatus. The medium was maintained at a temperature of 37 ± 2 °C, and the time was noted for the entire tablet to disintegrate completely.
2.5.8. In vitro dissolution
The USP dissolution test apparatus (Electrolab TDT - 08 L Dissolution testers USP) type 2 (paddle) was used for the study. First, 900 mL of the phosphate buffer pH 6.8 was taken in a vessel, and the temperature was maintained at 37 ± 0.5 °C. The speed of the paddle was fixed at 50 rpm. Dissolution samples were withdrawn at 2-minute intervals, and drug content was determined by measuring the absorbance at 250 nm.34 Drug concentration was calculated from the standard calibration curve and expressed as cumulative percent drug dissolved. In vitro dissolution study was also performed similarly on a conventional tablet formulation (Valzaar, Torrent Pharmaceuticals Ltd., India).
2.5.9. Drug–excipient interaction study
The pure drug, mixture of drug with polysaccharide (1:1), and the optimized formulations (mixture of drug with various excipients used in the preparation of ODT formulation) were characterized by Fourier transform infrared spectroscopy so the compatibility can be determined. The scanning range was 500–4000 cm–1 and the IR spectra of samples were obtained using the KBr disk method.
2.5.10. Stability studies
The stability study of the tablets was carried out by keeping the samples in the stability chamber at 40 ± 20 °C/75 ± 5% RH for 3 months as per the ICH (International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use) guidelines. The optimized batch was selected for stability studies. The tablets were evaluated for hardness, friability, drug content (Assay), disintegration time, and in vitro drug release profile after a 1-month interval.
3. Results
The polysaccharide isolated from C. tora seeds was a light brown colored powder (yield = 18.56% w/w). The polysaccharide was soluble in hot water forming a colloidal solution and practically insoluble in organic solvents. The pH of 1% w/w solution of polysaccharide was found to be near neutral. The polysaccharide showed good swelling and water absorption capacity. The Carr's index and angle of repose indicated that the polysaccharide has a good flow with moderate compressibility. The losses on drying and ash values were well within official limits. The results of the physicochemical characterization of polysaccharide are reported in Table 2.
Table 2.
Physicochemical parameters of polysaccharide
| Parameters | Results |
|---|---|
| State | Solid |
| Color | Light brown |
| PH | 7.2–7.5 |
| Solubility | Soluble in hot water forming colloidal solution insoluble in organic solvent |
| Swelling factor | 11.5 mL |
| Practical yield | 18.56% w/w |
| Total ash | 1.47 |
| Moisture content | 1.2% |
| Total polysaccharide content | 72.12% w/w |
| Angle of repose | 29.24° |
| Bulk density | 0.64 g/cm3 |
| Tapped density | 0.53 g/cm3 |
| Carr's index | 17.16 |
| Hausner's ratio | 1.20 |
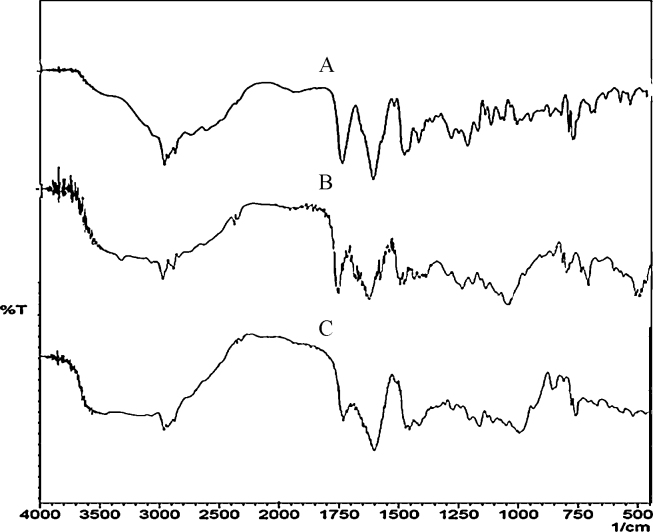
Drug–excipient interaction studies revealed that there was no physicochemical interaction between valsartan and other excipients (Fig. 1). All the major peaks of valsartan were found in the sample. The functional peaks include C–H stretch (aliphatic) at 2962.66 cm−1, N–H stretch at 3444.87 cm−1, carboxylic acid stretch at 1732.08 cm−1, C = O stretch at 1600.02 cm−1, and C–N stretch at 1273.02 cm−1, 1205.51 cm−1, and 1064.71 cm−1. The complex region of 900–600 cm−1 indicates skeletal vibration and an aromatic ring in the drug structure. The weights of all the prepared tablets were within the acceptable limits for uncoated tablets as per the United States Pharmacopoeia. The results of precompression parameter evaluation indicated good free flowing properties of the powder blend (Table 3).
Fig. 1.
Fourier transform infrared spectrum of (A) pure drug, (B) pure drug + polysaccharide, and (C) optimized formulation F4.
Table 3.
Precompression parameters of powder blend
| Formulation code | Parameters |
||||
|---|---|---|---|---|---|
| Angle of repose (θ) | Bulk density (g/cm3) | Tapped density (g/cm3) | Carr's index (%) | Hausner's ratio | |
| F1 | 30.15 ± 1.21 | 0.50 ± 0.005 | 0.646 ± 0.009 | 22.67 ± 1.40 | 1.28 ± 0.025 |
| F2 | 29.20 ± 0.42 | 0.508 ± 0.002 | 0.637 ± 0.0098 | 20.37 ± 1.61 | 1.24 ± 0.031 |
| F3 | 28.78 ± 1.29 | 0.493 ± 0.03 | 0.654 ± 0.012 | 24.7 ± 1.66 | 1.32 ± 0.030 |
| F4 | 28.03 ± 1.20 | 0.491 ± 0.015 | 0.651 ± 0.009 | 24.54 ± 0.78 | 1.32 ± 0.015 |
| F5 | 27.65 ± 1.27 | 0.522 ± 0.003 | 0.649 ± 0.008 | 19.55 ± 1.40 | 1.23 ± 0.025 |
| F6 | 26.28 ± 0.91 | 0.515 ± 0.005 | 0.635 ± 0.005 | 18.89 ± 0.39 | 1.22 ± 0.005 |
| F7 | 30.84 ± 0.81 | 0.547 ± 0.003 | 0.627 ± 0.004 | 13.12 ± 0.005 | 1.14 ± 0.010 |
| F8 | 29.20 ± 1.78 | 0.539 ± 0.003 | 0.629 ± 0.004 | 14.39 ± 0.54 | 1.16 ± 0.005 |
Results are presented as mean ± SD, n = 5.
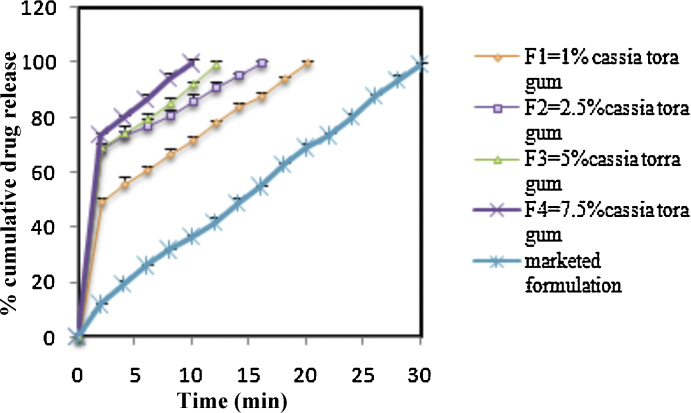
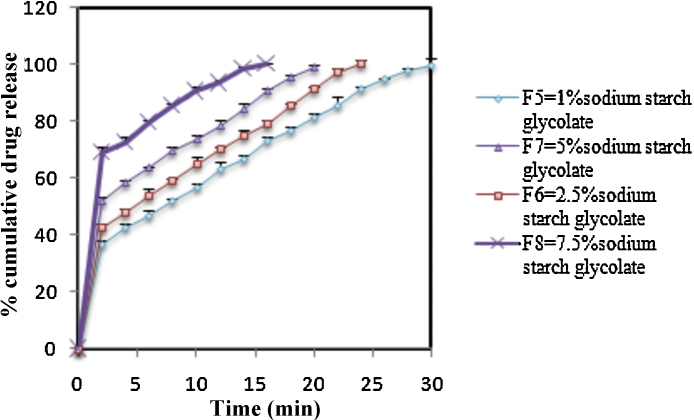
The hardness of tablets was determined and was found to be in the range of 4.20–4.56 kg/cm2. Friability was observed to be between 0.49% and 0.79%, which was less than 1%, indicating that the tablets had a good mechanical resistance. The percentage drug content of all the formulations was found to be between 98.36% w/w and 100.04% w/w. The results of postcompression parameters are summarized in Table 4, Table 5. The wetting time and water absorption ratio are important criteria for understanding the capacity of a disintegrant to swell in the presence of a small amount of water. The wetting time for all formulations was found to be between 26.2 ± 0.25 seconds and 54.17 ± 0.48 seconds (Table 5). The in vitro disintegration time for formulations F1 to F8 is summarized in Table 5. The in vitro drug release rate from the formulations containing polysaccharide was found to be rapid as compared to the formulations containing SSG. The in vitro drug release profiles of the formulations F1 to F8 are represented in Fig. 2, Fig. 3. The results of the stability study indicated that there was no significant change in the physical and chemical characteristics of the tablet, and the optimized formulation F4 containing 7.5% of polysaccharide was stable at 40° C/75% RH for 3 months (Table 6).
Table 4.
Postcompression parameters of orodispersible tablets of valsartan
| Formulation code | Parameters |
|||
|---|---|---|---|---|
| Thickness (mm) a |
Hardness (kg/cm2) a |
Friability (%) b |
Weight variation b | |
| F1 | 2.43 ± 0.11 | 4.56 ± 0.208 | 0.68 ± 0.01 | 200.2 ± 0.63 |
| F2 | 2.33 ± 0.15 | 4.40 ± 0.173 | 0.71 ± 0.01 | 200.2 ± 0.61 |
| F3 | 2.46 ± 0.05 | 4.43 ± 0.11 | 0.77 ± 0.01 | 199.7 ± 1.05 |
| F4 | 2.40 ± 0.12 | 4.2 ± 0.251 | 0.79 ± 0.01 | 200.5 ± 1.35 |
| F5 | 2.2 ± 0.11 | 4.33 ± 0.15 | 0.49 ± 0.015 | 200.2 ± 0.63 |
| F6 | 2.23 ± 0.20 | 4.53 ± 0.05 | 0.57 ± 0.017 | 200.2 ± 0.61 |
| F7 | 2.23 ± 0.057 | 4.46 ± 0.152 | 0.58 ± 0.069 | 199.7 ± 1.05 |
| F8 | 2.26 ± 0.014 | 4.3 ± 0.057 | 0.70 ± 0.015 | 200.5 ± 1.35 |
All values are presented as mean ± S D, n = 5 a/20 b.
Table 5.
Postcompression parameters of orodispersible tablets of valsartan
| Formulation code | Parameters |
|||
|---|---|---|---|---|
| In vitro disintegration time (s) a | Wetting time (s) a |
Water absorption ratio a | Drug content b | |
| F1 | 60.27 ± 0.62 | 46.73 ± 0.39 | 110.88 ± 0.24 | 100.00 ± 002 |
| F2 | 57.29 ± 0.62 | 42.39 ± 0.35 | 119.04 ± 0.33 | 99.86 ± 0.1 |
| F3 | 47.68 ± 0.53 | 37.05 ± 0.56 | 126.80 ± 1.02 | 99.81 ± 0.18 |
| F4 | 32.34 ± 0.78 | 26.2 ± 0.25 | 133.55 ± 0.28 | 98.36 ± 0.43 |
| F5 | 70.56 ± 1.82 | 54.17 ± 0.48 | 99.67 ± 0.49 | 98.54 ± 0.85 |
| F6 | 61.32 ± 0.58 | 50.46 ± 0.76 | 99.24 ± 0.24 | 99.5 ± 0.77 |
| F7 | 55.16 ± 1.44 | 47.90 ± 0.44 | 104.66 ± 0.70 | 99.84 ± 0.33 |
| F8 | 46.96 ± 0.70 | 39.18 ± 0.43 | 109.52 ± 0.99 | 100.04 ± 0.20 |
All values are presented as mean ± SD, n = 6 a/10 b.
Fig. 2.
In vitro drug release profile of formulations (F1–F4 and marketed formulation).
Fig. 3.
In vitro drug release profile of formulations (F5–F8).
Table 6.
Stability study data for F4 batch
| Parameters | 1 mo | 2 mo | 3 mo |
|---|---|---|---|
| Hardness (kg/cm2) | 4.08 ± 0.09 | 4.22 ± 0.1 | 4.1 ± 0.12 |
| % Friability | 0.76 ± 0.09 | 0.79 ± 0.03 | 0.77 ± 0.05 |
| Drug content (%) | 99.53 ± 0.45 | 100.3 ± 0.1 | 99.95 ± 0.19 |
| Disintegration time(s) | 31.84 ± 0.6 | 30.12 ± 0.9 | 32.28 ± 0.1 |
4. Discussion
It was reported that seeds of C. tora contain galactomannans.35 Certain gums having galactomannan such as locust bean gum and Guar gum have been used as superdisintegrants.35, 36 Hence, on a similar basis, C. tora gum has been studied for its superdisintegrant property. It was observed that C. tora has good swelling properties.37 Therefore, we have attempted to develop ODTs from polysaccharide isolated from the seeds of C. tora in order to investigate its potential as a superdisintegrant. There are various reported mechanisms of superdisintegrants such as swelling, wicking, deformation, and electrostatic repulsion.38 ODTs of valsartan were prepared by direct compression method using different concentrations of C. tora gum as a natural disintegrant and SSG as a synthetic superdisintegrant in the same concentration. The excipients were selected depending on preformulation studies, and their concentrations were established on the basis of an extensive literature survey. Microcrystalline cellulose was selected as a directly compressible diluent.39 Aspartame was selected as a sweetening agent,40 and vanilla was used as flavor. Because a direct compression method was used for the preparation of tablets, magnesium stearate was chosen as the lubricant to improve the flow properties of the blend.41
Tablets prepared using polysaccharide isolated from C. tora took a lesser time for wetting of the tablet as compared to the formulations containing SSG. This might be attributable to the rapid penetration of water into the pores of the tablets. The water absorption ratio of polysaccharide was higher compared to that of SSG. It was observed that the water absorption ratio increased with an increase in the concentration of the superdisintegrant. Because of their higher swelling property, formulations containing polysaccharide disintegrated quickly and completely as compared to formulations containing SSG. A rapid increase in the dissolution of drugs with an increase in polysaccharide content may be attributed to the swelling of the polysaccharide powder, which leads to the penetration of water in the pores of the tablets and the generation of hydrodynamic pressure for a quick and complete disintegration of tablets. However, in the case of tablets prepared by SSG, disintegration takes place by the rapid uptake of water followed by quick and enormous swelling into smaller particles, but dissolution occurs slowly owing to the formation of a viscous gel layer by SSG.42
The drug release of F4 formulation was rapid and better compared to the marketed formulations. The formulation F4 containing 7.5% of polysaccharide showed a rapid wetting time and disintegration time as compared with the formulation prepared using a synthetic superdisintegrant at the same concentration level. Hence, batch F4 was considered the optimized formulation.
In conclusion, the present work revealed that C. tora seed polysaccharide is a potential candidate for use as a disintegrant in the formulation of ODTs. Because C. tora polysaccharide is inexpensive compared to synthetic superdisintegrants, nontoxic, compatible, and easy to manufacture, it can be used in place of commercially available synthetic superdisintegrants. The prepared tablets also offer further advantages in terms of patient compliance, quick onset of action, high bioavailability, and good stability, all of which make these tablets a better dosage form for the treatment of hypertension.
Conflicts of interest
The authors declare that there is no conflict of interests regarding the publication of this article.
Acknowledgments
The authors are very grateful to Dr. Parag Gide, Principal of Hyderabad Sindhi National Collegiate Boards (HSNCB), Dr. L.H. Hiranandani College of Pharmacy, Ulhasnagar for, continuous support and encouragement.
References
- 1.Raghaendra RNG, Ketan T, Sumanji B. Formulation and evaluation of fast dissolving tablet of metoprolol tartrate. Int J Pharm Clin Res. 2010;2:40–45. [Google Scholar]
- 2.Sreenivas SA, Dandagi PM, Gadad AP, Godbole AM, Hiremath SP, Mastiholimath VS. Orodispersible tablets: new-fangled drug delivery system — a review. Ind J Pharm Educ Res. 2005;39:177–180. [Google Scholar]
- 3.Setty CM, Prasad DV, Gupta VRM. Development of fast dispersible acelofenac tablets: effect of functionality of superdisintegrants. Ind J Pharma Sci. 2008;70:180–185. doi: 10.4103/0250-474X.41452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karthikeyan M, Mukhthar UAK, Megha M, Shadeer HP. Formulation of diclofenac tablets for rapid pain relief. Asia Pacific J Trop Dis. 2012;2(Suppl):S308–S311. [Google Scholar]
- 5.Shweta SG, Tapar KK, Borse MD, Ghuge RA. Taste masking and characterization of diphenhydramine hydrochloride by spray-drying technique. Int J Pharm Res Dev. 2010;1:17. [Google Scholar]
- 6.Anupama K, Shelly K, Neena B. Formulation and evaluation of mouth dissolving tablets of oxcarbazepine. Intl J Pharm Sci. 2009;1:12–23. [Google Scholar]
- 7.Kibbe A.H., editor. Handbook of pharmaceutical excipient. 3rd ed. Pharmaceutical Press; London UK: 2000. [Google Scholar]
- 8.Ravi K, Swati P, Patil MB, Patil SR, Paschapur MS. Isolation and evaluation of disintegrant properties of fenugreek seed mucilage. Int J Pharm Tech Res. 2009;1:982–996. [Google Scholar]
- 9.Tripathy S, Promod K, Banthia AK. Novel delivery system for acelofenac. Scientific abstract, 56th Int Pharm Cong. 2004:A71. [Google Scholar]
- 10.Poddar SS, Saini CR, Paresh A, Singh R. The microencapsulation of ibuprofen by gelatincarrageenan complex coacervation. Scientific abstract, 56th Int Pharm Cong. 2004:AP111. [Google Scholar]
- 11.Bharadia PD, Patel MM, Patel GC, Patel GN. A preliminary investigation on sesbania gum as a pharmaceutical excipient. Int J Pharm Excip. 2004;3:99–102. [Google Scholar]
- 12.Srinivas K, Prakash K, Kiran HR, Prasad PM, Rao MEB. Study of Ocimum basilicum and Plantago ovata as disintegrants in the formulation of dispersible tablets. Ind J Pharm Sci. 2003;65:180–183. [Google Scholar]
- 13.Gilbert VL. Tagatose, the new GRAS sweetener and health product. J Med Food. 2002;5:23–36. doi: 10.1089/109662002753723197. [DOI] [PubMed] [Google Scholar]
- 14.Khanna M, Nandi RC, Singh S, Jain GK, Sarin JPS. Standardization of pure isapgol (Plantago ovata) mucilage for pharmaceutical use. Ind J Pharm Sci. 1988;50:238–240. [Google Scholar]
- 15.Gowthamarajan K, Kulkarni GT, Muthukumar A, Mahadevan N, Samantha MK, Suresh B. Evaluation of fenugreek mucilage as gelling agent. Int J Pharma Excip. 2002;3:16–19. [Google Scholar]
- 16.Chetan GP, Shivprasad HM. Comparative success of natural superdisintegrant in fast disintegrating tablets. Asian J Biomed Pharm Sci. 2012;2:69–72. [Google Scholar]
- 17.Wade A, Paul J. 2nd ed. Pharmaceutical Press; London, UK: 1994. Handbook of pharmaceutical excipients. [Google Scholar]
- 19.Harshal AP, Priscilla DM. Spectophotometric estimation of total polysaccharides in Cassia tora gum. J Appl Pharm Sci. 2011;01:93–95. [Google Scholar]
- 20.Harshal A, Pawar, Priscilla M. D’mello. Cassia tora Linn: an overview. Int J Pharm Res. 2011;2:2286–2291. [Google Scholar]
- 21.Shibata S, Morishita E, Kaheda M, Kimura Y, Takido M, Takashashi S. Torachrysone. Chem Pharm Bull. 1969;17:454. doi: 10.1248/cpb.17.454. [DOI] [PubMed] [Google Scholar]
- 22.Raghunathan K, Hariharan V, Rangaswami S. Chrysophanol-1-β-gentiobioside, a new anthraquinone glycoside from Cassia tora Linn. Indian J Chem. 1974;12:1251–1253. [Google Scholar]
- 23.Lohar DL, Chawan DD, Garg SP. Phytochemical studies on Cassia species of Indian arid zone. Curr Sci. 1975;44:67. [Google Scholar]
- 24.Maitya TK, Mandal SC, Saha BP, Pal M. Evaluation of hepatoprotective potential of Cassia tora leaf extract. Nat Prod Sci. 1998;4:226. [Google Scholar]
- 25.Asolkar LV, Kakkar KK, Chakre OJ. PID, CSIR; New Delhi: 1992. Second supplement to glossary of Indian medicinal Plants. p. 180–81. [Google Scholar]
- 26.Tripathi KD. 6th ed. Jaypee Brothers Medical Publishers; Delhi: 2008. Essential of medical pharmacology. p. 489–90. [Google Scholar]
- 27.Parmar B, Mandal S, Petkar KC, Patel LD, Sawant KK. Valsartan loaded solid lipid nanoparticles: development characterization and in vitro and ex vitro evaluation. Int J Pharm Sci Nanotechnol. 2011;4:1483–1490. [Google Scholar]
- 28.Khandelwal KR. Practical pharmacognosy techniques and experiments. Nirali Prakashan. 2008:159. [Google Scholar]
- 29.Indian Pharmacopoeia. Controller of publications. 4th ed. Ministry of Health and Family Welfare, Govt. of India. New Delhi, India; 1996, 1:78.
- 30.United States Pharmacopoeia 30/NF25. Asian edition, The official compendia of standard United States Pharmacopoeia. Rockville, MD: Convection Inc.; 2007, 277:644–45.
- 31.Leon L, Herbert AL, Joseph LK. 3rd ed. Varghese Publishing House; Bombay: 2008. The theory and practices of industrial pharmacy. p. 296–303, 430–56. [Google Scholar]
- 32.Zhao N, Augsburger LL. Functionality comparison of three classes of super-disintegrants in promoting aspirin tablets disintegration and dissolution. AAPS Pharm Sci Tech. 2005;6:634–640. doi: 10.1208/pt060479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaudhari PD, Chaudhari SP, Kohle SR, Kumar SRS, Thirupathi AT. Formulation and evaluation of fast dissolving tablets of famotidine. Ind Drug. 2005;42:641–649. [Google Scholar]
- 34.Rangole US, Kawtikwar PS, Sakarkar DM. Formulation and in vitro evaluation of rapidly disintegrating tablets using hydrochlorothiazide as a model drug. Res J Pharm Technol. 2008;1:349–352. [Google Scholar]
- 35.Bala R, Khanna S, Pawar P. Polymers in fast disintegrating tablets – a review. Asian J Pharm Clinical Res. 2012;5:8–14. [Google Scholar]
- 36.Shaikh T, Kumar SS. Pharmaceutical and pharmacological profile of guar gum: an overview. Int J Pharm Pharm Sci. 2011;3:38–40. [Google Scholar]
- 37.Pawar HA, Lalitha KG. Isolation, purification and characterization of galactomannans as an excipient from Senna tora seeds. Int J Biol Macromol. 2014;65:167–175. doi: 10.1016/j.ijbiomac.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 38.Shrivastava P, Sethi V. A review article on: superdisintegrants. Int J Drug Res Technol. 2013;3:76–87. [Google Scholar]
- 39.Chamarthi H. Formulation and evaluation of orodispersible tablet of escitalopram oxalate by super disintegrants addition method. Int J Pharm Res Dev. 2011;3:65–72. [Google Scholar]
- 40.Paul Y, Tyagi S, Singh B. Formulation and evaluation of oral dispersible tablets of zidovudine with different superdisintegrants. Int J Curr Pharm Rev Res. 2011;2:81–91. [Google Scholar]
- 41.Prajapati BG, Patel B. Formulation, evaluation and optimization of orally disintegrating tablet of piroxicam. Int J Pharm Tech Res. 2010;2:1893–1899. [Google Scholar]
- 42.Shihora H, Panda S. Superdisintegrants, utility in dosage forms: a quick review. J Pharm Sci Biosci Res. 2011;1:148–153. [Google Scholar]