Abstract
The major dithiothreitol-resistant phospholipase A2 activity present in the cytosol of U937 cells has been purified greater than 200,000-fold by sequential chromatography on phenyl-5PW, heparin-Sepharose CL-6B, high-performance hydroxylapatite, TSK-gel G3000-SW, and Mono Q columns. This 110-kDa cytosolic phospholipase A2 is distinct from the relatively small (14-kDa) dithiothreitol-sensitive phospholipases A2 that are secreted from many cell types. This additional phospholipase A2 selectively hydrolyzes fatty acid at the sn-2 position of the glycerol and favors phospholipids containing arachidonic acid, which is the rate-limiting precursor for prostaglandin and leukotriene production. Interestingly, a greater than 5-fold increase in phospholipase A2 activity is noted as the calcium concentration increases from the levels found in resting cells to those observed in activated macrophages. We suggest that this enzyme and not the previously described secretory phospholipase A2 is activated by cytosolic effectors such as GTP-binding regulatory proteins and protein kinases to initiate the production of prostaglandins, leukotrienes, and platelet-activating factor. To distinguish this cytosolic enzyme from the previously described secretory ones, we suggest referring to it as cPLA2 for cytosolic phospholipase A2 and collectively referring to the secretory phospholipases A2 as sPLA2s.
Full text
PDF
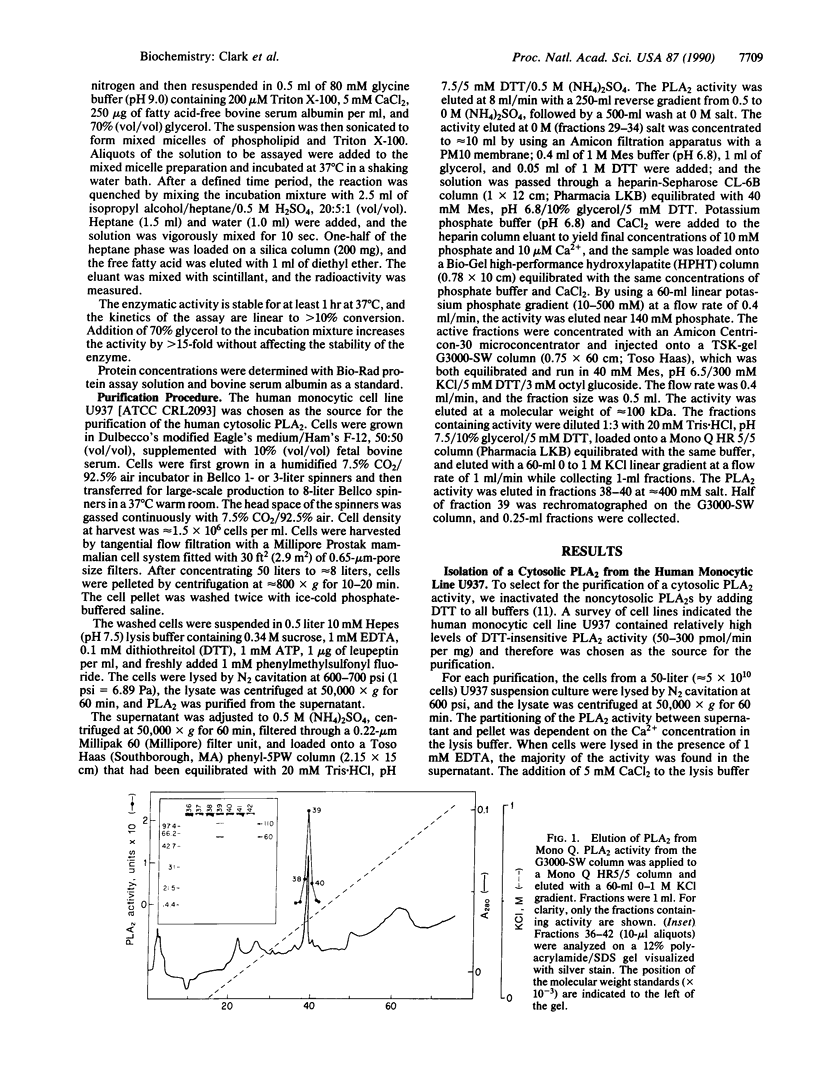
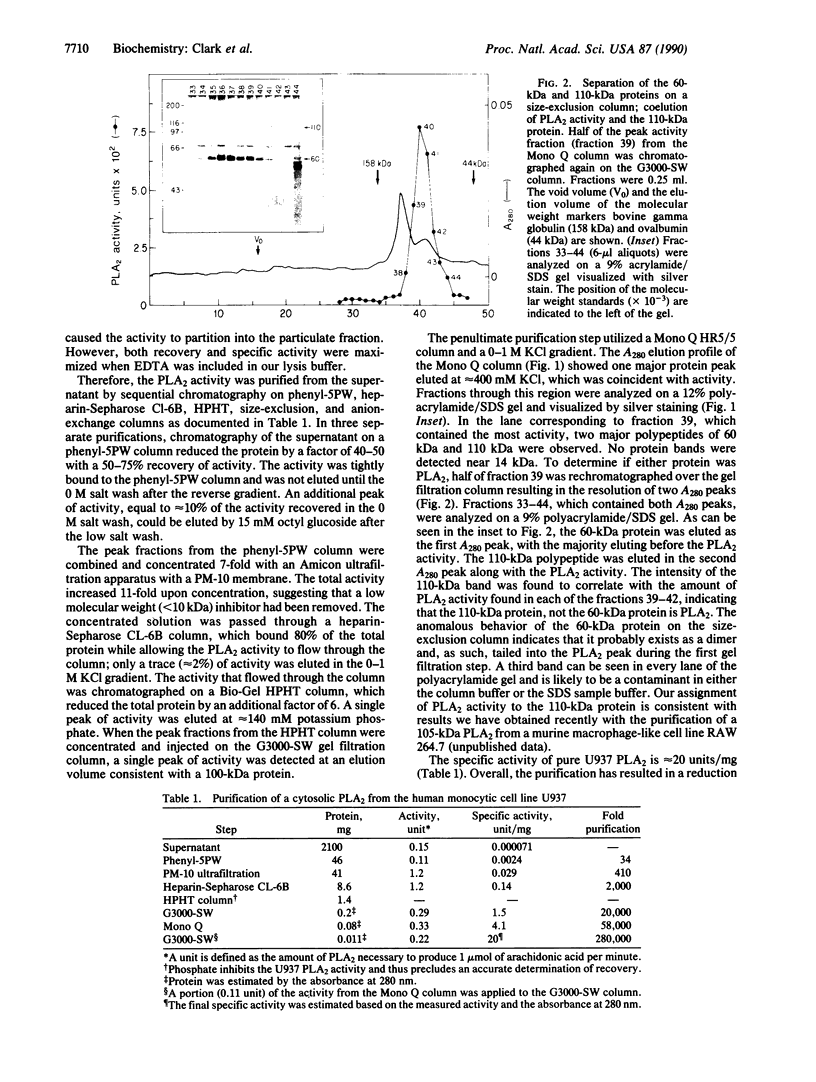
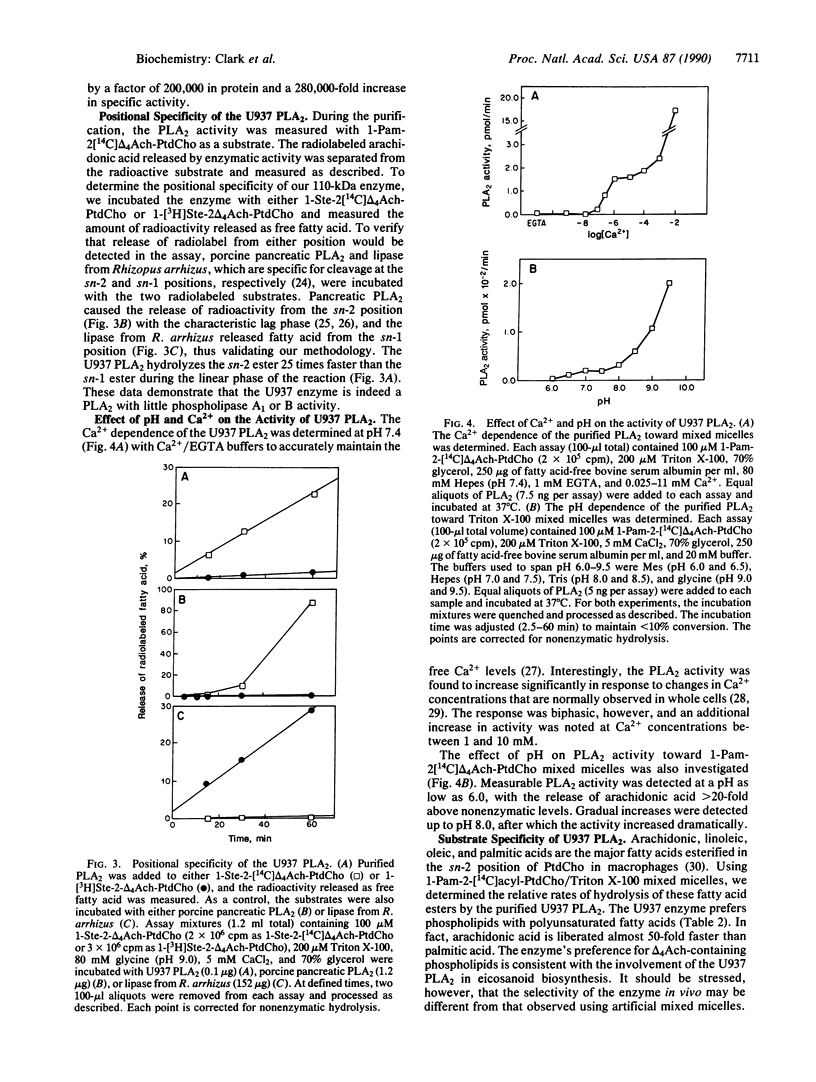

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benjamin C. W., Tarpley W. G., Gorman R. R. Loss of platelet-derived growth factor-stimulated phospholipase activity in NIH-3T3 cells expressing the EJ-ras oncogene. Proc Natl Acad Sci U S A. 1987 Jan;84(2):546–550. doi: 10.1073/pnas.84.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonventre J. V., Gronich J. H., Nemenoff R. A. Epidermal growth factor enhances glomerular mesangial cell soluble phospholipase A2 activity. J Biol Chem. 1990 Mar 25;265(9):4934–4938. [PubMed] [Google Scholar]
- Burch R. M., Axelrod J. Dissociation of bradykinin-induced prostaglandin formation from phosphatidylinositol turnover in Swiss 3T3 fibroblasts: evidence for G protein regulation of phospholipase A2. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6374–6378. doi: 10.1073/pnas.84.18.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch R. M., Connor J. R., Axelrod J. Interleukin 1 amplifies receptor-mediated activation of phospholipase A2 in 3T3 fibroblasts. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6306–6309. doi: 10.1073/pnas.85.17.6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channon J. Y., Leslie C. C. A calcium-dependent mechanism for associating a soluble arachidonoyl-hydrolyzing phospholipase A2 with membrane in the macrophage cell line RAW 264.7. J Biol Chem. 1990 Apr 5;265(10):5409–5413. [PubMed] [Google Scholar]
- Cho W., Tomasselli A. G., Heinrikson R. L., Kézdy F. J. The chemical basis for interfacial activation of monomeric phospholipases A2. Autocatalytic derivatization of the enzyme by acyl transfer from substrate. J Biol Chem. 1988 Aug 15;263(23):11237–11241. [PubMed] [Google Scholar]
- Deems R. A., Dennis E. A. Phospholipase A2 from cobra venom (Naja naja naja). Methods Enzymol. 1981;71(Pt 100):703–710. doi: 10.1016/0076-6879(81)71083-8. [DOI] [PubMed] [Google Scholar]
- Felder C. C., Kanterman R. Y., Ma A. L., Axelrod J. Serotonin stimulates phospholipase A2 and the release of arachidonic acid in hippocampal neurons by a type 2 serotonin receptor that is independent of inositolphospholipid hydrolysis. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2187–2191. doi: 10.1073/pnas.87.6.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W., Heinz E., Zeus M. The suitability of lipase from Rhizopus arrhizus delemar for analysis of fatty acid distribution in dihexosyl diglycerides, phospholipids and plant sulfolipids. Hoppe Seylers Z Physiol Chem. 1973 Sep;354(9):1115–1123. doi: 10.1515/bchm2.1973.354.2.1115. [DOI] [PubMed] [Google Scholar]
- Gorecka-Tisera A. M., Snowdowne K. W., Borle A. B. Implications of a rise in cytosolic free calcium in the activation of RAW-264 macrophages for tumor cell killing. Cell Immunol. 1986 Jul;100(2):411–421. doi: 10.1016/0008-8749(86)90040-7. [DOI] [PubMed] [Google Scholar]
- Hanahan D. J. Platelet activating factor: a biologically active phosphoglyceride. Annu Rev Biochem. 1986;55:483–509. doi: 10.1146/annurev.bi.55.070186.002411. [DOI] [PubMed] [Google Scholar]
- Hara S., Kudo I., Chang H. W., Matsuta K., Miyamoto T., Inoue K. Purification and characterization of extracellular phospholipase A2 from human synovial fluid in rheumatoid arthritis. J Biochem. 1989 Mar;105(3):395–399. doi: 10.1093/oxfordjournals.jbchem.a122675. [DOI] [PubMed] [Google Scholar]
- Irvine R. F. How is the level of free arachidonic acid controlled in mammalian cells? Biochem J. 1982 Apr 15;204(1):3–16. doi: 10.1042/bj2040003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer R. M., Hession C., Johansen B., Hayes G., McGray P., Chow E. P., Tizard R., Pepinsky R. B. Structure and properties of a human non-pancreatic phospholipase A2. J Biol Chem. 1989 Apr 5;264(10):5768–5775. [PubMed] [Google Scholar]
- Kuwae T., Schmid P. C., Johnson S. B., Schmid H. H. Differential turnover of phospholipid acyl groups in mouse peritoneal macrophages. J Biol Chem. 1990 Mar 25;265(9):5002–5007. [PubMed] [Google Scholar]
- Leslie C. C., Voelker D. R., Channon J. Y., Wall M. M., Zelarney P. T. Properties and purification of an arachidonoyl-hydrolyzing phospholipase A2 from a macrophage cell line, RAW 264.7. Biochim Biophys Acta. 1988 Dec 16;963(3):476–492. doi: 10.1016/0005-2760(88)90316-5. [DOI] [PubMed] [Google Scholar]
- McIntyre T. M., Reinhold S. L., Prescott S. M., Zimmerman G. A. Protein kinase C activity appears to be required for the synthesis of platelet-activating factor and leukotriene B4 by human neutrophils. J Biol Chem. 1987 Nov 15;262(32):15370–15376. [PubMed] [Google Scholar]
- Murayama T., Kajiyama Y., Nomura Y. Histamine-stimulated and GTP-binding proteins-mediated phospholipase A2 activation in rabbit platelets. J Biol Chem. 1990 Mar 15;265(8):4290–4295. [PubMed] [Google Scholar]
- Nieuwenhuizen W., Kunze H., de Haas G. H. Phospholipase A2 (phosphatide acylhydrolase, EC 3.1.1.4) from porcine pancreas. Methods Enzymol. 1974;32:147–154. doi: 10.1016/0076-6879(74)32018-6. [DOI] [PubMed] [Google Scholar]
- RAAFLAUB J. Applications of metal buffers and metal indicators in biochemistry. Methods Biochem Anal. 1956;3:301–325. doi: 10.1002/9780470110195.ch10. [DOI] [PubMed] [Google Scholar]
- Renetseder R., Brunie S., Dijkstra B. W., Drenth J., Sigler P. B. A comparison of the crystal structures of phospholipase A2 from bovine pancreas and Crotalus atrox venom. J Biol Chem. 1985 Sep 25;260(21):11627–11634. [PubMed] [Google Scholar]
- Samuelsson B., Dahlén S. E., Lindgren J. A., Rouzer C. A., Serhan C. N. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987 Sep 4;237(4819):1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- Silk S. T., Clejan S., Witkom K. Evidence of GTP-binding protein regulation of phospholipase A2 activity in isolated human platelet membranes. J Biol Chem. 1989 Dec 25;264(36):21466–21469. [PubMed] [Google Scholar]
- Sisson J. H., Prescott S. M., McIntyre T. M., Zimmerman G. A. Production of platelet-activating factor by stimulated human polymorphonuclear leukocytes. Correlation of synthesis with release, functional events, and leukotriene B4 metabolism. J Immunol. 1987 Jun 1;138(11):3918–3926. [PubMed] [Google Scholar]
- Van der Wiele F. C., Atsma W., Roelofsen B., van Linde M., Van Binsbergen J., Radvanyi F., Raykova D., Slotboom A. J., De Haas G. H. Site-specific epsilon-NH2 monoacylation of pancreatic phospholipase A2. 2. Transformation of soluble phospholipase A2 into a highly penetrating "membrane-bound" form. Biochemistry. 1988 Mar 8;27(5):1688–1694. doi: 10.1021/bi00405a046. [DOI] [PubMed] [Google Scholar]
- Wright G. W., Ooi C. E., Weiss J., Elsbach P. Purification of a cellular (granulocyte) and an extracellular (serum) phospholipase A2 that participate in the destruction of Escherichia coli in a rabbit inflammatory exudate. J Biol Chem. 1990 Apr 25;265(12):6675–6681. [PubMed] [Google Scholar]
- Young J. D., Ko S. S., Cohn Z. A. The increase in intracellular free calcium associated with IgG gamma 2b/gamma 1 Fc receptor-ligand interactions: role in phagocytosis. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5430–5434. doi: 10.1073/pnas.81.17.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]