Abstract
Background
Phikud Navakot (PN), a combination of nine herbs, has been used traditionally in Thai medicinal formulas to relieve circulatory disorder. The present study aimed to compare the synergistic antioxidant efficacy and toxicity of the hydroethanolic and water extracts of PN at cellular level.
Methods
PN and its nine herbs were extracted with either 50% ethanol or water. All extracts were tested for in vitro antioxidant potential using standard antioxidant assays. Evaluation of cytotoxicity, genotoxicity, and intracellular reactive oxygen species were performed using human endothelial ECV304 cells.
Results
Antioxidant assays in cell-free systems showed that the hydroethanolic extract of PN scavenged superoxide, hydroxyl, nitric oxide radicals, and hydrogen peroxide more effectively than its water extract. Combination indices were calculated to show that the ingredients of the hydroethanolic extract acted synergistically to exhibit antioxidant activities against all tested radicals, whereas, in the case of water extract, this effect was observed only against 2,2-diphenyl-1-picrylhydrazyl, superoxide, and hydroxyl radicals. A cell-based assay also revealed that the hydroethanolic extract concentration-dependently attenuated hydrogen peroxide-induced stress more effectively than the water extract. At the antioxidant and cytotoxic concentrations of both extracts, no genotoxicity was found.
Conclusion
Our findings demonstrate that the synergistic antioxidant action of PN ameliorates endothelial stress, which may provide some clues for understanding the traditional use of PN for the treatment of circulatory disorder. Additionally, the selection of a suitable solvent for the extraction of PN herbal combination is essential for maximal efficacy and safety.
Keywords: antioxidant, endothelial cell, oxidative stress, Phikud Navakot, synergism
1. Introduction
Many medicinal formulas prepared from different combinations of herbal ingredients have been widely used in Thai traditional medicine. These combinations were believed to produce maximal therapeutic efficacy with less associated side effects or toxicity. “Yahom” (meaning “aromatic medicine” in Thai) is a category of more than 300 Thai polyherbal formulas primarily prescribed as an antiflatulent, a cardiotonic, and a treatment for circulatory disorders. Several Yahom formulas have been included in Thailand's List of Herbal Medicinal Products and have become an integral part of the country's primary healthcare system.1 A number of experimental studies on Yahom have been performed, and evidence from these experiments tended to support its traditional uses. For example, its water extract could increase aortic ring and atrial contraction in rats, and could initially decrease and then increase blood pressure via an adrenergic pathway.2, 3 Yahom in its powder form, given orally to healthy women, was found to significantly increase the diastolic blood pressure and decrease the pulse pressure.4
Phikud Navakot (PN) is a major ingredient in “Yahom Navakot,” a Yahom formula comprising 54 herbs.1 PN is a mixture of nine herbs present in equal weight ratios, including the roots of Angelica dahurica (AD), Angelica sinensis (AS), and Saussurea costus (SC); the rhizomes of Atractylodes lancea (AL), Ligusticum chuanxiong (LC), and Picrorhiza kurrooa (PK); the roots and rhizomes of Nardostachys jatamansi (NJ); the aerial parts of Artemisia pallens (AP); and the galls of Terminalia chebula (TC). The hydroethanolic extract of PN has been shown to attenuate significantly carbachol-induced vasorelaxation in endothelium-intact rat aorta, partly through its antagonistic effect on the muscarinic receptor.5 As an ingredient in Yahom, PN may therefore be effective in relieving circulatory disorder. Moreover, its use is relatively safe, since no treatment-related mortality was observed in both acute and subchronic toxicity studies in PN-fed rats.6
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are products of normal cellular metabolism, and act as secondary messengers. Overproduction of ROS, such as superoxide anion (O2•−), hydrogen peroxide (H2O2), and hydroxyl radical (OH•), and RNS, such as nitric oxide radical (NO•), can cause oxidative stress, leading to acceleration of endothelial dysfunction followed by the pathogenesis of many cardiovascular diseases.7 PN may help in the maintenance of circulatory homeostasis by counteracting or quenching excess ROS and RNS, thereby reducing the harmful effects of these pro-oxidants on cellular components. A combination of herbal ingredients, as in PN, may produce synergistic, additive, or antagonistic effects, which could be vitally important to the therapeutic efficacy of phytomedicine. In this study, we selected the combination index (CI) theorem of Chou–Talalay, which is based on isobologram analysis, as the method of choice to analyze the interaction among the nine herbal materials of PN.8
Thus, the present study aimed to investigate and compare the antioxidant potential of PN and each of its nine ingredients when extracted with either 50% ethanol or water in cell-free and cell-based systems. To ascertain the safety of this polyherbal formula toward human endothelial ECV304 cells, cytotoxicity and genotoxic effect of the extracts were also investigated.
2. Methods
2.1. Plant materials and preparation of the extracts
The roots of A. dahurica (Fisch.) Benth. & Hook.f. (Apiaceae), A. sinensis (Oliv.) Diels (Apiaceae), and S. costus (Falc.) Lipsch. (Asteraceae); the rhizomes of A. lancea (Thunb.) DC. (Asteraceae), L. chuanxiong Hort. (Apiaceae), and P. kurrooa Royle ex Benth. (Scrophulariaceae); the roots and rhizomes of N. jatamansi (D. Don) DC. (Valerianaceae); the aerial parts of A. pallens Walls ex DC. (Asteraceae); and the galls of T. chebula Retz. (Combretaceae) were purchased in October 2009 from traditional drugstores in Bangkok, Thailand, and examined by Dr Sanya Hokputsa of the Research and Development Institute, Government Pharmaceutical Organization. Voucher specimens (NVK10-52) have been deposited at the Phytochemical Research Group, Research and Development Institute, Government Pharmaceutical Organization, Thailand. All herbal materials were examined according to the quality control parameters in Thai Herbal Pharmacopoeia and compared with the authentic specimens, generously provided by Associate Professor Dr Noppamas Soonthornchareonnon of the Faculty of Pharmacy, Mahidol University, Thailand.
Each dried plant material was powdered and sieved through a no. 40 mesh. The powdered herbs (1 kg each) were extracted with 2 × 5 L of either 50% ethanol or water under reflux for 3 hours. PN, as a combination of nine herbs mixed in equal weight proportions, was prepared and extracted using the same procedure. Then the extracts were either spray dried or freeze dried. Stock solutions were prepared by dissolving 1 g of the extract in 5 mL of water to a concentration of 200 mg/mL. Extract stocks were aliquoted and stored at –20 °C.
2.2. Antioxidant assays
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging assay was performed, according to a previously described method.9 The ability of the extract to quench O2•− in the reaction mixture was measured using the nitrotetrazolium blue system.10 The H2O2 scavenging assay was determined using the guaiacol method, with slight modifications.11 The OH• scavenging assay was performed using the deoxyribose method.12 The NO• scavenging assay was performed using the Griess reagent, according to a previous report.13 Quercetin and mannitol (Sigma-Aldrich, St Louis, MO, USA) were used as positive controls.
The interaction among herbal materials was evaluated by standard isobologram analysis, as described previously, and using the CompuSyn synergism/antagonism analysis software (ComboSyn Inc., Paramus, NJ, USA).8 Briefly, herbal interaction was quantified by determining the CI, where CI < 1, CI = 1, and CI > 1 indicate synergistic, additive, and antagonistic effects, respectively.
2.3. High-performance liquid chromatography analysis
The chemical profile of the extract was recorded using a high performance liquid chromatography–photodiode array system (HPLC-PDA) (Shimadzu, Kyoto, Japan), consisting of a binary pump, an autosampler, and a column oven. The extract (20 mg) was dissolved in methanol (10 mL) and filtered through a 0.45 μm membrane filter prior to injecting (20 μL) into a Phenomenex C18 column (250 × 4.6 mm, 5 μm; Phenomenex, Cheshire, UK) and monitored at 254 nm, 270 nm, and 320 nm. The column temperature was set at 25 °C. The mobile phase consisted of acetonitrile (solvent A) and 1% (v/v) acetic acid in water (solvent B). A gradient system was developed at a flow rate of 1.0 mL/minute for 60 minutes: 0–5 minutes, isocratic gradient 100% (B); 5–35 minutes, linear gradient 100–60% (B); 35–45 minutes, linear gradient 60–20% (B); 45–50 minutes, isocratic gradient 20% (B); 50–55 minutes, linear gradient 20–100% (B); and 55–60 minutes, isocratic gradient 100% (B). Calibration curves were obtained using ferulic acid, gallic acid, rutin, and vanillic acid (Sigma-Aldrich, St Louis, MO, USA) as standards. The standard stock solution was prepared in methanol at a concentration of 1 mg/mL. The presence of ferulic acid, gallic acid, rutin, and vanillic acid in PN extracts was detected by HPLC-PDA, observing the retention time (tR) and UV-VIS spectra.
2.4. Cell culture
Human umbilical vein endothelial ECV304 cells obtained from Cell Lines Service (Eppelheim, Germany) were maintained in an M199 medium containing 10% fetal bovine serum and 1% penicillin-streptomycin in a humidified atmosphere of 5% CO2 at 37 °C. Dimethyl sulfoxide (DMSO) (0.5% final concentration) was used as a vehicle control in all experiments.
2.5. MTT assay
Cell viability was determined by a modified 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, following a previously described method.14 Cells (5 × 105 cells/mL) were seeded into each well of a 96-well plate and incubated for 24 hours. The cells were exposed to the various concentrations of the extract for an additional 24 hours. The medium was then replaced by MTT (0.4 mg/mL) and incubated for a further 4 hours at 37 °C. Purple formazan crystals were dissolved in DMSO. The relative number of viable cells was assessed by measuring the absorbance of the formazan product at 570 nm with a microplate reader (Perkin Elmer, Waltham, MA, USA). H2O2 (Fisher Scientific, Leicestershire, UK) was used as a positive control.
To determine the effect of PN on cell viability in H2O2-treated cells, the cells were pretreated with the extract at various concentrations, ferulic acid, or vanillic acid for 24 hours prior to an exposure to H2O2 (100 μM) for 30 minutes. Thereafter, cell viability was measured by the MTT assay.
2.6. Alkaline comet assay
The alkaline comet assay was performed with a slight modification.15 Cells were exposed to various concentrations of the extract for 24 hours. After trypsinization, the cells were suspended in 1% low-melting agarose on a glass slide previously covered with 0.8% normal-melting agarose. After solidification of agarose, the slide was treated with a lysis buffer [2.5 M NaCl, 0.1 M EDTA, 10 mM Tris, pH 10, 1% (v/v) Triton X-100, and 10% (v/v) DMSO; pH 12.3] at 4 °C for 1 hour in the dark. Then the slide was equilibrated in electrophoresis buffer (300 mM NaOH and 1 mM EDTA, pH 13) for 5 minutes to allow the unwinding of DNA and electrophoresed at 25 V for an additional 5 minutes. The slide was washed with a neutralizing buffer (0.4 M Tris, pH 7.5) prior to dehydrating with methanol and finally stained with SYBR Green. Images were photographed using a fluorescence microscope (Olympus, Tokyo, Japan) and the percent of DNA in the tail was analyzed using the CometScore 1.5 software (TriTek Corporation, Sumerduck, VA, USA). H2O2 was used as a positive control.
2.7. DCFH-DA assay
Intracellular ROS was determined using the 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) assay, as previously described.16 Briefly, cells were treated with the extract (at various concentrations), ferulic acid, or vanillic acid for 24 hours. Thereafter, the cells were washed twice with cold phosphate-buffered saline and incubated with DCFH-DA (5 μM) for 30 minutes at 37 °C. After the dye solution was removed, the cells were washed twice with cold phosphate-buffered saline and further incubated with or without H2O2 (100 μM) for 30 minutes. The fluorescent intensity of 2′,7′-dichlorofluorescein was measured at an excitation wavelength of 485 nm and emission wavelength of 535 nm. Quercetin was used as a positive control.
2.8. Statistical analysis
All data are reported as mean ± standard error of the mean from three independent experiments. The Student's t-test was used to compare the significant difference between two groups. Differences among groups were evaluated by one-way analysis of variance, followed by Duncan's test.
3. Results
3.1. Evaluation of antioxidant potential
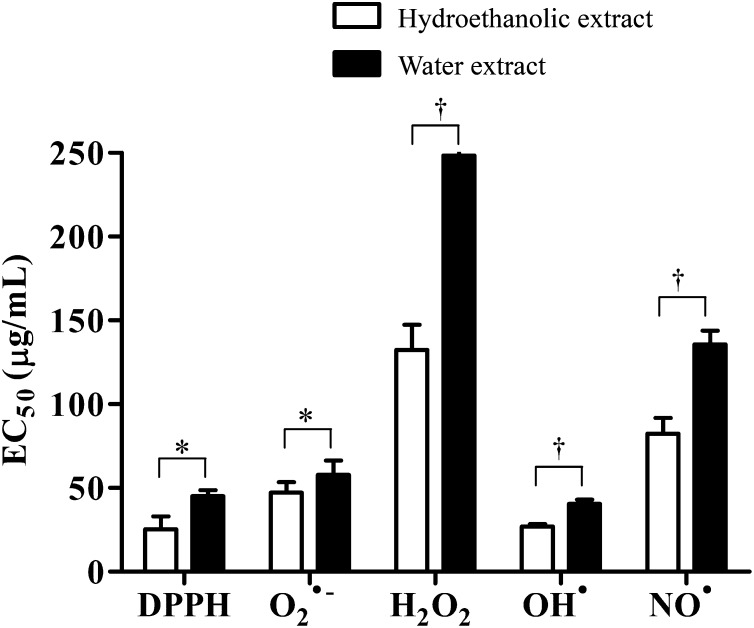
Cell-free antioxidant assays revealed that the hydroethanolic and water extracts of PN concentration-dependently exhibited strong antioxidant potential against DPPH, O2•−, OH•, and NO• and were moderately potent against H2O2, compared with quercetin and mannitol as positive controls (Table 1). The ability of PN to scavenge OH• was even higher than that of mannitol. Moreover, antioxidant activities of the hydroethanolic extract were significantly higher than that of the water extract (Fig. 1).
Table 1.
Comparison between antioxidant (EC50) and cytotoxic (IC50) effects of the hydroethanolic and water extracts of PN and its nine herbs
| Extract | EC50 (μg/mL) |
IC50 (μg/mL) | |||||
|---|---|---|---|---|---|---|---|
| DPPH | O2•− | H2O2 | OH• | NO• | Cytotoxicity | ||
| A. dahurica | E | 183.67 ± 8.33 | 201.20 ± 23.82 | 629.32 ± 22.91 | 183.70 ± 44.37 | 30.67 ± 4.16 | 3340.00 ± 98.90 |
| W | 397.33 ± 31.09 | 355.60 ± 13.02 | 896.71 ± 25.33 | 296.67 ± 15.31 | 70.00 ± 13.53 | 4436.33 ± 394.37 | |
| A. lancea | E | 891.67 ± 6.66 | 1599.84 ± 45.64 | 3016.00 ± 170.94 | 868.77 ± 150.63 | 353.33 ± 85.05 | >5000 |
| W | 845.67 ± 67.16 | 1392.30 ± 26.66 | 2726.00 ± 272.19 | 584.89 ± 87.24 | 368.67 ± 36.64 | >5000 | |
| A. pallens | E | 169.33 ± 32.58 | 75.29 ± 4.65 | 515.34 ± 29.84 | 308.71 ± 18.73 | 106.00 ± 20.66 | 3195.00 ± 101.90 |
| W | 192.67 ± 24.54 | 61.16 ± 2.30 | 559.14 ± 19.42 | 218.84 ± 36.51 | 83.00 ± 10.54 | 3494.00 ± 86.57 | |
| A. sinensis | E | 398.00 ± 34.77 | 358.78 ± 21.76 | 1190.00 ± 113.65 | 489.94 ± 51.33 | 546.67 ± 102.55 | >5000 |
| W | 511.33 ± 10.02 | 378.31 ± 6.80 | 994.67 ± 141.85 | 487.12 ± 48.38 | 781.33 ± 46.69 | >5000 | |
| L. chuanxiong | E | 268.00 ± 15.87 | 150.54 ± 5.79 | 733.67 ± 60.52 | 440.62 ± 57.37 | 81.67 ± 11.68 | 3741.00 ± 194.30 |
| W | 291.00 ± 11.79 | 130.99 ± 5.06 | 708.67 ± 17.21 | 474.70 ± 42.39 | 76.33 ± 9.87 | 3682.67 ± 166.26 | |
| N. jatamansi | E | 218.67 ± 11.93 | 80.69 ± 0.06 | 645.36 ± 18.70 | 325.54 ± 12.78 | 226.00 ± 28.84 | 3317.00 ± 57.60 |
| W | 485.00 ± 33.06 | 67.16 ± 1.45 | 645.64 ± 11.96 | 310.40 ± 13.12 | 195.00 ± 22.11 | >5000 | |
| P. kurrooa | E | 90.67 ± 12.50 | 45.35 ± 3.87 | 501.91 ± 14.95 | 216.33 ± 14.79 | 269.33 ± 33.08 | 584.00 ± 20.80 |
| W | 103.67 ± 16.17 | 28.70 ± 3.51 | 579.58 ± 31.36 | 231.37 ± 20.63 | 182.00 ± 26.89 | 641.33 ± 65.73 | |
| S. costus | E | 538.00 ± 66.70 | 1083.73 ± 41.01 | 1774.67 ± 152.06 | 80.86 ± 2.81 | 288.00 ± 28.93 | 2788.00 ± 252.00 |
| W | 496.67 ± 13.61 | 1559.22 ± 97.18 | 1133.00 ± 92.59 | 109.06 ± 3.65 | 217.00 ± 26.23 | 4509.33 ± 529.73 | |
| T. chebula | E | 4.47 ± 0.12 | 10.50 ± 1.05 | 21.28 ± 0.29 | 9.99 ± 1.03 | 68.33 ± 13.65 | 67.00 ± 1.70 |
| W | 5.90 ± 0.26 | 12.00 ± 1.14 | 22.35 ± 0.48 | 7.63 ± 0.36 | 75.67 ± 12.22 | 41.33 ± 25.04 | |
| Phikud Navakot | E | 25.40 ± 7.71 | 47.26 ± 6.23 | 132.32 ± 15.16 | 26.99 ± 1.47 | 82.33 ± 9.45 | 444.00 ± 20.10 |
| W | 45.00 ± 3.61 | 57.81 ± 8.56 | 248.52 ± 7.65 | 40.42 ± 2.51 | 135.67 ± 8.14 | 617.67 ± 16.52 | |
| Positive control | 2.28 ± 0.09* (6.75 ± 0.25 μM) |
14.28 ± 0.42* (42.21 ± 1.26 μM) |
15.62 ± 0.06* (46.18 ± 1.88 μM) |
442.51 ± 2.22† (2.43 ± 0.012 mM) |
11.06 ± 0.63* (32.68 ± 1.86 μM) |
1.42 ± 0.01‡ (41.73 ± 0.34 μM) |
|
All data represent mean ± SEM from at least three independent experiments performed in triplicate.
E, hydroethanolic extract; EC50, half maximal effective concentration; IC50, half maximal inhibitory concentration; PN, Phikud Navakot; SEM, standard error of the mean; W, water extract.
Quercetin.
Mannitol.
Hydrogen peroxide exposure for 24 hours.
Fig. 1.
Antioxidant activities against DPPH, O2•−, H2O2, OH•, and NO• of hydroethanolic and water extracts of PN, expressed as EC50 values. Results are expressed as mean ± SEM, based on at least three independent experiments performed in triplicate. Significant differences between the hydroethanolic and water extracts are indicated as * p < 0.05 and †p < 0.01.
DPPH, 2,2-diphenyl-1-picrylhydrazyl; PN, Phikud Navakot; SEM, standard error of the mean.
To identify the herbal components of PN responsible for the antioxidant activities, the half maximal effective concentration or EC50 values of PN and each herb were evaluated (Table 1). PN and four individual herbal ingredients displayed antioxidant activity against O2•−, with EC50 values of lower than 100 μg/mL, and their activity was in the following order: TC > PK > PN > AP > NJ. The ability of PN to scavenge OH• was mainly dependent on TC and SC, while its ability to scavenge NO• was found to be dependent on AD, TC, and LC.
We used the EC50 data in Table 1 to calculate the CI values of both extracts, to clarify the interaction inherent in PN. The hydroethanolic extract of nine herbal ingredients of PN exhibited significant synergistic antioxidant activities (CI < 1.0) against all tested ROS and RNS (Table 2). In contrast, the water extract showed a somewhat irregular pattern of antioxidant activities. It displayed synergistic effect against DPPH, O2•−, and OH•; additive effect (CI ∼ 1.0) against NO•; and antagonistic effect (CI > 1) against H2O2.
Table 2.
CI values of antioxidant activities of PN as computed by CompuSyn‡
| Hydroethanolic extract | Water extract | |
|---|---|---|
| DPPH | 0.563 ± 0.019† | 0.774 ± 0.006† |
| O2•− | 0.765 ± 0.014† | 0.752 ± 0.015† |
| H2O2 | 0.564 ± 0.019† | 1.555 ± 0.055* |
| OH• | 0.421 ± 0.005† | 0.752 ± 0.017† |
| NO• | 0.831 ± 0.022* | 1.156 ± 0.041 |
CI, combination index; PN, Phikud Navakot.
p < 0.05 as compared to CI = 1.
p < 0.005 as compared to CI = 1.
CI < 1 indicates synergistic; CI = 1 indicates additive; and CI > 1 indicates antagonistic effect.
3.2. HPLC profile
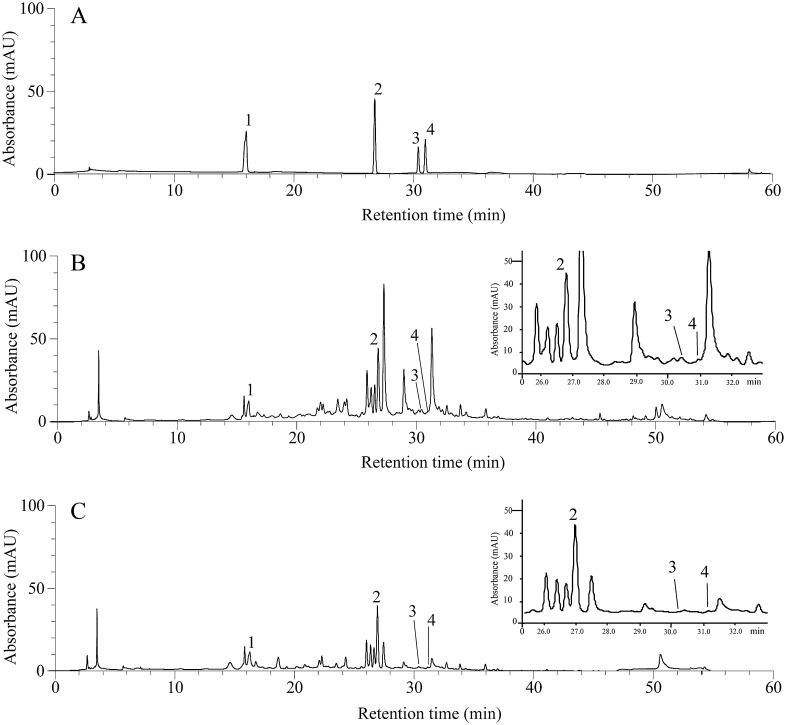
In order to detect active components responsible for the antioxidant activities of PN, HPLC profiles of both extracts were compared. HPLC fingerprints showed many common peaks, including those of the antioxidant constituents gallic acid (tR 16.42 ± 0.41 minutes), vanillic acid (tR 27.04 ± 0.52 minutes), rutin (tR 30.13 ± 0.61 minutes), and ferulic acid (tR 31.05 ± 0.24 minutes) (Fig. 2). Content of gallic acid, vanillic acid, rutin, and ferulic acid were 0.143, 0.396, 0.004, and 0.024 (% w/w) in the hydroethanolic extract and 0.136, 0.362, 0.002, and 0.003 (% w/w) in the water extract of PN, respectively (Table 3).
Fig. 2.
HPLC chromatograms monitored at 270 nm. (A) Mixture of standards containing gallic acid (1), vanillic acid (2), rutin (3) and ferulic acid (4), (B) the hydroethanolic and (C) water extracts of PN were used. Inserts show a zoomed image of the area from 25 to 32 minutes. HPLC, high performance liquid chromatography; PN, Phikud Navakot.
Table 3.
Regression, LOD, LOQ, % RSD, and content of gallic acid, vanillic acid, rutin, and ferulic acid in the extracts
| LOD (μg/mL) | LOQ (μg/mL) | % RSD | Amount (% w/w extract) |
|||
|---|---|---|---|---|---|---|
| Compounds | Regression equation | Hydroethanolic extract | Water extract | |||
| Gallic acid |
y = 3207.9x – 17356 (R2 = 0.9954) |
0.91 | 3.05 | 0.55 | 0.143 ± 0.005 | 0.136 ± 0.004 |
| Vanillic acid |
y = 2962.5x + 5567.8 (R2 = 0.9996) |
0.67 | 2.23 | 1.99 | 0.396 ± 0.025 | 0.362 ± 0.030 |
| Rutin |
y = 701.33x – 129.81 (R2 = 0.9925) |
0.06 | 0.20 | 1.73 | 0.004 ± 0.0002 | 0.002 ± 0.0004 |
| Ferulic acid |
y = 87.821x + 94.756 (R2 = 0.9977) |
0.16 | 0.54 | 0.83 | 0.024 ± 0.002 | 0.003 ± 0.002 |
LOD, limit of detection; LOQ, limit of quantitation; RSD, relative standard deviation.
3.3. Cytotoxicity
To investigate the safety of PN as an ingredient of herbal preparations, cytotoxicity of its extracts and each of its components on ECV304 cells was evaluated. Most of the single herbs were noncytotoxic (IC50 > 2500 μg/mL) (Table 1). Both extracts of TC showed the highest cytotoxicity, while the extracts of PK and PN showed moderate toxicity (IC50 < 1000 μg/mL).
3.4. Genotoxicity
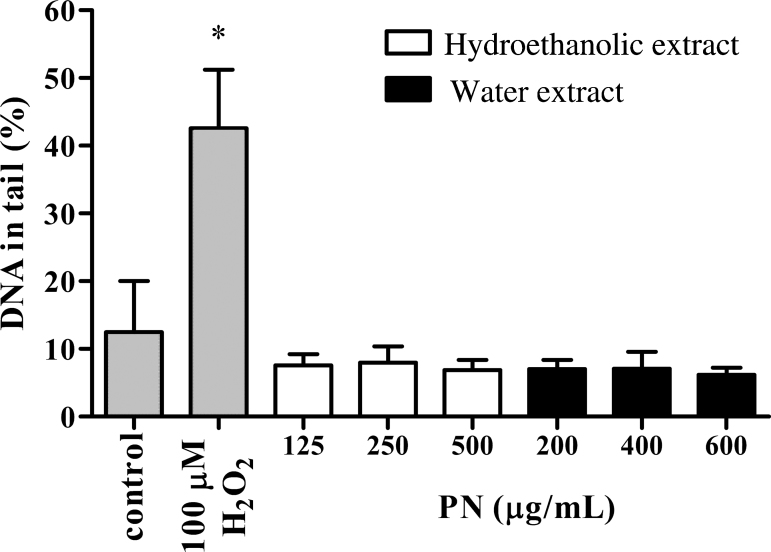
The alkaline comet assay was performed to screen the genotoxic potential of PN. Both extracts of PN at their IC10, IC25, and IC50 values did not produce a significant increase in the percentage of DNA in the tail, compared to a positive control, H2O2 (Fig. 3). Thus, the use of PN as an ingredient of herbal medicine should be relatively safe.
Fig. 3.
Effects of the hydroethanolic and water extracts of PN at their IC10, IC25, and IC50 values on DNA damage detected by an alkaline comet assay. Percentage of DNA in comet tail is presented as mean ± SEM from three independent experiments. H2O2 (100 μM) was used as the positive control. *p < 0.05 as compared to vehicle control.
PN, Phikud Navakot; SEM, standard error of the mean.
3.5. Intracellular ROS level
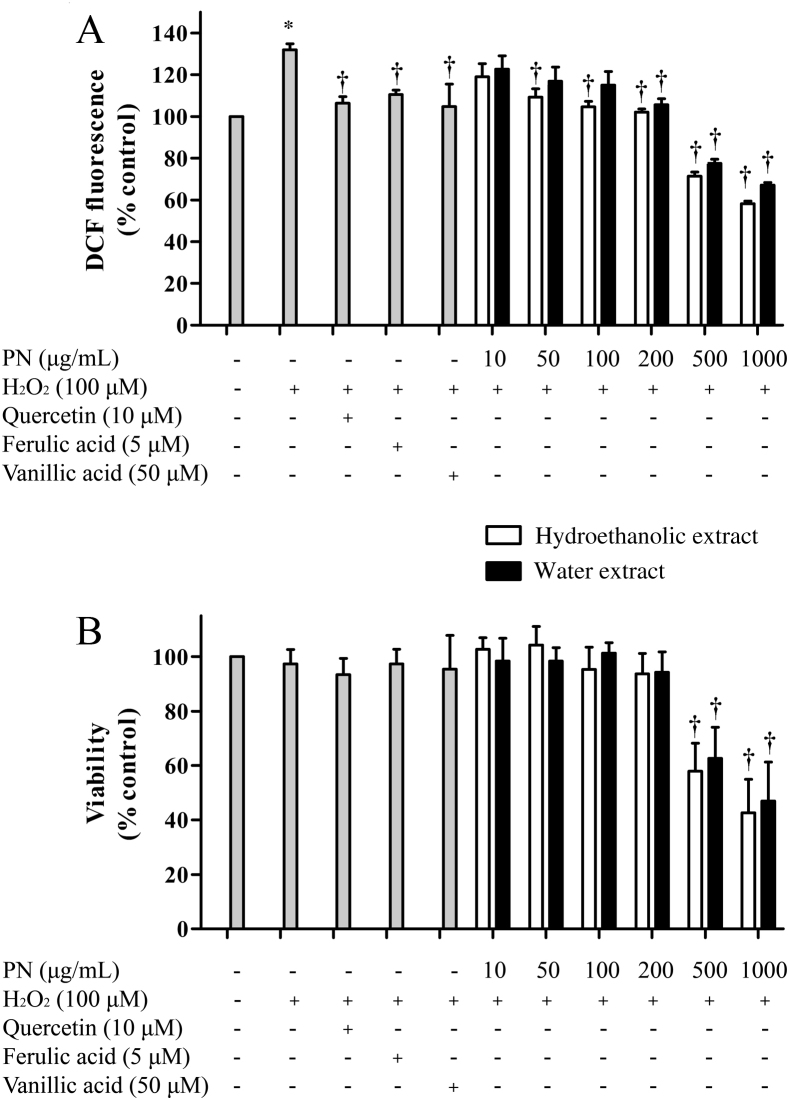
To confirm the antioxidant efficacy of PN at a cellular level, endothelial cells were treated with PN prior to a brief exposure to H2O2. The result showed that, compared to H2O2-treated cells, the hydroethanolic extract (50–200 μg/mL) attenuated intracellular ROS more effectively than the water extract (200 μg/mL) (Fig. 4A), without affecting cell viability (Fig. 4B). It should be noted that the hydroethanolic extract of PN (50 μg/mL) was relatively equipotent to quercetin (10 μM or 3.38 μg/mL), a well-known antioxidant, ferulic acid (5 μM or 0.97 μg/mL), and vanillic acid (50 μM or 8.41 μg/mL). Ferulic and vanillic acids were among the chemical markers found in PN (Fig. 2). Noticeably, a reduction of intracellular ROS induced by PN at the concentrations of 500 μg/mL and 1000 μg/mL was due to the loss of cell viability (Fig. 4B).
Fig. 4.
Effects of the hydroethanolic and water extracts on intracellular ROS level and viability in H2O2-treated cells. Cells were pretreated with the extracts, quercetin, ferulic acid, or vanillic acid at the indicated concentrations for 24 hours prior to an exposure to H2O2 (100 μM) for 30 minutes. (A) Intracellular ROS level and (B) viability were determined using DCFH-DA and MTT assays, respectively. *p < 0.05 as compared to vehicle control. †p < 0.05 as compared to H2O2-treated cells.
DCF, 2′,7′-dichlorofluorescein; DCFH-DA, 2′,7′-dichlorodihydrofluorescein diacetate; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; PN, Phikud Navakot.
4. Discussion
To support the traditional knowledge that PN, a combination of nine herbs originated from traditional Chinese medicine and Ayurveda, demonstrates better therapeutic efficacy for circulatory disorder, the antioxidant action of PN and its nine herbal ingredients was investigated. Our results suggested that almost all herbal ingredients, i.e., AD, AP, LC, NJ, PK, SC, and TC, served as scavengers of specific ROS and RNS, which was further supported by previous evidence.17, 18, 19, 20, 21, 22, 23, 24 To the best of our knowledge, the scavenging properties of AP against O2•− and of SC against OH• have not been reported elsewhere. It can be observed that, with the exception of TC, when individually assayed for their antioxidant activities and cytotoxicity, both the hydroethanolic and the water extract of each herbal ingredient in PN appeared to be less active than the polyherbal combination of PN. Herbal interaction within the multi-ingredient PN analyzed by the calculation of CI values revealed that the hydroethanolic extract showed significant synergistic interaction with stronger potency than the water extract, confirming the importance of solvent used in preparing herbal remedies. We also observed that the percentage yield of the extract obtained from combining the powders of all nine herbs, in equal weight proportions, was higher than the sum of those obtained from extracting each herb alone. This implied that by combining these herbs, the extraction environment might have been altered due to the induction of complexation or polymerization, resulting in the alteration of the solubility of the constituents.25, 26
Furthermore, HPLC profiles of both extracts of PN also showed the presence of gallic acid, vanillic acid, rutin, and ferulic acid. Gallic acid was previously found in the galls of TC and the aerial parts of AP.23, 24, 27 Vanillic acid was found in AS, while rutin, a well-known antioxidant flavonoid, was found in AP.27, 28 Ferulic acid, an effective scavenger of not only NO•, but also OH•, was obtained from the roots of AD and AS, the aerial parts of AP, and the rhizomes of LC.19, 27, 29, 30, 31, 32 Thus, the comparatively larger quantities of these compounds and other components present in the hydroethanolic extract might account for its stronger synergistic activities over those of the water extract.
Antioxidant activities in a cell-free system correlated well with the result obtained from the cell-based assay, revealing that the hydroethanolic extract of PN could concentration-dependently ameliorate hydrogen peroxide-induced stress in human endothelial cells more effectively than the water extract, without affecting DNA damage. The highest cytotoxicity was observed with both extracts of TC, which was also the ingredient of PN that showed the most potent antioxidant activity. Toxicity of TC might arise from its major constituent gallic acid, which can act as either an antioxidant or a pro-oxidant, depending on its concentration and cell types tested. Gallic acid was previously reported to exert its cytotoxicity through the generation of H2O2 in testicular cells.33 Furthermore, the hydroethanolic extract of PN became cytotoxic at 10-fold higher concentrations, resulting in a safety factor of approximately 10. Meanwhile, the water extract of PN showed a narrow margin of safety, with a safety factor of approximately 2. The considerably less cytotoxic effect of PN suggested that the extraction of the combined herbs with 50% ethanol could convey the synergistic antioxidant activities of the polyherbal formula while reducing the overall toxicity, supporting the traditional wisdom in the use of polyherbal formula.
In conclusion, results of our study using cell-free and cell-based systems demonstrated that the hydroethanolic extract of PN scavenged ROS and RNS more effectively than the water extract. Moreover, the solvent used in the preparation of polyherbal formulas is important in providing either synergistic or antagonistic effect. Our findings encourage future preclinical and clinical studies using PN in patients with circulatory disorders.
Conflicts of interest
All authors declare no conflicts of interest.
Acknowledgments
This work was financially supported by Grants-in-Aid for Scientific Research from the National Research Council of Thailand (NRCT 2011-33), TRF-MAG Window I (MRG545S094), and the 90th Anniversary of Chulalongkorn University Fund (Ratchadaphiseksomphot Endowment Fund). We thank Dr Sanya Hokputsa for technical and scientific advice in the characterization of Phikud Navakot and Associate Professor Dr Rutt Suttisri for reviewing and criticizing the manuscript.
References
- 1.National Drug Committee of Thailand . National Drug Committee of Thailand; Bangkok: 2011. List of Herbal Medicinal Products A.D. 2011. [Google Scholar]
- 2.Suvitayavat W, Tunlert S, Thirawarapan SS, Kitpati C, Bunyapraphatsara N. Actions of Ya-hom, a herbal drug combination, on isolated rat aortic ring and atrial contractions. Phytomedicine. 2005;12:561–569. doi: 10.1016/j.phymed.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 3.Suvitayavat W, Tunglert S, Thirawarapan SS, Bunyapraphatsara N. Effects of Ya-hom on blood pressure in rats. J Ethnopharmacol. 2005;97:503–508. doi: 10.1016/j.jep.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Suvitayavat W, Praputtam S, Viriyarumpanon A, Chomsiri P, Phutkeaw P, Thirawarapan SS. Cardiovascular effects of Ya-hom in human. Thai J Phytopharm. 2005;12:1–10. [Google Scholar]
- 5.Nusuetrong P, Sotanaphun U, Tepareenan P. Effects of Phikud Navakot extract on vascular reactivity in the isolated rat aorta. J Med Assoc Thai. 2012;95(Suppl 12):S1–S7. [PubMed] [Google Scholar]
- 6.Kengkoom K, Chaimongkolnukul K, Cherdyu S, Inpunkaew R, Ampawong S. Acute and sub-chronic oral toxicity studies of the extracts from herbs in Phikud Navakot. Afr J Biotechnol. 2012;11:10903–10911. [Google Scholar]
- 7.Higashi Y, Noma K, Yoshizumi M, Kihara Y. Endothelial function and oxidative stress in cardiovascular diseases. Circ J. 2009;73:411–418. doi: 10.1253/circj.cj-08-1102. [DOI] [PubMed] [Google Scholar]
- 8.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 9.Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28:25–30. [Google Scholar]
- 10.Fernandes E, Costa D, Toste SA, Lima JL, Reis S. In vitro scavenging activity for reactive oxygen and nitrogen species by nonsteroidal anti-inflammatory indole, pyrrole, and oxazole derivative drugs. Free Radic Biol Med. 2004;37:1895–1905. doi: 10.1016/j.freeradbiomed.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Choi EH, Chang HJ, Cho JY, Chun HS. Cytoprotective effect of anthocyanins against doxorubicin-induced toxicity in H9c2 cardiomyocytes in relation to their antioxidant activities. Food Chem Toxicol. 2007;45:1873–1881. doi: 10.1016/j.fct.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Halliwell B, Gutteridge JM, Aruoma OI. The deoxyribose method: a simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem. 1987;165:215–219. doi: 10.1016/0003-2697(87)90222-3. [DOI] [PubMed] [Google Scholar]
- 13.Mandal S, Hazra B, Sarkar R, Biswas S, Mandal N. Assessment of the antioxidant and reactive oxygen species scavenging activity of methanolic extract of Caesalpinia crista leaf. Evid Based Complement Alternat Med. 2011;173768:1–11. doi: 10.1093/ecam/nep072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987;47:936–942. [PubMed] [Google Scholar]
- 15.Bony S, Carcelen M, Olivier L, Devaux A. Genotoxicity assessment of deoxynivalenol in the Caco-2 cell line model using the comet assay. Toxicol Lett. 2006;166:67–76. doi: 10.1016/j.toxlet.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med. 1999;27:612–616. doi: 10.1016/s0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- 17.Lee YS, Kim NW. Antioxidant activity and irritation test of extracts obtained from Angelica dahurica. J Food Sci Nutr. 2011;16:8–11. [Google Scholar]
- 18.Ruikar A, Khatiwora E, Ghayal N, Misar A, Mujumdar A, Puranik V. Studies on aerial parts of Artemisia pallens wall for phenol, flavonoid and evaluation of antioxidant activity. J Pharm Bioallied Sci. 2011;3:302–305. doi: 10.4103/0975-7406.80768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li W, Wang H, Tang Y, Guo J, Qian D, Ding A. The quantitative comparative analysis for main bio-active components in Angelica sinensis, Ligusticum chuanxiong, and the herb pair Gui-Xiong. J Liq Chromatogr R T. 2012;35:2439–2453. [Google Scholar]
- 20.Sharma SK, Singh AP. In vitro antioxidant and free radical scavenging activity of Nardostachys jatamansi DC. J Acupunct Meridian Stud. 2012;5:112–118. doi: 10.1016/j.jams.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Chander R, Kapoor NK, Dhawan BN. Picroliv, picroside-I and kutkoside from Picrorhiza kurrooa are scavengers of superoxide anions. Biochem Pharmacol. 1992;44:180–183. doi: 10.1016/0006-2952(92)90054-m. [DOI] [PubMed] [Google Scholar]
- 22.Chang KM, Choi SI, Kim GH. Anti-oxidant activity of Saussurea lappa C.B. Clarke roots. Prev Nutr Food Sci. 2012;17:306–309. doi: 10.3746/pnf.2012.17.4.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee HS, Jung SH, Yun BS, Lee KW. Isolation of chebulic acid from Terminalia chebula Retz. and its antioxidant effect in isolated rat hepatocytes. Arch Toxicol. 2007;81:211–218. doi: 10.1007/s00204-006-0139-4. [DOI] [PubMed] [Google Scholar]
- 24.Manosroi A, Jantrawut P, Akazawa H, Akihisa T, Manosroi J. Biological activities of phenolic compounds isolated from galls of Terminalia chebula Retz. (Combretaceae) Nat Prod Res. 2010;24:1915–1926. doi: 10.1080/14786419.2010.488631. [DOI] [PubMed] [Google Scholar]
- 25.Li M, Xu Y, Yang W, Li J, Xu X, Zhang X. In vitro synergistic anti-oxidant activities of solvent-extracted fractions from Astragalus membranaceus and Glycyrrhiza uralensis. LWT Food Sci Technol. 2011;44:1745–1751. [Google Scholar]
- 26.van Acker SA, van den Berg DJ, Tromp MN, Griffioen DH, van Bennekom WP, van der Vijgh WJ. Structural aspects of antioxidant activity of flavonoids. Free Radic Biol Med. 1996;20:331–342. doi: 10.1016/0891-5849(95)02047-0. [DOI] [PubMed] [Google Scholar]
- 27.Niranjan A, Barthwal J, Lehri A, Singh DP, Govindrajan R, Rawat AKS. Development and validation of an HPLC–UV–MS–MS method for identification and quantification of polyphenols in Artemisia pallens L. Acta Chromatogr. 2009;21:105–116. [Google Scholar]
- 28.Lu JL, Zhao J, Duan JA, Yan H, Tang YP, Zhang LB. Quality evaluation of Angelica sinensis by simultaneous determination of ten compounds using LC-PDA. Chromatographia. 2009;70:455–465. [Google Scholar]
- 29.Maurya DK, Devasagayam TPA. Antioxidant and prooxidant nature of hydroxycinnamic acid derivatives ferulic and caffeic acids. Food Chem Toxicol. 2010;48:3369–3373. doi: 10.1016/j.fct.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Saija A, Tomaino A, Lo Cascio R, Trombetta D, Proteggente A, De Pasquale A. Ferulic and caffeic acids as potential protective agents against photooxidative skin damage. J Sci Food Agric. 1999;79:476–480. [Google Scholar]
- 31.Kwon YS, Kobayashi A, Kajiyama SI, Kawazu K, Kanzaki H, Kim CM. Antimicrobial constituents of Angelica dahurica roots. Phytochemistry. 1997;44:887–889. doi: 10.1016/s0031-9422(96)00634-6. [DOI] [PubMed] [Google Scholar]
- 32.Fan Q, Xia PF, Liu X, Gu JH, Wu XY, Zhao L. Simultaneous quantification of two major active components in radix Angelica sinensis by high-performance liquid chromatography with an internal standard correction method. Instrum Sci Technol. 2012;40:416–428. [Google Scholar]
- 33.Park W, Chang MS, Kim H, Choi HY, Yang WM, Kim DR. Cytotoxic effect of gallic acid on testicular cell lines with increasing H2O2 level in GC-1 spg cells. Toxicol In Vitro. 2008;22:159–163. doi: 10.1016/j.tiv.2007.08.010. [DOI] [PubMed] [Google Scholar]