Abstract
Background
The purpose of this study was to investigate the effect of hydroxypropyl methyl cellulose phthalate (HPMCP) coating on the digestive stability and intestinal transport of green tea catechins (GTCs).
Methods
Two types of HPMCP coating were prepared: one type with size smaller than 500 μm (S-HPMCP) and the other with size larger than 500 μm (L-HPMCP). An in vitro gastrointestinal model system coupled with Caco-2 cells was used for estimating the bioavailability of GTCs. Ultraperformance liquid chromatography with a photodiode array detector was performed to analyze GTCs.
Results
The digestive stability of GTCs was enhanced up to 33.73% and 35.28% for S-HPMCP and L-HPMCP, respectively. Intestinal transport of the GTCs was increased to 22.98% and 23.23% for S-HPMCP and L-HPMCP, respectively. Overall, the bioavailability of GTCs increased by 4.08 and 11.71 times for S-HPMCP and L-HPMCP, respectively.
Conclusion
The results of this study confirm that coating with HPMCP could be a way to improve the digestive stability and intestinal transport of GTCs.
Keywords: digestive stability, green tea catechin, hydroxypropyl methyl cellulose phthalate, intestinal transport
1. Introduction
Green tea is one of most popular beverages worldwide, and it contains a series of polyphenols known as catechins.1 Although green tea catechins (GTCs) provide a wide range of beneficial health effects, the oral bioavailability of catechins has been suggested to be low in humans.1, 2, 3, 4 Differences in pH levels and varying oxygen conditions during digestion are major factors that degrade GTCs.5
An enteric coating material [i.e., hydroxypropyl methyl cellulose phthalate (HPMCP)] has been used to protect drugs or flavonoids from degradation by gastric acid or to prevent them from causing side effects in the stomach.6, 7 For example, HPMCP, frequently used as a matrix for oral dosage forms, was expected to enhance the bioavailability of flavonoids, because it has the ability to protect it from various levels of pH in the gastrointestinal tract of humans.8
Therefore, we aimed to examine the digestive stability and intestinal transport of catechins by coating them with HPMCP.
2. Methods
2.1. Chemicals and standards
Standards of epigallocatechin, epigallocatechin gallate, epicatechin (EC), and epicatechin gallate were purchased from Wako (Osaka, Japan). Digestive enzymes (α-amylase from human saliva, pepsin from porcine gastric mucosa, porcine lipase, pancreatin from porcine pancreas, and bile extract porcine) were obtained from Sigma-Aldrich (St. Louis, MO, USA). High-performance liquid chromatography–grade solvent of acetic acids, water, and methanol were obtained from Sigma-Aldrich and J.T.Baker (Phillipsburg, NJ, USA).
2.2. Sample preparation
Two types of HPMCP coating were prepared: one with a size smaller than 500 μm (S-HPMCP) and another type with a size larger than 500 μm (L-HPMCP). The ratio of the catechin to HPMCP coating for L-HPMCP was 7:3 and for S-HPMCP it was 2:8. The origin of catechin for the coating of S-HPMCP was China and that for the coating of L-HPMCP was Korea.
2.3. Digestive stability of catechins using the in vitro digestion model system
The method used in this study was designed by Lee et al.9 The in vitro digestion model system, including the human gastrointestinal tract, including salivary, gastric, and small intestinal phases, was simulated as described previously.5 Approximately 5 mg of GTC and 5 mg of HPMCP-coated GTCs were suspended in 5-mL aliquots of 20 mM phosphate buffer. For the salivary phase, 60 μL of α-amylase (0.2 mg/mL in 20 mM phosphate buffer) was added, and the initial pH was controlled to 6.9 by adding 20 mM phosphate buffer. Samples were shaken at a constant speed of 150 rpm in a shaking water bath at a temperature of 37 °C for 5 minutes. The gastric phase was initiated with 120 μL of porcine pepsin (3 mg/mL in 100 mM sodium bicarbonate solution), and the pH was maintained at 2.0 by adding 0.1 M hydrogen chloride. Solutions were then incubated in a shaking water bath for 30 minutes, following which a 0.1 M solution of sodium bicarbonate was added to neutralize the pH to 5.3. For the small intestinal phase, the pH was regulated to 7.0 by adding 0.1 M sodium hydroxide solution, followed by the addition of 60 μL pancreatic enzyme mixture. The solution mixture was then incubated in a shaking water bath at 37 °C and 150 rpm for 1 hour. All samples were brought to the final volume of 5 mL using 20 mM phosphate buffer and a gentle stream of nitrogen gas was passed in each step. Finally, the supernatant from the digesta was obtained after centrifuging the solution mixture at 4 °C and 3000 rpm for 30 minutes for LCQ-Fleet ultraperformance liquid chromatography using a photodiode array detector (UPLC–PDA; Thermo Fisher Scientific, Waltham, MA, USA).
2.4. Observation of microscopy
The residues after continuous digestion were observed with an optical microscope (CKX41, Olympus, Japan). The magnification scale was 40×.
2.5. Study of intestinal transport of catechins using Caco-2 human intestinal cell culture
Passage numbers 32–36 of the Caco-2 cell cultures were obtained from the Korean Cell Line Bank (Seoul, South Korea) for this study. A 12-transwell plate (Corning, NY, USA) was used for Caco-2 cell cultures seeded in a growth medium consisting of Dulbecco's modified Eagle's medium (DMEM; Gibco Rockville, MD, USA) with 10% fetal bovine serum (FBS; Gibco, NE, USA), 1% nonessential amino acid (Sigma), 1% penicillin (Gibco), and 0.1% gentamicin (Gibco). The cells were maintained at 37 °C in an incubator with 5% CO2 and 95% air. A 10% FBS-supplemented DMEM was used to change the medium of the apical and basal compartments each day. Cellular transport of catechins was assessed when the cells grew between 2 and 3 weeks after being confluent. The value of transepithelial electric resistance (TEER) was measured by the Millicell ERS-2 system (Millipore, New Bedford, MA, USA) to ensure rigidity. When the cells had a TEER value higher than 350 Ω, they were used for the transport study. Each treatment mixture with a basal cell culture medium was dispensed to the apical Caco-2 human intestinal cells, which were then incubated at 37 °C for 2 hours. The basal medium was then collected for the liquid chromatography–mass spectrometry analysis.
2.6. UPLC–PDA analysis
The method used for the UPLC–PDA analysis was designed by Chung et al.5 The amount of catechins in the supernatant after in vitro digestion and the transported amount of catechin from the apical to basal compartment was quantified using a UPLC–PDA/electrospray ionization/multistage mass spectrometry. Methanol was added into the collected supernatant and basal media, and it was sonicated for 3 minutes followed by vortexing and centrifugation. The supernatant was filtered through a 0.45-μm polyvinyl difluoride syringe filter (Millipore, MA, USA) before the analysis. Chromatographic separation was performed on a Hypersil GOLD C18 (2.1 × 50 mm, 1.9 μm) column with mobile phases of solvents A and B (vol:vol, 0.1% acetic acid in water:methanol). A gradient elution was performed by varying the proportion of solvents A and B with a flow rate of 200 μL/minute and with an initial phase of 5% solvent B. The gradient increased linearly to 20% of solvent B for 15 minutes, increased linearly to 50% for next 5 minutes, and remained at 50% for 5 minutes until injection of the next sample for 10 minutes. The injection volume was 2 μL. The wavelength of the ultraviolet spectrum was set at 280 nm. Chromatographic peaks and mass spectrums in the samples were identified using comparative retention times and molecular weights of pure standards. Quantitative analysis was conducted using a standard curve.
2.7. Statistical analysis
Values were reported as a mean ± standard deviation from at least three different experiments. T-test was performed to measure significant differences between the control and treatment groups at the significant value p < 0.05 using GraphPad Prism 3.0 software (GraphPad, CA, USA).
3. Results
3.1. Disruption of HPMCP coating during digestion
Optical microscopy of HPMCP coating for GTCs after saliva–gastrointestinal digestion is shown in Fig. 1. Regardless of the HPMCP coating size, the surface of the HPMCP coating was slightly ruptured after digestion.
Figure 1.
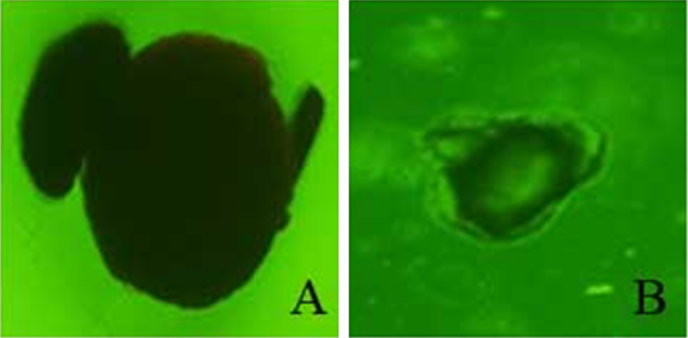
Optical microscopic image of HPMCP coating for GTCs after saliva–gastrointestinal digestion at a magnification of 40×. (A) L-HPMCP; (B) S-HPMCP. HPMCP, hydroxypropyl methyl cellulose phthalate; L-HPMCP, HPMCP size > 500 μm; S-HPMCP, HPMCP size < 500 μm.
3.2. Digestive stability of GTCs by HPMCP coating
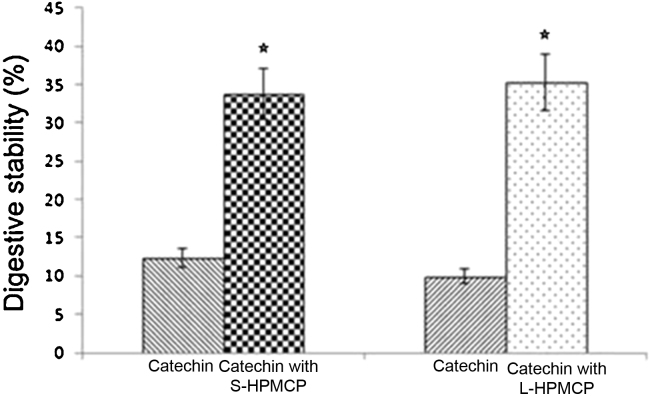
The released amount of GTCs was 12.33% and 33.73% for noncoated catechin and S-HPMCP, respectively. There was a remarkable increase in the percentage of GTCs released in catechin with L-HPMCP (Fig. 2). The digestive stability was enhanced by 2.74 and 3.56 times for S-HPMCP and L-HPMCP, respectively.
Figure 2.
Digestive stability of the GTCs according to the HPMCP coating size. S-HPMCP and L-HPMCP. There was significant difference between the treatment group and the control group. Both S-HPMCP and L-HPMCP had significantly higher values than catechin (p < 0.05). GTC, green tea catechin; HPMCP, hydroxypropyl methyl cellulose phthalate; L-HPMCP, HPMCP size > 500 μm; S-HPMCP, HPMCP size < 500 μm. The star shows the significant difference between control and treatment group.
3.3. Intestinal transport of catechins by HPMCP coating
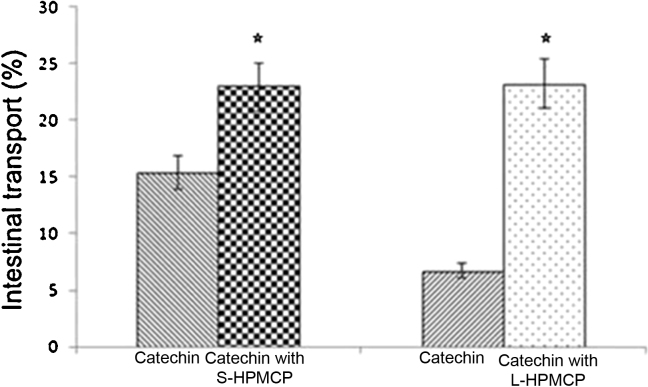
The Caco-2 cell was used to evaluate intestinal transport of GTCs using different sizes of HPMCP coating (Fig. 3). The intestinal transport of GTCs without S-HPMCP coating was 15.36% and GTCs without L-HPMCP coating was 6.70%, respectively. Their treatment groups were 22.98% and 23.23% for S-HPMCP and L-HPMCP, respectively. The increase in the ratio of the intestinal transport of S-HPMCP and L-HPMCP was 1.50 times and 3.47 times, respectively. The intestinal transport rate of L-HPMCP was better than S-HPMCP. However, ultimately there were no significant differences between the S-HPMCP and L-HPMCP (p > 0.05). Overall, the estimated bioavailability of GTCs increased by 4.08 and 11.71 times for S-HPMCP and L-HPMCP, respectively.
Figure 3.
Small intestinal transport of the GTCs according to the HPMCP coating size. S-HPMCP and L-HPMCP. There was a significant difference between the treatment group and the control group. Both S-HPMCP and L-HPMCP had significantly higher values than catechin (p < 0.05). GTC, green tea catechin; HPMCP, hydroxypropyl methyl cellulose phthalate; L-HPMCP, HPMCP size > 500 μm; S-HPMCP, HPMCP size < 500 μm. The star shows the significant difference between control and treatment group.
4. Discussion
The HPMCP is widely used as an enteric coating material in the pharmaceutical industry as it is not dissolved in a gastric acid solution (pH ∼3); however, it is dissolved in an alkaline environment such as that present in the small intestine (pH 7–9).10 Therefore, coating with HPMCP may protect GTCs from different pH levels and oxygen exposure during digestion. The GTCs were known to be affected by digestive enzymes, water activity, oxygen, and various pH values during digestion. However, coating with HPMCP can protect GTCs from digestive environments such as different pH values, activities of various enzymes, and oxygen, thereby providing digestive stability for the catechin.6, 7, 11 Among the catechins, EC has been reported to show efflux pattern.5 However, HPMCP protects EC from the efflux in the small intestine by the efflux protein instead of changes in transport mechanisms.5, 12 The HPMCP coating showed the efficiency in transport of GTCs in the small intestine than catechin without HPMCP coating, regardless of its size.
In conclusion, this study confirmed that the HPMCP coating increased the digestive stability and intestinal transport of GTCs, resulting in enhancing bioavailability of the GTCs.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Acknowledgments
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (Grant No.2012-0003388). This study was supported by AmorePacific R&D Center.
References
- 1.Baba S., Osakabe N., Natsume M., Muto Y., Takizawa T., Terao J. In vivo comparison of the bioavailability of (+)-catechin, (–)-epicatechin and their mixture in orally administered rats. J Nutr. 2001;131:2885–2891. doi: 10.1093/jn/131.11.2885. [DOI] [PubMed] [Google Scholar]
- 2.Chow H.H., Hakim I.A., Vining D.R., Crowell J.A., Ranger-Moore J., Chew W.M. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of Polyphenon E in healthy individuals. Clin Cancer Res. 2005;11:4627–4633. doi: 10.1158/1078-0432.CCR-04-2549. [DOI] [PubMed] [Google Scholar]
- 3.Yang C.S., Chen L., Lee M.J., Balentine D., Kuo M.C., Schantz S.P. Blood and urine levels of tea catechins after ingestion of different amounts of green tea by human volunteers. Cancer Epidemiol Biomarkers Prev. 1998;7:351–354. [PubMed] [Google Scholar]
- 4.Zhu M., Chen Y., Li R.C. Oral absorption and bioavailability of tea catechins. Planta Med. 2000;66:444–447. doi: 10.1055/s-2000-8599. [DOI] [PubMed] [Google Scholar]
- 5.Chung J.H., Kim S., Lee S.J., Chung J.O., Oh Y.J., Shim S.M. Green tea formulations with vitamin C and xylitol on enhanced intestinal transport of green tea catechins. J Food Sci. 2013;78 doi: 10.1111/1750-3841.12112. C685–C90. [DOI] [PubMed] [Google Scholar]
- 6.Sanders G.H.W., Booth J., Compton R.G. Quantitative rate measurement of the hydroxide driven dissolution of an enteric drug coating using atomic force microscopy. Langmuir. 1997;13:3080–3083. [Google Scholar]
- 7.Toress D., Encina G.G., Jato J.L.V. Formulation and in vitro evaluation of HPMCP-microencapsulated drug-resin complexes for sustained release of diclofenac. Int J Pharm. 1995;121:239–243. [Google Scholar]
- 8.Lee H.B., Ha H. Mechanisms of epithelial-mesenchymal transition of peritoneal mesothelial cells during peritoneal dialysis. J Korean Med Sci. 2007;22:943–945. doi: 10.3346/jkms.2007.22.6.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee H.R., Cho S.D., Lee W.K., Kim G.H., Shim S.M. Digestive recovery of sulfur-methyl-l-methionine and its bioaccessibility in Kimchi cabbages using a simulated in vitro digestion model system. J Sci Food Agric. 2014;94:109–112. doi: 10.1002/jsfa.6205. [DOI] [PubMed] [Google Scholar]
- 10.Wang X.Q., Zhang Q. pH-sensitive polymeric nanoparticles to improve oral bioavailability of peptide/protein drugs and poorly water-soluble drugs. Eur J Pharm Biopharm. 2012;82:219–229. doi: 10.1016/j.ejpb.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 11.Nanjo F., Goto K., Seto R., Suzuki M., Sakai M., Hara Y. Scavenging effects of tea catechins and their derivatives on 1,1-diphenyl-2-picrylhydrazyl radical. Free Radic Biol Med. 1996;21:895–902. doi: 10.1016/0891-5849(96)00237-7. [DOI] [PubMed] [Google Scholar]
- 12.Baluom M., Friedman M., Rubinstein A. Improved intestinal absorption of sulpiride in rats with synchronized oral delivery systems. J Control Release. 2001;70:139–147. doi: 10.1016/s0168-3659(00)00337-0. [DOI] [PubMed] [Google Scholar]