Abstract
Background
The transcription factor signal transducer and activator of transcription 3 (Stat3) is constitutively activated in many human cancers. It promotes tumor cell proliferation, inhibits apoptosis, induces angiogenesis and metastasis, and suppresses antitumor host immune responses. Therefore, Stat3 has emerged as a promising molecular target for cancer therapies. In this study, we evaluated the Stat3-suppressive activity of 38 herbal medicines traditionally used in Korea.
Methods
Medicinal herb extracts in 70% ethanol were screened for their ability to suppress Stat3 in the A549 human lung cancer cell line. A Stat3-responsive reporter assay system was used to detect intracellular Stat3 activity in extract-treated cells, and Western blot analyses were performed to measure the expression profiles of Stat3-regulated proteins.
Results
Fifty percent of the 38 extracts possessed at least mild Stat3-suppressive activities (i.e., activity less than 75% of the vehicle control). Ethanol extracts of Bupleurum falcatum L., Taraxacum officinale Weber, Solanum nigrum L., Ulmus macrocarpa Hance, Euonymus alatus Sieb., Artemisia capillaris Thunb., and Saururus chinensis (Lour.) Baill inhibited up to 75% of the vehicle control Stat3 activity level. A549 cells treated with these extracts also had reduced Bcl-xL, Survivin, c-Myc, and Mcl-1 expression.
Conclusion
Many medicinal herbs traditionally used in Korea contain Stat3 activity-suppressing substances. Because of the therapeutic impact of Stat3 inhibition, these results could be useful when developing novel cancer therapeutics from medicinal herbs.
Keywords: anticancer, herbal extract, Korean medicine, Stat3
1. Introduction
The signal transducer and activator of transcription (STAT) proteins comprise a family of seven closely related transcription factors with major roles in cytokine signaling.1 Of these proteins, Stat3 is well recognized in the field of cancer research because persistent Stat3 activation occurs in a variety of human cancer cell lines and in primary tumors (e.g., lymphoid/myeloid malignancies and solid tumors)2 and in cells transformed with c-Abl and v-Src oncogenes.3 Many genetic studies have suggested that the inhibition of this oncogenic transcriptional regulator could provide an effective treatment opportunity.
Stat3 is activated through tyrosine phosphorylation, which is mediated by members of the Jak tyrosine kinase family in response to numerous growth factors such as epidermal growth factor (EGF), interleukin-6 (IL-6),4 and other cytokines.5 These growth factors are closely related to tumorigenesis and cancer progression. In particular, EGF signaling has been linked to nearly 30% of tumor proliferation events, partly through Stat3 activation. Hence, Stat3 is a major mediator of tumorigenesis, especially because it affects many parameters of malignancy development and progression such as cell proliferation, apoptosis inhibition, and angiogenesis. Examples of Stat3-regulated genes include c-myc6 and cyclin D17 (which mediate cell proliferation); Mcl-1, c-IAP2, Bcl-2,8 and Survivin9 (which suppress apoptosis); and matrix metalloproteinase-9 (MMP-9)10 (which mediates cellular invasion).
In addition to regulating genes essential for tumor cell survival and proliferation, Stat3 also mediates immune suppression by inhibiting the expression of proinflammatory cytokines and chemokines necessary for dendritic cell activation, by negatively regulating T helper-1 cell-mediated inflammation,11 and by regulating vascular endothelial growth factor (VEGF) and IL-10-mediated crosstalk between tumor cells,12 thereby resulting in immune evasion by cancer cells.
Stat3 plays a critical role in tumorigenesis, inflammation, and crosstalk between tumor and normal cells within the tumor microenvironment. Thus, Stat3 inhibitors could be potentially useful for cancer prevention and treatment.5 Numerous studies have been conducted to develop a safe and efficient strategy of suppressing Stat3 activity in cancers. One of the best-known Stat3 inhibitors is AG490, which inhibits Jak2, the activation partner of Stat3.13 Some small peptides,14, 15 oligonucleotides,16 and small molecules17, 18 have anti-Stat3 activity. Several plant-derived polyphenols such as curcumin,19 resveratrol,20 and magnolol21 also have Stat3 inhibitory properties.
In our study, we demonstrated that elevated Stat3 signaling is a common feature of human lung cancer cell lines, and we evaluated the Stat3 inhibitory activities of 38 ethanol extracts of plants that have been traditionally used in Korea. These data could be helpful to the development of novel cancer chemotherapeutic reagents for lung cancers from naturally available and confirmed safe medicinal herbs.
2. Methods
2.1. Herbal materials
Table 1 lists the medicinal herbs and parts used in this study. Each herbal material was selected based on its traditional use for cancer-like symptoms and/or symptoms that accompany various types of cancers. Each was purchased from one of three sources: Kwangmyungdang Medicinal Herbs (Ulsan, Korea), Omniherb (Daegu, Korea), or UKHERB (Busan, Korea). The identification of each medicinal herb was confirmed by Dr Go Ya Choi of the Herbal Medicine Resources Group (Daejeon, South Korea). Voucher specimens were deposited with the Cancer Research Team of the KM-based Herbal Drug Development Group of the Herbal Medicine Research Division at the Korea Institute of Oriental Medicine (Daejeon, Republic of Korea). All herbal materials used in this study were grown and processed in Korea.
Table 1.
List of medicinal plants and parts used in the study.
| Scientific name | Parts used |
|---|---|
| Acanthopanax sessiliflorum Seeman | root, bark |
| Achyranthes japonica Nakai | root |
| Alisma orientale Juzepzuk | rhizome |
| Artemisia capillaris | aerial part |
| Astragalus membranaceus Bunge | root |
| Bupleurum falcatum L. | root |
| Carpesium abrotanoides | aerial part |
| Citrus unshiu Markovich | fruit skin |
| Coix lacryma-jobi L. var. ma-yuen Stapf | seed |
| Cornus officinalis Sieb. et Zucc. | fruit |
| Cudrania tricuspidata (Carr.) Bureau ex Lavallee | root |
| Cynanchum wilfordii (Maxim.) Hemsl. | rhizome |
| Dianthus chinensis L. | aerial part |
| Dioscorea batatas Decaisne | rhizome |
| Duchesnea indica (Andr.) Focke | whole |
| Euonymus alatus Sieb. | stem cork |
| Houttuynia cordata Thunb. | aerial part |
| Laminaria japonica | leaf |
| Liriope platyphylla Wang et Tang | root |
| Lithospermum erythrorhizon Sieb. et Zucc. | root |
| Orostachys japonicus A. Berger | whole |
| Panax ginseng C. A. Mey. | root |
| Patrinia scabiosaefolia Fisch. ex Trevir | root |
| Petasites japonicus (Sieb. et Zucc.) Maxim. | root |
| Saururus chinensis (Lour.) Baill | aerial part |
| Schisandra chinensis Baillon | fruit |
| Scutellaria baicalensis Georgi | root |
| Selaginella tamariscina Spring | whole |
| Smilax china L. | rhizome |
| Solanum nigrum L. | whole |
| Sophora flavescens Sol. ex Ait. | root |
| Taraxacum officinale Weber | whole |
| Torreya nucifera Sieb. et Zucc. | fruit |
| Ulmus macrocarpa Hance | bark |
| Vitex rotundifolia | fruit |
| Zanthoxylum schinifolium Siebold et Zuccarini | fruit |
| Zingiber officinale Roscoe | rhizome |
| Zizyphus jujuba var. inermis Rehder | fruit |
2.2. Preparation of ethanol extracts
The dried and ground plant materials were extracted with 70% ethanol (100 g/L) by sonication twice each for 1 hour. After extraction, each extract was filtered through 3 MM paper and concentrated in vacuo to obtain a dried extract. Each powdered herbal extract was first dissolved in dimethyl sulfoxide (DMSO) at a concentration of 20 mg/mL, and then diluted to 2 mg/mL in phosphate buffered saline (PBS; DMSO concentrations in the stock solutions were 10%) and stored at –20 °C until further use.
2.3. Cell cultures
Human lung cancer cell lines (A549 and NCI-H23), human colorectal cancer cell lines [KM12(SM) and HCT116], human renal cancer cell lines (Caki-1 and ACHN), human primary lung cell lines (MRC9 and IMR90), and human normal colon cell lines (CCD-112CoN) were purchased from the American Type Culture Collection (ATCC; Manassas, VA. USA). The human stomach cancer cell lines SNU1 and SNU638 were obtained from the Korean Cell Line Bank (Seoul, Korea). The cell lines A549, NCI-H23, SNU1, and SNU638 were cultured in Roswell Park Memorial Institute (RPMI) medium (Invitrogen, Carlsbad, CA, USA). The cell lines MRC9, IMR90, CCD-112CoN, KM12 (SM), and ACHN were cultured in DMEM medium (Invitrogen). The cell lines Caki-1 and HCT116 were grown in McCoy's 5A medium (Invitrogen). Each medium was supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen), 100 U/mL of penicillin, and 100 μg/mL of streptomycin (Invitrogen). The cells were cultured in a humidified atmosphere with 5% CO2 at 37 °C.
2.4. In vitro Stat3 activity assay
A Cignal Stat3 reporter kit (SABiosciences, Valencia, CA, USA) was used to measure the in vitro Stat3 activity of the A549 cells that had been treated with various herbal extracts. The cells were plated onto 96-well flat-bottom microwell plates at a density of 1 × 104 cells/well and cultured for 16–24 hours. They were then transfected with a Stat3-responsive luciferase or with a control (positive or negative) construct in accordance with the manufacturer's instructions. After 24 hours, the cells were treated either with 100 μg/mL of herbal extract or with 50 μM of AG490 (Sigma, St. Louis, MO, USA) for 16 hours and lysed for the luciferase assay. Stat3 activity, as measured by Stat3-responsive construct luciferase activity in herbal extract-treated A549 cell lines, was determined with the Dual Luciferase Reporter Assay System (Promega, Madison, WI, USA). Luciferase activities were measured on a GloMax 20/20 Luminometer (Promega).
2.5. Western blot analysis
Changes in intracellular protein levels in response to Stat3-regulating herbal extract treatments were determined by Western blot analysis. A549 human lung cancer cells were plated in 60-mm culture dishes at a density of 1 × 106 cells/dish 16–24 hours prior to drug treatment. The cells were treated with 100 μg/mL of each herbal extract for 24 hours. The total protein was extracted with an ice-cold radioimmunoprecipitation assay (RIPA) buffer (Thermo Scientific, Rockford, IL, USA) that contained Complete™ Protease Inhibitor Cocktail (Roche, Mannheim, Germany) and phosphatase inhibitor cocktails 2 and 3 (Sigma). The protein concentrations were quantified on the basis of the BCA colorimetric method (Thermo Scientific). For the next step, 25 μg of the proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. The protein-blotted membranes were blocked with a 5% (w/v) skim milk solution in Tris-buffered saline (TBS) with 0.1% Tween 20 for 1 hour at room temperature (RT). They were then probed with primary antibodies at 4 °C overnight. The primary antibodies were detected with species-specific horseradish peroxidase-conjugated secondary antibodies for 1 hour at RT. The membranes were visualized with the SuperSignal West Femto Kit (Thermo Scientific), and chemiluminescence was detected on a FUSION SL (Vilber Lourmat, Torcy, France). Primary antibodies against Bcl-xL, Mcl-1, and Survivin were purchased from Cell Signaling Technology (Danvers, MA, USA). Antibodies against phospho-Jak2, phospho-Stat3 (Y705), and Stat3 were obtained from Thermo Scientific. Anti-actin antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
3. Results
3.1. Elevated basal Stat3 activity levels in human lung and kidney cancer cell lines
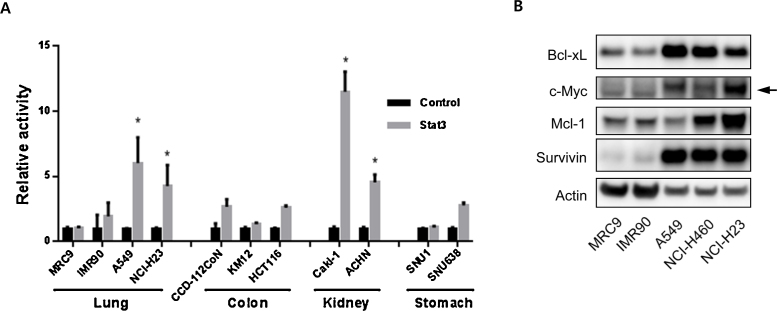
Stat3 was previously reported to be constitutively active in many biological tumors, including lung cancer.3 To test whether basal Stat3 signaling was elevated in various cancer cell lines in comparison to normal cells, eight human cancer cell lines from four different tissue origins (e.g., lung, colon, kidney, and stomach) and three human primary cell lines from lung and colon origins were selected for a comparison of Stat3 activation levels. Each cell line was transfected either with a Stat3-responsive luciferase construct or a noninducible negative control construct. The intracellular luciferase activity was measured 24 hours after transfection. The relative Stat3 activity was calculated by the fold increase in luciferase activity in the cells transfected with the Stat3-responsive element over the negative control. As Fig. 1A shows, all human lung cancer cell lines (i.e., A549 and NCI-H23) and renal cancer cell lines Caki-1 and ACHN showed elevated basal Stat3 activity levels (p < 0.03), compared to normal cell lines. By contrast, the cancer cell lines derived from the colon (KM12 and HCT116) and from the stomach (SNU1 and SNU638) did not show increased basal Stat3 activity levels in vitro. Of these cell lines, the human renal carcinoma cell line Caki-1 and the human lung cancer cell line A549 exhibited the highest basal Stat3 activity levels, which were approximately 7–12-fold higher than the negative control promoter activity. Even though both renal cancer cell lines that we tested showed highly elevated basal Stat3 levels, the lack of a normal counterpart prevented us from further investigating Stat3 signaling in these cell lines. Lung cancer cell lines also exhibited elevated basal Stat3 activity in comparison to normal lung cell lines; therefore, by using actin as an internal control, we further confirmed the overall Stat3 signaling activity levels among the lung cell lines by evaluating the expression levels of key proteins that are transcriptionally regulated by Stat3 (Fig. 1B). All cancer cell lines expressed high levels of Bcl-xL, c-Myc, Mcl-1, and Survivin, but had lower actin expressions in comparison to the normal cell lines. This indicates that hyperactive Stat3 signaling is a common feature in human lung cancer cell lines.
Fig. 1.
Intracellular Stat3 activity levels in human lung cell lines. (A) Relative Stat3 activity levels in various primary and cancer cell lines. The data are representative of one of three independent experiments that were performed in triplicate. (B) The expression levels of Stat3-regulated proteins in human lung cell lines. *p < 0.03.
3.2. Screening of herbal extracts for Stat3 inhibitory properties
We next used a Stat3-responsive reporter assay system to screen the 38 herbal extracts for their ability to suppress Stat3 activity in A549 cells. We selected the A549 cell line from among the lung cancer cell lines for this study because of its highly elevated intracellular Stat3 activity levels (Fig. 1A). A549 cells were transfected with a Stat3-responsive luciferase construct and subsequently treated with 100 μg/mL of various herbal medicine extracts for 16 hours, at which point changes in Stat3 activity in the treated cells were compared to the activity in the vehicle (0.5% DMSO)-treated cells. AG490 (50 μM), a well-known Stat3 inhibitor, was the positive control. As Table 2 shows, A549 cells treated with AG490 had approximately 50% basal Stat3 activity, compared to the vehicle control. Most extracts exhibited at least mild Stat3-suppressing activity, whereas only a few extracts exhibited Stat3-activating properties. The extracts were grouped into three categories based on their effect on Stat3 activity, as follows: “I” was specifically inhibitory (< 75% of the vehicle control); “N” was no effect (between 75% and 125%); and “S” was stimulatory (> 125%). Nineteen herbal extracts were found to have Stat3-inhibitory effects and only one herbal extract, the root of Scutellaria baicalensis Georgi, exhibited a Stat3-stimulatory effect. Of the 19 herbal extracts that exhibited anti-Stat3 activity, Bupleurum falcatum L.(BL), Taraxacum officinale Weber, Solanum nigrum L., Ulmus macrocarpa Hance, Euonymus alatus Sieb., Artemisia capillaris Thunb., and Saururus chinensis (Lour.) Baill inhibited more than 50% of the Stat3 activity in A549 human lung cancer cells.
Table 2.
Effect of herbal medicine ethanol extracts on STAT3 activity.
| Scientific name | Parts used | STAT3 activity (% of control) * | Category |
|---|---|---|---|
| Acanthopanax sessiliflorum Seeman | root, bark | 67.34 ± 6.80 | I |
| Achyranthes japonica Nakai | root | 70.19 ± 2.99 | I |
| Alisma orientale Juzepzuk | root tuber | 76.64 ± 3.56 | N |
| Artemisia capillaris Thunb. | aerial part | 44.35 ± 1.10 | I |
| Astragalus membranaceus Bunge | root | 82.45 ± 2.28 | N |
| Bupleurum falcatum L. | root | 31.63 ± 4.04 | I |
| Carpesium abrotanoides L. | aerial part | 89.84 ± 9.95 | N |
| Citrus unshiu Markovich | fruit skin | 92.92 ± 4.05 | N |
| Coix lacryma-jobi L. var. ma-yuen Stapf | seed | 81.09 ± 14.47 | N |
| Cornus officinalis Sieb. et Zucc. | fruit | 67.12 ± 8.75 | I |
| Cudrania tricuspidata (Carr.) Bureau ex Lavallee | root | 122.44 ± 5.43 | N |
| Cynanchum wilfordii (Maxim.) Hemsl. | root tuber | 80.53 ± 4.26 | N |
| Dianthus chinensis L. | aerial part | 53.03 ± 3.16 | I |
| Dioscorea batatas Decaisne | root tuber | 92.49 ± 2.62 | N |
| Duchesnea indica (Andr.) Focke | whole | 58.55 ± 9.18 | I |
| Euonymus alatus Sieb. | stem cork | 43.86 ± 6.68 | I |
| Houttuynia cordata Thunb | aerial part | 63.93 ± 6.01 | I |
| Laminaria japonica | leaf | 98.30 ± 18.22 | N |
| Liriope platyphylla Wang et Tang | root | 75.08 ± 22.21 | N |
| Lithospermum erythrorhizon Sieb. et Zucc. | root | 76.50 ± 13.66 | N |
| Orostachys japonicus A. Berger | whole | 63.33 ± 2.94 | I |
| Panax ginseng C. A. Mey. | root | 75.51 ± 6.86 | N |
| Patrinia scabiosaefolia Fisch. ex Link | root | 110.27 ± 1.42 | N |
| Petasites japonicus (Sieb. et Zucc.) Maxim. | root | 81.78 ± 8.43 | N |
| Saururus chinensis (Lour.) Baill | aerial part | 45.86 ± 6.63 | I |
| Schisandra chinensis Baillon | fruit | 69.84 ± 3.72 | I |
| Scutellaria baicalensis Georgi | root | 163.39 ± 13.84 | S |
| Selaginella tamariscina Spring | whole | 74.18 ± 4.26 | I |
| Smilax china L. | root tuber | 120.80 ± 24.92 | N |
| Solanum nigrum L. | whole | 33.83 ± 1.36 | I |
| Sophora flavescens Sol. ex Ait. | root | 117.30 ± 11.48 | N |
| Taraxacum officinale Weber | whole | 33.79 ± 5.02 | I |
| Torreya nuncifera Sieb. et Zucc. | fruit | 56.23 ± 8.93 | I |
| Ulmus macrocarpa Hance | bark | 39.56 ± 6.38 | I |
| Vitex rotundifolia | fruit | 79.10 ± 5.88 | N |
| Zanthoxylum schinifolium Siebold et Zuccarini | fruit | 60.33 ± 15.39 | I |
| Zingiber officinale Roscoe | root tuber | 97.81 ± 5.58 | N |
| Zizyphus jujuba var. inermis Rehder | fruit | 81.59 ± 11.81 | N |
| AG490 (Stat3 inhibitor) | – | 52.72 ± 5.38 |
* Mean ± S.D. of one representative experiment performed in triplicate (out of three independent experiments).
I, inhibitory (< 75%); N, no effect (between 75% and 125%); S = stimulatory (> 125%).
3.3. Regulation of Stat3 signaling components by herbal extracts
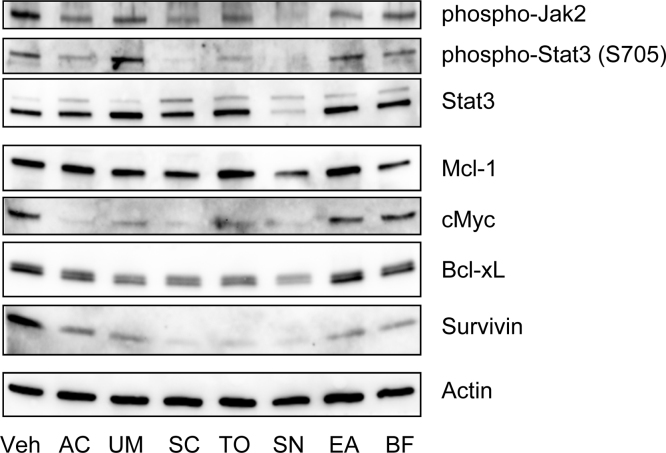
Because Stat3 activation is primarily mediated by Jak2,5 the levels of Jak2 phosphorylation, Stat3 tyrosine 705 phosphorylation (a primary activating phosphorylation site on Stat3), and the expression levels of Stat3 were assessed in the cells treated with selected strong anti-Stat3 herbal extracts. As Fig. 2 shows, Jak2 phosphorylation was effectively inhibited by all seven extracts, and Stat3 phosphorylation at Tyr705 was reduced by A. capillaris, S. chinensis, T. officinale, and S. nigrum treatment. The Stat3 expression level was interestingly nearly abrogated in S. nigrum-treated A549 cells, which suggests that an ethanol extract of S. nigrum can effectively suppress Stat3 gene transcription and/or translation or enhance Stat3 degradation.
Fig. 2.
The expression of Stat3 and related proteins in Stat3-inhibiting Korean medicine (KM) extract-treated A549 human lung cancer cells.
AC, Artemisia capillaris Thunb.; BF, Bupleurum falcatum L.; EA, Euonymus alatus Sieb; SC, Saururus chinensis (Lour.) Baill.; SN, Solanum nigrum L.; TO, Taraxacum officinale Weber; UM, Ulmus macrocarpa Hance.
As previously mentioned, the expression levels of many downstream target proteins are regulated by Stat3, including well-known oncogenes (such as c-Myc) and apoptosis-inhibitory proteins (such as Survivin and Bcl-xL). Survivin expression levels were decreased in A549 cells that had been treated with any of the seven extracts, and c-Myc expression levels were reduced in response to A. capillaris, U. macrocarpa, S. chinensis, T. officinale, and S. nigrum (Fig. 2). The expression levels of Bcl-xL, a Bcl-2 family member that inhibits apoptosis, were mildly decreased in response to the extracts of A. capillaris, U. macrocarpa, S. chinensis, T. officinale, and S. nigrum (Fig. 2).
4. Discussion
The major hallmarks of cancer include uncontrolled cell growth, growth suppressor evasion, cell death inhibition, angiogenesis induction, activation of invasion and metastasis, and host immunity avoidance.22 Since the introduction of mechanism-based targeted therapies to treat human malignancies, many efforts have been made to develop chemotherapeutic agents that can control each and/or all of these hallmarks of cancer. In this light, Stat3 is an attractive novel target for cancer therapies because it can regulate most of these hallmarks of cancer.23, 24
In this study, we first demonstrated that the basal Stat3 signaling levels are elevated in some types of human cancer cell lines such as lung and kidney cancers (Fig. 1A). The protein levels of genes regulated by Stat3 (e.g., Bcl-xL, Survivin, and c-Myc) consequently are much higher in human lung cancer cell lines than in normal lung cell lines (Fig. 1B). All of these proteins are important mediators of tumorigenesis that can enhance cell growth, inhibit apoptosis, and induce drug resistance. These findings, combined with other numerous reports about Stat3 hyperactivity in human cancers,2 led us to search for Stat3 signaling inhibitors in traditionally used herbal medicinal materials.
Thus, 38 medicinal herbs grown and processed in Korea were selected, extracted with 70% ethanol, and evaluated for their Stat3-inhibiting activities in the A549 human lung cancer cell line, which has elevated intrinsic Stat3 activity. All of these medicinal herbs have been traditionally used for various cancer-like symptoms. Of these 38 medicinal herbal extracts, 50% (19 extracts) exhibited at least moderate Stat3-inhibiting activities, whereas only one extract (Scutellaria baicalensis Georgi root) exhibited Stat3-stimulating activity (Table 2). We further selected the seven most potent extracts to test whether the Stat3-inhibiting activities of these extracts could suppress the key hallmarks of cancer proteins that are regulated by Stat3 (Fig. 2). When treated with these extracts, the A549 cells, as anticipated, showed inhibited activity of Stat3 and Jak2 (its activation partner) and showed reduced expression levels of some or all of the Stat3-regulated proteins.
Solanum nigrum L. (SN) has been widely used in Traditional Chinese Medicine (TCM) as an elemental ingredient of clinical TCM cancer therapies.25 A water extract of SN leaves was reportedly induces apoptosis and autophagy and enhances the cytotoxic activity of various chemotherapeutic agents in various human cancer cell lines, including colorectal cancer, prostate cancer, and breast cancer;20, 26, 27 however, little is known about the mechanism of its action besides the fact that it contains many plant polyphenols and flavonoids. Our preliminary study indicated that the ethanol extract of SN only exhibited moderate cytotoxicity in a single lung cancer cell line, NCI-H23, among the four cell lines we used in this study (data not shown). This clearly indicated that the mechanism behind the possible antitumor activity that we observed for SN is unrelated to its cytotoxic property. As Fig. 2 and Table 2 show, the SN extract has potent Stat3-inhibitory activity. The Stat3 transcriptional activity was reduced by more than 65%, and the expression levels of Stat3 and the phosphorylation of Jak2 were almost completely abolished. Furthermore, the expression levels of Mcl-1, c-Myc, Bcl-xL, and Survivin were reduced.
Another medicinal herb that we tested, BF, is a widely used herbal remedy in Korea, China, and Japan that possesses antidepressant,28 antioxidative,29 and antiulcerative30 properties. In our study, an ethanol extract of BF also exhibited a potent Stat3-inhibitory effect. However, unlike the SN extract, the BF extract had little effect on the Stat3 expression level and only mildly inhibited the phosphorylation of Stat3 and Jak2. The BF extract efficiently inhibited Mcl-1 and Survivin expression, although it had little effect on the expression levels of c-Myc and Bcl-xL.
U. macrocarpa Hance. (UM)—also called by its common name, “large-fruited elm”—is known for its antioxidative and anti-inflammatory properties.31, 32 In our study, the UM extract exhibited potent Stat3-inhibitory activity, yet had little effect on activating the phosphorylation of Stat3 and Jak2. The UM extract inhibited c-Myc, Survivin, and Bcl-xL expression but not Mcl-1 expression.
In conclusion, we evaluated the in vitro Stat3-inhibitory activity levels of 70% ethanol extracts from 38 herbal medicines. Of these extracts, seven herbal medicinal plants—including BL., T. officinale Weber, S. nigrum L., UM, E. alatus Sieb., A. capillaris Thunb., and S. chinensis (Lour.) Baill.—exhibited potent Stat3 inhibition and reduced the expression of many cancer-related proteins. Stat3 inhibition is the common feature exhibited by these plant materials; however, further research into the mechanism of Stat3 suppression should follow because each extract potentiated a different level of Stat3 downstream target expression. To our knowledge, this is the first report to demonstrate Stat3-inhibitory activity in plant extracts. These data could be a valuable source for the development of novel and safe chemotherapeutic agents from traditional herbal remedies.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
This study was supported by a grant (K13062) from the Korea Institute of Oriental Medicine (Daejeon, Republic of Korea).
References
- 1.Levy DE, Darnell JE., Jr. Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 2.Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–2488. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- 3.Calo V, Migliavacca M, Bazan V, Macaluso M, Buscemi M, Gebbia N. STAT proteins: from normal control of cellular events to tumorigenesis. J Cell Physiol. 2003;197:157–168. doi: 10.1002/jcp.10364. [DOI] [PubMed] [Google Scholar]
- 4.Zhong Z, Wen Z, Darnell JE., Jr. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal BB, Sethi G, Ahn KS, Sandur SK, Pandey MK, Kunnumakkara AB. Targeting signal-transducer-and-activator-of-transcription-3 for prevention and therapy of cancer. Ann N Y Acad Sci. 2006;1091:151–169. doi: 10.1196/annals.1378.063. [DOI] [PubMed] [Google Scholar]
- 6.Bowman T, Broome MA, Sinibaldi D, Wharton W, Pledger WJ, Sedivy JM. Stat3-mediated Myc expression is required for Src transformation and PDGF-induced mitogenesis. Proc Natl Acad Sci U S A. 2001;98:7319–7324. doi: 10.1073/pnas.131568898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masuda M, Suzui M, Yasumatu R, Nakashima T, Kuratomi Y, Azuma K. Constitutive activation of signal transducers and activators of transcription 3 correlates with cyclin D1 overexpression and may provide a novel prognostic marker in head and neck squamous cell carcinoma. Cancer Res. 2002;62:3351–3355. [PubMed] [Google Scholar]
- 8.Bhattacharya S, Ray RM, Johnson LR. STAT3-mediated transcription of Bcl-2. Mcl-1 and c-IAP2 prevents apoptosis in polyamine-depleted cells. Biochem J. 2005;392:335–344. doi: 10.1042/BJ20050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aoki Y, Feldman GM, Tosato G. Inhibition of STAT3 signaling induces apoptosis and decreases Survivin expression in primary effusion lymphoma. Blood. 2003;101:1535–1542. doi: 10.1182/blood-2002-07-2130. [DOI] [PubMed] [Google Scholar]
- 10.Dechow TN, Pedranzini L, Leitch A, Leslie K, Gerald WL, Linkov I. Requirement of matrix metalloproteinase-9 for the transformation of human mammary epithelial cells by Stat3-C. Proc Natl Acad Sci U S A. 2004;101:10602–10607. doi: 10.1073/pnas.0404100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang T, Niu G, Kortylewski M, Burdelya L, Shain K, Zhang S. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004;10:48–54. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- 12.Kortylewski M, Kujawski M, Wang T, Wei S, Zhang S, Pilon-Thomas S. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med. 2005;11:1314–1321. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen M, Kaltoft K, Nordahl M, Ropke C, Geisler C, Mustelin T. Constitutive activation of a slowly migrating isoform of Stat3 in mycosis fungoides: tyrphostin AG490 inhibits Stat3 activation and growth of mycosis fungoides tumor cell lines. Proc Natl Acad Sci U S A. 1997;94:6764–6769. doi: 10.1073/pnas.94.13.6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung CD, Liao J, Liu B, Rao X, Jay P, Berta P. Specific inhibition of Stat3 signal transduction by PIAS3. Science. 1997;278:1803–1805. doi: 10.1126/science.278.5344.1803. [DOI] [PubMed] [Google Scholar]
- 15.Nagel-Wolfrum K, Buerger C, Wittig I, Butz K, Hoppe-Seyler F, Groner B. The interaction of specific peptide aptamers with the DNA binding domain and the dimerization domain of the transcription factor Stat3 inhibits transactivation and induces apoptosis in tumor cells. Mol Cancer Res. 2004;2:170–182. [PubMed] [Google Scholar]
- 16.Mora LB, Buettner R, Seigne J, Diaz J, Ahmad N, Garcia R. Constitutive activation of Stat3 in human prostate tumors and cell lines: direct inhibition of Stat3 signaling induces apoptosis of prostate cancer cells. Cancer Res. 2002;62:6659–6666. [PubMed] [Google Scholar]
- 17.Turkson J, Zhang S, Mora LB, Burns A, Sebti S, Jove R. A novel platinum compound inhibits constitutive Stat3 signaling and induces cell cycle arrest and apoptosis of malignant cells. J Biol Chem. 2005;280:32979–32988. doi: 10.1074/jbc.M502694200. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Jiang B, Brecher P. Selective inhibition of STAT3 phosphorylation by sodium salicylate in cardiac fibroblasts. Biochem Pharmacol. 2002;63:1197–1207. doi: 10.1016/s0006-2952(02)00853-5. [DOI] [PubMed] [Google Scholar]
- 19.Bharti AC, Donato N, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J Immunol. 2003;171:3863–3871. doi: 10.4049/jimmunol.171.7.3863. [DOI] [PubMed] [Google Scholar]
- 20.Nawab A, Thakur VS, Yunus M, Ali Mahdi A, Gupta S. Selective cell cycle arrest and induction of apoptosis in human prostate cancer cells by a polyphenol-rich extract of Solanum nigrum. Int J Mol Med. 2012;29:277–284. doi: 10.3892/ijmm.2011.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen SC, Chang YL, Wang DL, Cheng JJ. Herbal remedy magnolol suppresses IL-6-induced STAT3 activation and gene expression in endothelial cells. Br J Pharmacol. 2006;148:226–232. doi: 10.1038/sj.bjp.0706647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Crowe PJ, Goldstein D, Yang JL. STAT3 inhibition, a novel approach to enhancing targeted therapy in human cancers (Review) Int J Oncol. 2012;41:1181–1191. doi: 10.3892/ijo.2012.1568. [DOI] [PubMed] [Google Scholar]
- 24.Yu H, Jove R. The STATs of cancer — new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 25.An L, Tang JT, Liu XM, Gao NN. [Review about mechanisms of anti-cancer of Solanum nigrum]. Zhongguo Zhong Yao Za Zhi 2006; 31: 1225-6, 1260.[In Chinese]. [PubMed]
- 26.Tai CJ, Wang CK, Tai CJ, Lin YF, Lin CS, Jian JY. Aqueous extract of Solanum nigrum leaves induces autophagy and enhances cytotoxicity of cisplatin, doxorubicin, docetaxel, and 5-fluorouracil in human colorectal carcinoma cells. Evid Based Complement Alternat Med. 2013;2013:514719. doi: 10.1155/2013/514719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang HC, Syu KY, Lin JK. Chemical composition of Solanum nigrum linn extract and induction of autophagy by leaf water extract and its major flavonoids in AU565 breast cancer cells. J Agric Food Chem. 2010;58:8699–8708. doi: 10.1021/jf101003v. [DOI] [PubMed] [Google Scholar]
- 28.Lee B, Yun HY, Shim I, Lee H, Hahm DH. Bupleurum falcatum prevents depression and anxiety-like behaviors in rats exposed to repeated restraint stress. J Microbiol Biotechnol. 2012;22:422–430. doi: 10.4014/jmb.1110.10077. [DOI] [PubMed] [Google Scholar]
- 29.Kim SM, Kim SC, Chung IK, Cheon WH, Ku SK. Antioxidant and protective effects of Bupleurum falcatum on the l-thyroxine-induced hyperthyroidism in rats. Evid Based Complement Alternat Med. 2012;2012:578497. doi: 10.1155/2012/578497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun XB, Matsumoto T, Yamada H. Effects of a polysaccharide fraction from the roots of Bupleurum falcatum L. on experimental gastric ulcer models in rats and mice. J Pharm Pharmacol. 1991;43:699–704. doi: 10.1111/j.2042-7158.1991.tb03461.x. [DOI] [PubMed] [Google Scholar]
- 31.Oh KS, Ryu SY, Oh BK, Seo HW, Kim YS, Lee BH. Antihypertensive, vasorelaxant, and antioxidant effect of root bark of Ulmus macrocarpa. Biol Pharm Bull. 2008;31:2090–2096. doi: 10.1248/bpb.31.2090. [DOI] [PubMed] [Google Scholar]
- 32.Kwon JH, Kim SB, Park KH, Lee MW. Antioxidative and anti-inflammatory effects of phenolic compounds from the roots of Ulmus macrocarpa. Arch Pharm Res. 2011;34:1459–1466. doi: 10.1007/s12272-011-0907-4. [DOI] [PubMed] [Google Scholar]