Abstract
Background
Photodynamic therapy (PDT) is a new modality in the treatment of cancer. This study thus aims to examine whether the PDT is effective against in vivo hepatocellular carcinoma.
Methods
In vivo efficacy of PDT on hepatocellular carcinoma was tested in xenografted mice with human hepatocellular carcinoma cell lines (Huh7) by utilizing a gold nanoparticles (GNPs) conjugate of new photosensitizer (PS), purpurin-18-N-butylimide-N-methyl-D-glucamine (Pu-18-N-butylimide-NMGA). The conjugate (PS-GNPs) was synthesized from the reaction between Pu-18-N-butylimide-NMGA and chloroauric acid (HAuCl4). Mice were arbitrarily assigned into one of three groups. First group received saline alone, second group received PS-GNPs alone, and the last group received both PS-GNPs and irradiation. PS-GNPs was injected directly into the tumor mass and irradiations were performed 24 hours after injection of PS-GNPs.
Results
Tumor volume was significantly smaller in the group which received both PS-GNPs and irradiation compared with other two groups. Western blot and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay revealed that the group which received both PS-GNPs and irradiation showed larger amount of apoptotic protein and DNA fragmentation compared with other two groups.
Conclusion
This study suggests that Pu-18-N-butylimide-NMGA-GNP conjugate is an effective agent for PDT in the treatment of hepatocellular carcinoma.
Keywords: hepatocellular carcinoma, irradiation, liver cancer, photodynamic therapy, photosensitizer
1. Introduction
Liver cancer is the sixth most common malignancy worldwide and it constitutes about 5.7% of new cases. The prognosis is so poor that number of cases (626,000) is almost same as that of deaths (598,000).1 Therefore, it ranks the third in total number of deaths from cancer and the survival rate ranges between 3 and 5%.1 Majority of liver cancer is hepatocellular carcinoma which originates from hepatocytes.2 Hepatocellular carcinoma has also very poor survival rate for its incidence as well as other types of liver cancer, which means medical treatment is not so successful in improving the survival rate of patients. So far the most effective treatment to improve survival rate is surgical excision plus liver transplantation.2 Although surgical excision is a choice of treatment for higher survival rate, accompanying conditions such as hepatic cirrhosis, vascular infiltration, and extrahepatic metastasislimit indication of surgery lower than 20% of patients.2 Liver transplantation was known to improve the survival rate but finding a donor is not easy al all.2 As an adjunctive therapy, chemotherapy or radiotherapy has been employed. However, those adjunctive therapies inadvertently cause toxicity or adverse reactions limiting long-term treatment. In order to overcome those limitations, the PDT, a new promising treatment modality, was recently developed.3 PDT generates singlet oxygen to selectively destroy target carcinoma cells by a combination of visible light, photosensitizer (PS), and oxygen.4 Although PDT is not currently employed in clinical treatment of hepatocellular carcinoma, it is expected to improve survival rate and quality of life without adverse reactions or side effects commonly seen in chemotherapy or radiotherapy.5 This study thus aims to examine whether the PDT is effective against in vivo hepatocellular carcinoma.
2. Method
2.1. Materials
Reagents used to synthesize PS-gold nanoparticles (PS-GNPs) including methylene chloride (MC), methanol (MeOH), and diethyl ether acetone were purchased from SK chemical (Korea). All other reagents including potassium hydroxide (KOH), 1-propanol, N-methyl-D-glucamine (NMGA), tetrahydrofuran (THF), and chloroauric acid were purchased from Sigma-Aldrich and used without further purification. 6-weeks old BALB/c-nu slc mice (female) were used for xenograft. The PDT was carried out using a diode laser generator apparatus (BioSpec LED, Russia) equipped witha halogen lamp, a band-pass filter (640-710 nm).
2.2. Synthesis of photosensitizing agent
2.2.1. Methyl Pheophorbide-a (MPa) from spirulina pacific algae
500 g of dried spirulina is dissolved in 2 L of acetone and stirred at room temperature for two hours under nitrogen atmosphere in dark. Supernatants are filtered more than twice and acetone is removed by evaporation. 400 mL of 5% sulfuric acid and 7,600 mL of methanol are then added and kept overnight. It is further purified by extraction of organic phase using CH2Cl2 and water. Silica gel is added to the organic phase to yield chlorophyll-a. Silica gel column chromatography is finally used to extract pure MPa from chlorophyll-a.
2.2.2. Purpurin-18 (Pu-18)
2 g of MPa is dissolved in a mixture solution composed of 800 mL diethyl ether, 80 mL KOH, and 24 mL 1-propanol. 30 mL of pyridine is added to the solution to be stirred for 1∼3 hours under air atmosphere at room temperature. pH is adjusted to 2∼4 by using 10% sulfuric acid. 400 mL of solution containing THF and MC with a ratio of 3:1 is added to separate organic phase and dried to yield residue. Silica gel column chromatography is finally used to extract pure Pu-18.
2.2.3. Pu-18-N-butylimide
Pu-18 is dissolved in 50 mL of toluene and 10 molar N-butylamine is added to be stirred for 24 hours under nitrogen in dark. Silica gel chromatography is used to extract pure purpurin-18-N-butylimide.
2.2.4. N-methyl-D-glucamine (NMGA) salt of Pu-18-N-butylimide(Pu-18-N-butylimide-NMGA)
Pu-18-N-butylimide and NMGA are under reaction for 4 hours. 20 μm filter paper is then used to collect Pu-18-NMGA dissolved in aqueous phase.
2.2.5. Synthesis of PS-GNPs conjugate
A seed solution is prepared by allowing 5 mL of PS (0.002 M) to react with 2.5 mL of HAuCl4 (0.001 M) at room temperature. The seed solution is heated until its color turns into dark brown and kept at room temperature. A mixture solution containing 25 mL of PS (0.002 M), 25 mL of HAuCl4 (0.001 M), and 1 mL of AgNO3 (0.005 M) is prepared and 100 μL of seed solution is added. The reaction mixture is centrifuged at 10,000 rpm for 10 min and resuspended in water.
2.3. Cell culture
Human hepatocellular carcinoma cell line, Huh7 cells were obtained from the Korean Cell Line Bank (Seoul, Korea) and cultured in Dulbecco's Modified Eagle's medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Sigma) and 1% penicillin/streptomycin (Lonza, USA) at 37 °C in a humidified atmosphere of 5% CO2.
2.4. Xenograft studies
2.4.1. Cells injection
Animal studies were approved by the committee of the Inje University. Two x 106 Huh7 cells per mouse were injected subcutaneously into the right flanks of 6-week-old female nude mice. The mice were incubated to form a tumor mass (50-100 mm3) for 4 days. After tumor formation, 18 mice were randomly assigned into one of 3 groups (6 mice/group) to examine whether the new modality of treatment is effective against hepatocellular carcinoma. The treatment group received photoirradiation after local injection of PS-GNPs into the tumor mass (PS-GNPs plus irradiation group). The control group was further divided into two groups: one received no particular treatment other than saline injection (saline alone group) whereas the other group received only injection of PS-GNPs solution without photoirradiation (PS-GNPs alone group).
2.4.2. Drug administration and light exposure
Size of tumor was measured every 4 days after establishment of xenograft. Synthesized PS-GNPs were dissolved in PBS (0.1 mg/mL) 24 hours before irradiation and kept dark in an aluminum foil. PS-GNPs (0.03 mg/kg) were directly injected into the mass of cancer cells by using a Hamilton syringe when the mass size reached 100 mm3. Mice were kept at dark room for 24 hours and an LED (wavelength: 640-710 nm) was used to irradiate the mass of cancer cells. These procedures were repeated after 16 days of observation with increased amount of PS-GNPs (0.07 mg/kg).
2.4.3. Measurement of tumor size
The efficacy of photodynamic therapy was evaluated by measurement of tumor mass using a digital caliper. The tumor volume was determined by measuring the length (l) and the width (w). Tumor size was recorded every 4 days. The mice were killed after tumor formation, and the tumor samples were analyzed by the In Situ Apoptosis TUNEL system (Milipore, USA) and by Western blot analysis for specific cleavage of caspase-3 (Cell Signaling Technology, USA).
2.4.4. Western blot and TUNEL assay
In order to quantify proteins related with apoptosis, the Western blot was carried out. Tumors were lysed in lysis buffer A [20 mM N-2-hydroxyethylpiperazine-N’-2-ethanesulfonic acid (pH 7.5), 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid, 2 mM ethylene glycol tetraacetic acid, 1% Triton X-100, 10% glycerol and protease cocktail I/II (Sigma)], and debris was removed by centrifugation at 15,000 rpm for 10 min. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred onto nitrocellulose membranes, blocked with 5% skim milk in 0.01 M Tris-buffered saline containing 0.5% Tween 20 and blotted with the appropriate primary antibodies. Membranes were then incubated with horseradish peroxidase-conjugated goat anti-rabbit-IgG or goat anti-mouse IgG secondary antibodies for 1 hour at room temperature and detected by chemiluminescence (Abclon, Korea). The Multi gauge software was used to quantify the results of Western blot6. TUNEL assay was employed to quantify the degree of apoptosis in tumor mass. The tumors were removed and frozen in OCT embedding medium, and then a series of tissue sections (10 μm in thickness) was obtained in cryostat (Leica). The sections were incubated in 50 μL of terminal deoxynuclotidyl transferase-mediate uridine 5’-triphosphate-biotin nick-end labeling (TUNEL) reaction mixture (Milipore, USA) for 60 min at 37 °C, washed and subsequently counterstained with 4’, 6’-diamidino-2-phenylindole (DAPI, 1 μg/ml, Sigma) for 30 min. The sections were mounted using the Vectashield mounting medium and examined using a LSM700 confocal laser-scanning microscope (Carl Zeiss, Germany).
2.4.5. Statistical analysis
Data were analyzed using either the student's t-test or F-test, and Sigma-Stat software; the p value was derived to assess the statistical significance and is indicated as *p < 0.05; **p < 0.01 (Fig. 2).
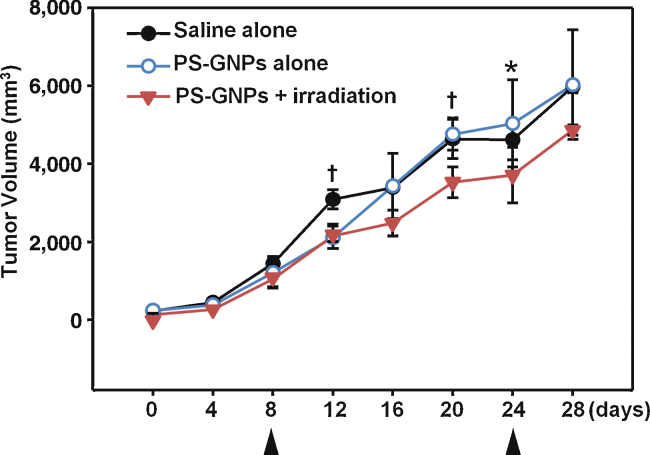
Fig. 2.
Change in tumor volume (mm3) during 28 days after first measurement of tumor mass. The growth of tumor was significantly lower (p < 0.05) in PS-GNPs plus irradiation group from 4 days after first irradiation compared with those of the other two groups. First and second irradiations were indicated by arrows. *p < 0.05, †p < 0.01. PS-GNPs, photosensitizer gold nanoparticles.
3. Results
3.1. Change in size of tumor and survival rate of mice
Size of tumor was quantified by measurement of length (l) and width (w) using a digital caliper. The volume was calculated as follows:
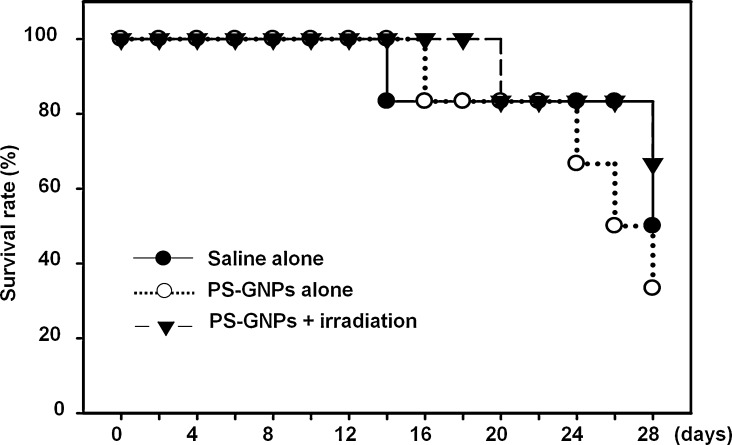
where L is length (in mm), and w is width of mass. Measurements were carried out twice until the growing volume of mass reaches 100 mm3. Thereafter photodynamic therapy began. The PS-GNPs were injected into the tumor mass and the Biospec LED (wavelength: 640-710 nm) was used to irradiate it for 30 min after 24 hours. There was no significant size difference among 3 groups until 8-days before irradiation (Table 1 and Fig. 1, Fig. 2). Volume of mass was still rapidly growing in all 3 groups on the day of irradiation. On the 4th day after first irradiation, the volume of mass in the PS-GNPs plus irradiation group was significantly smaller than those in two other groups (saline alone or PS-GNPs alone group). Second irradiation was given to mice on 16th day after first irradiation. The mean volume of PS-GNPs plus irradiation group was again significantly smaller than those of other groups (4863.50 mm3 vs 6027.88, 5977.22 mm3) indicating that the photodynamic therapy is effective in slowing down the rate of tumor growth. Survival rate was also different among them (Fig. 3). On 20th day after first irradiation, there were 4 mice alive in PS-GNPs plus irradiation group whereas 3 and 2 mice were alive in saline alone and PS-GNPs alone group, respectively.
Table 1.
Change in tumor size during 28 days after first measurement of tumor mass (unit: mm3).
| Groups | Day 0 | Day 4 | Day 8 (first irradiation) | Day 12 | Day 16 | Day 20 | Day 24 (second irradiation) | Day 28 |
|---|---|---|---|---|---|---|---|---|
| Saline alone | 232.81 | 448.06 | 1,447.06 | 3,090.30 | 3,393.55 | 4,635.60 | 4,616.85 | 5,977.22 |
| PS-GNPs alone | 244.67 | 386.18 | 1,215.09 | 2,115.86 | 3,434.48 | 4,758.79 | 5,036.23 | 6,027.88 |
| PS-GNPs + irradiation | 134.57 | 267.75 | 1,046.29 | 2,151.49 | 2,482.10 | 3,529.44 | 3,710.33 | 4,863.50 |
GNPs, gold nanoparticles; PS, photosensitizer.
Fig. 1.
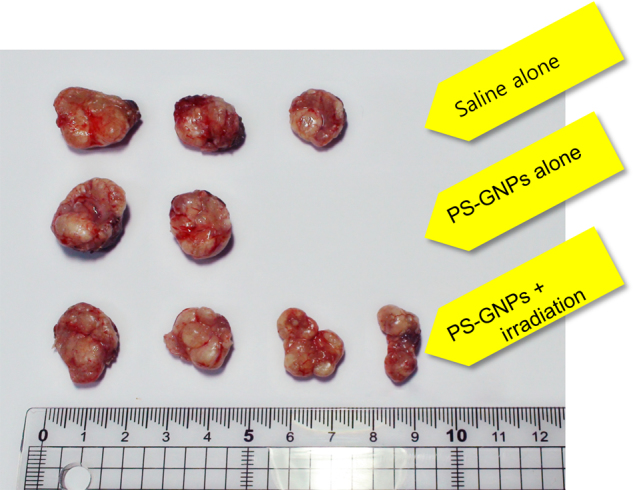
Photographs displaying tumor masses excised from right flanks of mice. Tumor masses of saline alone, PS-GNPs alone, and PS-GNPs plus irradiationgroup were excised from right flanks of mice on 32nd day afier injection of Huh7 cells. They are all similar in texture and color. The ruler is given to estimate the size. PS-GNPs, photosensitizer gold nanoparticles.
Fig. 3.
Survival rates of mice over time were plotted and compared. PS-GNPs, photosensitizer gold nanoparticles.
3.2. Cleavage of caspase-3 triggered by PS-GNPs and irradiation
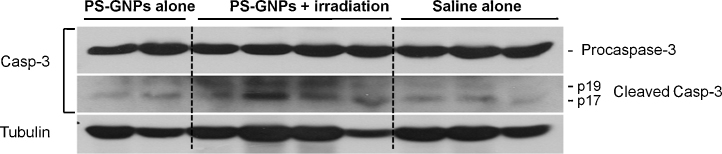
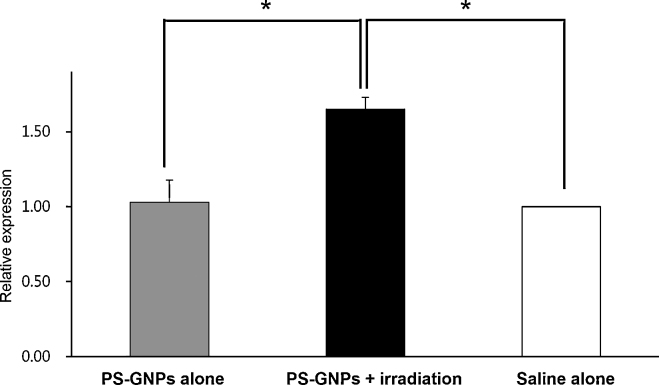
The procaspase-3 cleavage into p17 and p19 activated caspase-3 and appears as dual band. The activated caspase-3 is generally used for marker of apoptosis since it is involved in the progress of cellular apoptosis by destroying DNA. The cleaved form of caspase-3 was higher in PS-GNPs plus irradiation group than saline alone or PS-GNPs alone group. The latter two groups were similar in the relative amount of cleaved caspase-3 (Fig. 4). The tubulin, used for loading control, was not significantly different among those groups. When the amount of P17, p19 cleaved caspase-3 (QL/area) was normalized to the amount of tubulin (QL/area), the ratio was significantly different between PS-GNPs plus irradiation group and saline alone or PS-GNPs alone group as shown in Fig. 5 indicating that the photodynamic therapy is indeed effective in triggering apoptosis of cells in hepatocellular carcinoma.
Fig. 4.
Western blot analysis of activated caspase-3 quantifying the degree of apoptosis arising from the tumor mass in each group. Procaspase-3 cleavage into p17 and p19 activated caspase-3 which subsequently destroy DNAs. α-tubulin was used as a loading control. PS-GNPs, photosensitizer gold nanoparticles.
Fig. 5.
Relative amount of cleaved caspase-3 in PS-GNPs plus irradiation group compared to saline alone or PS-GNPs alone group. Protein amount in saline alone group was set to 1.00 while those of PS-GNPs alone and PS-GNPs plus irradiation group were expressed as relative values. *p < 0.05. PS-GNPs, photosensitizer gold nanoparticles.
3.3. DNA fragmentation by PS-GNPs and irradiation
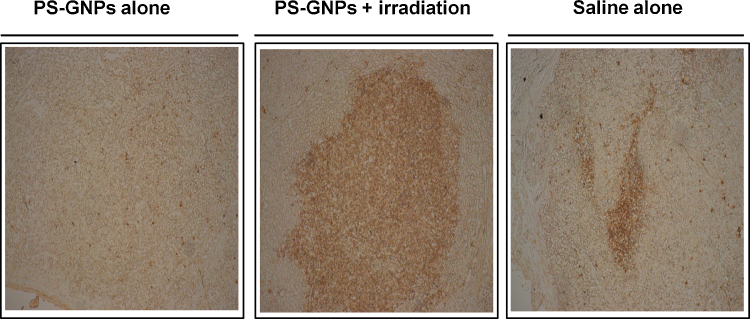
TUNEL assay is a common technique to detect DNA fragmentation which results from apoptotic signaling cascades. TUNEL immunohistochemistry of hepatic cells in tumor revealed a distinctive pattern of nuclear staining (Fig. 6). The number of TUNEL positive cells was significantly higher in the PS-GNPs plus irradiation group compared to the saline alone or PS-GNPs alone group (Fig. 6, p < 0.01).
Fig. 6.
Photographs showing TUNEL staining of tumor samples from mice in each group. TUNEL(+) cells were detected prominently in PS-GNPs plus irradiation group compared with other groups. PS-GNPs, photosensitizer gold nanoparticles.
4. Discussion
We performed a xenograft protocol to establish an animal model of hepatocellular carcinoma using Huh7 cells. We previously confirmed that the xenografts inoculated onto the right flanks of nude mice retained the genomic characteristics of hepatocellular carcinoma7. Having identified growing mass of hepatocellular carcinoma, we performed PDT twice and examined its efficacy in treatment of cancer by comparing volume of tumor mass among groups, counting alive individuals in each group, carrying out Western blot and TUNEL assay to quantify apoptosis-related proteins and degree of DNA fragmentation, respectively. All the results support the idea that the PDT using PS-GNPs may be effective in slowing down the progress of hepatocellular carcinoma. Application of PS-GNPs alone failed to inhibit the growth of tumor compared with no treatment. There was no evidence of cytotoxicity by PDT in normal tissue of mice. The photosensitizing agent, PS-GNPs, has several distinctive advantages over other PSs. First of all, it could be sensitized by relatively long light wavelength which can penetrate into deep tissues. In PDT, wavelengths shorter than 650 nm usually do not allow penetration into deep tissues but cause unwanted photosensitive reactions. Too long wavelengths (>850 nm) are not able to generate singlet oxygen which is a key molecule triggering production of ROS to destroy cancer cells. The range of wavelengths allowing deep penetration and ensuring therapeutic potential is called the “phototherapeutic window”.8 The PS-GNPs used in our experiment is therefore suitable for treatment of deep-seated tumor mass since its photosensitive wavelengths lie in the “phototherapeutic window”. In order to selectively destroy tumors without affecting normal tissues, the ideal PS should preferentially accumulate in tumor mass. The GNPs conjugate, one of which we employed as a carrier molecule, is so far most effective in delivery of PSs in that it allows PSs being used in photothermal therapy as well as acquiring enhanced permeability and retention in tumor mass.9, 10 The GNPs conjugate also makes PSs amphipathic resulting in better cellular uptake and solubility in aqueous phase.
The interval between injection of PSs and irradiation of a laser affects the outcome of PDT.11 PSs are initially localized to the vasculature of tumor mass after injection. If the irradiation is given to a tumor mass shortly after injection of a PS, the therapeutic effect is probably mediated by destroying blood supply to the tumor mass. If the PSs are allowed to perfuse into vasculature of tumor mass more than a couple of hours, they would probably begin to diffuse into most of compartments of tumor mass including intracellular compartment. Delayed irradiation after injection of PSs is therefore considered to directly destroy tumor cells rather than shut down blood supply to them. We employed a longer drug-injection interval (24 hours) to achieve maximal uptake of a PS into tumor mass. Although the longer interval of drug-injection is thought to be ideal for complete regression of tumor, it also has a potential danger to destroy normal tissues as well. The therapeutic response of the tumor mass in our experiment was at best inhibition of tumor growth rather than complete regression in spite of employing the longer drug-injection interval. A possible explanation for this is that PDT alone is not sufficient for regression of tumor mass or that hepatocellular carcinoma is relatively resistant to PDT. A combination therapy such as PDT after surgical excision might be a solution.
The efficacy of PDT on hepatocellular carcinoma has been demonstrated by several investigators.12, 13, 14, 15, 16, 17, 18, 19 Among them, Shao et al. (2012) used metal phthalocyanines, a new class of PSs developed for cancer treatment.19 They used cell lines in vitro to test efficacy of PDT rather than using a xenograft protocol. They concluded that the phthalocyanines can induce apoptosis of cancer cells via reducing mitochondrial membrane potential, producing ROS, activating caspase-3, and causing cell arrest at G2/M stage after localizing into mitochondria and lysosome. The efficacy of PDT on hepatocellular carcinoma in vivo, however, was rarely tried without assessment on tumor growth or survival.14 We believe that the efficacy of PDT on hepatocellular carcinoma is now more convinced with our in vivo results.
So far, three different mechanisms are proposed how PDT causes cell death of cancer cells.20 They are direct cell damage, vascular shut down, and activation of immune response. Among them, direct cell damage related with mitochondria is believed to be the major cell death modality in cells responding to PDT. Irradiation of cancer cells highly loaded with PSs in the appropriate wavelengths range results in production of ROS which can subsequently damage mitochondrial membrane to release cytochrome C into cytosol. In the cytosol, cytochrome C binds to apoptotic protease activating factor-1 (Apaf-1) and ATP, which in turn binds to procaspase-9 resulting in formation of apoptosome.21 The apoptosome then triggers caspase cascades to cleave nuclear lamins and causes DNA fragmentation.22 Our results are in accordance with this cell death mechanism in that there was a significant increase in caspase-3 (in Western blot) as well as DNA fragmentation (TUNEL assay).
Although we proved that our PDT using a newly developed PS is effective in slowing down the growth of tumor and improving survival rate of mice, there are several limitations in our study. For example, the tumor mass we made was located subcutaneously which is not the real clinical situation in hepatocellular carcinoma. Irradiation onto deep-seated organ might not be sufficient to expect the same degree of therapeutic effect as in our particular experimental condition. Furthermore, there is no evidence that our PDT is more profitable than conventional treatment such as surgical excision or chemotherapy in inhibiting tumor growth and preventing adverse effects. The alternative mechanism, for example, impairment of Bcl-2 by PDT-induced oxidation was not addressed, either. In addition, the most effective combination of PDT should be investigated. In spite of those issues, the efficacy of our PDT on hepatocellular carcinoma is quite convincing and the absence of side effects ensures us it is one of very promising therapeutic modalities against hepatocellular carcinoma.
Conflict of interest
No competing financial interests exist.
Acknowledgments
We would like to acknowledge the help of Seong-Jin Park, Yong-Woo Shim, Soo-Young Choi, Tae Heon Lee, Min A Jang, Jung Hoon Kim, Su-Young Kim, and Hye-Jin Heo. This work was supported by Inje University Student Research Grant and the Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0020224).
References
- 1.Parkin D.M., Bray F., Ferlay J., Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Bae S.H. Up-to-date Information for Hepatocellular Carcinoma Treatment. J Korean Med Assoc. 2008;51:457–474. [Google Scholar]
- 3.Hopper C. Photodynamic therapy: a clinical reality in the treatment of cancer. Lancet Oncol. 2000;1:212–219. doi: 10.1016/s1470-2045(00)00166-2. [DOI] [PubMed] [Google Scholar]
- 4.Jheon S. Photodynamic Therapy. J Korean Med Assoc. 2007;50:1119–1129. [Google Scholar]
- 5.Choi Y.D., Yoon S.G., Chea M.J. Cancer Imaging and Photodynamic Therapy Using Photosensitizers. Polymer Science and Technology. 2008;19:139–145. [Google Scholar]
- 6.Wang H., Wu K., Sun Y., Li Y., Wu M., Qiao Q., Wei Y., Han Z.G., Cai B. STC2 is upregulated in hepatocellular carcinoma and promotes cell proliferation and migration in vitro. BMB Rep. 2012;45:629–634. doi: 10.5483/BMBRep.2012.45.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song I.S., Jun S.Y., Na H.J., Kim H.T., Jung S.Y., Ha G.H., Park Y.H., Long L.Z., Yu D.Y., Kim J.M., Kim J.H., Ko J.H., Kim C.H., Kim N.S. Inhibition of MKK7-JNK by the TOR signaling pathway regulator-like protein contributes to resistance of HCC cells to TRAIL-induced apoptosis. Gastroenterology. 2012;143:1341–1351. doi: 10.1053/j.gastro.2012.07.103. [DOI] [PubMed] [Google Scholar]
- 8.Szaciłowski K., Macyk W., Drzewiecka-Matuszek A., Brindell M., Stochel G. Bioinorganic photochemistry: frontiers and mechanisms. Chem Rev. 2005;105:2647–2694. doi: 10.1021/cr030707e. [DOI] [PubMed] [Google Scholar]
- 9.Torchilin V.P. Multifunctional nanocarriers. Adv Drug Deliv Rev. 2006;58:1532–1555. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Jain P.K., Huang X., El-Sayed I.H., El-Sayed M.A. Noble metals on the nanoscale: optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc Chem Res. 2008;41:1578–1586. doi: 10.1021/ar7002804. [DOI] [PubMed] [Google Scholar]
- 11.Brown D.M., Kaiser P.K., Michels M., Soubrane G., Heier J.S., Kim R.Y., Sy J.P., Schneider S. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 12.Kawamoto C., Ido K., Terada T., Horiguchi M., Kimura K., Manaka K. Photodynamic effects of various types of porphyrins on cultured hepatocellular carcinoma cells (JTC-16): a fundamental study of porphyrin photodynamic therapy. Nihon Shokakibyo Gakkai Zasshi. 1985;82:261–269. [PubMed] [Google Scholar]
- 13.Yamashita Y., Moriyasu F., Tamada T., Kawasaki T., Ono S., Kimura T., Kajimura K., Someda H., Hamato N., Uchino H. Evaluation of the efficacy of photodynamic therapy on experimental hepatocellular carcinoma–using local injection of photosensitizer. Nihon Gan Chiryo Gakkai Shi. 1990;25:770–775. [PubMed] [Google Scholar]
- 14.Egger N.G., Schoenecker J.A., Jr., Gourley W.K., Motamedi M., Anderson K.E., Weinman S.A. Photosensitization of experimental hepatocellular carcinoma with protoporphyrin synthesized from administered delta-aminolevulinic acid: studies with cultured cells and implanted tumors. J Hepatol. 1997;26:913–920. doi: 10.1016/s0168-8278(97)80260-7. [DOI] [PubMed] [Google Scholar]
- 15.Li W.T., Tsao H.W., Chen Y.Y., Cheng S.W., Hsu Y.C. A study on the photodynamic properties of chlorophyll derivatives using human hepatocellular carcinoma cells. Photochem Photobiol Sci. 2007;6:1341–1348. doi: 10.1039/b704539e. [DOI] [PubMed] [Google Scholar]
- 16.Yow C.M., Wong C.K., Huang Z., Ho R.J. Study of the efficacy and mechanism of ALA-mediated photodynamic therapy on human hepatocellular carcinoma cell. Liver Int. 2007;27:201–208. doi: 10.1111/j.1478-3231.2006.01412.x. [DOI] [PubMed] [Google Scholar]
- 17.Tang P.M., Chan J.Y., Au S.W., Kong S.K., Tsui S.K., Waye M.M., Mak T.C., Fong W.P., Fung K.P. Pheophorbide a, an active compound isolated from Scutellaria barbata, possesses photodynamic activities by inducing apoptosis in human hepatocellular carcinoma. Cancer Biol Ther. 2006;5:1111–1116. doi: 10.4161/cbt.5.9.2950. [DOI] [PubMed] [Google Scholar]
- 18.Wang C.Y., Wang X., Wang Y., Zhou T., Bai Y., Li Y.C., Huang B. Photosensitization of phycocyanin extracted from Microcystis in human hepatocellular carcinoma cells: implication of mitochondria-dependent apoptosis. J Photochem Photobiol B. 2012;117:70–79. doi: 10.1016/j.jphotobiol.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Shao J., Xue J., Dai Y., Liu H., Chen N., Jia L., Huang J. Inhibition of human hepatocellular carcinoma HepG2 by phthalocyanine photosensitiser PHOTOCYANINE: ROS production, apoptosis, cell cycle arrest. Eur J Cancer. 2012;48:2086–2096. doi: 10.1016/j.ejca.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Agostinis P., Berg K., Cengel K.A., Foster T.H., Girotti A.W., Gollnick S.O., Hahn S.M., Hamblin M.R., Juzeniene A., Kessel D., Korbelik M., Moan J., Mroz P., Nowis D., Piette J., Wilson B.C., Golab J. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61:250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rustin P. Mitochondria, from cell death to proliferation. Nat Genet. 2002;30:352–353. doi: 10.1038/ng0402-352. [DOI] [PubMed] [Google Scholar]
- 22.Porter A.G., Jänicke R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]