Abstract
Background
Herbal drugs are considered alternative agents and have been used for several years around the world. Recurrent aphthous stomatitis (RAS) is one of the most common problems recognized by dentists and skin specialists. This problem is characterized by recurring, painful, small oral mucosal ulcers with a round or oval aspect that mostly appear in keratinized mucosa, cheeks, and on the surface of the mouth under the tongue.
Methods
In our experiment, the alcoholic and water extracts of Punica granatum var. pleniflora, P. granatum var. Sweet Alak, and P. granatum var. Saveh Black were tested on minor RAS. The study was carried out using the double-blind method. The study population consisted of 210 participants, of whom 69 were females (32%) and 141 were males (68%). In addition to checking several factors, the pain and the degree of the participant's satisfaction had been determined based on visual analog scale. Data analysis was done in the form of a nonparametric method using Kruskal–Wallis test and SPSS version 20 software.
Results
The results show that the alcoholic and water extracts of P. granatum var. pleniflora have a meaningful therapeutic effect on minor RAS. Results from the antioxidant activity and its relation to total phenolics show that P. granatum var. pleniflora and P. granatum var. Sweet Alak are rich in phenols.
Conclusion
The water and alcoholic extracts of P. granatum varpleniflora decreased the entire time of complete treatment, and the treatment was meaningfully satisfactory for patients who participated in this experiment.
Keywords: alcoholic extract, Punica granatum, recurrent aphthous stomatitis, water extract
1. Introduction
Recurrent aphthous stomatitis (RAS) is one of the most common problems recognized by doctors, skin specialists, and dentists. This problem is seen in children and adults. Studies showed that the occurrence of RAS ranges from 2% to 50% between different population groups. In high-risk population such as students and soldiers, the occurrence is reported to reach up to 50–60%.1, 2 There are several etiologic reasons responsible for this problem.3 The etiology of RAS is not perfectly clear, but a positive family tree is seen in about one-third of the patients, and there is also an increase in the “HLA” of A2, A11, B12, and DR2, which confirms a genetic background in some groups.4, 5, 6 In some of the patients (10–20%), an unusual hematologic background is seen as a decrease in Fe, ferritin, folate, and B12.5 The other etiologic reasons are stress, trauma, and cessation of smoking, whose mechanism is not yet accurately clarified. Some patients claim that RAS occurs in relation to the menstrual cycle and allergy to certain foods.4 The RAS is also seen in suite syndrome, Behcet's syndrome, and human immunodeficiency virus infection. RAS is rarely seen in children with fever pharyngitis and cervical adenitis,1, 7 but no autoimmune reason for RAS has been reported yet.4, 5, 6 Aside from explorations related to the clinical, immunological, microbiologic, hematological, and histological details of RAS, there are many unknown factors related to this problem. According to age, RAS is divided into three types: minor, major, and herpetiformis.8, 9 The most frequent type is minor, which is characterized by recurring, painful, small oral mucosal ulcers with a round or oval shape.8 Minor aphthous ulcers indicate that the lesion size is between 2 mm and 4 mm, and they frequently appear in keratinized mucosa, cheeks, and on the surface of the mouth under the tongue. However, they are not common in the gingiva and palate.4, 5, 6 There are several kinds of treatments for RAS by which the pain can be decreased. Although the drugs used for treatment of RAS have low side effects,10 nowadays antiseptic drugs, antibiotics, and immune modulators can be used for RAS treatment.4, 5, 6, 11, 12 Herbal drugs belonging to this category are considered alternative agents and have been used for several years around the world. These drugs include sage, carrot, cantaloupe, licorice, wild geranium, aloe vera, and oak.8, 11, 13, 14 P. granatum Linn., locally known as “Golnar-e-farsi,” is an important medicinal plant in some northern areas of Iran. Its flowers are used as their astringent, hemostatic, antibacterial, antifungal, and antiviral properties and also for treatment of wounds, bronchitis, diarrhea, digestive problems, male sex power reconstituent, and dermal infected wounds in Unani medicinal (Iranian traditional medicine) literature. The flowers of this plant are also effectively used for the treatment of fall injuries and premature graying of hair in traditional Chinese medicine.15 The topical use of pomegranate preparations is shown to be effectively useful for controlling oral inflammation, as well as bacterial and fungal counts in periodontal disease and Candida-associated denture stomatitis. A hydroalcoholic extract of P. granatum fruit, known as HAEP, is reported to be effective against Staphylococcus, Streptococcus, Klebsiella, and Proteus species, as well as Escherichia coli. It is believed that the ellagitannin, punicalagin, is the fraction responsible for pomegranate's antibacterial activity.16 The polyphenol composition of pomegranate arils and skins is characterized by a high proportion of punicalagin, punicalin, ellagitannins, and gallotannins in comparison to ellagic acid.17 To date, all outspread studies on minor aphthous cannot agree on a definite treatment. The plant possesses good properties in controlling bacteria growth and also acts as a fungicide. Therefore, herbal medicine as a kind of complementary medicine for the treatment of certain diseases such as aphthous lesions has a great value. The aim of this study is to identify the effect of the extracts of P. granatum var. pleniflora on minor RAS as a natural treatment.
2. Methods
2.1. Alcoholic extract
The flower of medical plants Punica granatum var. pleniflora, P. granatum var. Sweet Alak, and P. granatum var. Saveh Black leather were obtained from the Institute of Traditional Medicine and Herbal Plants of Iran in the Esfahan and Golestan provinces. They were dried in the shadow at laboratory temperature, and these plants were ground into powder by a hammer mill and passed through mesh with 80 and 100 sizes. To prepare the extract, the perculation method was used. In this method, 50 g of flower was powdered and placed in a perculator, and then a solution was added to it. (The solution was 70% ethanol, and the proportion of the solution to the herbal material was 5:1.) The preparation of extract was initiated after 24 hours. The pH of the alcoholic extracts of P. granatum var. pleniflora, P. granatum var. Sweet Alak and P. granatum var. Saveh Black was 5.85, 5.62, and 5.74, respectively.
2.2. Water extract
In order to prepare the water extract of various Punica flowers, the plants were gathered and after drying, they were ground and transferred from mesh (80 and 100). Next, 500 mL sterilized water was added to 50 g of the powder prepared from Punica flowers, and it was kept and soaked under laboratory conditions for 24 hours on a magnetic mixer. To avoid evaporation, the opening of the Erlenmeyer flask was blocked by parafilm. Then, the upper part and transparent liquid was separated using a filter, and the residues were discarded. The obtained liquid was centrifuged for 15 minutes at 3000 rpm, and then the upper part of the liquid located under hood laminar was separated using a Millipore filter and was kept in a dark container at 4 °C until use. The pH of the water extracts of P. granatum var. pleniflora, P. granatum var. Sweet Alak, and P. granatum var. Saveh Black was 6.15, 6, and 5.90, respectively.
2.3. Determination of total phenolics
The Folin–Ciocalteu procedure was used for the determination of the total phenolic amounts in extracts. The preparation of each extract was completed at a concentration of 1 mg/mL, and the absorbance of all samples was measured at 660 nm.18 Gallic acid was applied as the standard absorbance read. Results were expressed as milligram (mg) gallic acid equivalents per gram dry weight.19
2.4. Determination of free radical scavenging activity
The stable 2,2-diphenyl-picrylhydrazyl (DPPH) free radical-scavenging assay was used for determination of free radical-scavenging activity of the extracts. In this step, 0.1 mL of the flower extract of each sample was added to 1 mL DPPH. After 30 minutes, the absorbance was measured at 517 nm. The lower absorbance of the reaction mixture shows a higher free radical scavenging activity. The experiment was repeated three times. The capability to scavenge the DPPH radical was calculated using the following equation:
| (1) |
2.5. Effect of extract of herbal plants in treatment of aphthous stomatitis
This study was carried out using the double-blind method. In order to measure the level of Fe, B12, and folic acid, a blood test was carried out, and the result was compared with a group of 60 people without RAS. In this study, the 210 patients were aged between 17 years and 67 years. They have RAS with sizes ranging from 1 mm to 4 mm, and the maximum lesion number was 5. These 210 patients were divided into Groups a–g (n = 30 in each group). In this study, the alcoholic and water extracts of Punica flower were examined (both 10% in 100 mL) on RAS, in which Groups a–c received alcoholic extracts, and Groups d–f received water extracts of P. granatum var. pleniflora, P. granatum var. Sweet Alak, and P. granatum var. Saveh Black, respectively. Group g served as the negative control and received nothing. The extracts were labeled with digits on their containers, so that both the patients and researchers were not aware about the extract they used. The patients were taught to keep the mouthwash liquid formulation in their mouths for 10 minutes, four times a day, for 10 days; furthermore, after the treatment was completed, the size of lesions was measured in all groups at 1 day, 2 days, 4 days, 6 days, 8 days, and 10 days of treatment using a periodontal probe. The pain was calculated and noted for 10 days of treatment. Statistical analysis was performed in the form of a nonparametric method using Kruskal–Wallis test and SPSS 20 software (SPSS Inc., Chicago, IL, USA).
2.6. The pain satisfactory degree of patients
Some sheet forms were prepared during the data gathering stage, in which the degree of pain, based on visual analog scale (prior to treatment), immediate degree of pain just after the treatment, time of pain stopping (hour), and finally time interval of complete treatment were recorded. The patients were asked to rate their reaction from 0 (as no pain) to 10 (as the highest level of pain). After the experiment, the data were recorded in a computer, and the analysis was done using the SPSS 20 software (SPSS Inc.).
3. Results
3.1. Values of antioxidant activity and phenolic components of P. granatum flowers
Results shown in Table 1 indicate that the alcoholic extract has an antioxidant property and the extract carries greater amounts of phenol compared with the water extract. Comparison among the species also shows that the P. granatum var. pleniflora flower has a higher level of phenols and consequently, a higher antioxidant property (Table 1).
Table 1.
Values of antioxidant activity and phenolic components of Punica granatum flowers
| Antioxidant activity (%) |
Total phenolics (mg/L) |
||||
|---|---|---|---|---|---|
| No. | Water extract | Alcoholic extract | Alcoholic extract | Water extract | |
| 1 | P. granatum var. pleniflora | 35.3 ± 1.5 | 87.4 ± 2.8 | 258.1 ± 9.14* | 472.6 ± 23.4 |
| 2 | P. granatum var. Sweet Alak | 20.9 ± 4.1 | 57.10 ± 6.3 | 208.3 ± 17.2 | 315.7 ± 12.6 |
| 3 | P. granatum var. Saveh Black | 10.41 ± 1.9 | 27.9 ± 02 | 93.3 ± 11.5 | 163.1 ± 8.7 |
3.2. Comparison of herbal extracts effect, sex, and age concerning the time of complete treatment
Sex difference is not significant among the groups; the Chi-square value was 3.08 and p = 0.88, so the sex contribution is similar. A total of 210 people participated in the experiment, consisting of 69 females (32%) and 141 males (68%; Table 2).
Table 2.
Effect of sex and age on time intervals of complete treatment
| Treatment | No. of participants | No. of women (%) | No. of men (%) | Min. age | Max. age | Mean ± SD of age | Mean ± SD of aphthous in pretest |
|---|---|---|---|---|---|---|---|
| a | 30 | 11 (37) | 19 (63) | 17 | 63 | 32.23 ± 13.18 | 3.47 ± 0.65 |
| b | 30 | 11 (37) | 19 (63) | 17 | 59 | 27.4 ± 9.32 | 3.48 ± 0.63 |
| c | 30 | 9 (30) | 21 (70) | 17 | 63 | 28.77 ± 10.14 | 3.63 ± 0.46 |
| d | 30 | 8 (27) | 22 (73) | 18 | 61 | 32.93 ± 12.04 | 3.68 ± 0.53 |
| e | 30 | 7 (23) | 23 (77) | 17 | 63 | 30.36 ± 12.76 | 3.33 ± 0.63 |
| f | 30 | 9 (30) | 21 (70) | 17 | 62 | 32.93 ± 13.52 | 3.32 ± 0.58 |
| g | 30 | 9 (30) | 21 (70) | 19 | 52 | 30.47 ± 9.80 | 3.72 ± 0.54 |
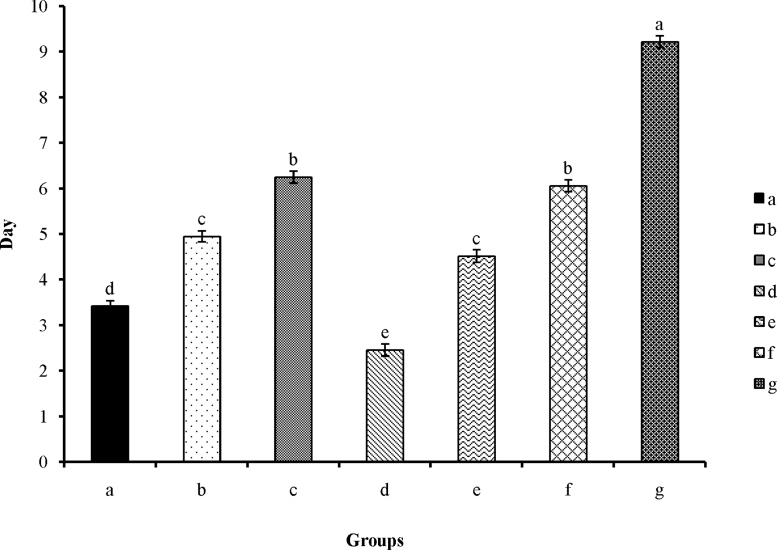
Results showed that the herbal extracts and age can be meaningfully effective on the process of treatment (p < 0.0001). The size of lesions was measured among the patients prior to the experiment so that the differences were meaningful (p < 0.0001), and by considering this variable as a covariant, its effect was duly noted. Sex and its relation to herbal extracts had no effect on rapidity of treatment (p > 0.05). This indicates that, in all patients, the effect of herbal extracts on the process of recovery is independent of their sex (Fig. 1).
Fig. 1.
Complete treatment of aphthous in different groups. Different letters represent the meaningful difference between herbal extracts (p < 0.0001). (a) Received alcoholic extract of Punica granatum var. pleniflora; (b) received alcoholic extract of P. granatum var. Sweet Alak; (c) received alcoholic extract of P. granatum var. Saveh Black; (d) received water extract of P. granatum var. pleniflora; (e) received water extract of P. granatum var. Sweet Alak; (f) received water extract of P. granatum var. Saveh Black; and (g) received nothing.
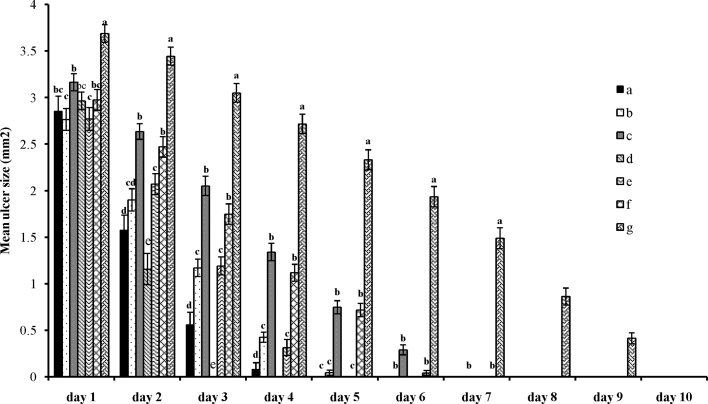
3.3. Daily change of aphthous using the herbal extracts
The curve shown in Fig. 2 represents the daily changes of aphthous under the effect of the herbal extracts. The different letters for each day show the difference between groups (p < 0.0001) and also represent a decrease in lesion size (Fig. 2). The figure shows that the water and alcoholic extracts of P. granatum var. pleniflora have a significant effect on decreasing the lesion size on the 2nd day, 3rd day, and 4th day so that during the last day we could see a complete recovery of lesions in Groups a and d. Also, the water and alcoholic extracts used by Groups b and e showed a significant change in lesion size, but complete recovery was seen on the 5th day and 6th day of treatment.
Fig. 2.
Daily changes in lesion sizes under the effect of herbal extracts. Lower-case letters on each column represent a meaningful difference (p < 0.0001). (a) received alcoholic extract of Punica granatum var. pleniflora; (b) received alcoholic extract of P. granatum var. Sweet Alak; (c) received alcoholic extract of P. granatum var. Saveh Black; (d) received water extract of P. granatum var. pleniflora; (e) received water extract of P. granatum var. Sweet Alak; (f) received water extract of P. granatum var. Saveh Black; and (g) received nothing.
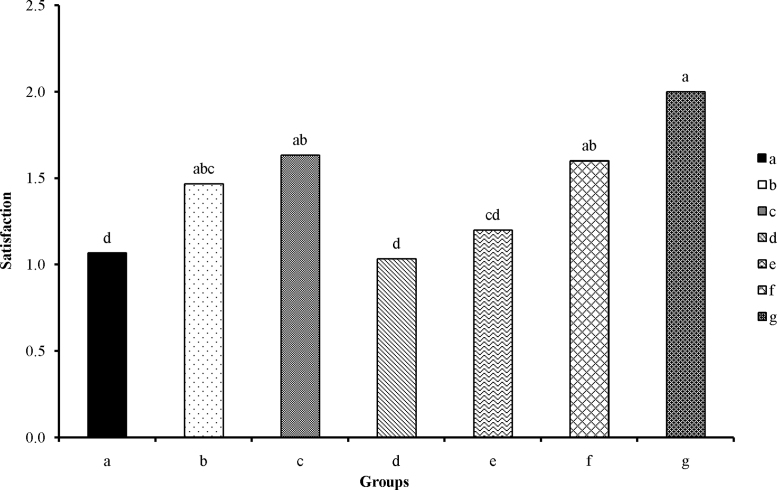
3.4. Pain and satisfaction through different days of treatment
Data analysis of pain degree in various days and the patient's satisfaction toward the herbal extracts as well as the time of complete recovery was carried out in the form of a nonparametric method using Kruskal–Wallis test and SPSS 20 software (SPSS Inc.). The pain degree and satisfaction among male and female patients using same herbal extracts were not significantly different. Results showed that the minimum pain degree and maximum satisfaction were related to treatments a, d, and e (Fig. 3). It should be noted that patients were more satisfied with the water extract; hence, it makes sense that the alcoholic extract caused some irritation because of the existence of alcohol in it.
Fig. 3.
Satisfaction toward using the herbal extracts among different groups (Kruskal-Wallis, p < 0.0001). Lower-case letters on each column represent a meaningful difference. (a) Received alcoholic extract of Punica granatum var. pleniflora; (b) received alcoholic extract of P. granatum var. Sweet Alak; (c) received alcoholic extract of P. granatum var. Saveh Black; (d) received water extract of P. granatum var. pleniflora; (e) received water extract of P. granatum var. Sweet Alak; (f) received water extract of P. granatum var. Saveh Black; and (g) received nothing.
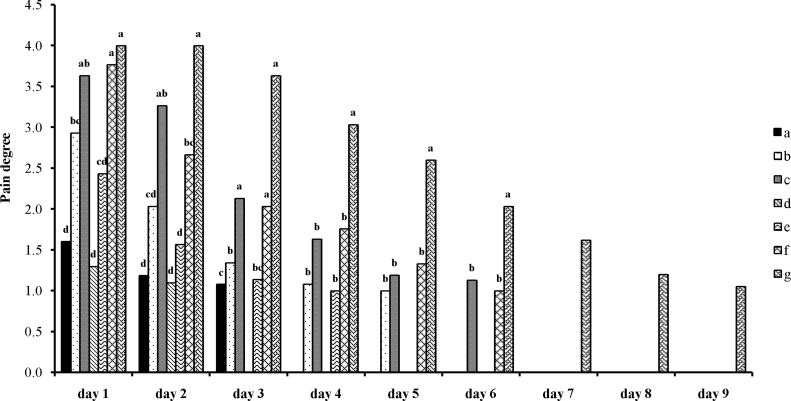
3.5. Pain degree in various days
In the first 2 days, patients in Groups a, d, and e reported the lowest pain compared to the other groups. Generally, treatment d had the best effect on aphthous recovery in terms of decrease in recovery time and reducing pain, which was the same in both pain degree and satisfaction compared to treatments a and e; however, the patients in Group d required a shorter period to achieve complete recovery (Fig. 4).
Fig. 4.
Effect of time on pain degree in different groups. Lower-case letters on each column represent a meaningful difference (p < 0.0001). (a) received alcoholic extract of Punica granatum var. pleniflora; (b) received alcoholic extract of P. granatum var. Sweet Alak; (c) received alcoholic extract of P. granatum var. Saveh Black; (d) received water extract of P. granatum var. pleniflora; (e) received water extract of P. granatum var. Sweet Alak; (f) received water extract of P. granatum var. Saveh Black; and (g) received nothing.
4. Discussion
RAS is one of the most common problems recognized by doctors, skin specialists, and dentists. Herbal drugs such as sage, carrot, cantaloupe, licorice, wild geranium, aloe vera, and oak are considered alternative agents and have been used for several years around the world.11, 12, 13, 14 In our preliminary study, both the water and alcoholic extracts of P. granatum var. pleniflora were examined by testing the effects of 5%, 10%, and 15% of the extracts on 60 participants. Results showed that the 5% concentration of extracts had no meaningful effect, but similar effects were observed for both 10% and 15% of the extracts. Thus, the concentration of 10% in 100 mL was chosen for the experiment. In this study, using the water and alcoholic extracts of P. granatum var. pleniflora, at the time of 3.07 ± 0.65 and 2.11 ± 0.85, led to the effective treatment of RAS, and these treatments produced the best effect in terms of a significant decrease in pain and time of recovery. Amanlou and his coworkers20 examined the effect of Satureja khuzistanica extract in the management of RAS pain. The mean time of pain elimination was 5.7 days in the control group compared to 3.4 days in the S. khuzistanica extract group. Their study showed the mean time of pain elimination using PG to be 3.4 days for the PG group, which was similar to the effect induced by the S. khuzistanica extract. Furthermore, there was a reduction in mean healing time from 10.4 days in the control group to 5.9 days in the S. khuzistanica extract group, which is similar to the findings of the present investigation.20 Another study showed that the complete healing time of RAS therapy with Zataria multiflora, Anthemis nobelis, mixture of these two agents, and Myrthus communis was 6 days, 8.5 days, 6.5 days, and 7.2 days, respectively. The results of the study suggest that PG with a mean healing time of 5.3 days can be a proper alternative for the clinical management of RAS. The results also showed that the mean time for pain elimination after therapy with Z. multiflora extract, A. nobelis extract, mixture of these two, and M. communis mouth rinse was 3 days, 5 days, 3.1 days, and 4 days, respectively.21 PG with a mean pain elimination time of 3.4 days was shown to have a comparable effect with other herbal agents in the management of RAS symptoms. A group of Thai researchers examined the effect of biodegradable chips impregnated with Centella asiatica and P. granatum pericarp on periodontal disease in which 20 patients with gum pocket depths of 5–8 mm were checked. A baseline test was carried out, and this was followed by root planing and scaling of the target teeth. Subgingival placement of the medicated chips was considered as the treatment group, and nonmedicated chips served as placebo/control group; in addition, indexes such as pocket depth, attachment level, bleeding, as well as gingival and plaque were measured at baseline and after 3 months and 6 months. The authors reported that all treatment places showed a development toward decreasing plaque, and significant improvements were tangible in terms of pocket depth and attachment level at 3 months when compared to the placebo group.22 In another study, 15 patients who had finished standard periodontal therapy but were still faced with pocket depths of 5–8 mm were tested with the same medicated chips used in the Thai researchers’ study. Again, the same indexes were measured at baseline and after 3 months and 6 months; however, the difference was that, the researchers also measured inflammatory markers interleukin-1s (IL-1β) and IL-6. All premeasured indexes were shown to have a significant improvement, and this was confirmed by the significant decreases in IL-1 β and IL-6 at 3 months and 6 months when compared to baseline.23 An in vitro test using four different testing methods have reported that pomegranate juice and seed extracts have 2–3 times the antioxidant capacity of either red wine or green tea.24 Pomegranate extracts have been proven to remove free radicals and reduce macrophage oxidative stress and lipid peroxidation in animals and to increase plasma antioxidant capacity in elderly humans.16, 25 The antioxidant properties of a pomegranate by-product extract that was made from whole fruit minus the juice have been demonstrated through several studies in rats and mice. Results show a 19% reduction in oxidative stress in mouse peritoneal macrophages, a 42% decrease in cellular lipid peroxide content, as well as a 53% increase in reduced glutathione levels.25 An in vitro analysis of a fermented pomegranate juice extract and a cold pressed seed oil extract reported that the antioxidant capacity of both extracts are higher compared with that of red wine and green tea extract.16 Another study in rats with CCl4-induced liver damage confirmed that pretreatment with pomegranate peel extracts improved or maintained the free-radical scavenging activity of the hepatic enzymes catalase, superoxide dismutase, and peroxidase. The experiment resulted in a 54% reduction in lipid peroxidation values compared to controls,26 and it was shown that pomegranate pulp (PPJ) has a higher antioxidant property compared to apple juice. Guo and his coworkers, using the ferric reducing/antioxidant power assay, reported that 250 mL PPJ (in a daily usage delivered to healthy elderly participants for 4 weeks) enhanced the plasma antioxidant capacity from 1.33 mmol to 1.46 mmol, whereas those who were treated with apple juice experienced no significant increase in antioxidant capacity. Moreover, participants using the PPJ showed considerable decreased plasma carbonyl content (a biomarker for oxidant/antioxidant barrier impairment in a range of inflammatory diseases) compared to participants consuming apple juice. Indexes such as plasma vitamin E, ascorbic acid, and reduced glutathione values did not show any significant difference between the groups, indicating that pomegranate phenolics may be responsible for the observed results.16 Previous studies show that the antimicrobial properties increase as phenolic components increase.27 In addition, some investigations proved that the antimicrobial properties are attributable to the existence of phenolics.28 Many researchers focused on the antifungal properties of some herbal extracts in contrast to some solutions.29 In a study in 2011, the antioxidant activity of seed, skin, and leaves of P. granatum were measured as 51.24%, 85.40%, and 22.10%, respectively. Moreover, the phenolic components of the extracts were 384.7 mg/L, 423.5 mg/L and 133.3 mg/L, respectively.30 Generally, the values of total phenolics and antioxidant activity, shown in Table 1, show that the alcoholic extract in all species possess antioxidant activity and higher phenol contents. The comparison of species also revealed that the flowers of P. granatum var. pleniflora have a higher value of phenolics and consequently more antioxidant activity. Results obtained from the alcoholic extract showed that the antioxidant activity of P. granatum var. pleniflora, P. granatum var. Sweet Alak, and P. granatum var. Saveh Black was 87.4 ± 0.8%, 57.10 ± 6.3%, and 27.9 ± 02%, respectively. The results showed that the alcoholic extract of P. granatum var. pleniflora has a higher antioxidant activity compared to that of plant organs used by Salahvarzi30 in 2011. In our study, the highest amounts of phenolic components were found in P. granatum var. pleniflora, at 472.6 ± 23.4 mg/L, higher than those used by Salahvarzi30 in 2011.
In conclusion, based on recent studies, several different herbal extracts are reported for the treatment of RAS, and each of them has different effects on RAS treatment. P. granatum var. pleniflora flowers possess a higher amount of phenolics, resulting in higher antioxidant activity. The water and alcoholic extracts of P. granatum var. pleniflora decreased the entire time of complete treatment, and the treatment was meaningfully satisfactory for patients who participated in this experiment.
Conflicts of Interest
All authors declare no conflicts of interest.
References
- 1.Gavanji S, Larki B, Doostmohammadi M, Mortezaeinezad F. Production of a new mixed herbal medicine for minor Aphthous Ulcers. Med Plants. 2012;4:49–51. [Google Scholar]
- 2.Ship JA, Levery K, Blomquist JE. Recurrent aphthous stomatitis. An update Oral Surg Oral Med Oral Pathol Oral Radiol. 1996;81:141–147. doi: 10.1016/s1079-2104(96)80403-3. [DOI] [PubMed] [Google Scholar]
- 3.Weusten B, van de Wiel A. Aphthous ulcer and vitamin B12 deficiency. Neth J Med. 1998;53:172–175. doi: 10.1016/s0300-2977(98)00096-5. [DOI] [PubMed] [Google Scholar]
- 4.Champion RH, Burton JL, Barns DA. 8th ed. Blackwell Sciences; Oxford: 2004. Textbook of dermatology. [Google Scholar]
- 5.Eversole LR. Immunopathology of oral mucosal ulcerative, desquamative and bullous diseases. Oral Surg Oral Med Oral Pathol Oral Radiol. 1994;77:555–571. doi: 10.1016/0030-4220(94)90312-3. [DOI] [PubMed] [Google Scholar]
- 6.Porter SR, Hegarty A, Kaliakasou F, Hodgson TA, Scully C. Recurrent aphthous stomatitis. Clin Dermatol. 2000;18:569–578. doi: 10.1016/s0738-081x(00)00147-4. [DOI] [PubMed] [Google Scholar]
- 7.Marshall GS, Edwards KM, Butler J. Syndrome of periodic fever, pharyngitis and aphthous stomatitis. J Investig Dermatol. 1987;110:43–46. doi: 10.1016/s0022-3476(87)80285-8. [DOI] [PubMed] [Google Scholar]
- 8.Burgess JA, Johnson BD, Sommers E. Pharmacological management of recurrent oral mucosal ulceration Drugs. Drugs. 1990;39:54–65. doi: 10.2165/00003495-199039010-00005. [DOI] [PubMed] [Google Scholar]
- 9.Rogers RS. Recurrent aphthous stomatitis: clinical characteristic and evidence for an immunopathogenesis. J Investig Dermatol. 1997;69:499–509. doi: 10.1111/1523-1747.ep12687958. [DOI] [PubMed] [Google Scholar]
- 10.Casiglia JM. Recurrent aphthous stomatitis: etiology diagnosis and treatment. Gen Dent. 2002;50:157–166. [PubMed] [Google Scholar]
- 11.McBride DR, MacBride DR, Krause LS. Management of aphthous ulcers. Am Fam Physician. 2000;62:149–154. [PubMed] [Google Scholar]
- 12.Rattan J, Schneider M, Arber N. Sucralfate suspension as a treatment of recurrent aphthous stomatitis. J Intern Med. 1994;236:341–343. doi: 10.1111/j.1365-2796.1994.tb00805.x. [DOI] [PubMed] [Google Scholar]
- 13.Rodu B, Mattingly G, Oral mucosal ulcers diagnosis and management. J Am Dent Assoc. 1992;123:83–86. doi: 10.14219/jada.archive.1992.0268. [DOI] [PubMed] [Google Scholar]
- 14.Weinberg MA, Insler MS, Campen RB. Mucocutaneous features of autoimmune blistering diseases. Oral Surg Oral Med Oral Pathol Oral Radiol. 1997;84:517–534. doi: 10.1016/s1079-2104(97)90269-9. [DOI] [PubMed] [Google Scholar]
- 15.Pirbalouti AG, Koohpayeh A, Karimi I. The wound healing activity of flower extracts of Punica granatum and Achillea kellalensis in Wistar rats. Acta Pol Pharm. 2010;67(1):107–110. [PubMed] [Google Scholar]
- 16.Jurenka JS. Therapeutic applications of pomegranate (Punica granatum L.): a review. Altern Med Rev. 2008;13:128–144. [PubMed] [Google Scholar]
- 17.Madrigal-Carballo S, Rodriguez G, Krueger CG, Dreher M, Reed JD. Pomegranate (Punica granatum) supplements: authenticity, antioxidant and polyphenol composition. J Funct Foods. 2009;1:324–329. [Google Scholar]
- 18.Singleton VL, Orthofer R, Lamuela Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of the Folin-Ciocalteu reagent. Methods Enzymol. 1965;299:152–178. [Google Scholar]
- 19.Mousavinejad G, Emam-Djomeh Z, Rezaei K, Khodaparast MHH. Identification and quantification of phenolic compounds and their effects on antioxidant activity in pomegranate juices of eight Iranian cultivars. Food Chem. 2009;115:1274–1278. [Google Scholar]
- 20.Amanlou M, Babaee N, Saheb-Jamee M, Salehinia A, Farsam H, Tohidast Z. Efficacy of Satureja khuzistanica extract ant its essential oil preparations in the management of recurrent aphthous stomatitis. DARU. 2007;15:231–235. [Google Scholar]
- 21.Ghalayani P, Zolfaghary B, Farhad AR, Tavangar A, Soleymani B. The efficacy of Punica granatum extract in the management of recurrent aphthous stomatitis. J Res Pharm Pract. 2013;2:88–92. doi: 10.4103/2279-042X.117389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sastravaha G, Yotnuengnit P, Booncong P, Sangtherapitikul P. Adjunctive periodontal treatment with Centella asiatica and Punica granatum extracts. A preliminary study. J Int Acad Periodontol. 2003;5:106–115. [PubMed] [Google Scholar]
- 23.Sastravaha G, Gassmann G, Sangtherapitikul P, Grimm WD. Adjunctive periodontal treatment with Centella asiatica and Punica granatum extracts in supportive periodontal therapy. J Int Acad Periodontol. 2005;7:70–79. [PubMed] [Google Scholar]
- 24.Gil MI, Tomas-Barberan FA, Hess-Pierce B, Holcroft DM, Kader AA. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem. 2000;48:4581–4589. doi: 10.1021/jf000404a. [DOI] [PubMed] [Google Scholar]
- 25.Rosenblat M, Volkova N, Coleman R, Aviram M. Pomegranate byproduct administration to apolipoprotein e-deficient mice attenuates atherosclerosis development as a result of decreased macrophage oxidative stress and reduced cellular uptake of oxidized low-density lipoprotein. J Agric Food Chem. 2006;54:1928–1935. doi: 10.1021/jf0528207. [DOI] [PubMed] [Google Scholar]
- 26.Chidambara Murthy KN, Jayaprakasha GK, Singh RP. Studies on antioxidant activity of pomegranate (Punica granatum) peel extract using in vivo models. J Agric Food Chem. 2002;50:4791–4795. doi: 10.1021/jf0255735. [DOI] [PubMed] [Google Scholar]
- 27.Bragaa LC, Shupp JW, Cummings C, Jett B, Takahashi JA, Carmod LS. Pomegranate extract inhibits Staphylococcus aureus growth and subsequent enterotoxin production. J Ethnopharmacol. 2005;96:335–339. doi: 10.1016/j.jep.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 28.Cushnie TPT, Lamb AJ. Review antibacterial activity of flavonoids. Int J Antimicrobial Agents. 2005;26:343–356. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi NH, Choi GJ, Jang KS, Choi YH, Lee SO, Choi JE. Antifungal activity of the methanol extract of Myristica malabarica fruit rinds and the active ingredients malabaricones against phytopathogenic fungi. Plant Pathol J. 2008;24:317–321. [Google Scholar]
- 30.Salahvarzi Y, Tehranifar A, Jahanbakhsh V. Relation of antioxidant and antifungal activity of different parts of Pomegranate (Punica granatum L.) extracts with its phenolic content. Iran J Med Arom Plant. 2011;7:47–56. [Google Scholar]