Abstract
Background
The transient receptor potential cation channel subfamily V member 1 (TRPV1) channel has been proved to be a molecular integrator of inflammatory pain sensation. 2-Aminoethoxydiphenyl borate (2-APB) and its analogs have been noticed as attractive candidates for the development of a selective TRPV1 agonist and/or antagonist. However, selectivity and effectiveness, species dependence, and the binding site(s) of 2-APB on TRPV1 channel protein remain controversial.
Methods
The present study aimed to characterize acting sites of 2-APB on heterologously expressed rat TRPV1 (rTRPV1) channels in HEK 293 cells. Rat TRPV1 currents were recorded by cell-free, excised patch clamp techniques.
Results
In inside-out and outside-out patch modes, 2-APB applied either side of the membrane dose-dependently activated rTRPV1 channels. 2-APB dose-dependently potentiated rTRPV1 currents, that activated by capsaicin, protons, or noxious heat. 2-APB potentiated the capsaicin-activated rTRPV1 current from both side of the patch membrane. A structural analogue of 2-APB, diphenylboronic anhydride, showed the same potentiation effect on the capsaicin-activated rTRPV1 current.
Conclusion
It is suggested that 2-APB directly opens rTRPV1 channels from both sides of the membrane and potentiates the opening of channels by inflammatory stimuli.
Keywords: 2-aminoethoxydiphenyl borate, capsaicin, diphenylborinic anhydride, TRPV1
1. Introduction
Capsaicin, a pungent ingredient of chili peppers, excites nociceptive neurons by stimulating capsaicin receptors on the nerve endings.1, 2 Capsaicin receptor was cloned and named as vanilloid receptor 1 or transient receptor potential cation channel subfamily V member 1 (TRPV1).3 In addition to vanilloid compounds such as capsaicin, TRPV1 channels are activated by noxious heat (> 43 °C), extracellular protons, endogenous lipid products, and many inflammatory mediators.4 Interestingly, most of them are structurally unrelated but they all can cause pain. High expression of TRPV1 channels in dorsal root ganglionic neurons and trigeminal ganglionic neurons has been demonstrated, and the channels are now believed to be a molecular integrator of inflammatory pain sensation. The essential roles of TRPV1 for pain sensation have been strongly supported by mice lacking TRPV1 that were impaired in the detection of painful inflammatory stimuli, painful vanilloids, and heat.5
Because TRPV1 is a good target of analgesics for inflammatory pain, many pharmaceutical companies have tried to identify novel and potent TRPV1 modulators. TRPV1 targeting analgesics are classified into two groups: TRPV1 antagonists and TRPV1 agonists. Although small molecular synthetic TRPV1 antagonists with improved toxicity are the major focus of development, local treatment of TRPV1 agonists has been tried in clinical practice. The strong TRPV1 activators capsaicin and resiniferatoxin initially excite TRPV1 and then produce a strong desensitization of the channels or irreversibly destroy capsaicin-sensitive sensory neurons. For this reason, capsaicin-based TRPV1 agonists can be used when they are topically applied by injection or patches. Successful TRPV1-agonist-based therapy has been reported for osteoarthritis, postherpetic neuropathy, painful HIV-associated sensory neuropathy, cluster headache, migraine, urinary incontinence, and overactive bladder.6 Disadvantages of TRPV1 agonists include a burning sensation and the need for multiple applications to get sufficient therapeutic effects. Therefore, identifying and developing new TRPV1 agonists with reduced pungency compared with capsaicin-based agonists and a higher chemical stability have been a matter of great interest.
2-Aminoethoxydiphenyl borate (2-APB) has been described as an inhibitor of inositol 1,4,5-trisphosphate receptor (IP3-R)-induced Ca2+ release from intracellular stores.7 Later studies, however, have revealed that 2-APB is not IP3-R specific, but has many targets. For example, 2-APB inhibits a native store-operated calcium entry (SOCE) channels8, 9, 10 and many transient receptor potential cation (TRPC) and transient receptor potential melastatin channels,11, 12 endoplasmic reticulum Ca2+-ATPases13 and volume-regulated anion channels,14 but it activates phospholipase C.15 This complexity renders 2-APB as a poor tool for the separation of IP3-R and SOCEs from other Ca2+ entry pathways.
2-APB was found to be a common activator of three thermo-sensitive TRPV channels: TRPV1, TRPV2, and TRPV3.16, 17 With a whole-cell patch clamp recording, it was shown that 2-APB strongly activated mouse TRPV1 and stimulated the response to capsaicin, heat, and acid.16, 17 In addition, 2-APB sensitized native nociceptive neurons to heat in a mouse model.18 For this reason, it is suggested that 2-APB analogues could be attractive candidates for the development of a selective TRPV1 agonist or antagonist. However, it has been noticed that 2-APB has no effect on TRPV2 and shows a very weak effect on activating TRPV1 expressed in human embryonic kidney (HEK) cells.19 This discrepancy remains to be explained.
Therefore, we aimed to establish whether the heterogeneously expressed TRPV1 channels are directly activated by 2-APB and to prove the potentiation of 2-APB on the effects of capsaicin, heat, and protons on the channels. To this purpose, we recorded rat TRPV1 (rTRPV1) channels using excised membrane patches, because the previously reported were mainly achieved by whole-cell patch clamp recordings and mouse TRPV1.16, 19 Recording of TRPV1 current by whole-cell patch clamp cannot exclude the cytoplasmic components and signaling molecules that influence overall activity of the channels. Many cytoplasmic molecules including protein kinase C and calmodulin kinase modulate TRPV1 activity.20, 21 Assuming that these signaling molecules are relatively well conserved in whole-cell patch recordings, the demonstration of TRPV1 activation by 2-APB from a cell-free patches can rule out their contribution and can distinguish from an indirect activation mechanism.
2. Methods
2.1. Cell culture and expression of rTRPV1
HEK 293 cells were cultured at 37 °C, 5% CO2, in Dulbecco's minimal essential medium (DMEM, Gibco, Grand Island, NY, USA) containing 4.5 mg/mL glucose, 10% heat-inactivated fetal bovine serum, 100 units/mL penicillin, 100 μg/mL streptomycin. Rat TRPV1 cDNA was kindly provided Dr Uhtaek Oh (Seoul National University, Korea). The cells were passaged three times/week. Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) was used for the transfection of rTRPV1 cDNA. 35-mm dishes of HEK 293 cells at approximately 80% confluence were prepared for transfection. A 4-μL aliquot of Lipofectamine was added into 250 μL of Opti-MEM (Gibco) and incubated for 5 minutes at room temperature and 5 μg of rTRPV1 cDNA labeled with a PIRES-EGFP fluorescent tag in 250 μL of Opti-MEM were added. Lipofectamine and cDNA solution were mixed and incubated at room temperature for 20 minutes. The mixture was dropped on to HEK 293 cells and mixed gently. Cells were maintained in a humidified CO2 incubator at 37 °C for 24–48 hours.
2.2. Patch clamp
HEK cells that fluoresced green underlight of wavelength 588 nm were considered to be transfected with rTRPV1 and were selected for recording. Patch clamp recordings were performed at room temperature (23–26 °C) with the inside-out and outside-out configurations. Conventional whole-cell recordings were performed to verify the responses in outside-out configuration. Signals were amplified with an Axopatch 1D patch clamp amplifier and controlled with pClamp software 7.0 (Axon Instruments). Signals were filtered at 1 kHz and recorded at 5 kHz. For the inside-out patch clamp, pipettes were pulled from micropipette glass (World Precision Instruments Inc, Sarasota, FL, USA) to 1.5–2 M Ω tip resistance and filled with an extracellular (pipette) solution containing 140 mM NaCl and 10 mM Hepes (pH 7.3 with N-methyl-D-glucamin). The cytoplasmic (bath) solution contained 140 mM NaCl, 10 mM Hepes, and 2 mM EGTA (pH 7.2 with N-methyl-D-glucamin). The current was recorded at a holding potential of –60 mV. For outside-out patch clamp, the same cytoplasmic (pipette) and extracellular (bath) solutions, as used in the inside-out patches, were used. Whole-cell currents were recorded in a nominal Ca2+ free extracellular (bath) solution contained 145 mM NaCl, 5 mM KCl, 1 mM MgCl2, and 10 mM glucose(pH 7.4). Outside-out and whole-cell currents were recorded at a holding potential of –30 mV.
2.3. Chemicals and reagents
All chemicals used were purchased from Sigma–Aldrich (St Louis, MO, USA). Stock solutions of 2-APB, diphenylborinic anhydride (DPBA), capsaicin, and capsazepine were prepared in dimethyl sulfoxide. Drugs were diluted in the appropriate solutions to the desired final concentrations prior to use.
2.4. Statistics
Data are presented as mean ± standard error of the mean with n of sample number. Statistical comparison was tested using unpaired t test and significance was set at p < 0.05.
3. Results
3.1. Activation of rTRPV1 channels by 2-APB
To prove the functional expression of rTRPV1 channels in HEK 293 cells, we first recorded the capsaicin-activated current with the inside-out patch configuration. To prevent a rapid desensitization of the current, we perfused the patch membrane with a Ca2+-free cytoplasmic (bath) solution containing 1 mM EGTA and 140 mM Na+. At a holding potential of –60 mV, capsaicin dose-dependently activated an inward rTRPV1 current with no significant desensitization, indicating that the expressed channels are functional and are properly detected from excised patch membranes (Fig. 1). Capsaicin-activated rTRPV1 current was strongly inhibited by a TRPV1 antagonist capsazepine (10 μM; Fig. 1A). Half maximal concentration of capsaicin was 21.2 ± 5 μM (n = 8; Fig. 1B,C). In excised patches, the amplitudes of the capsaicin-activated currents were so variable that it was not possible to compare the current obtained from a different patch. Initially, we tried to measure density of the currents by normalizing the current with a measured membrane capacitance. However, the normalized data were also very variable, so we concluded that this was probably due to a different expression level of the channels. The patch membranes were easily broken at capsaicin concentrations > 50 μM, so we did not use capsaicin at > 10 μM.
Fig. 1.
Activation of rat transient receptor potential cation channel subfamily V member 1 (TRPV1) channels by capsaicin. In the inside-out patch configuration, rat TRPV1 channels expressed in human epithelial kidney cells were dose-dependently activated by capsaicin. (A) The capsaicin-induced current was inhibited by TRPV1 antagonist capsazepine (10 μM). (B) The concentration of capsaicin was raised from 1 μM to 50 μM to construct the dose-response curves. (C) Dose-response curve was plotted and the calculated half maximal effective concentration of capsaicin was 21.2 ± 5.1 μM (n = 8). The currents were measured at a holding potential of –60 mV at room temperature (∼24 °C).
We investigated whether 2-APB activates rTRPV1 channels in excised patch membranes. In the inside-out patch mode, cytoplasmic application of 2-APB activated an inward rTRPV1 current at a holding potential of –60 mV (Fig. 2Ai). Capsazepine (10 μM) strongly inhibited the 2-APB-activated currents suggesting that 2-APB activates rTRPV1 channels. 2-APB dose-dependently activated the current and the estimated half maximal effective concentration value for 2-APB was 277.6 ± 64.5 μM at a holding potential of –60 mV (n = 6, data not shown). In outside-out patches mode, inclusion of 1 mM 2-APB into the recording pipette weakly activated rTRPV1 current (Fig. 2Ai). The same results were obtained from whole-cell recordings. This was somewhat unexpected, because the same concentration of 2-APB strongly activated the current when applied from the cytoplasmic side of the inside-out patches (Fig. 2Ai). We therefore thought that if interference by unknown factors reduces available 2-APB, it could be overcome by raising the concentration of the compound in the pipette. To test this possibility, we raised the intrapipette 2-APB up to 5 mM and the outside-out patches were achieved. As shown in Fig. 2Aiii, 5 mM 2-APB slowly activated the capsaicin-sensitive current after achieving the outside-out patches. In this condition, application of 100–500 μM 2-APB into the extracellular space, which is much lower than intrapipette 2-APB, slightly activated the current. This indicates that, 2-APB has binding sites not only on the cytoplasmic but also extracellular side.
Fig. 2.
Activation of rat transient receptor potential cation channel subfamily V member 1 (rTRPV1) channels by 2-aminoethoxydiphenyl borate (2-APB) in excised patches. (A) To activate rTRPV1 channels from the cytoplasmic side of the membrane, the inside-out and outside-out patches were achieved. (i) In inside-out mode, the application of an increasing concentration of 2-APB, from the cytoplasmic side, activated the current, which is inhibited by capsazepine (CZP, 10 μM). (ii) In the outside-out patch mode, inclusion of 1 mM 2-APB into the recording pipette weakly activated rTRPV1 current. In the same patch, application of 1 mM or 5 mM 2-APB from the extracellular face activateda CZP-sensitive current. (iii) In the outside-out patches, inclusion of 5 mM 2-APB into the recording pipette activated the CZP-sensitive current, and the addition of 2-APB (0.1–0.5 mM) from the extracellular face further enhanced the current. (B) 2-APB activation of the channels from the extracellular face of the membrane. (i) In the outside-out patches, extracellular 2-APB (0.5–1 mM) activated rTRPV1. Capsaicin activated and capsazepine inhibited the current, respectively. (ii) In the inside-out mode, intrapipette 2-APB (5 mM) weakly activated the current and the additional 2-APB from the cytosolic face enhanced it. Outside-out patches were recorded at –30 mV. Each panel illustrates a representative trace from 5 or 6 recordings.
To determine whether the extracellular 2-APB activates rTRPV1, we recorded the current in outside-out patch mode. As shown in Fig. 2Bi, 0.5–1 mM 2-APB applied into the extracellular side of the outside-out patch membrane activated the capsaicin-sensitive current. In Fig. 2Bii, we included 5 mM 2-APB in the inside-out pipette solution. When the inside-out patch membranes were excised from the cells, a detectable current could be recorded. In the presence of 5 mM of extracellular 2-APB, addition of 2-APB (0.1–5 mM) into the cytoplasmic solution strongly activated the current (Fig. 2Bii). Therefore, the results confirm that 2-APB's binding sites are located on both sides of the membrane.
3.2. Potentiation of the capsaicin-activated rTRPV1 current by cytoplasmic 2-APB
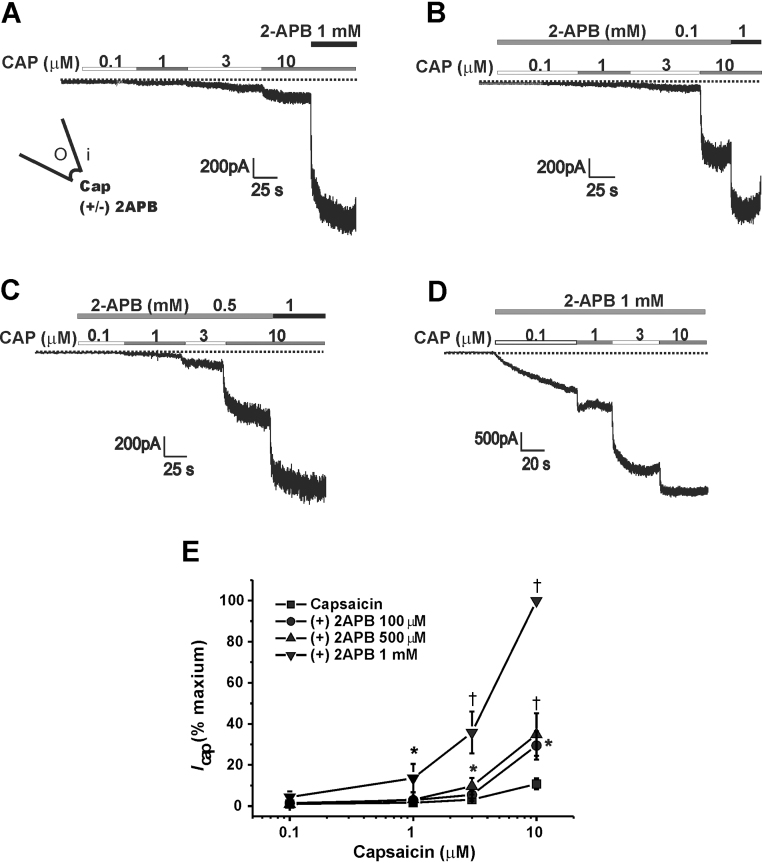
The effect of 2-APB on the capsaicin-activated rTRPV1 current (Icap) was investigated in the inside-out patches. To this end, we applied 2-APB either from the intracellular side or extracellular side of the inside-out patch membrane, and the changes in amplitudes of Icap were compared. For this comparison, we added 1 mM 2-APB simultaneously with 10 μM capsaicin at the end of each recording and the obtained maximum Icap was used to normalize a given current.
As shown in Fig. 3A, capsaicin (0.1–10 μM) dose-dependently activated Icap and 1 mM 2-APB added from cytoplasmic face further increased the current. Cytoplasmic application of 2-APB with increasing concentrations of 0.1 mM, 0.5 mM, and 1 mM increased Icap (Fig. 3). As illustrated in Fig. 3E, the Icap activated by 3 μM capsaicin was increased from 3.1 ± 0.7% (n = 11) to 5.5 ± 1.7% (n = 9), 9.7 ± 4.1% (n = 5, p < 0.05), and 35.8 ± 10.2% (n = 9, p < 0.01) by 0.1 mM, 0.5 mM, and 1 mM 2-APB, respectively. Icap activated by 10 μM capsaicin was increased from 10.8 ± 2.7% (n = 11) to 29.5 ± 6.8% (n = 9, p < 0.05), 34.8 ± 10.4% (n = 5, p < 0.01), and 100% (n = 9, p < 0.01) by 0.1 mM, 0.5 mM, and 1 mM 2-APB, respectively.
Fig. 3.
Potentiation of the capsaicin-activated rat transient receptor potential cation channel subfamily V member 1 (rTRPV1) current by the cytoplasmic 2-aminoethoxydiphenyl borate (2-APB). (A) Representative current traces showing the capsaicin-activated current. To normalize the current, 1 mM 2-APB was added at the end of the recording with 10 μM capsaicin (n = 11). (B–D) Potentiation of the capsaicin-activated currents by the cytoplasmic 2-APB was shown at a concentration of 0.1 mM (n = 9), 0.5 mM (n = 5), and 1 mM 2-APB (n = 9). (E) Summarized data are plotted. Detail values are described in text.
* p < 0.05, † p < 0.01 compare to the control current at a given concentration of capsaicin.
Icap, capsaicin-activated rTRPV1 current.
3.3. Potentiation of the 2-APB-activated rTRPV1 current by capsaicin
To prove the reciprocal stimulation between capsaicin and 2-APB, we tested whether the pretreatment of capsaicin potentiates the 2-APB-induced opening of rTRPV1 channels. As illustrated in Fig. 4, pretreatment of an increasing concentrations of capsaicin (0.3–1 μM) strongly potentiated the current activated by intracellular 2-APB. In the absence of capsaicin, the 1 mM 2-APB-evoked current (4.79 ± 1.4%, n = 4) increased to 10.37 ± 4.4% (n = 3), 12.8 ± 5.1% (n = 3), and 43.4 ± 13.2% (n = 6, p < 0.05) by 0.3 μM, 0.5 μM, and 1 μM capsaicin (Fig. 4E).
Fig. 4.
Potentiation of the 2-aminoethoxydiphenyl borate (2-APB)-activated rat transient receptor potential cation channel subfamily V member 1 (rTRPV1) current by capsaicin. (A) The cytoplasmic 2-APB dose-dependently activated rTRPV1 in the absence of capsaicin. As in Fig. 3, normalization of the current was performed with 1 mM 2-APB and 10 μM capsaicin. (B–D) Potentiation of the cytoplasmic 2-APB-activated currents by capsaicin (0.3–1 μM) are illustrated. (E) Summarized data are plotted. Detail values are described in text. IAPB: 2-APB-activated rTRPV1 current.
* p < 0.05 compare to the control.
Cap, capsaicin.
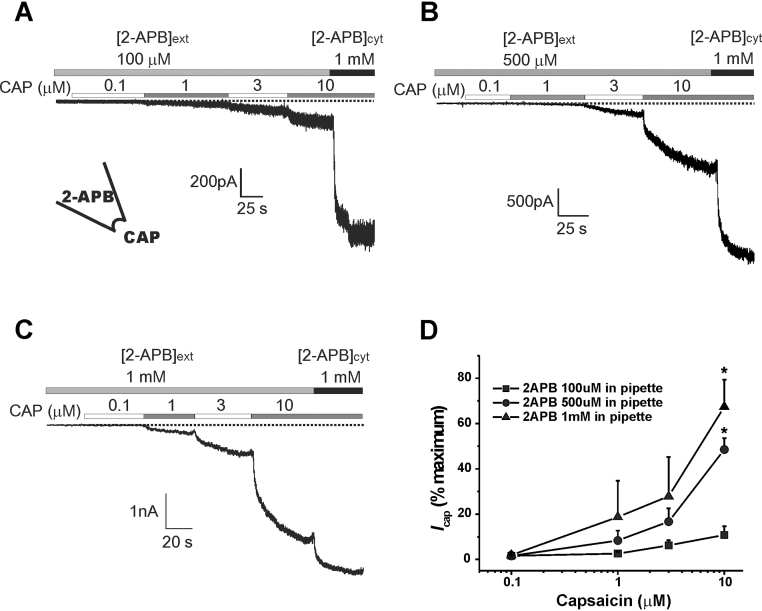
3.4. Potentiation of the capsaicin-activated rTRPV1 current by extracellular 2-APB
In Fig. 5, increasing concentrations of 2-APB (0.1–1 mM) were included in the inside-out patch pipette to stimulate the channels from the outside of the membrane. As previously indicated, < 1 mM of extracellular 2-APB weakly activated the channels and no significant current was monitored until capsaicin was added into the cytoplasmic solution. In this condition, extracellular 2-APB dose-dependently potentiated Icap as does in the cytoplasmic side. The 10 μM capsaicin-activated Icap in the presence of 100 μM 2-APB (10.8 ± 3.8%, n = 9) was not different from the control shown in Fig. 3 (10.8 ± 2.7%). As shown in Fig. 5B–D, 0.5 mM and 1 mM 2-APB significantly potentiated the Icap to 48.5 ± 4.9% (n = 5, p < 0.01) and 67.4 ± 11.8% (n = 4, p < 0.01), respectively.
Fig. 5.
Potentiation of the capsaicin-activated rat transient receptor potential cation channel subfamily V member 1 (rTRPV1) current by extracellular 2-aminoethoxydiphenyl borate (2-APB).(A–C) In the inside-out mode, the indicated concentration of 2-APB was included in the patch pipette ([2-APB]ext), and the amplitudes of capsaicin-activated currents were compared. For normalization, 1 mM 2-APB was applied from the cytoplasmic side ([2-APB]cyt) at the end of each recording. (D) Summarized data.
* p < 0.01.
Potentiation effect of 2-APB on Icap was further demonstrated by the whole-cell recordings (Fig. 6). In whole-cell mode, 2-APB activated the capsaicin-sensitive current (Fig. 6A), and Icap was strongly potentiated by 2-APB in a dose-dependent manner (Fig. 6B). These data strongly indicate that 2-APB and capsaicin reciprocally potentiate the opening of rTRPV1 channels.
Fig. 6.
2-Aminoethoxydiphenyl borate (2-APB) activation of the capsaicin-induced rat transient receptor potential cation channel subfamily V member 1 (rTRPV1) current in whole-cell mode. (A) In the whole-cell recording, extracellular 2-APB (0.01–1 mM) activated rTRPV1. (B) 2-APB dose-dependently potentiated the 0.5 μM capsaicin-induced current. In contrast to the inside-out current, the whole-cell current easily desensitized after treating 2-APB.
3.5. Potentiation of the heat- and H+-activated rTRPV1 current by 2-APB
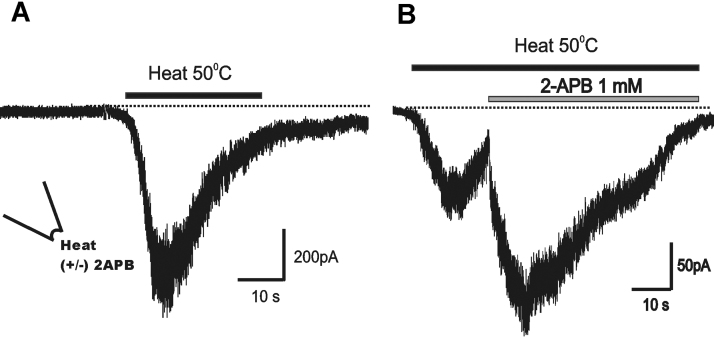
The effect of 2-APB on the heat-activated rTRPV1 current was investigated (Fig. 7). In the inside-out configuration, the perfusion of preheated (at 50 °C) bath solution rapidly activated rTRPV1 currents. Usually, the heat-gated current rapidly desensitizes and the channels hardly respond to the next challenge. For this reason, we compared the response to 2-APB from different patches. When heat-activated rTRPV1 current started to desensitize, we challenged 1 mM 2-APB-containing heated solution. As shown in Fig. 7B, 2-APB added in the middle of desensitization strongly activated the current and reduced the speed of desensitization.
Fig. 7.
2-aminoethoxydiphenyl borate potentiation of the heat-activated rat transient receptor potential cation channel subfamily V member 1 current. (A) The heat (50 °C)-activated current was recorded in the inside-out patches. A rapid desensitization was prominent. (B) The current was markedly enhanced by 1 mM 2-aminoethoxydiphenyl borate applied into the cytoplasmic side. Each recording was achieved from a different patch.
In the next step, we examined whether 2-APB-activated currents are potentiated by extracellular protons. To this end, we applied weak acid to the inside-out pipette solution by preparing the solution at pH 6.3, and the effect of acid on the 2-APB-activated current was compared to the control at pH 7.3 (Fig. 7A). As expected, the response of channels to 2-APB was strongly enhanced by extracellular acid (Fig. 7B). At extracellular pH 6.3, normalized amplitudes of the 0.1 mM, 0.5 mM, and 1 mM 2-APB-evoked currents were 8–10-fold larger than the control responses. At pH 7.3 the currents activated by 10 μM, 100 μM, 500 μM, and 1000 μM 2-APB were 0.7 ± 0.2%, 0.9 ± 0.4%, 1.7 ± 0.5%, and 4.8 ± 1.5%, respectively (n = 4). At pH 6.3, they were increased to 1.8 ± 0.8%, 7.0 ± 3.0%, 35.3 ± 12.1% (p < 0.05), and 52.2 ± 12.5% (p < 0.01), respectively (n = 6; Fig. 8C). The data clearly demonstrate that 2-APB stimulates the activation of rTRPV1 channels by heat and protons, i.e., they reciprocally sensitized each other.
Fig. 8.
Extracellular acid (proton) potentiate the 2-aminoethoxydiphenyl borate (2-APB)-induced rat transient receptor potential cation channel subfamily V member 1 (rTRPV1) current. (A) At extracellular pH of 7.3, a dose-response of 2-APB activation of the current was recorded. (B) With the inside-out pipette solution of pH 6.3, the intracellularly applied 2-APB (10 μM–1 mM) produced a significant increase in current. (C) Summarized data are illustrated.
* p < 0.05, † p < 0.01.
IAPB, 2-APB-induced current.
3.6. Effect of 2-APB analogue DPBA
To define the pharmacological requirements for the 2-APB activation, the effect of structural analogue DPBA was examined on rTRPV1 with inside-out patches. As shown in Fig. 9A, Icap was enhanced by the cytoplasmic addition of 1 mM DPBA. Inclusion of 1 mM DPBA in the pipette solution increased Icap and the currents were further activated by 1 mM DPBA applied from the cytoplasmic side (Fig. 9B,Cw). Icapat 10 μM capsaicin (13.8 ± 4.6%) was significantly potentiated by extracellular DPBA (51.6 ± 8.1%, n = 7, p < 0.05). Similar to the effect of acid on the 2-APB current (Fig. 8), intrapipette acid enhanced the DPBA-induced currents (recordings not shown). The summarized data are shown in Fig. 9D. At pH 6.3, 1 mM DPBA-induced currents were significantly higher than control at pH 7.3 (1.16 ± 0.18%, n = 5 vs. 35.1 ± 14.0%, n = 4; p < 0.05; Fig. 9D). Therefore, these data suggest that DPBA is an rTRPV1 agonist and shows similar response to that of 2-APB.
Fig. 9.
Activation of rat transient receptor potential cation channel subfamily V member 1 (rTRPV1) current by diphenylborinic anhydride (DPBA), a 2-aminoethoxydiphenyl borate analog. (A) Control response to capsaicin in the inside-out patches. 1 mM DPBA applied into the cytoplasmic solution ([DPBA]cyt) strongly enhanced the current by capsaicin. (B) 1 mM DPBA was included in the recording pipette ([DPBA]ext). Capsaicin-induced currents were greatly enhanced by 1 mM of intrapipette DPBA ([DPBA]ext = 1 mM). (C) Summarized data on the effect of 1 mM DPBA. (D) The effect of extracellular pH (7.3 vs. 6.3) on the cytoplasmic DPBA-induced current was summarized.
* p < 0.05.
IAPB, 2-aminoethoxydiphenyl borate-induced current.
4. Discussion
A synthetic organic compound, 2-APB has been commonly used as a general inhibitor of SOCE and TRP channels. Recently, it was shown that 2-APB is not only an inhibitor of many native and expressed TRPC and TRPM channels but also an activator of TRPV1, TRPV2, and TRPV3 channels.16, 17 It has been postulated that TRP channels have a common 2-APB binding domain but the channel activity is differently affected by 2-APB. Among TRP channels, 2-APB at 10–100 μM inhibits TRPC1, TRPC3, TRPC5, TRPC6, TRPM3, TRPM7, TRPM8, and TRPP2.12, 16, 22, 23 At high concentrations (> 100 μM), 2-APB selectively activates TRPV1, V2, and V3 channels.16, 19 However, the stimulatory effect of 2-APB on these thermo-TRPV channels is not generally accepted, because it is suggested that the compound has no effect on TRPV2 and hardly activates TRPV1 expressed in HEK cells.19 This discrepancy has been explained by the different concentrations of 2-APB used or by the different expression level of the channels.16, 19
The present study attempted to demonstrate the effects of 2-APB on rTRPV1 channels expressed in HEK cells. Instead of whole-cell patch clamp recording, by which the 2-APB activation of TRPV1 was first reported, our results were obtained from excised patches, which excludes an intervention by cytosolic components. TRPV1 modulating molecules that might affect the response to 2-APB included protein kinase C,20 protein kinase A,24, 25, 26 calmodulin kinase II,21 and regulators of phosphatidylinositol 4,5-bisphosphate (PIP2) metabolism.27, 28 TRPV1 channels are easily rundown or desensitize in response to stimuli, and the contribution of protein kinase C and calmodulin kinase II on the desensitization has been reported.20 PIP2 is a substrate of phospholipase C and is known to inhibit TRPV1 through a specific domain of the channel. Furthermore, it is known that 2-APB activates phospholipase C in B lymphocytes and releases the stored Ca2+ from endoplasmic reticulum.15
The plasma membrane side of action for 2-APB has been suggested to be extracellular, because the intracellular application of 2-APB failed to activate TRPV1 and TRPV3 current in Xenopus oocytes and conventional whole-cell recorded HEK293 cells, respectively.16, 29 To determine the 2-APB-binding sites, we applied 2-APB from either side of the membrane in the inside-out or outside-out patch configuration (Fig. 2). We clearly demonstrated that 2-APB activates rTRPV1 channels not only from inside but also from the outside of the membrane. In the inside-out and outside-out modes, the perfusion of 2-APB-contained bath solution activated the channels in a dose-dependent manner, i.e., it stimulates the channels from the inside as well as the outside of the membrane. In whole-cell configuration, 2-APB also activated TRPV1 channels (Fig. 6). Therefore, it clearly shows that 2-APB-binding sites are located on both sides of the membrane. To further confirm this, we included 2-APB inside of the recording pipettes of the inside-out or outside-out mode, thereby stimulating the channels from outside or inside of the membrane, respectively. To our surprise, however, it needed a higher concentration of intrapipette 2-APB (> 5 mM) to activate the channels sufficiently (Fig. 2). This was an unexpected result, because < 500 μM of 2-APB applied from the bath solution of the inside-out or outside-out patches readily activated a discernible current, regardless of the expression level of the channels. In the present study, we could not thoroughly explain the reason for this phenomenon. Chelation or adsorption of 2-APB by the glass pipette may be suggested but needs to be proved. When 2-APB was included in the patch pipette solution for the excised patches, accumulation of the membrane permeable 2-APB at the extrapipette side (bath solution) is hard to achieve as the drug will be rapidly diluted by the bath solution and will be washed away by perfusion. Therefore, activation of TRPV1 current by the intrapipette 2-APB in the excised patches (inside-out or outside-out modes) further supports that 2-APB can activate TRPV1 from both sides of the plasma membrane. Conversely, the membrane permeable 2-APB can accumulate at the pipette side even though it is applied to the extrapipette side of the excised patch membrane.30
Activation of TRPV1, TRPV2, and TRPV3 channels by 2-APB has been reported by whole-cell recordings and [Ca2+]i measurement.16 Because 2-APB commonly activated TRPV1, TRPV2, and TRPV3, it is suggested that these channels share structural similarity and are gated by similar mechanisms. Interestingly, the sensitivity of channels to 2-APB, revealed by the order of half maximal effective concentration values, follows the order of temperature threshold of each channel: TRPV3 (> 32 °C), TRPV1 (> 43 °C), and TRPV2 (> 52 °C). To find the 2-APB-binding site, Hu et al16 applied 1 mM 2-APB to the inside of the whole-cell recording pipette and waited more than 6 minutes after breaking the membrane of TRPV3-expressing HEK cells. However, they failed to record the TRPV3 current in this condition. Furthermore, they failed to activate TRPV1 expressed in Xenopus oocytes by the intracellular injection of 40 mM 2-APB. Based on these results, they suggested that 2-APB-binding sites are located at the extracellular side of the membrane. This is, however, clearly contradictory to our present data. We recorded TRPV1 current mainly by inside-out patches, and the cytoplasmic perfusion of 2-APB activated the channels with no exception. In the outside-out patches, cytoplasmic application of 2-APB through the patch pipette activated the current, although it requires a relatively higher concentration. More interestingly, even in the presence of a higher concentration of 2-APB in the recording pipette (i.e., 5 mM 2-APB in the inside-out or outside-out patch pipettes), a low concentration of 2-APB applied from the opposite side of the membrane further activated the current (Fig. 2Aii, Bii). There are two possible explanations for the responses. First, intracellular and extracellular 2-APB molecules cooperate or potentiate each other when they simultaneously occupy their binding sites, thus they produce a supra-additive response. As 2-APB does on capsaicin, heat, and protons to potentiate TRPV1, each 2-APB molecule from the opposite side of the membrane can potentiate each other to open the channels. Second, a simple additive effect of TRPV1 opening by 2-APB can be postulated. When 2-APB binds to the channel simultaneously from the inside and outside of the membrane, they independently activate the channels without any interruption to the opposite. We think that the former seems plausible, because the current activated by the first arrived 2-APB is always smaller than the current activated by the latter (Fig. 2).
In contrast to our results that show two 2-APB-binding sites on rTRPV1, it has been suggested that the blocking site of TRPC3, C5, V1, and V3 channels by 2-APB is located only at the extracellular face.12, 16, 23 In expressed human TRPC5 channels, it was suggested that one 2-APB molecule binds per channel with mild voltage-dependence and the extracellular blocking site is exclusive for 2-APB without sharing with other Ca2+ channel blockers.12 We could not completely explain the reason for this discrepancy, and it remains to be elucidated.
It has been shown that 2-APB stimulates the response to capsaicin, heat, acid, and sensitizes native TRPV1-expressing nociceptive neurons to heat in a mouse model.16, 18 Our present study strongly supports this suggestion. We clearly demonstrate evidence supporting the stimulatory roles of 2-APB on the heterologously expressed TRPV1 by using the cell-free, excised patch clamp: (1) 2-APB directly activates rTRPV1 (Fig. 2, 10); (2) 2-APB and capsaicin reciprocally potentiate the stimulatory effect on rTRPV1 (Fig. 3–6); (3) 2-APB potentiates the response to heat and protons (Fig. 7, Fig. 8); and (4) DPBA potentiates the response to capsaicin and protons (Fig. 9).
It still remains a mystery how 2-APB activates and sensitizes the capsaicin-activated TRPV1 channel. In Xenopus oocytes expressing TRPV1, 2-APB left-shifted the dose-response curves for capsaicin and the pH dependence by several fold. Conversely, capsaicin left-shifted the dose-response curve to 2-APB several-folds.16 We showed that 2-APB applied from the cytoplasmic side (Fig. 3) or extracellular side (Fig. 5) sensitized capsaicin-evoked rTRPV1 current and shifted the dose-response curve to the left, which is in accordance with previous suggestions.16 TRPV1 can be activated by a number of lipophilic molecules.31 Increasing the content of the plasma membrane cholesterol lowers the temperature sensitivity and shifts the threshold of TRPV1 to higher temperature range, and the membrane PIP2 hold TRPV1 in an inhibitory state.28, 30 2-APB is a lipophilic molecule and can accumulate in the plasma membrane bilayer at higher concentrations. Therefore, 2-APB could disrupt the interaction between TRPV1 and the inhibitory membrane lipids (PIP2 and cholesterol) and sensitize the channel to various agonists.30
Because the effects of 2-APB on TRPV1 channels require a higher concentration and are relatively nonspecific, at present, it is unlikely to be used for therapeutic and clinical uses. Chemical modification of the structure of 2-APB can generate various analogs with greater differences in the affinity and selectivity to TRPV1 channel and/or TRPV1-interacting molecules to enhance or reduce the channel activity. If the chemically modified 2-APB analogs have highly specific TRPV1 inhibitory activities at lower concentrations, they can be used as therapeutic tools to reduce TRPV1-mediated inflammatory pain. In addition, 2-APB analogs can be used for the development of a specific antagonist for SOCE channels, which are pivotal for many immunological and cardiovascular diseases. Recent progress on the synthesis of 2-APB analogs with higher specificity for SOCE channel should certainly accelerate the discovery of therapeutic drugs for many TRP channel-related diseases.32
In conclusion, our data suggest that 2-APB directly opens rTRPV1 channels and strongly potentiates the effects of well-known TRPV1 agonists. Binding sites of 2-APB on TRPV1 channels are judged to be located on both sides of the membrane. Because DPBA shows the same effects, it is suggested that 2-APB and its analogs could be attractive candidates for the development of a selective TRPV1 agonist and/or antagonist.
Conflict of interest
No conflicts of interest, financial or otherwise, are declared by the author(s).
Acknowledgments
We thank Professor Uhtaek Oh (Seoul National University, Korea) for providing rTRPV1 cDNA. This study was supported by the National Research Foundation, the Ministry of Education, Science, and Technology (Korea) Grants (NRF-2011-0030629).
References
- 1.Baumann T.K., Smone D.A., Shain C.N., LaMotte R.H. Neurogenic hyperalgesia: the search for the primary cutaneous afferent fibers that contribute to capsaicin-induced pain and hyperalgesia. J Neurophysiol. 1991;66:212–227. doi: 10.1152/jn.1991.66.1.212. [DOI] [PubMed] [Google Scholar]
- 2.LaMotte R.H., Lundberg L.E.R., Torebjörk H.E. Pain hyperalgesia and activity in nociceptive C units in humans after intradermal injection of capsaicin. J Physiol. 1992;448:749–764. doi: 10.1113/jphysiol.1992.sp019068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caterina M.J., Shumacher M.A., Tominaga M., Rosen T.A., Levine J.D., Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 4.Caterina M.J., Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Ann Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- 5.Caterina M.J., Leffler A., Malmberg A.B., Martin W.J., Trafton J., Petersen-Zeltz K.R. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 6.Szallazi A., Cortright D.N., Blum C.A., Eid S.R. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov. 2007;6:357–372. doi: 10.1038/nrd2280. [DOI] [PubMed] [Google Scholar]
- 7.Maruyama T., Kanaji T., Nakade S., Kanno T., Mikoshiba K. 2-APB, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J Biochem (Tokyo) 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- 8.Dobrydneva Y., Blackmore P. 2-Aminoethoxydipenyl borate directly inhibits store-operated calcium entry channels in human platelets. Mol Pharmacol. 2001;3:541–552. [PubMed] [Google Scholar]
- 9.Prakriya M., Lewis R.S. Potentiation and inhibition of Ca2+ release-activated Ca2+ channels by 2-aminoethoxydipenyl borate (2-APB) occurs independently of IP3 receptors. J Physiol. 2001;536:3–19. doi: 10.1111/j.1469-7793.2001.t01-1-00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bootman M.D., Collins T.J., Mackenzie L., Roderick H.L., Berridge M.J., Pepiatt C.M. 2-aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J. 2002;16:1145–1150. doi: 10.1096/fj.02-0037rev. [DOI] [PubMed] [Google Scholar]
- 11.Montell C., Birnbaumer L., Flockerzi V. The TRP channels, a remarkably functional family. Cell. 2002;108:595–598. doi: 10.1016/s0092-8674(02)00670-0. [DOI] [PubMed] [Google Scholar]
- 12.Xu S.Z., Zeng F., Boulay G., Grimm C., Harteneck C., Beech D.J. Block of TRPC channels by 2-aminoethoxydipehylborate: a differential, extracellular and voltage-dependent effect. Br J Pharmacol. 2005;145:405–414. doi: 10.1038/sj.bjp.0706197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bilmen J.G., Wootton L.L., Michelangeli F. The inhibition of the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase by macrocyclic lactones and cyclosporin A. J Biochem. 2002;366:255–263. doi: 10.1042/BJ20020431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemonnier L., Prevarskaya N., Shuba Y., Abeele F.V., Nilius B., Skryma R. Ca2+ modulation of volume-regulated anion channels: evidence for co localization with store-operated channels. FASEB J. 2002;16:222–224. doi: 10.1096/fj.01-0383fje. [DOI] [PubMed] [Google Scholar]
- 15.Ma H.T., Venkatachalam K., Rys-SikoraKE, He L.P., Zeng F., Gill D.L. Modification of phospoholipase C gamma–induced Ca2+ signal generation by 2-aminoethoxydiphenylborate. J Biochem. 2003;376:667–676. doi: 10.1042/BJ20031345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu H.Z., Gu Q., Wang S., Colton C.K., Tang J., Kinoshita-Kawada M. 2-Aminoethoxydiphenyl borate is a common activator of TRPV1. TRPV2 and TRPV3. J Biol Chem. 2004;279:35741–35748. doi: 10.1074/jbc.M404164200. [DOI] [PubMed] [Google Scholar]
- 17.Zhu W., Galoyan S.M., Petrushka J.C., Oxford G.S., Mendell L.M. A developmental switch in acute sensitization of small dorsal root ganglion neurons to capsaicin or noxious heating by NGF. J Neurophys. 2004;92:3148–3152. doi: 10.1152/jn.00356.2004. [DOI] [PubMed] [Google Scholar]
- 18.Zimmermann K., Leffler A., Fischer M.J., Messlinger K., Nau C., Reeh P.W. The TRPV1/2/3 activator 2-aminoethoxydipenyl borate sensitizes native nociceptive neurons to heat in wild type but not TRPV1 deficient mice. Neuroscience. 2005;135:1277–1284. doi: 10.1016/j.neuroscience.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 19.Chung M.K., Lee H., Mizuno A., Suzuki M., Caterina M.J. 2-Aminoethoxydipenyl borate activates and sensitizes the heat-gated ion channel TRPV3. J Neurosci. 2004;24:5177–5182. doi: 10.1523/JNEUROSCI.0934-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vellani V., Mapplebeck S., Moriondo A., Davis J.B., McNaughton P.A. Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and an andamide. J Physiol. 2001;534:813–825. doi: 10.1111/j.1469-7793.2001.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung J., Shin J.S., Lee S.V., Hwan S.W., Koo J., Cho H. Phosphorylation of vanilloid receptor 1 by Ca2+/calmodulin dependent-kinase II regulates its vanilloid binding. J Biol Chem. 2004;279:7048–7054. doi: 10.1074/jbc.M311448200. [DOI] [PubMed] [Google Scholar]
- 22.Ma H.T., Patterson R.L., Van Rossum D.B., Birnbaumer L., MikoshibaK, Gill D.L. Requirement of the inositol trisphosphate receptor for activation of store-operated calcium channels. Science. 2000;287:1647–1651. doi: 10.1126/science.287.5458.1647. [DOI] [PubMed] [Google Scholar]
- 23.Trebak M., Bird G.S., McKay R.R., Putney J.W. Comparison of human TRPV3 channels in receptor-activated and store-operated modes. Differential sensitivity to channel blockers suggests fundamental differences in channel composition. J Biol Chem. 2002;277:21617–21623. doi: 10.1074/jbc.M202549200. [DOI] [PubMed] [Google Scholar]
- 24.Mohapatra D.P., Nau C. Desensitization of capsaicin-activated current in the vanilloid receptor TRPV1 is decreased by the cyclic AMP-dependent protein kinase pathway. J Biol Chem. 2003;278:50080–50090. doi: 10.1074/jbc.M306619200. [DOI] [PubMed] [Google Scholar]
- 25.Mohapatra D.P., Nau C. Regulation of Ca2+ dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP dependent protein kinase. J Biol Chem. 2005;280:13424–13432. doi: 10.1074/jbc.M410917200. [DOI] [PubMed] [Google Scholar]
- 26.Rathee P.K., Distler C., Obreja O., Neuhuber W., Wang G.K., Wang S.Y. PKA/AKAP/VR1 module: A common link of Gs-mediated signaling to thermal hyperalgesia. J Neurosci. 2002;22:4740–4745. doi: 10.1523/JNEUROSCI.22-11-04740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chuang H.H., Prescott E.D., Kong H., Shields S., Jordt S.E., Basbaum A.I. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns (4,5) P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- 28.Liu B., Zhang C., Qin F. Functional recovery from desensitization of vanilloid receptor TRPV1 requires resynthesis of phosphatidylinositol 4,5-bisphosphate. J Neuroscience. 2005;25:4835–4843. doi: 10.1523/JNEUROSCI.1296-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung M.K., Güler A.D., Caterina M.J. Biphasic currents evoked by chemical or thermal activation of the heat-gated ion channel. TRPV3. J Biol Chem. 2005;280:15928–15941. doi: 10.1074/jbc.M500596200. [DOI] [PubMed] [Google Scholar]
- 30.Colton C.K., Zhu M.X. 2-Aminoethoxydiphenyl borate as a common activator of TRPV1. TRPV2, and TRPV3 channels. Handb Exp Pharmacol. 2007;179:173–187. doi: 10.1007/978-3-540-34891-7_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calixto J.B., Kassuya C.A., André E., Ferreira J. Contribution of natural products to the discovery of the transient receptor potential (TRP) channels family and their functions. Pharmacol Ther. 2005;106:179–208. doi: 10.1016/j.pharmthera.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 32.Zhou H., Iwasaki H., Nakamura T., Nakamura K., Maruyama T., Hamano S., Ozaki S., Mizutani A., Mikoshiba K. 2-Aminoethyl diphenylborinate analogues: selective inhibition for store-operated Ca2+ entry. Biochem Biophys Res Commun. 2007;352:277–282. doi: 10.1016/j.bbrc.2006.10.174. [DOI] [PubMed] [Google Scholar]