Abstract
Background
While dichotomous criteria for the metabolic syndrome (MetS) appear heritable it is not known whether MetS severity as assessed by a continuous MetS score is heritable and whether this varies by race.
Methods and Results
We used SOLAR to evaluate heritability of ATP-III MetS and a sex- and race-specific MetS severity Z-score among three large familial cohorts: the Jackson Heart Study (JHS, 1404 black participants), Take-Off-Pounds-Sensibly (TOPS, 1947 white participants) and Princeton Lipid Research Study (PLRS, 229 black and 527 white participants). Heritability estimates were larger for ATP-III MetS among black- compared to white cohort members (JHS 0.48 [95% confidence interval 0.28, 0.68], PLRS-blacks 0.93 [0.73, 1.13] vs. TOPS 0.21 [−0.18, 0.60], PLRS-whites 0.27 [−0.04, 0.58]). The difference by race narrowed when assessing heritability of the MetS severity score (JHS 0.52 [0.38, 0.66], PLRS-blacks 0.64 [0.13, 1.15] vs. TOPS 0.23 [0.15, 0.31], PLRS-whites 0.60 [0.33, 0.87]). There was a high degree of genetic and phenotypic correlation between MetS severity and the individual components of MetS among all groups, though the genetic correlations failed to reach statistical significance among PLRS-blacks. Meta-analyses revealed a combined heritability estimate for ATP-III MetS of 0.24 [0.11, 0.36] and for the MetS severity score of 0.50 [−0.05, 0.99].
Conclusions
MetS severity appears highly heritable among whites and blacks. This continuous MetS severity Z-score may provide a more useful means of characterizing phenotypic MetS in genetic studies by minimizing racial differences.
Keywords: metabolic syndrome, heritability, insulin resistance, genetics, race and ethnicity
Introduction
Successfully addressing the adverse health outcomes related to the current obesity epidemic relies on identifying individuals and their family members who are at risk for future disease, including future type 2 diabetes (T2DM) and cardiovascular disease (CVD). The metabolic syndrome (MetS) is a cluster of cardiovascular risk factors that are associated with insulin resistance and future disease risk. MetS is classically identified using criteria such as the Adult Treatment Panel (ATP)-III as the presence of abnormalities in at least 3 of the 5 individual components of elevated waist circumference, high blood pressure, elevated fasting glucose, hypertriglyceridemia, and low HDL cholesterol.1 Using these criteria, researchers have evaluated cohorts composed of predominantly white participants and reported that MetS is heritable, 10–30%,2–5 indicating both environmental and genetic influences. Twin studies from Asian and European cohorts have demonstrated similar high degrees of heritability in the individual components of MetS,6–9 while another comparison of white cohorts with Hispanic and Asian populations revealed similarities in the genetic correlation between MetS and its underlying factors.2 However, direct comparisons between cohorts with white and black participants are lacking.
There is potential that the genetic basis of MetS may differ between white and black populations, as MetS appears to be manifested differently by race. Blacks as a group have a low prevalence of MetS despite having high prevalence of insulin resistance, T2DM and CVD.10–13 This appears to be due to low rates of dyslipidemia among blacks, who have low levels of triglycerides that are nevertheless positively correlated with insulin resistance.14, 15 It is for this reason that we developed a MetS severity Z-score that is specific to sex and race/ethnicity.16–18 This score is correlated with insulin resistance19 and predicted future T2DM20 and CVD21, 22 events in cohorts with white and black participants. Additionally, as a linear score, this provides a more powerful way to assess genetic underpinnings than traditional binary criteria.
The goal of this study was to evaluate the heritability of the MetS severity score and its individual components, as well as the genetic and phenotypic correlations between MetS severity and these components, in cohorts composed of white and black participants. For this, we utilized data from the Jackson Heart study (JHS, a cohort of black individuals from the area around Jackson, MS), the TOPS study (a cohort of white individuals from Minnesota), and the Princeton Lipid Research Cohort (PLRS, a cohort of white and black individuals in the Cincinnati area). We were particularly interested in potential differences in the heritability of MetS severity between groups, both to help guide future efforts at risk identification and to assess whether heritability differences have been minimized in the use of the continuous MetS Z-score.
Methods
Cohorts evaluated
Jackson Heart Study (JHS)
JHS is the largest longitudinal, single-site study of cardiovascular risk in black individuals in and around Jackson, MS. JHS was approved by the Institutional Review Board of the University of Mississippi Medical Center, and participant provided informed consent. The cohort consists of 5,301 participants age 21–95 years23 of whom 1,500 are part of a family component of the study. They were recruited by identifying index participants who provided extensive family information. We utilized data from JHS Visit 1 (2000–2004). MetS components measured using standardized protocols as described previously.23–25
TOPS
Families were recruited from the TOPS (Take Off Pounds Sensibly, Inc.) membership. In 1994 TOPS provided mailing material on membership attending its chapters in 10 states (Wisconsin, Illinois, Michigan, Iowa, Minnesota, Ohio, West Virginia, Missouri, Kentucky, and Indiana). Questionnaire data received from 60,000 respondents were verified and entered into the TOPS Obesity and Metabolic Research Center databases at the Medical College of Wisconsin. Families with at least two obese siblings [body mass index (BMI) ≥ 30 mg/kg2], availability of one (preferably both) parents, as well as at least one never-obese sib and/or parent [BMI ≤ 27 mg/kg2] were identified and contacted for ascertainment. Families were scheduled to visit satellites (4–6 per state), where an experienced team undertook the phenotypic procedures. Research protocols were approved by the Institutional Review Board of the Medical College of Wisconsin and participants provided informed consent. Data for the genetic analyses presented here included 2,209 individuals distributed across 507 Caucasian families of predominantly northern European ancestry and residing in the United States. Data regarding MetS-associated data were collected via standard protocols as published previously.26
Princeton Lipid Research Study (PLRS)
Participants were originally recruited as part of the Cincinnati Clinic of the National Heart Lung and Blood Institute Lipid Research Clinic (LRC) Prevalence Program (1972–1978), a multistage survey of lipids and other CVD risk factors.27, 28 In 1973–1976, the LRC enrolled students in grades 1–12 in the Princeton City School District near Cincinnati, OH, and a random sample of their parents. The Institutional Review Boards of NHLBI, the University of Cincinnati, and Cincinnati Children’s Hospital Medical Center approved the study and/or its analysis and participants provided informed consent. The Princeton Follow-up Study (PFS, 2000–2004) was a 25–30-year follow-up of these student and parent-participants to prospectively assess changes in CVD risk factors from childhood into the 4th–5th decades of life.29 PFS visit eligibility required participation in LRC visits where lipoproteins were measured and participation of at least one first-degree relative at those same visits. In this analysis, the family-based data from the PFS study visit was used. MetS-associated data were collected at the PFS study visit via standard protocols as published previously.30
MetS determination
Traditional MetS was defined using the ATP-III criteria for adults;1 participants had to meet ≥3 of the following 5 criteria: concentration of triglycerides ≥1.69 mmol/L (150 mg/dL), HDL-C <1.04 mmol/L (40 mg/dL) for men and <1.3 mmol/L (50 mg/dL) for women, WC ≥102cm for males and 88cm for females, glucose concentration ≥5.55 mmol/L (100 mg/dL), and systolic BP ≥130 mmHg or diastolic BP ≥85 mmHg. Traditional MetS is classified as meeting vs. not meeting the established criteria.
MetS severity Z-score was calculated using formulas published previously by our group.16, 31 Briefly, these scores were formed using confirmatory factor analysis of the 5 traditional components of MetS (as above) to determine the weighted contribution of each of these components to a latent MetS “factor” on a sex- and race/ethnicity-specific basis. Confirmatory factor analysis was performed on data from the National Health and Nutrition Examination Survey (NHANES) for adults age 20–64 years31 divided into six sub-groups based on sex and the following self-identified race/ethnicities: non-Hispanic white, non-Hispanic black and Hispanic. For each of these six population sub-groups, loading coefficients for the 5 MetS components were determined toward a single MetS factor. The loading coefficients were then used to generate equations to calculate a standardized MetS severity score for each sub-group (http://mets.health-outcomes-policy.ufl.edu/calculator/).32 These MetS severity scores are Z-scores (with mean=0 and SD=1, with 99.75% of values ranging from −3 to 3) of relative MetS severity on a sex- and race/ethnicity-specific basis, with higher scores indicating worse MetS severity. These scores correlate with other CHD risk factors, including hsCRP, uric acid, insulin17 and adiponectin33 and with long-term disease risk20, 21
Correlations and Heritability analysis
We used a variance component approach implemented in SOLAR34 to estimate heritability for MetS severity score as well as its phenotypic and genetic correlations with its components (blood pressure, HDL, triglycerides, waist circumference and fasting glucose). While the genetic correlation was directly estimated from the bivariate modeling, the phenotypic correlation was derived from the estimated proportion of variance attributed to genetic and non-genetic factors as well as the correlation due to genetic and non-genetic factors35. We did not include dominance deviations; while dominance deviations can be estimated, the contribution of dominance deviations apart from siblings is quite low; thus most studies such as this, which include nuclear and extended pedigrees rarely estimate this component36. Heritability analysis on MetS as defined in the ATP-III guidelines was also conducted to allow for comparison purposes. Due to the non-normal distribution of most continuous phenotypes, we applied the t-distribution option. Covariates included age, age*age, sex, age*sex and age*age*sex interactions. Heritability estimates for each study were computed independently, which were then meta-analyzed across racial groups using a random effects model based on inverse variance weighting approach to reduce bias associated with meta-analysis of few studies.
Results
We evaluated data from 4,107 individuals: 1404 from JHS, 756 from PLRS and 1947 from TOPS. Participant characteristics are shown in Table 1. There was a high proportion of obesity in each of the cohorts, consistent with US population data.37 The prevalence of ATP-III MetS was higher in TOPS (48.2% of women and 41.3% of men) and white male PLRS participants (37.1%) than in JHS (31.0% of women and 28.3% of men) and black PLRS participants (31.6% of women and 34.5% of men). MetS severity scores were generally higher in males vs. females (except in JHS) and were highest in TOPS.
Table 1.
Participant characteristics by cohort.
| Jackson HS | Princeton LRS | TOPS* | ||||||
|---|---|---|---|---|---|---|---|---|
| Black | White | |||||||
| Women | Men | Women | Men | Women | Men | Women | Men | |
| N | 935 | 469 | 142 | 87 | 268 | 259 | 1492 | 455 |
| Race (% black) | 100 | 100 | 100 | 100 | 0 | 0 | 0 | 0 |
| Age, Yrs | 51±14 | 49±15 | 38.9±4.5 | 38.8±5.6 | 39.5±4.5 | 39.9±4.7 | 47±14 | 53±17 |
| BMI, kg/m2 | 33.5±8.2 | 30.0±6.8 | 31.0±8.4 | 29.8±6.4 | 27.4±6.7 | 28.8±5.4 | 32.8±7.5 | 28.5±5.0 |
| Waist Circumference | 100.8±17.7 | 100.4±16.0 | 100.6±20.4 | 99.2±17.7 | 93.1±16.2 | 100.0±13.5 | 101.2±17.8 | 102.1±13.0 |
| Obesity (BMI ≥ 30) | 63.7 | 38.8 | 45.1 | 45.3 | 26.5 | 35.1 | 61.9 | 31.6 |
| Systolic BP, mmHg | 124.9±19.1 | 126.4±17.3 | 122.3±18.2 | 125.3±13.6 | 116.1±13.9 | 124.4±13.5 | 128.8±18.4 | 132.2±17.8 |
| Diastolic, BP mmHg | 78.1±10.3 | 81.3±10.4 | 80.2±11.5 | 82.5±10.5 | 76.0±10.8 | 82.9±10.1 | 80.4±10.6 | 81.9±10.1 |
| High BP, n (%) | 59.6 | 51.5 | 38.8 | 44.2 | 24.7 | 45.5 | 20.0 | 19.8 |
| Triglycerides, mg/dL† | 85.0 (58.0, 116.0) | 96.5 (66.0, 146.0) | 72 (52, 109) | 104 (74,169) | 99 (67,152) | 142 (87,219) | 111 (75, 153) | 112 (78, 166) |
| HDL, mg/dL | 53.8±14.1 | 45.2±12.1 | 50.6±14.9 | 44.3±17.0 | 48.8±13.6 | 38.4±11.2 | 40.3±11.4 | 34.1±9.7 |
| Total Chol, mg/dL | 197.5±40.7 | 195.2±39.4 | 182.6±30.2 | 196.0±52.4 | 188.6±36.1 | 199.0±41.8 | 198.8±43.8 | 197.1±40.5 |
| Fasting Glucose† | 90.0 (84.0, 98.0) | 91.0 (86.0, 98.0) | 86 (79, 95) | 89 (81, 97) | 87 (79, 92) | 91 (83, 98) | (85 (79, 94) | 87 (79, 96) |
| MetS Z Score† | 0.04 (−0.53, 0.69) |
0.04 (−0.53, 0.60) |
−0.10 (−0.95, 0.66) |
0.12 (−0.80, 0.75) |
−0.27 (−0.86, 0.34) |
0.52 (−0.19, 1.20) |
0.30 (−.36, 0.91) |
0.48 (−0.04, 0.97) |
| MetS (ATP-III criteria) | 31.0 | 28.3 | 31.6 | 34.5 | 24.2 | 37.1 | 48.2 | 41.3 |
Hypertension for TOPS – those on medications
Median (25th, 75th) percentiles;
ATP-III: Adult Treatment Panel III; BP: blood pressure; HS: Heart Study; LRS: Lipid Research Study; MetS: metabolic syndrome; TOPS: Take Off Pounds Sensibly
Table 2 shows heritability estimates for the MetS severity score and ATP-III MetS for each of the cohorts, with the PLRS cohort divided into white and black participants. MetS severity score had a significantly (p<0.001) high heritability (> 0.50) estimate in all cohorts except in TOPS where the estimate (±SE) was lower 0.23 (±0.04). ATP-III MetS demonstrated similar or lower heritability estimates in most cohorts (0.21 in TOPS, 0.27 in PLRS whites, and 0.48 in JHS; p<0.01 for JHS and TOPS), but a higher heritability estimate among PLRS blacks (0.93, p<0.001).
Table 2.
Estimated Heritability for Metabolic Syndrome Severity Score and ATP-III Metabolic Syndrome for all Cohorts
| Cohort | Metabolic Syndrome Score
|
ATP-III MetS
|
|||
|---|---|---|---|---|---|
| h2±SE | P Value | Phenotypic varianceŦ | h2±SE | P Value | |
| JHS | 0.52 (0.05) | < 0.001 | 0.09 | 0.48 (0.10) | < 0.001 |
| PLRS-Blacks | 0.64 (0.26) | 0.005 | 0.21 | 0.93 (0.10) | 0.001 |
| PLRS-Whites | 0.60 (0.14) | < 0.001 | 0.00 | 0.27 (0.16) | 0.085 |
| TOPS | 0.23 (0.04) | < 0.001 | 0.28 | 0.21 (0.20) | < 0.001 |
h2: Heritability estimate; SE: Standard Error of the estimate; JHS: Jackson Heart Study; PLRS: Princeton Lipid Research Study; TOPS: Take Off Pounds Sensibly
Proportion of phenotypic variance explained by significant covariates
Table 3 presents heritability of the individual MetS components by cohort. Each of the individual components of MetS had a significant degree of heritability, with the exception of SBP and FG among blacks in the PFS cohort. Of the individual MetS components, SBP and FG had lower heritability estimates (< 0.40) while waist circumference, HDL and log TG showed higher heritability (>0.47) estimates across all cohorts except in TOPS, where all estimates were less than 0.35. Heritability estimates did not differ consistently by race, although blacks in PLRS showed greater variability, likely due to the smaller sample size.
Table 3.
Estimated Heritability for Individual Components of Metabolic Syndrome
| Cohort | SBP | WC | LnTG | HDL | FG |
|---|---|---|---|---|---|
| JHS | 0.09 (0.05) | 0.55 (0.07) | 0.48 (0.07) | 0.58 (0.20) | 0.22 (0.06) |
| PLRS-Blacks | 0.39 (0.28)ns | 0.51 (0.21) | 0.61 (0.25) | 0.85 (0.21) | 0.33 (0.25)ns |
| PLRS-Whites | 0.35 (0.14) | 0.49 (0.14) | 0.54 (0.14) | 0.47 (0.14) | 0.49 (0.15) |
| TOPS | 0.32 (0.05) | 0.26 (0.04) | 0.33 (0.05) | 0.28 (0.04) | 0.20 (0.03) |
All heritability estimates significant (p<0.05) unless otherwise noted;
not significant (p > 0.05)
SBP: Systolic Blood Pressure; WC: Waist Circumference; LnTG: natural logarithm of Triglycerides, HDL: High Density Lipoprotein; FG: Fasting Glucose; JHS: Jackson Heart Study; PLRS: Princeton Lipid Research Study; TOPS: Take Off Pounds Sensibly
Genetic correlations between the individual components and MetS severity were overall similar between cohorts, with high correlation coefficients for each of the individual components (Table 4). SBP had the lowest correlation coefficients, particularly among black cohorts (0.39 and 0.27 for JHS and the black participants of PLRS) while WC had the highest (range 0.74 in TOPS to 0.87 in JHS). With the exception of SBP, there were not consistent differences in genetic correlation coefficients by race, although genetic correlations among blacks in PLRS were not significant.
Table 4.
Estimated Genetic and Phenotypic Correlations between Metabolic Syndrome Severity Score and Individual Components
| Cohort | SBP | WC | LnTG | HDL | FG |
|---|---|---|---|---|---|
| Genetic
| |||||
| JHS | 0.39 (0.17) | 0.87 (0.04) | 0.71 (0.06) | −0.56 (0.07) | 0.90 (0.05) |
| PLRS-Blacks | 0.27 (0.38) ns | 0.83 (0.12) ns | 0.47 (0.25) ns | −0.69 (0.24) ns | 1.0 ns |
| PLRS-Whites | 0.67 (0.16) | 0.77 (0.08) | 0.90 (0.05) | −0.71 (0.10) | 0.61 (0.13) |
| TOPS | 0.56 (0.10) | 0.74 (0.06) | 0.71 (0.06) | −0.51 (0.10) | 0.49 (0.10) |
|
| |||||
| Phenotypic*
| |||||
| JHS | 0.15 | 0.77 | 0.54 | −0.43 | 0.80 |
| PLRS-Blacks | 0.53 | 0.82 | 0.46 | −0.50 | 0.65 |
| PLRS-Whites | 0.47 | 0.74 | 0.82 | −0.67 | 0.51 |
| TOPS | 0.43 | 0.74 | 0.68 | −0.45 | 0.55 |
All correlations significant (p<0.05) unless otherwise noted;
not significant (p > 0.05)
Phenotypic correlations are estimated in SOLAR without SE, so SE is not presented; All correlation coefficients were significant (P < 0.01).
SBP: Systolic Blood Pressure; WC: Waist Circumference; LnTG: natural logarithm of Triglycerides, HDL: High Density Lipoprotein; FG: Fasting Glucose; JHS: Jackson Heart Study; PLRS: Princeton Lipid Research Study; TOPS: Take Off Pounds Sensibly
Phenotypic correlations between the individual components and MetS severity were again overall similar between cohorts, with high correlation coefficients for each of the individual components with the overall MetS severity score (Table 4). There were no consistent differences in phenotypic correlation coefficients by race.
Meta-analysis
Figure 1 panels A and B displays the heritability estimates of ATP-III MetS and MetS severity score both by cohort and combined across races. Due to the small number of studies within each group, we pooled the estimates across groups using a random effects model based on inverse variance weighting. The heterogeneity among the studies was significant for h2 of both MetS phenotypes, suggesting that the studies are significantly different. We obtained the 95% confidence intervals by ignoring heterogeneity and forest plots are presented in Figure 1 panels A for ATP-III MetS and panel B for MetS severity score. Confidence intervals were estimated assuming that the studies were similar, which is a major assumption.
Figure 1.
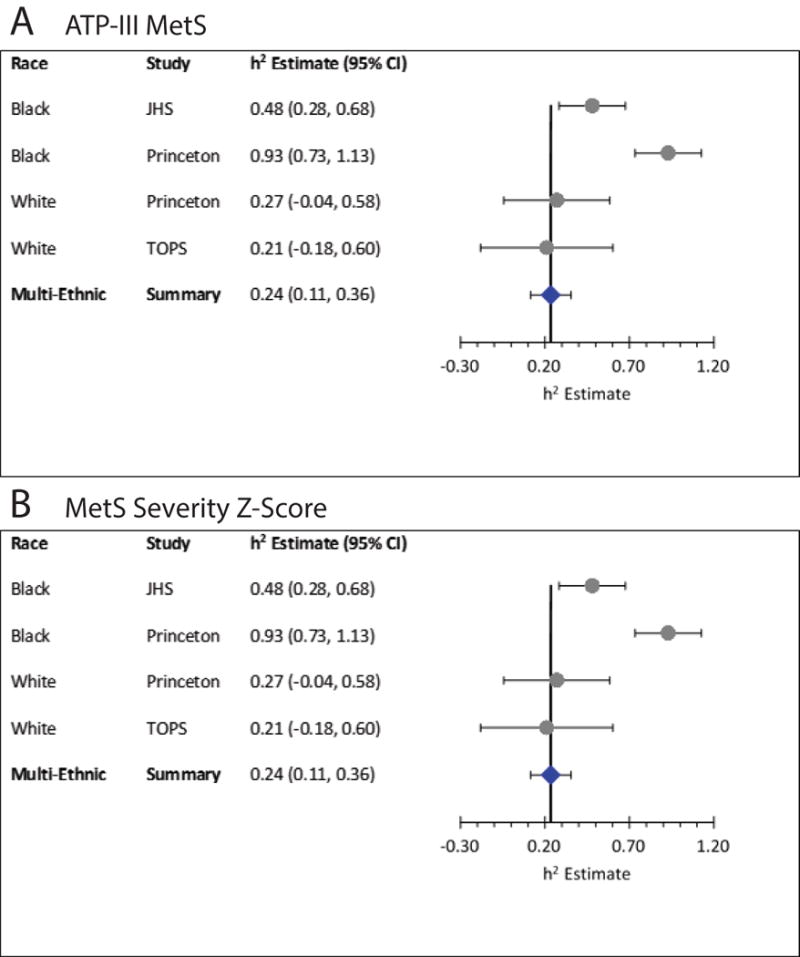
Meta-analysis of Heritability estimates for Metabolic Syndrome. A. Combined cohort estimate of heritability of ATP-III MetS. B. Combined cohort estimate of MetS severity Z-score. Abbreviations: JHS = Jackson Heart Study; TOPS = Take Off Pounds Sensibly.
Discussion
Using traditional MetS criteria, blacks often have a lower prevalence of MetS than whites despite higher rates of T2DM and CVD, raising the potential that there could be differences between races in the genetics and environmental factors associated with MetS.10–12 We evaluated data from three large familial cohorts of whites and blacks. We indeed found a separation in heritability of ATP-III MetS by race, with a higher degree of heritability among blacks (estimates of 0.48 and 0.93 for JHS and PLRS, respectively) compared to whites (0.21 and 0.27 for TOPS and PLRS, respectively). Interestingly, in evaluating heritability of a sex- and race-specific MetS severity score, we report a much smaller difference, with blacks (estimates 0.52 and 0.64) and whites (0.23 and 0.60) exhibiting significant overlap in heritability estimates. It is important to note that heritability estimates are inherently variable by population, but this study demonstrated that the use of the MetS severity scoring reduced the degree of variability among studies, and also reduced the racial disparities that were seen in heritability estimates for the ATP-III MetS definition. This score further exhibited a relatively consistent and high degree of genetic correlation with the individual components of MetS for each of the cohorts, supporting the presence of strong genetic underpinnings that are not race-specific. Taken together, this score may be able to overcome some of the racial/ethnic discrepancies that are noted in MetS, and potentially provide a more useful means of characterizing phenotypic MetS in genetic studies by minimizing these racial/ethnic differences.
The high degree of heritability of ATP-III MetS among the cohorts of black individuals may be surprising given that prior studies have suggested lower-than-expected prevalence of MetS among blacks. Prior research has suggested that by the time blacks are categorized with ATP-III MetS, there is already a more advanced condition of metabolic abnormalities, with more extreme levels of inflammation, insulin resistance and oxidative stress than seen in other racial/ethnic groups.10, 13, 38–40 Much of this potential under-diagnosis of MetS among blacks has been because of a lower prevalence of lipid abnormalities.10, 14, 15 The lower overall prevalence of ATP-III MetS among blacks may have resulted in more family-level clustering of genetic factors of disease, increasing the within-family similarity and thus the high heritability estimate, with particularly high heritability estimates for lipid abnormalities among PLRS individuals (low HDL: 0.85 and high ln(TG): 0.61). It is unclear whether this characteristic of ATP-III MetS contributed to the higher degree of heritability of ATP-III MetS among the cohorts of black individuals in this study and whether a different threshold for determining the presence of MetS would yield different estimates. Unique MetS-associated gene variants have been identified in African American populations, providing potential mechanisms for genetic contribution to heritability.41 The higher heritability estimate of ATP-III among PLRS black individuals may also have been due to chance alongside a smaller sample size.
One key reason for the low prevalence of ATP-III MetS among blacks is the lower rates of dyslipidemia compared to whites.14, 15 Blacks are less likely to have hypertriglyceridemia and, in adolescence, less likely to have low HDL.10 This raised the possibility that there may be differences in how triglyceride levels relate to MetS overall. However, we noted that while the black cohorts had lower triglycerides overall, the genetic correlation coefficients for triglycerides and MetS severity were similar between white and black cohorts, suggesting a similar link between relative elevations in triglycerides and MetS, especially when using a race-specific severity score. This is consistent with a study of white, black and Hispanic individuals that did not find racial/ethnic differences in the relationship between triglycerides and insulin resistance.14
One MetS component that did appear to differ by race in its genetic correlation with MetS was SBP, for which black cohorts had correlation coefficients approximate half that of the white cohorts. Blacks have a high prevalence of high blood pressure, much of which may not be related to the pathophysiologic processes underlying MetS. Interestingly, participants of JHS had a low heritability of SBP overall (0.09), suggesting a lower contribution of genetic factors in the development of SBP in this cohort.
Prior twin studies from European and Asian cohorts had revealed overall similar heritability of MetS between racial/ethnic groups, with heritability estimates for the individual MetS components among Chinese6 and Korean7 individuals of 0.45–0.71 compared to estimates among Dutch8 and Hungarian9 individuals of 0.36–0.76—and without a clear difference between cohorts. Similarly, Povel et al.2 performed a meta-analysis of MetS components using data from white, Asian and Hispanic populations, reporting a high degree of genetic correlation between components but without clear racial/ethnic difference. This supports the concept of overall similarity of MetS heritability between other racial/ethnic groups.
We noted some variability in heritability estimates for MetS severity between studies irrespective of race. In particular, the TOPS study had lower heritability of MetS (0.23) compared to the other cohorts (0.52 to 0.60). This may relate to the selection criteria for participants of TOPS, which specifically targeted families with both obese and non-obese members, possibly contributing to lower within-family correlations. Nevertheless, a prior analysis did not reveal significant concerns for selection bias in this cohort, and these results may also relate to the greater geographical (and possibly genetic) diversity in TOPS compared to the JHS and PLRS, which were regionally-based and may have a higher degree of shared lifestyle factors.
As heritability is the proportion of variation that can be attributed to genetics and shared behavior/environment, these differences could be driven by differences in the overall phenotypic variability.35 The Princeton black participants had some of the highest heritability estimates. This may be because the families attended the same school district and 30 years later many still resided within the same geographic area. In contrast, the Jackson Heart participants were located in the region around a single city and the TOPS participants primarily were sampled from a single state. These findings may overall illustrate that while genes clearly play a strong role in relationships between factors, environment also plays a sizable role. Whereas prior investigators had questioned the utility of performing genetic studies among different groups by race/ethnicity, this study showed that cohorts of blacks can provide data that are highly informative. In this case, the black cohorts had a strong inheritance pattern that was overall similar to the white cohorts. Thus, while initial discovery for association studies must stratify by race in order to control for population stratification, attention should be paid toward inclusion of cohorts of all backgrounds.
This study had several weaknesses. Foremost, the high degree of heterogeneity between these cohorts—in terms of racial differences and measurement methods—limits the conclusions one can make from direct comparisons of heritability between the groups. In addition, there was variation in the dichotomous and continuous MetS measures that may have further contributed to the differences in heritability estimates by racial group. The MetS severity score we used was formulated to reflect how MetS severity was manifest among each sex- and racial/ethnic sub-group by using differential weights for each of the MetS components in calculating MetS severity. This is to say that our use of a sex- and race/ethnicity specific score resulted in evaluating different phenotypes in each group, thus lacking a common measure. Nevertheless, this did not create an obvious bias, as it resulted in both increases in heritability estimates vs. ATP-III MetS (in the case of blacks in JHS and whites in TOPS and PLRS) and decreases (among blacks in PLRS). These cohorts were also assessed at different times and using different protocols, which may have also contributed to some of the variability.
In conclusion, while heritability estimates differed, the largest differences were not due to race, rather were study specific. Further, the current study found overall similar degrees of genetic correlation between MetS and its individual components in a diverse group of cohorts, suggesting that the biologic relationships among genes may not differ significantly by race. For gene discovery efforts, this suggests that the identification of how genes influence multiple traits is likely to persist across races. Understanding the unique and shared contributions of genes across traits may provide critical insight on how to manage the sequelae associated with metabolic dysregulation.
Clinical Perspective.
The metabolic syndrome (MetS) is a cluster of cardiovascular risk factors that is associated with insulin resistance and an increase risk of cardiovascular disease (CVD) and Type 2 diabetes mellitus (T2DM). Prior studies have demonstrated that MetS, like insulin resistance itself, is heritable, with heritability estimates of 10–30%, mostly from cohorts of white individuals. These studies all assessed MetS using standard dichotomous ATP-III MetS criteria, which has been shown to exhibit some racial discrepancies, with black individuals less likely to be diagnosed with MetS despite having more T2DM and death from CVD. We evaluated 3 large cohorts of white and black individuals for the heritability of MetS as assessed both by ATP-III criteria and by a continuous sex- and race/ethnicity-specific MetS severity Z-score. We found that using the ATP-III criteria, MetS had a higher heritability among black (estimates of 0.48 and 0.93 in the cohorts with black participants) compared to white individuals (estimates of 0.21 and 0.27 in the cohorts with white participants). However, using the sex- and race/ethnicity-specific severity score, there was less of a difference between blacks (estimates of 0.52 and 0.64) compared to whites (estimates of 0.23 and 0.60). This demonstrates that among blacks, similar to whites, there is a high degree of heritability of MetS. This heritability of MetS seemed to be more similar between groups when assessed using a race/ethnicity-specific Z-score. Because this score is continuous, it also provides improved power for statistical comparisons and may be a better way of following MetS within families.
Acknowledgments
Sources of Funding: This work was supported by NIH grant 1R01HL120960 (MJG and MDD). The Jackson Heart Study is supported by contracts HHSN268201300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C, HHSN268201300050C from the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities. The PLRS cohort was funded by NHLBI grants HL62394 N01HV22914 and American Heart Association grant 9750129.
Footnotes
Journal Subject Terms: Epidemiology; Risk Factors; Cardiovascular Disease
Disclosures: None.
References
- 1.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome - An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 2.Povel CM, Boer JM, Feskens EJ. Shared genetic variance between the features of the metabolic syndrome: heritability studies. Mol Genet Metab. 2011;104:666–669. doi: 10.1016/j.ymgme.2011.08.035. [DOI] [PubMed] [Google Scholar]
- 3.Bosy-Westphal A, Onur S, Geisler C, Wolf A, Korth O, Pfeuffer M, et al. Common familial influences on clustering of metabolic syndrome traits with central obesity and insulin resistance: the Kiel obesity prevention study. Int J Obes (Lond) 2007;31:784–790. doi: 10.1038/sj.ijo.0803481. [DOI] [PubMed] [Google Scholar]
- 4.Henneman P, Aulchenko YS, Frants RR, van Dijk KW, Oostra BA, van Duijn CM. Prevalence and heritability of the metabolic syndrome and its individual components in a Dutch isolate: the Erasmus Rucphen Family study. J Med Genet. 2008;45:572–577. doi: 10.1136/jmg.2008.058388. [DOI] [PubMed] [Google Scholar]
- 5.Bellia A, Giardina E, Lauro D, Tesauro M, Di Fede G, Cusumano G, et al. “The Linosa Study”: epidemiological and heritability data of the metabolic syndrome in a Caucasian genetic isolate. Nutr Metab Cardiovasc Dis. 2009;19:455–461. doi: 10.1016/j.numecd.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Duan H, Pang Z, Zhang D, Li S, Kruse TA, Kyvik KO, Christensen K, Tan Q. Genetic and environmental dissections of sub-phenotypes of metabolic syndrome in the Chinese population: a twin-based heritability study. Obes Facts. 2011;4:99–104. doi: 10.1159/000327735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song YM, Sung J, Lee K. Genetic and environmental relationships of metabolic and weight phenotypes to metabolic syndrome and diabetes: the healthy twin study. Metab Syndr Relat Disord. 2015;13:36–44. doi: 10.1089/met.2014.0087. [DOI] [PubMed] [Google Scholar]
- 8.van Dongen J, Willemsen G, Chen WM, de Geus EJ, Boomsma DI. Heritability of metabolic syndrome traits in a large population-based sample. J Lipid Res. 2013;54:2914–2923. doi: 10.1194/jlr.P041673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jermendy G, Horváth T, Littvay L, Steinbach R, Jermendy AL, Tárnoki AD, Tárnoki DL, Métneki J, Osztovits J. Effect of genetic and environmental influences on cardiometabolic risk factors: a twin study. Cardiovasc Diabetol. 2011;10:96. doi: 10.1186/1475-2840-10-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker SE, Gurka MJ, Oliver MN, Johns DW, DeBoer MD. Racial/ethnic discrepancies in the metabolic syndrome begin in childhood and persist after adjustment for environmental factors. Nutr Metab Cardiovasc Dis. 2012;22:141–148. doi: 10.1016/j.numecd.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cowie CC, Rust KF, Byrd-Holt DD, Gregg EW, Ford ES, Geiss LS, et al. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988–2006. Diabetes Care. 2010;33:562–568. doi: 10.2337/dc09-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Writing Group Members. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 13.DeBoer MD, Dong L, Gurka MJ. Racial/Ethnic and Sex Differences in the Ability of Metabolic Syndrome Criteria to Predict Elevations in Fasting Insulin Levels in Adolescents. J Pediatr. 2011;159:975–981. doi: 10.1016/j.jpeds.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sumner AE, Cowie CC. Ethnic differences in the ability of triglyceride levels to identify insulin resistance. Atherosclerosis. 2008;196:696–703. doi: 10.1016/j.atherosclerosis.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 15.Sumner AE. Ethnic Differences in Triglyceride Levels and High-Density Lipoprotein Lead to Underdiagnosis of the Metabolic Syndrome in Black Children and Adults. J Pediatr. 2009;155:e7–e11. doi: 10.1016/j.jpeds.2009.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gurka MJ, Ice CL, Sun SS, DeBoer MD. A confirmatory factor analysis of the metabolic syndrome in adolescents: an examination of sex and racial/ethnic differences. Cardiovasc Diabetol. 2012;11:128. doi: 10.1186/1475-2840-11-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurka MJ, Lilly CL, Norman OM, DeBoer MD. An Examination of Sex and Racial/Ethnic Differences in the Metabolic Syndrome among Adults: A Confirmatory Factor Analysis and a Resulting Continuous Severity Score. Metabolism. 2014;63:218–225. doi: 10.1016/j.metabol.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee AM, Gurka MJ, DeBoer MD. A metabolic syndrome severity score to estimate risk in adolescents and adults: current evidence and future potential. Expert Rev Cardiovasc Ther. 2016;14:411–3. doi: 10.1586/14779072.2016.1143360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeBoer MD, Gurka MJ, Morrison JA, Woo JG. Inter-relationships between the severity of metabolic syndrome, insulin and adiponectin and their relationship to future type 2 diabetes and cardiovascular disease. Int J Obes (Lond) 2016;40:1353–9. doi: 10.1038/ijo.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeBoer MD, Gurka MJ, Woo JG, Morrison JA. Severity of the metabolic syndrome as a predictor of type 2 diabetes between childhood and adulthood: the Princeton Lipid Research Cohort Study. Diabetologia. 2015;58:2745–52. doi: 10.1007/s00125-015-3759-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeBoer MD, Gurka MJ, Woo JG, Morrison JA. Severity of Metabolic Syndrome as a Predictor of Cardiovascular Disease Between Childhood and Adulthood: The Princeton Lipid Research Cohort Study. J Am Coll Card. 2015;66:755–757. doi: 10.1016/j.jacc.2015.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeBoer MD, Gurka MJ, Golden SH, Musani SK, Sims M, Vishnu A, et al. Independent Associations Between Metabolic Syndrome Severity and Future Coronary Heart Disease by Sex and Race. J Am Coll Card. 2017;69:1204–1205. doi: 10.1016/j.jacc.2016.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor HA, Wilson JG, Jones DW, Sarpong DF, Srinivasan A, Garrison RJ, et al. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis. 2005;15:S6-4–17. [PubMed] [Google Scholar]
- 24.Taylor H, Liu J, Wilson G, Golden SH, Crook E, Brunson CD, et al. Distinct component profiles and high risk among African Americans with metabolic syndrome: the Jackson Heart Study. Diabetes Care. 2008;31:1248–1253. doi: 10.2337/dc07-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gurka MJ, Vishnu A, Okereke OI, Musani S, Sims M, DeBoer MD. Depressive symptoms are associated with worsened severity of the metabolic syndrome in African American women independent of lifestyle factors: A consideration of mechanistic links from the Jackson heart study. Psychoneuroendocrinology. 2016;68:82–90. doi: 10.1016/j.psyneuen.2016.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kissebah AH, Sonnenberg GE, Myklebust J, Goldstein M, Broman K, James RG, et al. Quantitative trait loci on chromosomes 3 and 17 influence phenotypes of the metabolic syndrome. Proc Natl Acad Sci U S A. 2000;97:14478–83. doi: 10.1073/pnas.97.26.14478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrison J, Degroot I, Kelly K, Mellies M, Glueck CJ. PARENT-CHILD ASSOCIATIONS - CHOLESTEROL AND TRIGLYCERIDE. Circulation. 1977;56:20–20. [Google Scholar]
- 28.Woo JG, Morrison JA, Stroop DM, Aronson Friedman L, Martin LJ. Genetic architecture of lipid traits changes over time and differs by race: Princeton Lipid Follow-up Study. J Lipid Res. 2014;55:1515–1524. doi: 10.1194/jlr.M049932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrison JA, Friedman LA, Wang P, Glueck CJ. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. J Pediatr. 2008;152:201–6. doi: 10.1016/j.jpeds.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 30.Morrison JA, Friedman LA, Gray-McGuire C. Metabolic syndrome in childhood predicts adult cardiovascular disease 25 years later: The Princeton Lipid Research Clinics follow-up study. Pediatrics. 2007;120:340–345. doi: 10.1542/peds.2006-1699. [DOI] [PubMed] [Google Scholar]
- 31.Gurka MJ, Lilly CL, Oliver MN, DeBoer MD. An examination of sex and racial/ethnic differences in the metabolic syndrome among adults: A confirmatory factor analysis and a resulting continuous severity score. Metabolism. 2014;63:218–225. doi: 10.1016/j.metabol.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeBoer MD, Gurka MJ. Clinical Utility of Metabolic Syndrome Severity Scores: Considerations for Practitioners. Diabetes Metab Syndr Obes. 2017;10:65–72. doi: 10.2147/DMSO.S101624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeBoer MD, Gurka MJ, Morrison JA, Woo JG. Inter-relationships between the severity of metabolic syndrome, insulin and adiponectin and their relationship to future type 2 diabetes and cardiovascular disease. Int J Obes (Lond) 2016;40:1353–1359. doi: 10.1038/ijo.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams JT, Duggirala R, Blangero J. Statistical properties of a variance components method for quantitative trait linkage analysis in nuclear families and extended pedigrees. Genet Epidemiol. 1997;14:1065–1070. doi: 10.1002/(SICI)1098-2272(1997)14:6<1065::AID-GEPI84>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 35.Comuzzie AG, Blangero J, Mahaney MC, Mitchell BD, Stern MP, MacCluer JW. Genetic and environmental correlations among skinfold measures. Int J Obes Relat Metab Disord. 1994;18:413–418. [PubMed] [Google Scholar]
- 36.Docherty AR, Kremen WS, Panizzon MS, Prom-Wormley EC, Franz CE, Lyons MJ, et al. Comparison of Twin and Extended Pedigree Designs for Obtaining Heritability Estimates. Behav Genet. 2015;45:461–466. doi: 10.1007/s10519-015-9720-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogden CL, Carroll MD, Flegal KM. Prevalence of obesity in the United States. JAMA. 2014;312:189–190. doi: 10.1001/jama.2014.6228. [DOI] [PubMed] [Google Scholar]
- 38.Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2003;163:427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeBoer MD, Gurka MJ, Sumner AE. Diagnosis of the Metabolic Syndrome Is Associated With Disproportionately High Levels of High-Sensitivity C-Reactive Protein in Non-Hispanic Black Adolescents: An analysis of NHANES 1999–2008. Diabetes Care. 2011;34:734–740. doi: 10.2337/dc10-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeBoer MD, Dong L, Gurka MJ. Racial/ethnic and sex differences in the relationship between uric acid and metabolic syndrome in adolescents: an analysis of National Health and Nutrition Survey 1999–2006. Metabolism. 2012;61:554–561. doi: 10.1016/j.metabol.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carty CL, Bhattacharjee S, Haessler J, Cheng I, Hindorff LA, Aroda V, et al. Analysis of metabolic syndrome components in >15 000 african americans identifies pleiotropic variants: results from the population architecture using genomics and epidemiology study. Circ Cardiovasc Genet. 2014;7:505–513. doi: 10.1161/CIRCGENETICS.113.000386. [DOI] [PMC free article] [PubMed] [Google Scholar]


