Abstract
Background
In view of the large consumption of herbal medicine in Africa countries, it is likely that many adverse drugs reactions go unrecorded with either patients failing to present to health services, or no pharmacovigilance analysis being made, or the analysis not being reported centrally. This problem is of interest especially for those who are working in the general area of adverse drug reactions or stakeholders in the domain of herbal medicine for considering safety issues.
Methods
We are particularly interested in the way that the use of very well-known and highly valued plants is linked to the observation of adverse drug reactions in African countries. We investigated, through a literature review and using the Internet (with a semantic search strategy), some well-known or popular medicinal plants used in African herbal medicine (AHM). Other information on the properties related to use, and characteristics of medicinal plants was complemented by some interviews with stakeholders.
Results
Although substantial progress has been made in elucidating the mechanisms of action of many drugs, the pharmacological actions of many medicinal plants are generally not well understood. The results of a literature review suggest that the reported adverse drug reactions of herbal remedies are often due to a lack of understanding of their preparation and appropriate use. The results of stakeholders’ interviews suggest that there is a growing need to provide patients with correct information about the herbal medicines they consume.
Conclusion
An important aspect of herbal medicine is the correct, timely, and integrated communication of emerging data on risk as an essential part of pharmacovigilance, which could actually improve the health and safety of patients. This calls for improved collaboration between traditional practitioners and modern healthcare professionals, researchers, and drug regulatory authorities. In addition, there is a need for an adverse drug reaction reporting system to facilitate the collection, monitoring, and evaluation of adverse drug events.
Keywords: alternative medicines, complementary therapies, pharmacovigilance practice, toxicological risk assessment, traditional medicine
1. Introduction
Medicinal plants are important materials for the pharmaceutical and cosmetic industries. Neglected since the discovery of synthetic drugs, which can be produced in a wider scale, there has been a resurgence of interest in plant medicines in recent years. It is estimated that approximately half of all synthetic drugs have a natural origin.1 Any substance with a healing influence can also generate unwanted or adverse side reactions. As with synthetic drugs, the quality, efficacy, and safety of medicinal plants must also be assured. There is a non-negligible contribution of Africa to the world's medicinal plants’ stock. In effect, Africa provides approximately 60,000 of the world's higher plant species and yet contributes < 8% of the 1100 drugs placed on the market worldwide.2 Many plants used in herbal medicine provide valuable promising medical solutions to some diseases [pathologies and infections], since many medicinal plants are a source of new medicines and therefore they have an essential role in medical research.3 African traditional medicine is “the most economical and available system of health care for a large number of the African population in rural and semi-urban areas.”4 African herbal medicine (AHM) is a fundamental element of African traditional medicine practices.
However, there are some challenges and opportunities in the improvement of herbal medicine. The various adverse drug reactions of herbal medicine carry with them specific risk factors which can lead to increased vulnerability to human health difficulties. In recognition of the increasing possibilities of purchasing medicinal products on the street or online worldwide, improvement measures are needed to better inform people about the efficiency and vulnerabilities of purchasing medicines. An adverse drug reaction is defined by Edwards and Aronson as “an harmful or troublesome reaction, due to intervention related to the use of a healing substance, which envisages risk from future administration and requires prevention or explicit treatment, or alteration of posology and method of administration, or withdrawal of the medical substance.”5 The latency period between the use of a drug and the occurrence of an adverse reaction can help in its causality assessment in pharmacovigilance management.6 Such information can be invaluable in the interpretation of drug safety signals, and facilitate decisions on further protective actions being taken then and the type of further investigations about alteration in the posology, or removal of the substance.
2. Methods
We researched some well-known or popular AHM products by using a literature review and through the Internet (using a semantic search strategy).
A thorough literature search was made by referring to books, peer-reviewed papers, scientific databases such as Scopus, Science Direct, PubMed, and Google Scholar. Keywords such as adverse drug reactions, African traditional medicine, herbal or natural products, alternative therapy, surveillance, phytotherapy in relation to medicinal plants were the terms employed in the different semantic investigations. Other information on the properties of medicinal plants was complemented by interviews with stakeholders. Medicinal plants were identified based on their traditional use in different aspects of African traditional medicine.
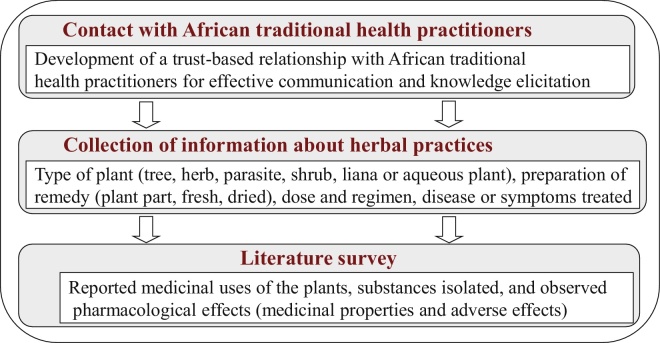
In our approach, the information-gathering process was based both on the details of stakeholders’ interviews and the outcomes of the literature review. More specifically, in order to obtain information about herbal practices and their associated effects, we went through the following process with three phases adapted from Kamsu-Foguem et al7 (Fig. 1):
-
•
Contact with African traditional health practitioners: development of a trust-based relationship with African traditional health practitioners for effective communication and knowledge elicitation. Trust is especially necessary for open and operative communication, since health practitioners keep their knowledge secret and do not wish to share their know-how outside the family environment.
-
•
Collection of information about herbal practices: the information is collected through questionnaires or structured interviews with stakeholders. The form includes the following information for considered herbal medicines: type of plant (tree, herb, parasite, shrub, liana or aqueous plant), preparation of remedy (plant part, fresh, dried), dose and regimen, disease or symptoms treated.
-
•
Literature survey with monographs, chemical abstracts and MEDLINE: we investigated the previously described medicinal uses of the plants, substances isolated and identified pharmacological effects (medicinal properties, alternative options for the use of the plant, and adverse effects).
Fig. 1.
The adopted information-gathering process for herbal practices.
3. Results
Our review as such was limited to highlighting the potential for adverse drug reactions in AHM. Thus, we have considered that it is essential to clearly delimit the scope of the study, which has been restricted to certain well-known and highly appreciated plants in AHM. The rich flora of Africa (e.g., the Paleotropical natural environment of Central Africa and the Capensis natural environment of South Africa) contains numerous toxic plants with ethnopharmacological uses (e.g., parasitic, bacterial, fungal or viral treatments, treatment of central nervous system-related conditions, aphrodisiacs, and for maternal health care, miscellaneous conditions such as cancer, arthritis, and heart conditions). Toxic constituents (e.g., neurotoxins, cytotoxins, and metabolic toxins) from these plants can harm the major systems of the human body (cardiovascular system, digestive system, endocrine system, urinary system, immune system, muscular system, nervous system, reproductive system, and respiratory system).8
Several causality assessment methods have been proposed to assess the relationship between drugs and the appearance of adverse events. Those methods belong to three categories: expert judgment, probabilistic methods, and algorithms or scales.9 In view of their attractive characteristics (successive evaluation of criteria, sum of scores or decision trees), scales and algorithms are usually used for operational assessments of adverse drug reactions and they enable an easy and convenient handling in various situations. For instance, the Council for International Organizations of Medical Sciences (CIOMS) scale, also referred to as the Roussel Uclaf Causality Assessment Method (RUCAM),10 is used by many expert hepatologists, researchers, and regulatory authorities to evaluate the possibility of supposed underlying causes of adverse drug reactions. Particularly, the CIOMS provides advantages [standardized scale (chronology, risk factors, concomitant drug(s) or herbs(s), search for nondrug/herb causes, previous information on hepatotoxicity of the drug/herb, response to unintentional readministration), validation for hepatotoxicity with excellent sensitivity, specificity, and predictive validity] for drug-induced liver injury and herb induced liver injury (HILI) causality assessment.11 Meanwhile, the CIOMS scale has certain weaknesses (e.g., retrospective use, dechallenge criteria, qualitatively graded risk factors, and limited exclusion of alternative causes). Some challenges (e.g., the quality of adverse reaction reports) influence the accuracy in causality assessment of herbal medicinal products. The exploration of omics technologies (genomics/transcriptomics, proteomics, and metabolomics) applied to toxicology (toxicogenomics, toxicoproteomics, and toxicometabolomics) is a valuable way of providing additional information related to predictive safety assessment of herbal medicinal products.12 The predictive toxicity of herbal constituents generates emerging opportunities that are essential to improve the knowledge base for integrated safety assessment.
The results are presented in two segments, one on the literature review and the other on the stakeholders’ interview. We obtained 100 reports from the literature review and > 50 stakeholders’ interviews. This research took place over a 2-year period from December 2012 to January 2014.
-
•
The literature review conducted in the area of AHM only reported adverse drug reactions often a negligible impact on the global health issues in a country or region. However, people are very often subject to safety risks related to herbal medicine which affect or may affect their vital organs (e.g., kidneys, lungs, liver, gastrointestinal tract) and could cause serious damage to other vulnerable areas of the human body. The survey reveals that adverse drug reactions may be caused by logistical and technical problems or questionable decisions by the traditional health practitioners. The logistical and technical problems include poor harvest, the inadequate identification of complex properties, inappropriate preparation, improper conservation or incorrect packing of natural products. The considered problems are not peculiar to the selected plants alone, but exist also in other herbal medicine products that are not included in this study, because of the nonexhaustive nature of the list of products encountered in AHM. Furthermore, a certain control range can be made to monitor the widespread practice of combining many different compound classes and herbal types or creating heterogeneous product class models for use in therapeutic procedures.
-
•
The stakeholders’ interview results in significant information that has highlighted (in addition to managerial and some regulatory problems) insufficient communication in relation to increased consumption of products from AHM. Traditional health practitioners are capable of identifying simple adverse drug reactions and taking preventive actions by learning ideas with experienced feedback processes. Meanwhile, for complex adverse drug reactions, the lack of apparent symptoms, coupled with the potential to cause severe damage to key organs, means that educational assistance for careful and safe use of herbal medicinal products must be encouraged, with special care for high-risk patients. The problem is typically manifested by the fact that information is often either inaccessible, incomplete, or of low quality about potential adverse drug reactions of used herbal products. The managerial and regulatory barriers are present in many African countries by the lack of clear enforced rules for bearing and sharing various experienced feedback processes, as well as by the absence of systems that can help to promote integrative policies and implementation.
Examples of potential adverse effects of some herbal medicines with their traditional use and their countries of origin are presented in Table 1.
Table 1.
Examples of medicinal plants with their potential adverse drug reactions.
| Medicinal plants | Traditional uses | Potential adverse effects | Origin |
|---|---|---|---|
| Pygeum africanum (Prunus africana) |
Prostate cancer, prostatitis, benign prostatic hyperplasia, other urinary tract infections, and aphrodisiac | Gastrointestinal upsets13 | Cameroon, South Africa, and Madagascar |
| Wild wisteria, violet tree (Securidaca longepedunculata) | Laxative, nervous system ailments (epilepsy), wounds, sores, coughs, venereal diseases, snakebites, bilharzias, headaches, fever related to malaria, erectile dysfunction or aphrodisiac, dysmenorrhea, and abortion induction | Acute kidney (cortical necrosis, acute interstitial nephritis), injury, diarrhea, dehydration, and collapse14 | Tanzania, Malawi, Soudan, Burkina Faso, Congo, Zambia, and Zimbabwe |
| Madagascar rosy periwinkle (Catharanthus roseus) | Cancer chemotherapy | Medullary aplasia, leucopoenia, incoordination of movements, convulsions, fatigue, mucositis, constipation, and neutropenia of short duration15 |
Madagascar |
| Round leaf Buchu (Agathosma betulina) | Diuretic and urinary tract antiseptic, arthritis, treatment of cellulite, cystitis, diarrhea, flatulence, kidney infections, nausea, rheumatism, and wounds | Gastrointestinal irritation, and centrilobular hepatic or hepatocellular necrosis16 | South Africa |
| Umckaloabo or South African Geranium (Pelargonium sidoides) | Coughs, upper respiratory tract irritations and infections (bronchitis, sinusitis, and pneumonia, tonsillitis, and rhinopharyngitis), gastrointestinal disorders, and pain |
Gastrointestinal complaints (nausea, heartburn, diarrhea), skin rashes, and allergic (hypersensitivity) reactions17 | South Africa |
|
Hoodia cactus (Hoodia gordonii) |
Appetite suppressant and anorectic action | Potentiation of diabetes mellitus (hyperglycemia), sometimes hypoglycemia and confusions18 | South Africa and Namibia |
| Red spinach (also known as Chinese spinach, Hon-toi-moi, Yin choy, Hsien tsai) or Spleen amaranth (Amaranthus dubius) | Diuresis [high blood pressure, kidney infections, obesity, and the edema associated with premenstrual syndrome (PMS) or traumatic injuries] | Hypotension, skin irritations, to extensive organ and tissue damage with death induction19 | Africa (e.g., Ethiopia, South Africa, Kenya, and Uganda), Asia, India, Europe, West Indies, North America, and South America |
| Bird flower (Crotalaria laburnifolia) | Dysmenorrhea and abortion induction20 | Acute kidney injury, hepatic veno-occlusive disease, pulmonary injury, and thrombocytopenia | Africa (e.g., Zimbabwe) and Asia (e.g., Sri Lanka) |
| Impila, ox-eye daisy (Callilepis laureola) | Impotence, evil spirits, and gastrointestinal complaints21 | Chronic renal disease, acute kidney injury, hyperkalemia, abdominal pain, vomiting, diarrhea, a disturbed level of consciousness, convulsions, and liver failure | North and South Africa, and Mediterranean Basin |
| Khat leaf (Catha edulis) | Central stimulant action (e.g., management of obesity and depression)22 | Chronic kidney disease, acute kidney injury (acute tubular necrosis), hepatotoxicity, cardiovascular diseases (hypertension, cerebrovascular ischemia, dilated cardiomyopathy, myocardial infarction, and thromboembolism), diabetes, sexual dysfunction, duodenal ulcer, and hepatitis | East Africa, Yemen |
| Licorice (Glycyrrhiza glabra) | Sore throat, cough, arthritis, and weight loss induction23 | Acute kidney injury (hypokalemic nephropathy), amenorrhea, pseudoaldosteronism, hypertension, heart failure, and rhabdomyolysis | Worldwide |
4. Discussion
Quality of herbal medicines is a matter that affects the whole world today, with a requirement for the safety monitoring of herbal remedies, and there is a growing necessity to provide consumers with the correct information about the medicinal products they consume. In this perspective, quality control of herbal medicine and good practices are indispensable for the advancement of the herbal medicine system.24 Quality problems of herbal medicines can be categorized into two classes: internal factors arising from the drug and external factors in clinical use.25 The internal safety factors related to drug quality include: raw herbals (species and origin, place of origin and collection, natural environment, processing, storage), inactive ingredients, manufacturing, defects of the prescribing information/package leaflet, limitations of premarketing safety studies, and adulterated and counterfeit drugs. The external safety factors in clinical use comprise: lack of traditional medicine theoretical guidance, failure to adhere to the prescribing information, inappropriate combination with allopathic medicines, inappropriate route and dosing and timing, incorrect preparation, and individual factors.
African regions are particularly concerned by these issues requiring an adverse drug reaction reporting system to reduce the risks of AHM. However, few African countries (e.g., South Africa, Nigeria, and Cameroon) have subsequently introduced herbal/traditional medicine as part of their pharmacovigilance system. South Africa has a well-built plant biotechnology community, as well as the thorough implementation of a legal and operational framework for the guideline of the manipulation of local botanic resources, and of genetically-modified organisms to improve human health.26 Nigeria is in a position to introduce advanced investigations (assessment of traditional medicine practitioners’ disposition and doctors’ attitudes) which effectively explore the opportunities for the integration of herbal medicine into the national health system with applicable regulations.27, 28 In Cameroon, the government, in collaboration with the World Health Organization (WHO), has set up a strategic platform for the harmonization of the AHM practices with an organizational framework that has integrated a national commission specializing in pharmacovigilance and traditional pharmacopoeia.3
There are also other regions of the world (e.g., China, South Pacific, and India) with some problems related to herbal adverse drug reactions. The characteristics of traditional Chinese medicine drugs and their associated adverse drug reactions require an accurate understanding of local cultural and social realities in order to establish a stringent quality control and a risk management model adapted to the pharmacovigilance system (current practice, risk factors, risk control, and future) in China.29 In this context, a recent toxicity evaluation (risk–benefit analysis, severity of toxic effects, and clinical and preclinical data) of Chinese herbal medicines has led to the proposition of four regulatory classes: prohibited for medicinal usage (e.g., aristolochia); restricted for medicinal usage, (e.g., ephedra); required warning label (e.g., coltsfoot); and over-the-counter herbs (i.e., safe toxicity profiles).30 In the South Pacific, we found the kava plant (Piper methysticum) consumed worldwide for its relaxing properties and used to treat general anxiety. Traditional aqueous extracts of this pacific herb used in New Caledonia, Australia, the USA, and Germany are associated with rare hepatotoxicity. The primary cause of the kava problem with this toxicity can be traced to poor quality of the raw material triggered by mold hepatotoxins.31, 32, 33
In the worldwide cases, the organs most notably affected by herbal medicine consumption are the liver and kidneys, as they functionally detoxify and excrete several toxic substances including metabolic wastes. Many renal syndromes have been reported following the use of medicinal plants, including tubular necrosis, acute interstitial nephritis, Fanconi's syndrome, hypokalemia or hyperkalemia, hypertension, papillary necrosis, chronic interstitial nephritis, nephrolithiasis, urinary retention, and cancer of the urinary tract.34 A recent United States research study, completed by the Einstein Medical Center, Philadelphia, reported that the use of herbal and dietary supplements (HDS) has a devastating impact on consumers’ quality of life, as they begin to damage the liver irreversibly and this requires a relevant clinical perspective to the diagnosis of HDS-induced liver injury (HILI).35
In fact, herbal and dietary supplements are generally used throughout the world. There is a large and varied quantity of herbal medicinal products (e.g., Ayurvedic and Chinese herbs, black cohosh, chaparral, germander, greater celandine, green tea, Herbalife, Hydroxycut, kava, pennyroyal, pyrrolizidine alkaloids, skullcap, and usnic acid), which are associated with a range of hepatotoxicity events.36, 37, 38, 39, 40, 41, 42 Progress in the understanding of the epidemiology of induced cell and organ injuries, the pathogenesis, clinical observations, and consequences is required to be able to advance herbal medicine safety.
5. Conclusion
In both developed and developing countries, self-medication and consulting community networks for many herbal medicines tend to decrease monitoring of treatment adherence, as do adverse effects, which patients may not always mention. More specifically, the African reality clearly shows that natural products are perceived as safe and secure with herbal medicines at sustainable low prices. In general, AHM products have some potential side effects (dose-related, time-related, or failure of therapy) that can damage a basic structural, functional, and biological unit of the organism, such as a cell (cytotoxicity) or an organ such as the kidney (nephrotoxicity) or the heart (cardiotoxicity) or the liver (hepatotoxicity). Particularly, the studied medicinal plants can cause problems in some systems of the human body (e.g., cardiovascular system, digestive system, and nervous system). Thus, with our research sample (Pygeum africanum, wild wisteria, Madagascar rosy periwinkle, round leaf Buchu, South African geranium, Hoodia cactus, red spinach, bird flower, Impila, ox-eye daisy, khat leaf, and licorice), we have noticed some adverse drug reactions with several clinical manifestations of toxic effects of plants that cause gastrointestinal, respiratory, cardiovascular, and neurological disorders.
Despite their lack of knowledge of physiopathology and physiomolecular processes, traditional medicine practitioners often possess information and experienced knowledge that is not yet widely or universally known and insufficiently used. However, these practitioners should be equipped with adequate knowledge of the pharmacological properties of medicinal plants to be able to serve as a guide for them to easily identify the information needed for the interpretation of adverse medical effects and counselling patients. Although many important plants of Africa with ethnopharmacological uses could have potential biological activities, there are a number of the active principles (e.g., alkaloids, cardiac glycosides, phorbol esters, lectins, and cyanogenic glycosides) in these medicinal plants that are extremely toxic. Finally, medicinal substances in the African traditional pharmacopoeia must comply with the international Pharmacopoeia and WHO recommendations, to offer good quality products and safe services. These improvements would have a considerable compact on the physical, mental, and social benefits of traditional medicine in development of both preventative and therapeutic health care services to patients in the field of integrative medicine.
Conflicts of interest
All authors declare no conflicts of interest.
Contributor Information
Bernard Kamsu-Foguem, Email: Bernard.Kamsu-Foguem@enit.fr.
Clovis Foguem, Email: cfoguem@yahoo.fr.
References
- 1.Gillespie A. Conservation, Biodiversity and International Law. New Horizons in Environmental and Energy Law series. Cheltenham, UK: Edward Elgar Publishing; 2013.
- 2.Dzoyem JP, Tshikalange E, Kuete V. 24 - Medicinal Plants Market and Industry in Africa. Medicinal Plant Research in Africa (Pharmacology and Chemistry) 2013:859–890. [Google Scholar]
- 3.Fokunang CN, Ndikum V, Tabi OY, Jiofack RB, Ngameni B, Guedje NM. Traditional Medicine: past, present and future research and development prospect and integration in the national health system of Cameroon. Afr J Trad Complement Altern Med. 2011;8:284–295. doi: 10.4314/ajtcam.v8i3.65276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kasilo OMJ, Trapsida J-M, Ngenda Mwikisa C, Lusamba-Dikassa PS. An overview of the traditional medicine situation in the African Region. The African Health Monitor, magazine of the World Health Organization Regional Office for Africa (WHOAFRO) 2010; Special Issue 14 African Traditional Medicine Day.
- 5.Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. The Lancet. 2000;356:1255–1259. doi: 10.1016/S0140-6736(00)02799-9. [DOI] [PubMed] [Google Scholar]
- 6.Shetti S, Kumar CD, Sriwastava NK, Sharma IP. Pharmacovigilance of herbal medicines: Current state and future directions. Pharmacognosy Magazine. 2011;7(25):69–73. doi: 10.4103/0973-1296.75905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamsu-Foguem B, Diallo G, Foguem C. Conceptual graph-based knowledge representation for supporting reasoning in African traditional medicine. Eng Appl Artif Intell. 2013;26:1348–1365. [Google Scholar]
- 8.Ndhlala AR, Ncube B, Okem A, Mulaudzi RB, van Staden J. Toxicology of some important medicinal plants in southern Africa. Food Chem Toxicol. 2013;62:609–621. doi: 10.1016/j.fct.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 9.García-Cortés M, Stephens C, Isabel Lucena M, Fernández-Castañer A, Andrade RJ. Causality assessment methods in drug induced liver injury: Strengths and weaknesses. J Hepatol. 2011;55:683–691. doi: 10.1016/j.jhep.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Danan G, Benichou C. Causality assessment of adverse reactions to drugs-I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323–1330. doi: 10.1016/0895-4356(93)90101-6. [DOI] [PubMed] [Google Scholar]
- 11.Teschke R, Wolff A, Frenzel C, Schwarzenboeck A, Schulze J, Eickhoff A. Drug and herb induced liver injury: Council for International Organizations of Medical Sciences scale for causality assessment. W J Hepatol. 2014;6:17–32. doi: 10.4254/wjh.v6.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jordan SA, Cunningham DG, Marles RJ. Assessment of herbal medicinal products: Challenges, and opportunities to increase the knowledge base for safety assessment. Toxicol Appl Pharmacol. 2010;243:198–216. doi: 10.1016/j.taap.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Kim T-H, Lim H-J, Kim M-S, Lee MS. Dietary supplements for benign prostatic hyperplasia: An overview of systematic reviews. Maturitas. 2012;73:180–185. doi: 10.1016/j.maturitas.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Luyckx VA. Nephrotoxicity of alternative medicine practice. Adv Chronic Kidney Dis. 2012;19:129–141. doi: 10.1053/j.ackd.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Morel A, Talbot D. Critical evaluation of vinflunine in the treatment of refractory metastatic urothelial carcinoma. Open Access J Urol. 2010;2:99–108. [PMC free article] [PubMed] [Google Scholar]
- 16.Moolla A, Viljoen AM. ‘Buchu’ – Agathosma betulina and Agathosma crenulata (Rutaceae): A review. J Ethnopharmacol. 2008;119:413–419. doi: 10.1016/j.jep.2008.07.036. [DOI] [PubMed] [Google Scholar]
- 17.Brendler T, van Wyk B-E. A historical, scientific and commercial perspective on the medicinal use of Pelargonium sidoides (Geraniaceae) J Ethnopharmacol. 2008;119:420–433. doi: 10.1016/j.jep.2008.07.037. [DOI] [PubMed] [Google Scholar]
- 18.Jain S, Singh SN. Metabolic effect of short term administration of Hoodia gordonii, an herbal appetite suppressant. S Afr J Bot. 2013;86:51–55. [Google Scholar]
- 19.Halberstein RA. Chapter 1 - Botanical medicines for diuresis: Cross-cultural comparisons. Stud Nat Prod Chem. 2012;37:1–41. [Google Scholar]
- 20.Colson CRD, De Broe ME. Kidney injury from alternative medicines. Adv Chron Kid Dis. 2005;12:261–275. doi: 10.1016/j.ackd.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Popat A, Shear NH, Malkiewicz I, Stewart MJ, Steenkamp V, Thomson S. The toxicity of Callilepis laureola, a South African traditional herbal medicine. Clin Biochem. 2001;34:229–236. doi: 10.1016/s0009-9120(01)00219-3. [DOI] [PubMed] [Google Scholar]
- 22.Al-Motarreb A, Al-Habori M, Broadley KJ. Khat chewing, cardiovascular diseases and other internal medical problems: The current situation and directions for future research. J Ethnopharmacol. 2010;132:540–548. doi: 10.1016/j.jep.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Strader DB, Navarro VJ, Seeff LB. Zakim and Boyer's Hepatology. 6th ed. Saunders; Philadelphia, PA: 2012. Hepatotoxicity of herbal preparations; pp. 462–475. [Google Scholar]
- 24.Sen S, Chakraborty R, Biplab D. Challenges and opportunities in the advancement of herbal medicine: India's position and role in a global context. J Herb Med. 2011;1:67–75. [Google Scholar]
- 25.Zhang J, Wider B, Shang H, Li X, Ernst E. Quality of herbal medicines: Challenges and solutions. Complement Ther Med. 2012;20:100–106. doi: 10.1016/j.ctim.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Rybicki EP, Chikwamba R, Koch M, Rhodes JI, Groenewald J-H. Plant-made therapeutics: An emerging platform in South Africa. Biotechnol Adv. 2012;30:449–459. doi: 10.1016/j.biotechadv.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 27.Awodele O, Agbaje EO, Ogunkeye FA, Kolapo AG, Awodele DF. Towards integrating traditional medicine (TM) into National Health Care Scheme (NHCS): Assessment of TM practitioners’ disposition in Lagos, Nigeria. J Herb Med. 2011;1:90–94. [Google Scholar]
- 28.Awodele O, Agbaje EO, Abiola OO, Awodele DF, Dolapo DC. Doctors’ attitudes towards the use of herbal medicine in Lagos, Nigeria. J Herb Med. 2012;2:16–22. [Google Scholar]
- 29.Zhang L, Yan J, Liu X, Ye Z, Yang X, Meyboom R. Pharmacovigilance practice and risk control of Traditional Chinese Medicine drugs in China: Current status and future perspective. J Ethnopharmacol. 2012;140:519–525. doi: 10.1016/j.jep.2012.01.058. [DOI] [PubMed] [Google Scholar]
- 30.Kim EJY, Chen Y, Huang JQ, Li KM, Razmovski-Naumovski V, Poon J. Evidence-based toxicity evaluation and scheduling of Chinese herbal medicines. J Ethnopharmacol. 2013;146:40–61. doi: 10.1016/j.jep.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 31.Teschke R, Schwarzenboeck A, Hennermann KH. Kava hepatotoxicity: a clinical survey and critical analysis of 26 suspected cases. Eur J Gastroenterol Hepatol. 2008;20:1182–1193. doi: 10.1097/MEG.0b013e3283036768. [DOI] [PubMed] [Google Scholar]
- 32.Teschke R, Qiu SX, Xuan TD, Lebot V. Kava and kava hepatotoxicity: requirements for novel experimental, ethnobotanical, and clinical studies based on a review of the evidence. Phytother Res. 2011;25:1262–1274. doi: 10.1002/ptr.3464. [DOI] [PubMed] [Google Scholar]
- 33.Teschke R, Sarris J, Schweitzer I. Kava hepatotoxicity in traditional and modern use: the presumed Pacific kava paradox hypothesis revisited. Br J Clin Pharmacol. 2012;73:170–174. doi: 10.1111/j.1365-2125.2011.04070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Isnard Bagnis C, Deray G, Baumelou A, Le Quintrec M, Vanherweghem JL. Herbs and the kidney. Am J Kid Dis. 2004;44:1–11. doi: 10.1053/j.ajkd.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 35.Rossi S, Navarro VJ. Herbs and liver injury: A clinical perspective. Clin Gastroenterol Hepatol 2014; in press. doi:10.1016/j.cgh.2013.07.030. [DOI] [PubMed]
- 36.Bunchorntavakul C, Reddy KR. Review article: herbal and dietary supplement hepatotoxicity. Aliment Pharmacol Ther. 2013;37:3–17. doi: 10.1111/apt.12109. [DOI] [PubMed] [Google Scholar]
- 37.Teschke R, Wolff A, Frenzel C, Schulze J, Eickhoff A. Herbal hepatotoxicity: A tabular compilation of reported cases. Liver Int. 2012;32:1543–1556. doi: 10.1111/j.1478-3231.2012.02864.x. [DOI] [PubMed] [Google Scholar]
- 38.Teschke R, Frenzel C, Schulze J, Schwarzenboeck A, Eickhoff A. Herbalife hepatotoxicity: Evaluation of cases with positive reexposure tests. World J Hepatol. 2013;5:353–363. doi: 10.4254/wjh.v5.i7.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teschke R, Schwarzenboeck A, Eickhoff A, Frenzel C, Wolff A, Schulze J. Clinical and causality assessment in herbal hepatotoxicity. Expert Opin Drug Saf. 2013;12:339–366. doi: 10.1517/14740338.2013.774371. [DOI] [PubMed] [Google Scholar]
- 40.Teschke R, Frenzel C, Glass X, Schulze J, Eickhoff A. Herbal hepatotoxicity: A critical review. Br J Clin Pharmacol. 2013;75:630–636. doi: 10.1111/j.1365-2125.2012.04395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teschke R, Bahre R. Severe hepatotoxicity by Indian Ayurvedic herbal products: A structured causality assessment. Ann Hepatol. 2009;8:258–266. [PubMed] [Google Scholar]
- 42.Woodward KN. The potential impact of the use of homeopathic and herbal remedies on monitoring the safety of prescription products. Human Exp Toxicol. 2005;24:219–233. doi: 10.1191/0960327105ht529oa. [DOI] [PubMed] [Google Scholar]