Abstract
Background
Since ancient times, various infectious diseases have been treated using herbal drugs. Today, efforts regarding the discovery of the effectual components of plants possessing antimicrobial properties are advanced. Herbal essential oils are widely used for treatment of various diseases, and they play an important role in health care considerations.
Methods
The antibacterial activity of Artemisia kermanensis, Lavandula officinalis, and Zataria multiflora Boiss essential oils against Staphylococcus aureus (ATCC 25923), Pseudomonas aeruginosa (PTCC 1310), and Klebsiella pneumonia (PTCC 1053) was evaluated using the disk diffusion method as well as determination of the minimal inhibitory concentration and minimal bactericidal concentration. The composition of the three essential oils was determined with gas chromatography-mass spectrometry. Variable amounts of different components (such as oxygenated monoterpenes, thymol, carvacrol, and 1,8-cineol) were found in all three oils. Among the tested bacteria, S. aureus was the most sensitive to the three essential oils.
Results
The obtained results showed that each of the three essential oils has an inhibitory effect on pathogenic strains. Of these three oils, Z. multiflora Boiss essential oil showed the highest inhibitory effect on microbial strains. Furthermore, comparison of the antibacterial effects of these three essential oils with ampicillin and tetracycline revealed that these antibiotics have a better effect in controlling pathogenic strains.
Conclusion
The essential oils used in the present study with different components showed antibacterial activity (especially Z. multiflora Boiss essential oil), and therefore they can be used as a new antibacterial substance.
Keywords: antibacterial activity, disk diffusion method, essential oil, minimal bactericidal concentrations, minimal inhibitory concentrations
1. Introduction
In recent decades, increasingly drug-resistant bacteria have been a major concern. Drug resistance is common among pathogenic staphylococci. Staphylococcus aureus is a facultative anaerobic Gram-positive coccobacillus naturally found in parts of the skin and nasal cavity.1 The inherent virulence of S. aureus and its ability to create a diverse array to life-threatening infections and capacity to adapt to different environmental conditions are the main concerns about this pathogen.2 Pseudomonas aeruginosa is Gram-negative aerobic motile basil.3, 4 This bacterium is commonly found in most environments in hospitals. P. aeruginosa often exist in small numbers in the normal intestinal flora and on human skin. It becomes pathogenic only when introduced into areas without normal defenses such as the skin and the mucus layer.3 This bacterium can express a variety of efficient efflux pump and antibiotic inactivating enzymes, so it can be resistant to antibiotics. Klebsiella pneumonia (which belongs to the Enterobacteriaceae family) is a nonmobile and encapsulated Gram-negative, facultative anaerobic bacillus, and is found in the normal flora of intestines.3 This bacterium causes infections in hospitals and communities.5 The majority of hospital infections caused by K. pneumonia are nosocomial pneumonia, urinary tract infections, diarrhea, and intra-abdominal infections.6 K. pneumonia may be attributable to multidrug efflux systems.7 The mounting concern about drug resistance has led researchers to focus more attention on natural products, including plants, with antimicrobial properties as the future source of antimicrobial agents.8, 9 For thousands of years, humans have been using natural products derived from plants for therapeutic purposes.9 The World Health Organization reported that in 2008, more than 80% of the world population depended on traditional medicine for their primary health care needs.10 Artemisia is a genus belonging to the Asteraceae family. Many members of this genus are important medicinal plants. Artemisia kermanensis is an endemic plant in Iran and important medicinal plant in the south of Kerman Province.11, 12 Lavandula officinalis (L. angustifolia) is an important species of the family Laminaceae, and is broadly distributed in the Mediterranean region.13 In Iranian flora, lavender is chiefly distributed in the northern parts of the country. Lavender oil is known for its excellent aroma and is widely used in flavor, perfume, and cosmetic industries; it is also recommended for its anti-inflammatory and anti-infectious effects.14 In Europe, lavender is used as an antispasmodic, carminative, and mild tranquilizer for digestive and mild nervous disorders. Lavender oil's antifungal and antibacterial activities oil have been reported.14, 15 Moreover, it has been found that lavender oil is active against many species of bacteria and fungi. For example, L. angustifolia oil was indicated to have in vitro antibacterial activity against methicillin-resistant S. aureus and vancomycin-resistant Enterococcus faecalis at a concentration of < 1%.16 Zataria multiflora Boiss, a member of the Labiatae family, is a native plant of Iran, Pakistan, and Afghanistan. It is traditionally used for anesthetic, antiseptic, and antispasmodic purposes. Z. multiflora has also been shown to have anti-inflammatory analgesic effects.17, 18 This plant is also used as a condiment and has many therapeutic applications in traditional folk medicine (Iranian Herbal Pharmacopoeia). In this study, we examined the antibacterial activity of A. kermanensis, L. officinalis, and Z. multiflora Boiss against three pathogenic bacteria (P. aeruginosa, S. aureus, and K. pneumonia).
2. Materials and methods
2.1. Origin and isolation of essential oils
Fresh Z. multiflora, L. officinalis, and A. kermanensis plants were gathered from Lorestan and Chaharmahal provinces in Iran (2012). Their scientific names were searched through the Herbarium part of Institution of Traditional Medicine in Iran (nos. 2359, 2360, and 2361, respectively). At first, the aerial parts of the herbs were kept at room temperature for 3 days, and after complete dryness was attained, the parts were powdered by mill. Making of essential oil was done with water using the essential making machine, Clevenger apparatus (model BP, Ashke Shisheh Co., Tehran, Iran & mantle model H610, Fater Electronic, Tehran, Iran) based on boiling point. For each batch, 100 g of the powder was placed in a 1-L balloon of Clevenger, and then water was added. After 5 hours of distillation, the essence—which was a yellow to green liquid with a good smell—was gathered.19 The oils were dried over anhydrous Na2SO4 and stored at 4 °C in sealed amber vials until use.20
2.2. Gas chromatography-mass spectrometry
Analysis was carried out using a GC-mass chromatograph with an HP-5MS column (30 m × 0.25 mm, film thickness 0.25 m). Helium was used as the carrier gas at a flow rate of 0.8 mL/minute. The column temperature was kept at 50 °C for 2 minutes, and then it was programmed to 200 °C at a rate of 3 °C/minute and kept constant at 200 °C for 10 minutes. The injection was performed in split mode with ratio of 50:1 at 250 °C. The compounds were identified by comparison of the relative retention indices with those reported in the literature and also by comparison of their mass spectra with published mass spectra.20, 21 The retention indices for all the components were determined according to the Van Den Dool method22 using n-alkanes as standards.
2.3. Antimicrobial activities assays
2.3.1. Preparation of bacterial cells
The bacterial species consisted of S. aureus (ATCC 25923), P. aeruginosa (PTCC 1310), and K. pneumonia (PTCC 1053), which were prepared at the Traditional Medicine Institute of Isfahan (Isfahan, Iran). First, the Muller-Hinton agar (MHA) medium was prepared and transferred in sterilized Petri dishes (5 cm thick). Under aseptic conditions, the samples of bacteria were taken from basal culture using an applicator and then inoculated in the medium.
2.3.2. Antibacterial assay
In order to evaluate the antimicrobial effect, the disk diffusion method (which is known as Kirby–Bauer and is the most common form of antimicrobial assay)23 and assessment of minimal inhibitory and minimal bactericidal concentrations (MIC and MBC, respectively), were applied. After 18 hours of culture, liquid containing bacteria, with a standard density (1 × 106 CFU/mL) of 0.5 McFarland in MHA, was prepared, and by using Sampler, 500 μL of the liquid was transferred to MHA. The liquid was gently distributed on the surface of MHA using sterile loop. There were blank disks with 6 mm in diameter containing 30 μL with concentrations of 0.08 μg/disk, 0.16 μg/disk, 0.31 μg/disk, 0.63 μg/disk, 1.25 μg/disk, 2.5 μg/disk, 5 μg/disk, 10 μg/disk, 20 μg/disk, 40 μg/disk, 60 μg/disk, 80 μg/disk, and 100 μg/disk on MHA. A disk containing ampicillin, penicillin, and tetracycline was used as positive control, and the diameter of inhibition of zone was measured after 24 hours of incubation at 37 °C at 24 hours, 48 hours, and 72 hours in triplicate. The microwell method24 was used for determining the MIC value (the lowest concentration of an antibacterial agent that prevents visible bacterial growth after 24 hours of incubation at 37 °C) and MBC value (the lowest concentration required to kill certain bacteria) for S. aureus, P. aeruginosa, and K. pneumonia. The suspension of bacterial strain was prepared from the liquid culture with a standard darkness of 0.5 McFarland. The essential oils were prepared, and different dilutions (6 dilutions) were added to the pipes containing 10 mL liquid culture medium. In this step, in order to determine MIC, the 96-well plate was used. To every well, 95 μL Mueller–Hinton broth and 5 μL microbial suspension were added. Next, 100 μL of the essential oil with a concentration of 500 μg/μL was added to the first well. Then, 100 μL was taken from the first well and transferred to the next well. This process went on until the sixth well. The last well contained 195 μL MHB culture medium and 5 μL of microbial suspension without any essential oil) as negative control). In the next step, the ingredients of every well were mixed using a rotary shaker for 20 minutes. Then it was put in an incubator for 24 hours at a suitable temperature (37 °C). The microbial growth was measured at 600 nm.25
2.4. In vitro analyses
2.4.1. Cell culture
The L929 fibroblast cell line of mouse (NCBI code 161 (was obtained from the Pasteur Institute of Iran (Tehran, Iran), then grown in Dulbecco's modified Eagle's medium supplemented with 5% fetal calf serum, 100 U/mL penicillin (Gibco), and 100 μg/mL streptomycin (Gibco, Carlsbad, CA, USA), at 37 °C in a humidified atmosphere containing 90% air and 5% CO2.
2.4.2. Cytotoxicity assay
In order to determine the cytotoxicity effect of essential oils, the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay was used. This method is based on a mitochondrial succinate dehydrogenase activity that changes the yellow dye of MTT to the violet dye of Formosan. Formosan can dissolve in dimethyl sulfoxide, and its optical density (OD) could be measured using enzyme-linked immunosorbent assay.26, 27 The cells were cultured in T25, and after 90% confluency, they were removed from the culture dishes using trypsinization and suspended in 10 mL culture medium. Next, they were seeded with a cell count of 5 × 103 for L929 cells per well in 96-well plates for 24 hours. After that, the cultured cells were treated with different concentrations of essential oils (0 μg/mL, 0.75 μg/mL, 1.56 μg/mL, 3.12 μg/mL, 6.25 μg/mL, 12.5 μg/mL, 25 μg/mL, 50 μg/mL, 100 μg/mL, 150 μg/mL, 200 μg/mL, 250 μg/mL, 300 μg/mL, and 350 μg/mL), and the plate was incubated for 48 hours in a CO2 incubator at 37 °C. Next, 20 μL MTT solution was added to the wells and these were incubated for 4 hours. For dissolving Formosan christa, 100 μL dimethyl sulfoxide was added. The absorbance of MTT was measured at 560 nm. The vital percent of cells in negative control was considered 100, and it can be obtained using the following equation. A concentration from the essential oil that reduces cells’ vitality to half is observed as CC50.
| [1] |
3. Results
Data analysis was performed using SPSS version 20 (SPSS Inc., Chicago, IL, USA), analysis of variance (ANOVA), and Tukey's comparison procedure. After ANOVA showed significantly different values between treatment groups, Turkey's HSD (Honestly Significant Difference) test was performed for all pairwise comparisons which allows to rank means and put them into significant treatment groups, while controlling maximum experiment-wise error rate under null hypothesis. The effects of different concentrations of A. kermanensis, L. officinalis, and Z. multiflora Boiss essential oils were examined against the three bacteria (S. aureus, K. pneumonia, and P. aeruginosa) at 24 hours, 48 hours, and 72 hours using the disk diffusion method (Table 1, Table 2, Table 3). The results showed that the concentration of 100 μg/disk of each of the three essential oils was more efficient compared with lower concentrations on the bacteria (p < 0.0001).
Table 1.
Antibacterial activity of different concentrations of Artemisia kermanensis essential oil against three bacteria using disk diffusion method (zone of inhibition)*
| Artemisia kermanensis (μg/disk) |
Staphylococcus aureus Mean ± SE |
Klebsiella pneumonia Mean ± SE |
Pseudomonas aeruginosa Mean ± SE |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 24 | 48 | 72 | 24 | 48 | 72 | 24 | 48 | 72 | |
| 0.08 | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a |
| 0.16 | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a |
| 0.31 | 0.50 ± 0.06a | 0.63 ± 0.07a | 0.63 ± 0.07a | 0.17 ± 0.17a | 0.27 ± 0.14a | 0.27 ± 0.14a | 0.10 ± 0.10a,b | 0.17 ± 0.17a | 0.17 ± 0.17a |
| 0.63 | 2.49 ± 0.09b | 2.67 ± 0.33b | 2.67 ± 0.03b | 1.20 ± 0.15b | 1.37 ± 0.18b | 1.50 ± 0.15b | 1.33 ± 0.18b | 1.60 ± 0.15b | 1.60 ± 0.15b |
| 1.25 | 4.20 ± 0.11c | 4.33 ± 0.17c | 4.40 ± 0.20c | 2.27 ± 0.12c | 2.50 ± 0.10c | 2.63 ± 0.18c | 3.33 ± 0.23c | 3.63 ± 0.14c | 3.63 ± 0.14c |
| 2.5 | 6.48 ± 0.02d | 6.51 ± 0.01d | 6.52 ± 0.01d | 4.00 ± 0.06d | 4.13 ± 0.13d | 4.37 ± 0.14d | 5.13 ± 0.09d | 5.37 ± 0.09d | 5.37 ± 0.09d |
| 5 | 7.90 ± 0.40e | 8.02 ± 0.38d | 8.11 ± 0.38e | 6.57 ± 0.18e | 6.73 ± 0.12e | 6.90 ± 0.10e | 7.57 ± 0.09e | 7.73 ± 0.12e | 7.73 ± 0.12e |
| 10 | 9.64 ± 0.54f | 10.02 ± 0.50e | 11.16 ± 0.13f | 8.27 ± 0.13f | 8.70 ± 0.11f | 8.70 ± 0.11f | 8.53 ± 0.23e | 9.13 ± 0.35f | 9.13 ± 0.35f |
| 20 | 12.13 ± 0.13g | 13.30 ± 0.21f | 13.40 ± 0.15g | 13.20 ± 0.11g | 13.80 ± 0.11g | 13.80 ± 0.11g | 10.80 ± 0.15f | 11.10 ± 0.15g | 11.33 ± 0.17g |
| 40 | 14.43 ± 0.12h | 14.70 ± 0.15f | 14.87 ± 0.01g | 15.17 ± 0.09h | 15.77 ± 0.27h | 15.97 ± 0.22h | 12.20 ± 0.53g | 12.40 ± 0.58h | 12.40 ± 0.58g |
| 60 | 16.70 ± 0.51i | 17.23 ± 0.68g | 17.50 ± 0.81h | 17.13 ± 0.33i | 17.57 ± 0.28i | 17.57 ± 0.28i | 13.83 ± 0.42h | 14.20 ± 0.11i | 14.20 ± 0.11h |
| 80 | 19.77 ± 0.23j | 20.10 ± 0.49h | 20.17 ± 0.44i | 19.97 ± 0.32j | 20.23 ± 0.18j | 20.40 ± 0.15j | 15.87 ± 0.47i | 16.10 ± 0.40j | 16.10 ± 0.40i |
| 100 | 22.27 ± 0.14k | 22.73 ± 0.18i | 22.73 ± 0.18j | 25.70 ± 0.32k | 26.37 ± 0.23k | 26.37 ± 0.23k | 17.43 ± 0.07j | 17.67 ± 0.03k | 17.67 ± 0.03j |
Different letters on every column represent a meaningful difference (p < 0.0001).
SE, standard error.
Table 2.
Antibacterial activity of different concentrations of Lavandula officinalis essential oil against three bacteria using disk diffusion method (zone of inhibition)*
| Lavandula officinalis (μg/disk) |
Staphylococcus aureus Mean ± SE |
Klebsiella pneumonia Mean ± SE |
Pseudomonas aeruginosa Mean ± SE |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 24 | 48 | 72 | 24 | 48 | 72 | 24 | 48 | 72 | |
| 0.08 | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a |
| 0.16 | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a |
| 0.31 | 0.37 ± 0.18a | 0.40 ± 0.20a | 0.40 ± 0.20a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.30 ± 0.15a | 0.37 ± 0.18a,b | 0.50 ± 0.25a,b |
| 0.63 | 1.50 ± 0.06b | 1.70 ± 0.06b | 1.70 ± 0.06b | 0.20 ± 0.20b | 0.23 ± 0.23b | 0.23 ± 0.23b | 1.30 ± 0.21a,b | 1.40 ± 0.15b,c | 1.40 ± 0.15b |
| 1.25 | 3.42 ± 0.21c | 3.62 ± 0.21c | 3.73 ± 0.09c | 1.43 ± 0.23c | 1.60 ± 0.21c | 1.63 ± 0.23c | 2.37 ± 0.18b,c | 2.70 ± 0.06c,d | 2.77 ± 0.03c |
| 2.5 | 5.39 ± 0.05d | 5.67 ± 0.12d | 5.72 ± 0.16d | 3.40 ± 0.25d | 3.53 ± 0.24d | 3.53 ± 0.24d | 3.57 ± 0.14c | 3.70 ± 0.11d | 3.77 ± 0.14c |
| 5 | 6.63 ± 0.20e | 6.77 ± 0.18e | 6.77 ± 0.18e | 5.23 ± 0.14e | 5.47 ± 0.09e | 5.57 ± 0.09e | 5.97 ± 0.03d | 6.23 ± 0.12e | 6.47 ± 0.14d |
| 10 | 8.47 ± 0.12f | 8.70 ± 0.12f | 8.80 ± 0.15f | 7.27 ± 0.14f | 7.40 ± 0.11f | 7.50 ± 0.11f | 7.50 ± 0.11e | 7.70 ± 0.06f | 7.83 ± 0.03e |
| 20 | 11.77 ± 0.19g | 12.33 ± 0.18g | 12.63 ± 0.18g | 9.00 ± 0.25g | 9.37 ± 0.28g | 9.87 ± 0.13g | 8.37 ± 0.18e | 8.77 ± 0.18f | 8.97 ± 0.18e |
| 40 | 14.67 ± 0.20h | 15.13 ± 0.09h | 15.13 ± 0.09h | 12.53 ± 0.09h | 13.03 ± 0.09h | 13.27 ± 0.14h | 10.43 ± 0.14f | 10.67 ± 0.09g | 10.73 ± 0.14f |
| 60 | 16.13 ± 0.07i | 16.50 ± 0.06i | 16.50 ± 0.06i | 15.07 ± 0.18i | 16.00 ± 0.40i | 16.47 ± 0.34i | 12.60 ± 0.17g | 12.77 ± 0.14h | 12.80 ± 0.17g |
| 80 | 18.50 ± 0.06j | 18.63 ± 0.09j | 18.63 ± 0.09j | 18.87 ± 0.09j | 19.10 ± 0.15j | 19.43 ± 0.22j | 14.17 ± 0.12h | 14.47 ± 0.14i | 14.60 ± 0.10h |
| 100 | 20.17 ± 0.09k | 20.47 ± 0.09k | 20.47 ± 0.09k | 21.23 ± 0.56k | 21.93 ± 0.54k | 21.93 ± 0.54k | 14.80 ± 0.81h | 15.03 ± 0.84i | 15.13 ± 0.75h |
Different letters on every column represent meaningful difference (p < 0.0001).
SE, standard error.
Table 3.
Antibacterial activity of different concentrations of Zataria multiflora Boiss essential oil against three bacteria using disk diffusion method (zone of inhibition)*
| Zataria multiflora (μg/disk) |
Staphylococcus aureus Mean ± SE |
Klebsiella pneumonia Mean ± SE |
Pseudomonas aeruginosa Mean ± SE |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 24 | 48 | 72 | 24 | 48 | 72 | 24 | 48 | 72 | |
| 0.08 | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a |
| 0.16 | 0.60 ± 0.23a | 0.87 ± 0.23a | 0.87 ± 0.23a | 0.53 ± 0.14a,b | 0.67 ± 0.14a | 0.67 ± 0.14a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a |
| 0.31 | 1.93 ± 0.29b | 2.37 ± 0.24b | 2.37 ± 0.24b | 1.23 ± 0.12b,c | 1.43 ± 0.27b | 1.67 ± 0.24b | 1.17 ± 0.17b | 1.33 ± 0.17b | 1.33 ± 0.17b |
| 0.63 | 3.56 ± 0.29c | 3.73 ± 0.18c | 3.73 ± 0.18c | 2.10 ± 0.10c | 2.40 ± 0.15c | 2.77 ± 0.14c | 2.37 ± 0.12c | 2.53 ± 0.09c | 2.70 ± 0.06c |
| 1.25 | 5.62 ± 0.06d | 5.87 ± 0.09d | 5.87 ± 0.09d | 3.40 ± 0.00d | 4.00 ± 0.00d | 4.00 ± 0.00d | 4.27 ± 0.18d | 4.47 ± 0.22d | 4.47 ± 0.22d |
| 2.5 | 7.81 ± 0.13e | 7.83 ± 0.12e | 7.87 ± 0.13e | 5.44 ± 0.02e | 6.47 ± 0.03e | 6.77 ± 0.03e | 6.43 ± 0.12e | 6.58 ± 0.09e | 6.65 ± 0.05e |
| 5 | 10.10 ± 0.45f | 10.40 ± 0.45f | 8.11 ± 0.38f | 7.90 ± 0.40f | 8.27 ± 0.37f | 8.44 ± 0.34f | 8.56 ± 0.23f | 8.73 ± 0.12f | 8.73 ± 0.12f |
| 10 | 11.54 ± 0.12g | 11.64 ± 0.07g | 11.80 ± 0.06g | 10.60 ± 0.24g | 11.29 ± 0.05g | 11.35 ± 0.05g | 10.35 ± 0.26g | 10.51 ± 0.13g | 10.63 ± 0.12g |
| 20 | 13.57 ± 0.12h | 14.50 ± 0.26h | 14.73 ± 0.13h | 14.30 ± 0.26h | 14.50 ± 0.29h | 14.70 ± 0.26h | 11.97 ± 0.20h | 12.30 ± 0.15h | 12.30 ± 0.15h |
| 40 | 16.43 ± 0.09i | 16.93 ± 0.12i | 17.30 ± 0.15i | 16.70 ± 0.11i | 16.93 ± 0.07i | 17.00 ± 0.11i | 13.27 ± 0.13i | 13.43 ± 0.18i | 13.53 ± 0.22i |
| 60 | 20.37 ± 0.20j | 20.93 ± 0.07j | 21.27 ± 0.14j | 20.80 ± 0.30j | 21.67 ± 0.09j | 21.77 ± 0.09j | 15.23 ± 0.14j | 15.63 ± 0.14j | 15.63 ± 0.14j |
| 80 | 24.67 ± 0.03k | 24.97 ± 0.03k | 25.20 ± 0.15k | 24.43 ± 0.12j | 25.13 ± 0.13k | 25.33 ± 0.20k | 17.20 ± 0.11k | 17.60 ± 0.30k | 17.83 ± 0.23k |
| 100 | 27.80 ± 0.20l | 28.67 ± 0.33l | 28.67 ± 0.33l | 27.83 ± 0.12k | 28.10 ± 0.21l | 28.10 ± 0.21l | 19.90 ± 0.27l | 20.40 ± 0.23l | 20.53 ± 0.18l |
Different letters on every column represent meaningful difference (p < 0.0001).
SE, standard error.
In the highest concentration of A. kermanensis essential oil (100 μg/disk), most of the antibacterial effects was observed against K. pneumonia. However, at lower concentrations (from 80 μg/disk to 20 μg/disk), a similar effect was observed on both S. aureus and K. pneumonia. At concentrations < 20 μg/disk, a minimal antibacterial effect was observed against K. pneumonia. In a broad range of A. kermanensis essential oil concentrations used, P. aeruginosa (with the smallest inhibition zone compared with other bacteria) showed more resistance toward this oil (Fig. 1).
Fig. 1.
Effect of essential oil of Artemisia kermanensis on three species of bacteria.
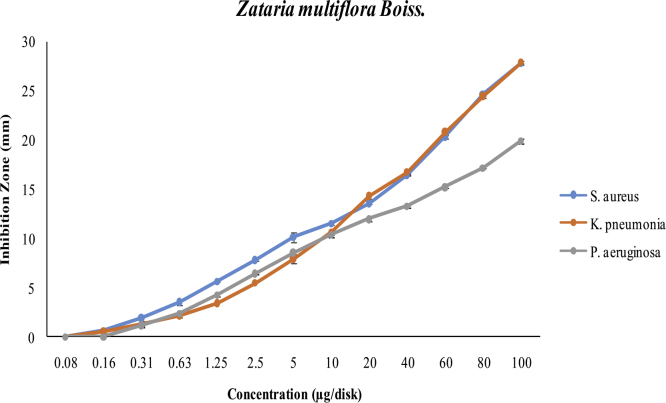
In concentrations < 20 μg/disk, Z. multiflora Boiss essential oil is more effective against S. aureus than against the two other bacteria (in these concentrations, the antibacterial effects against K. pneumonia and P. aeruginosa were rather similar). But with increasing concentrations (20–100 μg/disk), it had the same effect on both S. aureus and K. pneumonia. In a broad range of Z. multiflora Boiss essential oil concentrations used, P. aeruginosa, which has the smallest inhibition zone compared to other bacteria, showed more resistance toward this oil (Fig. 2).
Fig. 2.
Effect of Zataria multiflora Boiss essential oil on three species of bacteria.
At high concentrations (80 μg/disk and 100 μg/disk), the essential oil of L. officinalis performed more effectively against K. pneumonia compared with the two other bacteria. However, at concentrations < 80 μg/disk, it was found to be more effective against S. aureus. In a board range of L. officinalis essential oil concentrations used, P. aeruginosa, which has the smallest inhibition zone compared to the other bacteria, proved to be more resistant toward this oil (Fig. 3).
Fig. 3.
Effect of essential oil of Lavandula officinalis on three species of bacteria.
A comparison between the three plant essential oils (at a concentration of 100 μg/disk) and positive control antibiotics (ampicillin, penicillin, and tetracycline) demonstrated that Z. multiflora Boiss essential oil (at all time intervals) had a stronger antibacterial effect (bigger inhibition zone) against the three bacteria (Table 4).
Table 4.
Comparison of different common essential oils concentrations and antibiotics effect on Staphylococcus aureus, Klebsiella pneumonia, and Pseudomonas aeruginosa
| Treatment (μg/disk) |
Staphylococcus aureus Mean ± SE |
Klebsiella pneumonia Mean ± SE |
Pseudomonas aeruginosa Mean ± SE |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 24 | 48 | 72 | 24 | 48 | 72 | 24 | 48 | 72 | |
| Artemisia kermanensis (100) | 22.27 ± 0.14c | 22.73 ± 0.18d | 22.73 ± 0.18d | 25.70 ± 0.32d | 26.37 ± 0.23d | 26.37 ± 0.23d | 17.43 ± 0.07c | 17.67 ± 0.03c | 17.67 ± 0.03c |
| Lavandula officinalis (100) | 20.17 ± 0.09b | 20.47 ± 0.09c | 20.47 ± 0.09c | 21.23 ± 0.56c | 21.93 ± 0.54c | 21.93 ± 0.54c | 14.80 ± 0.81b | 15.03 ± 0.84b | 15.13 ± 0.75b |
| Zataria multiflora (100) | 27.80 ± 0.20d | 28.67 ± 0.33e | 28.67 ± 0.33e | 27.83 ± 0.12d | 28.10 ± 0.21d | 28.10 ± 0.21d | 19.90 ± 0.27d | 20.40 ± 0.23d | 20.53 ± 0.18d |
| Ampicillin (10) | 16.44 ± 0.29a | 16.80 ± 0.25a,b | 16.91 ± 0.31a,b | 6.40 ± 0.61a | 6.63 ± 0.59a | 6.63 ± 0.59a | 5.93 ± 0.35a | 6.17 ± 0.43a | 6.17 ± 0.43a |
| Penicillin (10) | 14.93 ± 0.52a | 14.93 ± 0.52a | 14.93 ± 0.52a | 5.87 ± 0.41a | 6.00 ± 0.29a | 6.00 ± 0.29a | 6.33 ± 0.67a | 6.50 ± 0.78a | 6.50 ± 0.78a |
| Tetracycline (30) | 19.47 ± 0.73b,c | 19.63 ± 0.63c | 19.63 ± 0.63c | 13.30 ± 0.70b | 13.40 ± 0.78b | 13.40 ± 0.78b | 6.00 ± 0.52a | 6.40 ± 0.45a | 6.40 ± 0.45a |
* Different letters on every column represent meaningful difference (p < 0.0001).
SE, standard error.
The MIC and MBC results demonstrated that Z. multiflora essential oil, with lower MIC and MBC values than L. officinalis and A. kermanensis essential oils, showed a higher antibacterial activity against the three bacteria (Table 5, Table 6, Table 7). Compared with L. officinalis essential oil, A. kermanensis essential oil had a higher antibacterial activity (lower MIC and MBC values) against the three bacteria (Table 5, Table 6, Table 7).
Table 5.
MIC and MBC of essential oils on Pseudomonas aeruginosa
| No. | Extract | MIC (μg/mL) |
MBC (μg/mL) | |
|---|---|---|---|---|
| MIC 50 | MIC 90 | |||
| 1 | Artemisia kermanensis | 37 | 62 | 71 |
| 2 | Zataria multiflora | 31 | 58 | 63 |
| 3 | Lavandula officinalis | 41 | 75 | 92 |
| 4 | Ampicillin | 12 | 21 | 25 |
| 5 | Penicillin | 12 | 20 | 25 |
| 6 | Tetracycline | 6 | 11 | 15 |
MBC, minimal inhibitory concentration; MIC, minimal bactericidal concentration; SE, standard error.
Table 6.
MIC and MBC of the essential oils on Klebsiella pneumonia
| No. | Extract | MIC (μg/mL) |
MBC (μg/mL) | |
|---|---|---|---|---|
| MIC 50 | MIC 90 | |||
| 1 | Artemisia kermanensis | 30 | 54 | 68 |
| 2 | Zataria multiflora | 25 | 47 | 51 |
| 3 | Lavandula officinalis | 39 | 63 | 76 |
| 4 | Ampicillin | 10 | 17 | 24 |
| 5 | Penicillin | 13 | 19 | 26 |
| 6 | Tetracycline | 11 | 17 | 22 |
MBC, minimal inhibitory concentration; MIC, minimal bactericidal concentration.
Table 7.
MIC and MBC of the essential oils on Staphylococcus aureus
| No. | Extract | MIC (μg/mL) |
MBC (μg/mL) | |
|---|---|---|---|---|
| MIC 50 | MIC 90 | |||
| 1 | Artemisia kermanensis | 28 | 48 | 57 |
| 2 | Zataria multiflora | 18 | 35 | 43 |
| 3 | Lavandula officinalis | 32 | 52 | 69 |
| 4 | Ampicillin | 4 | 6 | 9 |
| 5 | Penicillin | 6 | 9 | 10 |
| 6 | Tetracycline | 5 | 7 | 10 |
MBC, minimal inhibitory concentration; MIC, minimal bactericidal concentration.
3.1. Cytotoxic effects on viability of L929 cells
Results from the cytotoxicity test showed that Z. multiflora and A. kermanensis essential oils have no cellular toxic effect up to 6.25 μg/mL, and L. officinalis oil showed no cellular toxic effect up to 12.5 μg/mL. With the increase in essential oil concentration, cellular resistance is considerably decreased. The CC50 for Z. multiflora, L. officinalis, and A. kermanensis is 123 μg/mL, 218 μg/mL, and 154 μg/mL, respectively (Fig. 4).
Fig. 4.
Cytotoxic activity of different concentration of herbal medicine essential oils. Each bar represents the mean±S.D of three independent experiments.
4. Discussion
Nowadays, the resistance of bacteria is increasing against antibiotics. Consequently, research on exploration of new materials having antimicrobial properties is growing. As essences and herbal extracts have been historically used in treatment of diseases,28, 29 they can be good candidates in such studies. Herbal extracts possessing antimicrobial effects on a broad range of organisms, nutrient applicability, and fewer side effects (compared with common antibiotics), can be a replacement for antibiotics.30, 31, 32 Various studies have documented the antimicrobial activity of essential oils and plant extracts, such as A. kermanensis, L. officinalis, and Z. multiflora Boiss.33, 34, 35 In this study, we evaluated the antibacterial activity of A. kermanensis, L. officinalis, Z. multiflora Boiss essential oils against P. aeroginusa, S. aureus, and K. pneumonia. The results showed that the extract of Z. multiflora has the highest effect on all species of bacteria used. The highest effect was observed against S. aureus, with an MBC of 43 μg/mL (Table 7). Motevasel and coworkers36 reported that the high concentration of Zataria extract showed the best antimicrobial activities and killed numerous types of bacteria with no difference between pathogens and nonpathogens. In a research by Sharififar and coworkers,37 the effect of this extract was tested on K. pneumoniae and S. aureus, in which MIC was measured as 30 μg/mL and 21 μg/mL, respectively. In our study, the MIC90 of Z. multiflora against K. pneumonia and S. aureus was measured as 25 μg/mL and 18 μg/mL, respectively. Our results are compatible with those obtained by Sharififar et al.37 Owlia and coworkers38 tested the effect of the extract on P. aeruginosa, and their results showed an MIC of 64 μg/mL and an MBC of 128 μg/mL. In our study, the MIC and MBC of Z. multiflora essential oil on P. aeruginosa were measured as 31 μg/mL and 63 μg/mL, respectively. This indicates that the essential oil used in our study has a better effect. Moreover, in another study by El-Shouny and coworkers,39 the researchers determined the effect of Thymus vulgaris essential oil on P. aeruginosa, which is resistant to common antibiotics. The MIC was measured as 0.32 mg/mL (320 μg/mL). Another experiment reported the effect of T. vulgaris essential oil on S. aureus ATCC 25923, and yielded an MIC of 1.33 mg/mL.38, 40 Another experiment in 2008 reported the effect of Z. multiflora on K. pneumonia. The MIC was reported to be between 312 μg/mL and 624 μg/mL.41 Results of the study indicated that the essential oils possess a better effect on pathogens. The analysis of Z. multiflora Boiss essential oil with gas chromatography–mass spectrometry (GC-MS) showed 34 constituents representing 96.94% of the total oil. The major components consisted of thymol (33.05%), carvacrol (25.88%), and p-cymene (11.34%) (Table 8). Govaris and coworkers42 reported that carvacrol (80.15%) and thymol (4.82%) were the major components of Z. multiflora.37 Different studies indicated that essential oils containing thymol, carvacrol, or eugenol possess the highest antimicrobial properties.43 The antimicrobial effect of essential oil components such as thymol, menthol, and linalyl acetate may be caused by a perturbation of the lipid fractions of bacterial plasma membranes, which might be influenced by the membrane permeability and leakage of intracellular materials. Z. multiflora essential oil, with a high percentage of thymol and carvacrol, has a considerable antimicrobial activity.44 According to our results and previous research, thymol is the major compound of Z. multiflora oil. Sharif Roohani and coworkers (2007) (39.65%), Mahboubi and GhazianBidgoli45 (38.7%), and Saei-Dehkordi and coworkers46 (47.46%) reported thymol as the major component of Z. multiflora oil. The European Chemicals Agency reported that thymol has not shown chronic side effects or teratogenic effects in previous studies, and it can be considered a safe compound.35, 45
Table 8.
Chemical composition of the essential oil of Artemisia kermanensis
| No | Compositions | % | RI |
|---|---|---|---|
| 1 | Artemisiatriene | 0.41 | 926 |
| 2 | α-Pinene | 0.54 | 934 |
| 3 | Camphene | 0.93 | 949 |
| 4 | Verbenene | 1.88 | 954 |
| 5 | Benzaldehyde | 0.11 | 960 |
| 6 | β-Pinene | 0.08 | 977 |
| 7 | p-Menthatriene | 0.57 | 993 |
| 8 | Yomogi alcohol | 2.67 | 1001 |
| 9 | α-Terpinene | 0.2 | 1016 |
| 10 | p-Cymene | 1.88 | 1024 |
| 11 | 1,8-Cineole | 1.82 | 1030 |
| 12 | Artemisia ketone | 0.11 | 1032 |
| 13 | trans-Carane | 0.13 | 1050 |
| 14 | gamma-Terpinene | ‘ | 1056 |
| 15 | Artemesia alcohol | 1.48 | 1082 |
| 16 | Styrene | 0.82 | 1087 |
| 17 | α-Thujone | 13.83 | 1108 |
| 18 | β-Thujone | 6.23 | 1117 |
| 19 | trans-Pinocarveol | 1.39 | 1138 |
| 20 | Camphen | 4.13 | 1142 |
| 21 | Camphore | 10.23 | 1144 |
| 22 | p-Menth-1,5-dien-8-ol | 2.04 | 1147 |
| 23 | 1-Menthene | 0.49 | 1156 |
| 24 | Pinocarvone | 1.37 | 1160 |
| 25 | Borneol | 1.97 | 1164 |
| 26 | p-Mentha-1,5-dien-8-ol | 4.38 | 1166 |
| 27 | Terpinene-4-ol | 1.01 | 1175 |
| 28 | Naphthalene | 0.73 | 1178 |
| 29 | p-Cymen-3-ol | 1.26 | 1182 |
| 30 | α-Terpineol | 0.72 | 1188 |
| 31 | Verbenone | 1.53 | 1206 |
| 32 | Norbornane | 0.36 | 1215 |
| 33 | Cuminic aldehyde | 1.1 | 1235 |
| 34 | (+)-Carvone | 0.48 | 1239 |
| 35 | Carvotanacetone | 0.28 | 1243 |
| 36 | cis-Myrtanol | 0.15 | 1247 |
| 37 | Carvenone | 0.12 | 1253 |
| 38 | Chrysanthenyl acetate | 1 | 1256 |
| 39 | Cinnamic aldehyde-E | 0.16 | 1264 |
| 40 | Bornyl acetate | 2.3 | 1280 |
| 41 | Thymol | 1.29 | 1286 |
| 42 | Carvacrol | 1.78 | 1297 |
| 43 | α-Copaene | 0.23 | 1368 |
| 44 | Methyl cinnamate | 0.15 | 1375.7 |
| 45 | (Z)-Jasmone | 0.22 | 1393.1 |
| 46 | Methyleugenol | 0.15 | 1399.3 |
| 47 | trans-Caryophyllene | 0.3 | 1395.6 |
| 48 | α-Curcumen | 0.15 | 1475.4 |
| 49 | Spathulenol | 0.25 | 1569 |
| 50 | Caryophyllene | 0.07 | 1644.5 |
| Total | 75.84 | ||
Orhan and coworkers47 observed the effect of L. officinalis essential oil against P. aeruginosa, K. pneumonia, and S. aureus, and reported an MIC of 32 μg/mL, 128 μg/mL, and 128 μg/mL, respectively. In our study, the MIC of L. officinalis essential oil on the bacteria was 41 μg/mL, 63 μg/mL, and 52 μg/mL, respectively. The comparison between our study and that of Orhan et al47 indicated that in our study L. officinalis essential oil was more effective in terms of its inhibitory effect on K. pneumonia and S. aureus. Changes in the antimicrobial properties of the essential oil in different concentrations can be attributable to the different amounts of flavonoid compositions or different active forms of flavonoids.48 According to GC-MS results, 69 constituents were identified in L. officinalis essential oil, representing 83.99% of the total oil and major components, and these included 1,8-cineol (12.01%), camphore (9.16%), verbenone (8.47%), alpha-pinene (7.58%), thymol (6.23%) (Table 9). Soković and coworkers50 reported that linalyl acetate (27.54%) and linalool (27.21%) are the most abundant components in L. angustifolia (L. officinalis) oil. Meanwhile, Hamad and coworkers13 reported that the major components of L. langustifolia oil are linalool (24.63%) and camphor (13.58%). 1,8-Cineole and camphor, which are used as useful substances in producing numerous drugs, have antiseptic properties.49, 50
Table 9.
Chemical composition of the essential oil of Lavandula officinalis
| No | Compositions | % | RI |
|---|---|---|---|
| 1 | α-Pinene | 7.58 | 938 |
| 2 | Camphene | 4.51 | 952 |
| 3 | Verbenene | 0.64 | 956 |
| 4 | 1,3,5-Cycloheptatriene | 0.03 | 972 |
| 5 | β-Pinene | 0.49 | 979 |
| 6 | 3-Octanone | 2.19 | 988 |
| 7 | β-Myrcene | 1.18 | 993 |
| 8 | 3-Octanol | 0.36 | 997 |
| 9 | α-Phellandrene | 0.05 | 1007 |
| 10 | o-Isopropenyltoluene | 0.08 | 1014 |
| 11 | α-Terpinene | 0.12 | 1017 |
| 12 | p-Cymene | 2.96 | 1025 |
| 13 | 1,8-Cineol | 12.01 | 1033 |
| 14 | gamma-Terpinene | 0.08 | 1057 |
| 15 | Linalool oxide | 0.06 | 1072 |
| 16 | Methyl banzoate | 0.99 | 1088 |
| 17 | Linalool | 2.45 | 1100 |
| 18 | Thujancis | 0.81 | 1103 |
| 19 | D-Fenchyl alcohol | 0.28 | 1112 |
| 20 | Pinocarveol | 0.12 | 1138 |
| 21 | Camphore | 9.16 | 1144 |
| 22 | Isopinocamphone | 1.72 | 1158 |
| 23 | Pinocarvone | 0.13 | 1160 |
| 24 | Pinocamphone | 0.39 | 1171 |
| 25 | Terpene-4-ol | 1.27 | 1174 |
| 26 | naphtalene | 0.08 | 1177 |
| 27 | p-Cymen-8-ol | 0.23 | 1183 |
| 28 | α-Terpineol | 2.31 | 1188 |
| 29 | Myrtenol | 0.35 | 1194 |
| 30 | No pol (terpene) | 1.11 | 1203 |
| 31 | Verbenone | 8.47 | 1209 |
| 32 | trans-Carveol | 0.13 | 1215 |
| 33 | β-Citronellol | 0.1 | 1225 |
| 34 | Pulegone | 0.09 | 1235 |
| 35 | Piperitone | 0.04 | 1250 |
| 36 | Cinnamaldehyde | 0.05 | 1265 |
| 37 | Borneol acetate | 2.41 | 1281 |
| 38 | Thymol | 6.23 | 1288 |
| 39 | 2-Hydroxy-4-isopropyl-1-methylbenzene | 0.17 | 1290 |
| 40 | Carvacrol | 4.14 | 1297 |
| 41 | α-Terpinene | 0.15 | 1329 |
| 42 | Piperitenone | 0.37 | 1335 |
| 43 | α-Cubebene | 0.06 | 1343 |
| 44 | Thymyl acetate | 0.06 | 1349 |
| 45 | α-Copaene | 0.43 | 1369 |
| 46 | trans-Caryophyllene | 0.47 | 1412 |
| 47 | α-Humulene | 0.22 | 1446 |
| 48 | Farnesene | 0.08 | 1451 |
| 49 | β-Acoradiene | 0.09 | 1460 |
| 50 | gamma-Cadinene | 0.16 | 1473 |
| 51 | Zingiberene | 0.1 | 1488 |
| 52 | β-Himachalene | 0.28 | 1492 |
| 53 | delta-Cadinene | 0.27 | 1516 |
| 54 | α-Cedrene | 0.15 | 1524 |
| 55 | Germacrene B | 0.06 | 1548 |
| 56 | spathulenol | 0.26 | 1567 |
| 57 | Caryophyllene oxide | 0.29 | 1572 |
| 58 | α-Farnesene | 0.06 | 1587 |
| 59 | Butlidenephthalide | 0.15 | 1642 |
| 60 | 3N Butylphthalide | 4.62 | 1687 |
| 61 | Butylidene dihydro-phthalide | 0.09 | 1720 |
| Total | 83.99 | ||
Derakhshan and coworkers51 investigated the effect of Artemisia turcomanica, Artemisia khorassanica, Artemisia kopetdaghensis, and Artemisia ciniformis extracts against S. aureus ATCC 25923, and their results showed MIC to be 3 mg/mL, 2 mg/mL, 2 mg/mL, and 1.5 mg/mL, respectively. Another study reported on the effect of Artemisia absinthium extract on S. aureus, and reported an MIC of 52 μg/mL.52 We also investigated the effect of A. kermanensis extract on S. aureus. Our results yielded an MIC of 48 μg/mL (Table 7), which is compatible with that obtained by Blagojevic et al52 in 2006. Konatchiev and coworkers53 examined the effect of Artemisia distans extract on S. aureus. They reported an MIC of 20 μg/mL. A comparison between our results with those of Konatchiev et al53 shows that the A. distans extract possesses a better inhibitory effect on S. aureus compared with the A. kermanensis extract. Generally, in reference to different studies, it is revealed that the essential oil and extract of different species of Artemisia have an inhibitory effect against P. aeruginosa, K. kermanensis, and S. aureus.52 Generally, Gram-positive bacteria are more sensitive to herbal extracts when compared with Gram-negative ones. This can be attributed to their different cell wall structures. Gram-positive bacteria have mucopeptide compositions, whereas Gram-negative bacteria just have a thin layer of mucopeptide and most of their cell wall is made of lipoprotein and lipopolysaccharide. Hence, they are more resistant against antibacterial materials.54, 55 In A. kermanensis oil, 50 constituents were identified representing 75.84% of the total oil. The major components were alpha-thujone (13.83%), camphor (10.23%), and p-mentha-1,5-dien-8-ol (4.38%) (Table 10). Kazemi and coworkers56 determined the constituents of A. kermanensis oil using GC-flame ionization detection and GC-MS methods. They reported that the major components of this oil were isoborneol (21.5%) and camphor (9.8%). Sardashti and Pourramazani Harati49 also analyzed A. kermanensis oil with GC-MS, and reported the following results: in 1,8-cineole (56.55%), borneol (5.28%), and camphene (4.48%). Oxygenated monoterpenes (examples of this substance include linalool, α-terpineol, 1,8-cineole, borneol, camphor, and alpha, beta-thujone) are prevalent components of essential oils.49 Oxygenated monoterpenes have shown variable antibacterial activities.34 Based on season, geographical location, and the location of plants, the overall quality and quantity of the essential oil of species vary. Climate and soil conditions can also affect the composition of the oil, and differences in the major constituents of the different compounds of the essential oils can likely be attributable to differences in habitat conditions.57, 58
Table 10.
Chemical composition of the essential oil of Zataria multiflora Boiss
| No | Compositions | % | RI |
|---|---|---|---|
| 1 | α-Thujene | 0.34 | 931 |
| 2 | α-Pinene | 3.88 | 937 |
| 3 | Camphene | 0.18 | 951 |
| 4 | Verbenene | 0.02 | 956 |
| 5 | Sabinene | 0.02 | 974 |
| 6 | β-Pinene | 0.68 | 979 |
| 7 | β-Myrcene | 0.68 | 993 |
| 8 | α-Phellandrene | 0.11 | 1007 |
| 9 | Δ-3-Carene | 0.04 | 1012 |
| 10 | α-Terpinene | 1.32 | 1016 |
| 11 | p-Cymene | 11.34 | 1025 |
| 12 | Limonene | 0.67 | 1032 |
| 13 | 1,8-Cineole | 0.55 | 1030 |
| 14 | gamma-Terpinene | 4.73 | 1057 |
| 15 | trans-Sabinene hydrate | 0.27 | 1087 |
| 16 | Linalool | 1.46 | 1098 |
| 17 | Borneol | 0.37 | 1162 |
| 18 | Terpinen-4-ol | 0.82 | 1186 |
| 19 | α-Terpineol | 0.67 | 1191 |
| 20 | Carvacrol methyl ether | 0.77 | 1239 |
| 21 | Carvol | 0.77 | 1239 |
| 22 | trans-Anethole | 2.46 | 1281 |
| 23 | Thymol | 33.05 | 1285 |
| 24 | Carvacrol | 25.88 | 1297 |
| 25 | Thymyl acetate | 1.03 | 1311 |
| 26 | Carvacryl acetate | 0.69 | 1371 |
| 27 | β-Caryophyllene | 1.83 | 1412 |
| 28 | Aromadendrene | 0.84 | 1437 |
| 29 | α-Humulene | 0.09 | 1443 |
| 30 | Germacrene-D | 0.13 | 1473 |
| 31 | Ledene | 0.77 | 1491 |
| 32 | cis-α-Bisabolene | 0.09 | 1537 |
| 33 | (+)Spathulenol | 0.24 | 1579 |
| 34 | Caryophyllene oxide | 0.15 | 1589 |
| Total | 96.94 | ||
5. Conclusion
Essential oils possess a range of volatile molecules such as terpenes and terpenoids, and phenol-derived aromatic and aliphatic compounds, which might have bactericidal, virucidal, and fungicidal consequences. Essential oils directly affect the cell membrane of the pathogenic microorganism by causing an increase in permeability and leakage of vital intracellular elements, and finally disorder the cell respiration and microbial enzyme system.59, 60 In this study, three essential oils showed antibacterial effects against tested bacterial strains. Z. multiflora Boiss essential oil showed stronger antibacterial effects than the other essential oils. Z. multiflora oil has also been found to be active against clinical isolates of a broad spectrum of beta-lactamase-producing K. pneumoniae.61 It has been shown that Z. multiflora extract can inhibit the release of the deoxyribonuclease (DNase) enzyme and the making of enterotoxin in S. aureu.62 Due to the increasing problem of antibiotic resistance in bacteria, using these essential oils as natural and new antimicrobial substances can be useful.
Conflicts of interest
The authors declare no conflict of interest.
References
- 1.Kluytmans J, Van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10:505–520. doi: 10.1128/cmr.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lowy FD. Staphylococcus aureus infections. New Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 3.Brooks GF, Butel JB, Ornston LN. McGraw-Hill; New York: 1991. Jawetz, Melnick & Adelberg's medical microbiology. [Google Scholar]
- 4.Gavanji S, Larki B, Zand Jalali A, Mohammadi E, Mehrasa M, Taraghian AM. Comparative effects of propolis of honey bee on pathogenic bacteria. Afr J Pharm Pharacol. 2011;6:2408–2412. [Google Scholar]
- 5.Jayavanth P, Kaur K, Junainah AH. Antibacterial efficacy of chitosan, Manuka honey and chlorophyll against Klebsiella pneumoniae. J Nat Prod. 2011;4:94–99. [Google Scholar]
- 6.Podschun R, Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 1998;11:589–603. doi: 10.1128/cmr.11.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deguchi T, Kawamura T, Yasuda M, Nakano M, Fukuda H, Kato H. In vivo selection of Klebsiella pneumoniae strains with enhanced quinolone resistance during fluoroquinolone treatment of urinary tract infections. Antimicrob Agents Chemother. 1997;41:1609–1611. doi: 10.1128/aac.41.7.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butler MS, Buss AD. Natural products—the future scaffolds for novel antibiotics? Biochem Pharmacol. 2006;71:919–929. doi: 10.1016/j.bcp.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Butler MS. Natural products to drugs: natural product-derived compounds in clinical trials. Nat Prod Rep. 2008;25:475–516. doi: 10.1039/b514294f. [DOI] [PubMed] [Google Scholar]
- 10.Vital PG, Rivera WL. Antimicrobial activity and cytotoxicity of Chromolaena odorata (L. f.) King and Robinson and Uncaria perrottetii (A. Rich). Merr. extracts. J Med Plants Res. 2009;3:511–518. [Google Scholar]
- 11.Rechinger KH. Artemisia. In: Rechinger K.H., Hedge I.C., editors. Vol. 158. AcademischeDruck and Verlagsanstalt; Graz, Austria: 1980. pp. 185–216. (Flora of Iranica, compositae). [Google Scholar]
- 12.Lopez-Lutz D, Alviano SD, Alviano SC, Kolodziejczyk PP. Screening of chemical composition of microbial and antioxidant activities of Artemisia essential oils. Flavour Frag J. 2008;8:131–137. doi: 10.1016/j.phytochem.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 13.Hamad KJ, Al-Shaheen SJA, Kaskoos RA, Ahamad J, Mir SR. Essential oil composition and antioxidant activity of Lavandula angustifolia from Iraq. Int J Pharm. 2013;4:117–120. [Google Scholar]
- 14.Cavanagh H, Wilkinson J. Biological activities of lavender essential oil. Phytother Res. 2002;16:301–308. doi: 10.1002/ptr.1103. [DOI] [PubMed] [Google Scholar]
- 15.Mehrorosh H, Gavanji S, Larki B, Mohammadi MD, Karbasiun A, Bakhtari A. Essential oil composition and antimicrobial screening of some Iranian herbal plants on Pectobacterium carotovorum. Global NEST J. 2014;16:240–250. [Google Scholar]
- 16.Edwards-Jones V, Buck R, Shawcross SG, Dawson MM, Dunn K. The effect of essential oils on methicillin-resistant Staphylococcus aureus using a dressing model. Burns. 2004;30:772–777. doi: 10.1016/j.burns.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Hosseinzadeh H, Ramezani M, Salmani G. Antinociceptive, anti-inflammatory and acute toxicity effects of Zataria multiflora Boiss extracts in mice and rats. J Ethnopharmacol. 2000;73:379–385. doi: 10.1016/s0378-8741(00)00238-5. [DOI] [PubMed] [Google Scholar]
- 18.Ramezani M, Hosseinzadeh H, Samizadeh S. Antinociceptive effects of Zataria multiflora Boiss fractions in mice. J Ethnopharmacol. 2004;91:167–170. doi: 10.1016/j.jep.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 19.Ghani A, Ebrahimpour A, Tehranifar A, Hassanzadeh Khayat M. Evaluation of growth and development adaptability and medicinal–ornamental potential of Clary sage (Salvia sclarea L.) cultivated in Mashhad climatic conditions. J Plant Prod. 2010;17:77–90. [Google Scholar]
- 20.Adams RP. Identification of essential oil components by gas chromatography-quadropole mass spectroscopy. J Am Soc Mass Spectrom. 2005;16:1902–1903. [Google Scholar]
- 21.Sparkman OD. Identification of essential oil components by gas chromatography/quadrupole mass spectroscopy. J Am Soc Mass Spectrom. 1997;8:671–672. [Google Scholar]
- 22.Van Den Dool H, Kratz PD. A generalization of the retention index system including linear temperature programmed gas–liquid partition chromatography. J Chromatogr. 1963;11:463–471. doi: 10.1016/s0021-9673(01)80947-x. [DOI] [PubMed] [Google Scholar]
- 23.Khosravi AD, Behzadi A. Evaluation of the antibacterial activity of the seed hull of Quercus brantii on some Gram negative bacteria. Pakistan J Med Sci. 2006;22:429–432. [Google Scholar]
- 24.Şahin F, Güllüce M, Daferera D, Sökmen A, Sökmen M, Polissiou M. Biological activities of the essential oils and methanol extract of Origanum vulgare ssp. vulgare in the Eastern Anatolia region of Turkey. Food Control. 2004;15:549–557. [Google Scholar]
- 25.Rota C, Carraminana J, Burillo J, Herrera A. In vitro antimicrobial activity of essential oils from aromatic plants against selected foodborne pathogens. J Food Prot. 2004;67:1252–1256. doi: 10.4315/0362-028x-67.6.1252. [DOI] [PubMed] [Google Scholar]
- 26.Buttke TM, McCubrey JA, Owen TC. Use of an aqueous soluble tetrazolium/formazan assay to measure viability and proliferation of lymphokine-dependent. Cancer Commun. 1991;3:207–212. [Google Scholar]
- 27.Plumb JA, Milroy R, Kaye SB. Effects of the pH dependence of 3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyl-tetrazolium bromide-formazan absorption on chemosensitivity determined by a novel tetrazolium-based assay. Cancer Res. 1989;49:4435–4440. [PubMed] [Google Scholar]
- 28.Besharat M, Rahimian M, Ghaemi E, Besharat S. Effect of ethanolic extract of Adiantum capillus veneris in comparison with Gentamicine on 3 pathogenic bacteria in vitro. Pharm Sci. 2009;15:49–52. [Google Scholar]
- 29.Weinstine RA. Controlling antimicrobial resistance in hospitals: infection control and use of antibiotics. Emerg Infect Dis. 2001;7:188–192. doi: 10.3201/eid0702.010206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lanciotti R, Gianatti A, Patrignani F, Belletti N, Guerzoni ME, Gardini F. Use of natural aroma compounds to improve shelf life and safety of minimally processed fruits. J Food Sci Technol. 2004;15:201–208. [Google Scholar]
- 31.Murphy Cowan M. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;2:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gavanji S, Larki B, Bakhtari A. The effect of extract of Punica granatum var. pleniflora for treatment of minor recurrent aphthous stomatitis. Integr Med Res. 2014;3:83–90. doi: 10.1016/j.imr.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stanojević L, Stanković M, Cakić M, Nikolić V, Nikolić L, Ilić D. The effect of hydrodistillation techniques on yield, kinetics, composition and antimicrobial activity of essential oils from flowers of Lavandula officinalis L. Hem Ind. 2011;65:455–463. [Google Scholar]
- 34.Sajed H, Sahebkar A, Iranshahi M. Zataria multiflora Boiss. (Shirazi thyme)—an ancient condiment with modern pharmaceutical uses. J Ethnopharmacol. 2013;145:686–698. doi: 10.1016/j.jep.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 35.Mahboobi M, Shahcheraghi F, Feizabadi MM. Bactericidal effects of essential oils from clove, lavender and geranium on multi-drug resistant isolates of Pseudomonas aeruginosa. Iranian J Biotechnol. 2006;4:137–140. [Google Scholar]
- 36.Motevasel M, Zomorodian K, Ashraf Mansouri M, Farshad S, Haghighhat A, Hadaegh M. The anti-bacterial effects of Zataria multiflora extract on common pathogenic Gram positive cocci, pathogenic Gram negative bacilli and non-pathogenic bacteria. Afr J Microbiol Res. 2011;5:4993–4996. [Google Scholar]
- 37.Sharififar F, Moshafi MH, Mansouri SH, Khodashenas M, Khoshnoodi M. In vitro evaluation of antibacterial and antioxidant activities of the essential oil and methanol extract of endemic Zataria multiflora Boiss. Food Control. 2007;18:800–805. [Google Scholar]
- 38.Owlia P, Saderi H, Rasooli I, Sefidkon F. Antimicrobial characteristics of some herbal oils on Pseudomonas aeruginosa with special reference to their chemical compositions. Iranian J Pharmacol Res. 2009;8:107–114. [Google Scholar]
- 39.El-Shouny WA, Magaam S. Sensitivity of multi-drug resistant Pseudomonas aeruginosa isolated from surgical wound-infections to essential oils and plant extracts. World J Med Sci. 2009;4(2):104–111. [Google Scholar]
- 40.Imelouane B, Amhamdi H, Wathelet JP, Ankit M, Khedid K, El Bachiri A. Chemical composition and antimicrobial activity of essential oil of thyme (Thymus vulgaris) from Eastern Morocco. Int J Agric Biol. 2009;11:205–208. [Google Scholar]
- 41.Abbasgholizadeh N, Ettehad GH, Arab R, Nemati A, Barak M, Pirzadeh A. Antibacterial effect of Zataria multiflora Boiss (Shiraz oregano essence) on Entrobactericea species. Res J Biol Sci. 2008;3:345–347. [Google Scholar]
- 42.Govaris A, Solomakos N, Pexara A, Chatzopoulou PS. The antimicrobial effect of oregano essential oil, nisin and their combination against Salmonella enteritidis in minced sheep meat during refrigerated storage. Int J Food Microbiol. 2010;137:175–180. doi: 10.1016/j.ijfoodmicro.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 43.Yousefzadi M, Sonboli A, Ebrahimi SN, Hashemi SH. Atividade antimicrobiana do oleo essencial e principais constituintes dos Chloroleuca salvia. Z Naturforsch. 2008;63:337–340. doi: 10.1515/znc-2008-5-605. [DOI] [PubMed] [Google Scholar]
- 44.Shafiee A, Javidnia K, Tabatabai M. Volatile constituents and antimicrobial activity of Zataria multiflora, population Iran. Iranian J Chem Chem Eng. 1999;18:1–5. [Google Scholar]
- 45.Mahboubi M, GhazianBidgoli F. In vitro synergistic efficacy of combination of amphotericin B with Myrtus communis essential oil against clinical isolates of Candida albicans. Phytomedicine. 2010;17:771–774. doi: 10.1016/j.phymed.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 46.Saei-Dehkordi SS, Tajik H, Moradi M, Khalighi-Sigaroodi F. Chemical composition of essential oils in Zataria multiflora Boiss from different parts of Iran and their antioxidant and antimicrobial efficacy. Food Chem Toxicol. 2010;48:1562–1567. doi: 10.1016/j.fct.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 47.Orhan IE, Ozcelik B, Kartal M, Kan Y. Antimicrobial and antiviral effects of essential oils from selected Umbelliferae and Labiatae plants and individual essential oil components. Turk J Biol. 2012;36:239–246. [Google Scholar]
- 48.Hajhashemi V, Ghannadi A, Sharif B. Antiinflammatory and analgesic properties of the leaf extracts and essential oil of Lavandula angustifolia Mill. J Ethnopharmacol. 2003;89:67–71. doi: 10.1016/s0378-8741(03)00234-4. [DOI] [PubMed] [Google Scholar]
- 49.Sardashti AR, Pourramazani Harati M. Chemical composition of the essential oil of A. kermanensis from Taftanaera by GC/MS technique. Int J Agric Crop Sci. 2012;4:561–563. [Google Scholar]
- 50.Soković M, Marin PD, Brkić D, van Griensven LJLD. Chemical composition and antibacterial activity of essential oils of ten aromatic plants against human pathogenic bacteria. Food. 2007;1:220–226. [Google Scholar]
- 51.Derakhshan S, Sattari M, Bigdeli M, Zarei-Eskikand N. Antibacterial activity of essential oils from Artemisia and Cumin plants against Staphylococcus aureus, Escherichia coli and Vibrio cholera. J Qazvin Med Sci. 2011;1:6–14. [Google Scholar]
- 52.Blagojevic P, Radulovic N, Palic R, Stojanovic G. Chemical composition of the essential oils of Serbian wild-growing Artemisia absinthium and Artemisia vulgaris. J Agric Food Chem. 2006;54:4780–4789. doi: 10.1021/jf060123o. [DOI] [PubMed] [Google Scholar]
- 53.Konatchiev A, Todorova M, Mikhova B, Vitkova A, Najbensli H. Composition and antimicrobial activity of Artemisia distans essential oil. Nat Prod Commun. 2011;6:905–906. [PubMed] [Google Scholar]
- 54.Tassou CC, Nychas GJ. Antimicrobial activity of the essential oil of Mastic fum on gram positive and gram negative bacteria in broth and model food systems. Int Biodeter Biodegrad. 1995;36:411–420. [Google Scholar]
- 55.Zakarya D, Fkih-Tetouani S, Hajji F. Chemical composition antimicrobial activity relationship of Eucalyptus essential oils. Plants Med Phytother. 1993;26:319–333. [Google Scholar]
- 56.Kazemi M, Dakhili M, Dadkhah A, Yasrebifar Z, Larijani K. Composition, antimicrobial and antioxidant activities of the essential oil of Artemisia kermanensis Podl., an endemic species from Iran. J Med Plants Res. 2011;5:4481–4486. [Google Scholar]
- 57.Arnold N, Valentini G, Bellomaria B, Hocine L. Comparative study of the essential oils from Rosmarinus eriocalyx Jordan & Fourr from Algeria and R. officinalis L. from other countries. J Essent Oil Res. 1997;9:167–175. [Google Scholar]
- 58.Ghasemi E, Yamini Y, Bahramifar N, Sefidkon F. Comparative analysis of the oil and supercritical CO2 extract of Artemisia sieberi. J Food Eng. 2007;79:306–311. [Google Scholar]
- 59.Akthar MS, Degaga B, Azam T. Antimicrobial activity of essential oils extracted from medicinal plants against the pathogenic microorganisms: a review. Biol Sci Pharm Res. 2014;2:1–7. [Google Scholar]
- 60.Eftekhar F, Zamani S, Yusefzadi M, Hadian J, Nejad Ebrahimi S. Antibacterial activity of Zataria multiflora Boiss. essential oil against extended spectrum b lactamase produced by urinary isolates of Klebsiella pneumonia. Jundishapur J Microbiol. 2011;4:43–49. [Google Scholar]
- 61.Zaringhalam M, Sattari M, Zaringhalam J, Rezazadeh S. Effect of black pepper, red pepper and Zataria multiflora Boiss. alcoholic extracts on growth and DNase activity of Staphylococcus aureus. J Med Plants. 2007;6:17–21. [Google Scholar]
- 62.Parsaeimehr M, Basti AA, Radmehr B, Misaghi A, Abbasifar A, Karim G. Effect of Zataria multiflora Boiss. essential oil, nisin, and their combination on the production of enterotoxin C and alfa-hemolysin by Staphylococcus aureus. Foodborne Pathog Dis. 2010;7:299–305. doi: 10.1089/fpd.2009.0416. [DOI] [PubMed] [Google Scholar]