Abstract
The worldwide incidence of diabetes mellitus has reached alarming proportions. Persistent hyperglycemia due to impaired insulin activity and/or insulin resistance inversely affects the retina, cerebrovascular system, kidney, peripheral limbs, and other parts of the body, which leads to life-threatening complications. The causal role of oxidative stress in the development and progression of diabetic complications has been emphasized. Polyphenols present in natural products have gained much attention in recent decades in preventive studies against diabetes-associated pathologies. In the present review, we provide a comparative update on the role of quercetin, myricetin, and resveratrol—the major polyphenols present in red grapes—in intervening with diabetic complications, and a brief highlight on the molecular mechanisms underlying oxidative stress mediated hyperglycemia.
Keywords: diabetes, hyperglycemia, oxidative stress, polyphenols
1. Introduction
Diabetes mellitus is the most common metabolic disorder, being ranked as the fourth most common cause of mortality. According to the International Diabetes Federation, there are approximately 366 million diabetic individuals around the world.1 The Federation assumes that this figure could increase to 552 million by the year 2030.2
Diabetes mellitus is characterized by hyperglycemia due to partial or absolute lack of insulin activity and/or insulin resistance. Hyperglycemia-mediated oxidative stress and chronic inflammatory components in diabetic tissues cause the accumulation of advanced glycation end products (AGEs), lipid peroxidation products, protein carbonyls, and late-stage glycoxidation adducts of proteins that results in toxicity in the cardiovascular system, retina, kidney, peripheral limbs, and other parts of the body.3, 4 These impairments play a major role in the development of diabetic complications.5, 6
Many factors have been implicated to play crucial role in the development of hyperglycemia and progression of diabetes. Physical inactivity, sedentary lifestyle, flawed dietary attributes, being overweight, and obesity are some of the factors directly associated with insulin resistance, followed by state of impaired glucose metabolism, and eventually diabetes.7, 8 An evaluation of all the factors contributing to the development of diabetes has been conducted, wherein obesity and dietary attributes are considered major contributors.9, 10 It has been reported that glucose tolerance and insulin sensitivity may be modified by both the quantity as well as the quality of diet.11
Plant polyphenols are among the most abundant phytochemicals present in the human diet. Polyphenols are the secondary metabolites synthesized by plants as part of their defense mechanism for survival during adverse conditions and to provide resistance against microbial infections.12, 13 Based on the large quantity of data available through clinical and epidemiological studies, polyphenols have received considerable interest for their presumed role in the prevention of various degenerative diseases.14, 15, 16 Antidiabetic effect is one of the most intensely studied biological roles of polyphenols.15, 17 Grapes and their products, including red wine, are largely consumed dietary components all over the word. The observation of a lower incidence of coronary heart disease despite a high-fat diet in the French population, commonly referred to as the “French paradox”, has also been attributed to a copious consumption of red wine made from grapes. This has stimulated interest in investigating whether grape polyphenols may offer consequential health benefits, including improved insulin sensitivity.18, 19 The present review is a comparative study of the antidiabetic effects of quercetin, myricetin, and resveratrol, the major polyphenols present in red grapes, incorporating a brief account of the molecular mechanism involved in the prevalence of hyperglycemia. The criteria for selection of papers for the present study was adopted from the method described by Siwek et al.20 The paper search was carried out using online sources of evidence-based reviews databases such as the Center for Research Support, TRIP Database (http://www.tripdatabase.com/index.html), American College of Physicians Journal Club (http://acpjc.acponline.org), and Medline (http://www.ncbi.nlm.nih.gov/pubmed/).
2. Role of oxidative stress in hyperglycemia-induced diabetic complications
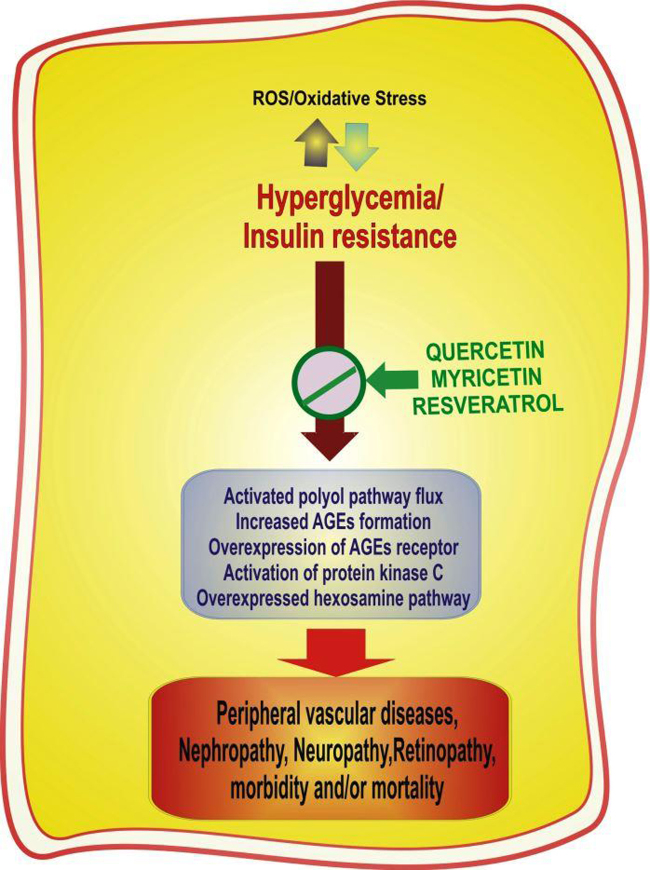
Ample evidence exists showing that oxidative stress plays a pivotal role in the development of diabetes and associated complications. Oxidative stress damages the vital cellular molecules including proteins, lipids, and DNA, resulting in functional loss and ultimately impaired cellular physiology.21 Studies have reported alterations in ion transporters, membrane integrity, and redox imbalance in erythrocytes of diabetic humans.6, 21 Oxidative stress mediated insulin resistance, dysfunctional pancreatic islets, and tissue damage lead to late pathological consequences of diabetes.22, 23 Because pancreatic islets possess a low level of intrinsic antioxidant enzymes such as superoxide dismutase, catalase, and glutathione peroxidase, they remain at greater risk of damage from reactive oxygen species (ROS).24, 25 Laboratory and clinical studies have radially reported that most of the mechanisms involved in the tissue damage and development of diabetic complications during hyperglycemia are activated by the overproduction of ROS in the cells.4, 6 Receptors for AGE binding have been reported to induce the production of ROS, which in turn activates the pleotropic transcription nuclear factor κB, causing multiple pathological changes in gene expression.26 ROS have also been reported to influence the polyol pathway and activate protein kinases C (PKC), which serve as further pathways mediating tissue damage during diabetes.6
2.1. Increased activity of the polyol pathway
The polyol pathway is a combination of aldo-ketoreductase enzymes that reduce glucose and other carbonyl compounds into respective sugar alcohols by using nicotinamide adenine dinucleotide phosphate (NADPH). In this pathway, glucose is first converted into sorbitol through an NADPH-dependent reaction by the enzyme aldose reductase and later oxidized to fructose by sorbitol dehydrogenase.6 Normally, this pathway contributes very little in the metabolism of glucose in blood; however, during hyperglycemia the activity of this system increases by many folds, resulting in excess conversion of glucose into sorbitol, which leads to enhanced consumption of NADPH. Excess consumption of NADPH leads to depletion of the reduced glutathione (GSH) level because NADPH is required to regenerate GSH. GSH, as a primary scavenger of ROS, plays a very crucial role in maintaining the cellular redox state. A diminished regeneration of GSH adds burden to oxidative stress during diabetes.22, 23 Moreover, decreased glutathiolation of cellular proteins has been related to the reduced availability of nitric oxide that would diminish S-nitrosoglutathione.6 Increased polyol pathway flux also leads to an increase in the cytosolic NADH/NAD+ ratio, thereby inhibiting the activity of the enzyme glyceraldehyde-3-phosphate dehydrogenase, which leads to an increase in the concentration of triose phosphate. Elevated levels of triose phosphate can increase the generation of methylglyoxal (precursor of AGEs) and diacylglycerol.23, 27 Moreover, the accumulation of sorbitol generates reciprocal depletion of taurine. Taurine is an intracellular osmolyte and endogenous antioxidant, the depletion of which may compromise the antioxidative defense in many ways. Thus, the activated polyol pathway may contribute to the oxidative burden through cofactor as well as osmotic mechanisms.28
2.2. Elevated AGEs and PKC
AGEs are generated by the nonenzymatic reaction of glucose with proteins.27 AGEs and their precursors are highly reactive; they interact with intracellular proteins as well as other matrix compounds and alter their functions. In addition, AGE receptors binding to endothelial cells appear to mediate, in part, increased vascular permeability and impaired wound healing.6, 27
AGEs are found in almost all tissues examined in diabetic rats and diabetic humans, but their susceptibility to AGE formation differs.21 Cells of the liver, kidney, and erythrocytes possess a higher susceptibility towards AGE formation. AGE-modified proteins adversely affect many cellular processes including cell adhesion and aggregation.29 AGEs appear to play a central role in heart failure and increased mortality after ischemic events in diabetic patients.21
PKCs are widely distributed in all mammalian tissues. They phosphorylate many target proteins. The activity of PKC depends on calcium ion, phosphatidyl serine, and diacylglycerol.30 It has been suggested that interaction between AGEs and cell surface receptors enhances the expression of PKC and their isoforms.31 Elevation in diacylglycerol concentration due to increased polyol pathway flux also causes activation in PKC. The activation of PKC has a number of pathogenic consequences including abnormalities in blood flow and permeability by affecting the expression of endothelial nitric oxide synthase.6, 32 The persistence of high glucose levels induces fatty acid oxidation, which contributes to the pathogenesis of diabetic complications through increasing the flux of fructose-6-phophate into the hexamine pathway.6, 23 In the hexamine pathway, fructose-6-phosphate is used to synthesize uridine diphosphate-N-acetylglucosamine, which is used by specific O-N-acetylglucosamine (GlcNAc) transferases for posttranslational modification of specific serine and threonine residues on cytoplasmic and nuclear proteins by O-GlcNAcylation. Hyperglycemia-mediated increased hexosamine pathway flux increases the O-GlcNAcylation of the transcription factors and gene transcription, leading to changes in both gene expression and protein function, which together contribute to the pathogenesis of diabetic complications.24, 33
3. Antidiabetic interventions and plant polyphenols
A large number of conventional drugs are used to manage hyperglycemia; however, most of them fail to provide long-term control. Antidiabetic agents include sulfonylureas, biguanides, metformin, glinides, and insulin, many of which have serious adverse side effects.34 The rising trend in the prevalence of diabetes and associated complications all over the world suggests that existing medical treatments for diabetic pathologies are not sufficient and use of supplementary/complimentary treatments such as functional foods and their nutraceuticals may enhance the effectiveness of diabetic management.35, 36 Among the known bioactive compounds and phytochemicals, plant polyphenols have gained much attention and popularity because of their antihyperglycemic effects and minimal side/adverse effects.37 The reported beneficial health effects of polyphenols have led to an upsurge in scientific interest in these natural compounds during the past decade. Many laboratory studies and clinical trials on animals and on humans have proposed plant polyphenols to be effective in a complimentary role for diabetes management.37, 38
The antihyperglycemic effects of polyphenols have been attributed to many biological properties including reduction in intestinal absorption of dietary carbohydrates, modulation of the activities of enzymes involved in glucose metabolism, protection of β cell from oxidative injury, and stimulation of insulin secretion and action.37, 39 Other significant complimentary roles of polyphenols have been observed in the treatment of cardiovascular problems in diabetic patients. Studies have revealed that the regulation of lipid and lipoprotein metabolism and improvement of dyslipidemia may be the factors behind the improvement of vascular functions in diabetic patients receiving polyphenol supplementation.40 Moreover, alleviation of oxidative stress and stress-sensitive pathways and inflammatory processes have also been reported that highlight the biological roles of plant polyphenols, which establish their putative complimentary role against diabetic complications.37 In the following sections, we review evidence-based studies on the antidiabetic effects of quercetin, myricetin, and resveratrol.
3.1. Quercetin
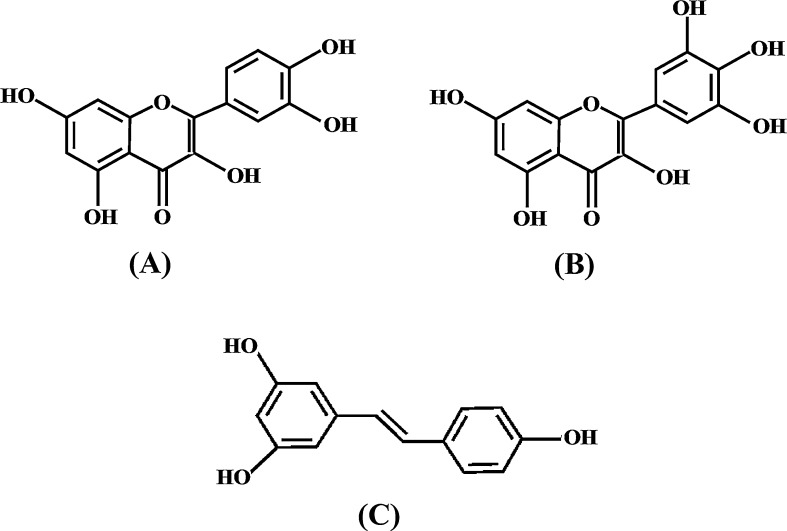
Quercetin (3,3′,4′,5,7-pentahydroxyflavone) is one of the most studied polyphenolic compounds because of its pleotropic biological properties including its anti-inflammatory, antioxidative, and neuroprotective activities.41, 42 Belonging to the flavonoid group of polyphenols, quercetin contains three rings and five hydroxyl groups in its structure (Fig. 1).
Fig. 1.
Chemical structures of (A) quercetin, (B) myricetin, and (C) resveratrol.
Many in vitro as well as in vivo studies have reported the significant protective potential of quercetin against diabetic complications.42, 43 Supplementation of quercetin up to 10 mg/kg body weight/day for 10 weeks improved the insulin resistance in genetically obese Zucker rats.44 In another study, Wistar rats on a high fructose diet supplemented with 25 mg quercetin/kg body weight/day were reported to show a higher expression of adiponectin in white adipose tissue and blood concentration, despite an inhibition of poly(ADP-ribose) polymerase γ expression.45 It is concluded that the effect of quercetin on adiponectin was poly(ADP-ribose) polymerase γ-independent. Because adiponectin is an adipokine, which facilitates insulin action, it is proposed that the increase in adiponectin level might be a factor responsible for the improvement in insulin sensitivity induced by quercetin.45
Recently, Kim et al46 reported a reduction in serum glucose level and glycated hemoglobin in C57BL/KsJdb–db mice supplemented with quercetin for 7 weeks. The study performed by Coskun et al.47 on diabetic rats showed that quercetin has the ability to protect streptozotocin-induced damage in β cells and thereby normalize the serum glucose level. Likewise, Kobori et al48 reported that quercetin in the diet led to the recovery of cell proliferation in streptozotocin-induced diabetic mice. Quercetin in micromolar quantities has been reported to protect lipid peroxidation in oxidatively stressed human erythrocytes obtained from diabetic individuals. Moreover, quercetin significantly restored the depleted level of cellular antioxidant molecules in diabetic cells during oxidative stress.43
Different mechanisms have been reported by which quercetin may elicit an antidiabetic effect. Glycemic control achieved by quercetin has been attributed to the inhibition of glucose uptake at the level of glucose transporters (GLUTs).42 The study performed by Kwon et al49 to evaluate the effect of quercetin on Caco-2E intestinal cells documented that the transport of fructose and glucose by GLUT2 was strongly inhibited by quercetin. Blockage of tyrosine kinase is another mechanism by which quercetin is reported to express effects against diabetes.49 Furthermore, mitogen-activated protein kinase pathway modulation is also a reported mechanism by which quercetin performs action against hyperglycemia and associated consequences.41
3.2. Myricetin
Myricetin, chemically known as 3,5,7,3′,4′,5′-hexahydroxyflavone (Fig. 1), belongs to the flavonol group of polyphenols. An array of health-promoting effects of myricetin has been demonstrated.50, 51 Apart from myricetin's antioxidative, antiviral, and anticarcinogenic activities, consumption of myricetin and reduced risk of diabetes have been reported in many studies.51, 52
Myricetin is used as a traditional medicine in northern Brazil to control hyperglycemia.52 Studies performed on diabetic rats revealed that myricetin prevents the condition of hyperglycemia through different mechanisms. Myricetin has the ability to promote glucose uptake in soleus muscles and the liver, and enhance hepatic glycogen synthase activity and thus glycogen synthesis in the hepatocytes of diabetic rats.53, 54
It has been observed that myricetin may also elicit antidiabetic effects via the amelioration of insulin resistance. Because insulin resistance is the most common etiology of diabetes, improving insulin sensitivity followed by amelioration of insulin resistance seems to be crucial for the management of diabetes.55 A study performed by Liu et al55 showed that repeated intravenous injection of myricetin for 14 days increased the whole-body insulin sensitivity and decreased the higher degree of insulin resistance in fructose chow-fed rats.54 Recently, Ding et al52 reported that myricetin-attenuated hyperinsulinemia induced insulin resistance in skeletal muscle cells by increasing AMP-activated protein kinase activity in C2C12 myotubes.52
During a drug screening study among 30 bioflavonoids on glucose uptake under normal and insulin-stimulated conditions in C2C12 myotubes, it was found that myricetin was the only compound that stimulated insulin-mediated lipogenesis.52 The insulin-mimicking effect of myricetin has also been reported on glucose transport in adipocytes of rats with noninsulin-dependent diabetes.53
Myricetin is reported to have a protective effect on many oxidative stress-induced cellular abnormalities during diabetes.51, 56 There is strong evidence that myricetin could effectively remove ROS owing to a large number of active hydroxyl groups in its structure.51 A study performed on diabetic human volunteers of both sexes with a mean age of 58 ± 7 years, showed that myricetin at micromolar concentrations significantly protected the peroxidation of erythrocyte membrane lipids and oxidation of proteins, which was increased during oxidative stress.56 Myricetin has also been reported to inhibit AGEs generation in diabetics.5 In addition, the antihyperlipidemic and inhibition of human pancreatic α-amylase is another effect through which myricetin may elicit a strong antidiabetic effect.55
3.3. Resveratrol
Resveratrol (3,4′,5-trihydroxystilbene), which is a phytoalexin, belongs to the stilbene class of polyphenolic compounds (Fig. 1).57 Fresh grape skin contains about 50–100 μg of resveratrol per gram wet weight; its concentration is especially high in red wine, which is made from grapes.58 Despite being discovered in 1940, resveratrol was not a popular polyphenol in laboratory studies until the 1990s. In 1992, Renaud and de Lorgeril59 first postulated that resveratrol present in the red wine might play a major role in the prevention of cardiovascular diseases among those who consume red wine regularly. Later experimental evidence proved that resveratrol possesses strong antioxidative, anti-inflammatory, cardioprotective, and neuroprotective effects.60, 61
Studies performed on a number of laboratory models and on human volunteers have shown that resveratrol can elicit a strong antidiabetic effect.62, 63 In 2006, Orhan et al64 reported that the extract of Vitis vinifera leaves, in which resveratrol is present in considerable amounts, exhibited significant antihyperglycemic and antioxidant activity equipotent with the reference hypoglycemic agent, tolbutamide. Another study performed by Fujii et al65 in the same year documented that polyphenolic compounds including resveratrol obtained from grape seeds, showed protection against high glucose-induced oxidative stress generally observed under diabetic conditions. Resveratrol has also been shown to provide protection against diabetic nephropathy (Fig. 2).66 Treatment of diabetic rats with resveratrol demonstrated the amelioration of renal dysfunction and oxidative stress.66 Impaired cellular homeostasis is a common condition in diabetes, responsible for the development of many associated cellular as well as organ dysfunctions. Resveratrol has been reported to restore cellular homeostasis significantly via the activation of the plasma membrane redox system, which operates in the cell as a compensatory mechanism to maintain the redox state.67, 68 The administration of resveratrol to diabetic rats resulted in diminished levels of glycosylated hemoglobin.69
Fig. 2.
Schematic representation of the factors and mechanisms involved in the development of complications in diabetes mellitus and the role of quercetin, myricetin, and resveratrol in the prevention of diabetic pathologies.
It has been proposed that the antihyperglycemic effect of resveratrol observed in diabetic animals is attributable to the stimulatory action of resveratrol on intracellular glucose transport. The presence of resveratrol caused increased glucose uptake by different cells isolated from diabetic rats.70 The study on diabetic rats showed increased expression of the insulin-dependent glucose transporter (GLUT4) after resveratrol ingestion.70, 71 Resveratrol has also been reported to modulate the activity of sirtuin-1, which improves whole-body glucose homeostasis and insulin sensitivity in diabetic rats.70
4. Conclusion
Diabetes mellitus can compromise life quality and expectancy in many ways. Molecular mechanisms involved in the degenerative consequences of hyperglycemia are mediated by oxidative stress. Studies performed on model systems as well as human trails provide evidence that quercetin, myricetin, and resveratrol possess a strong ability to ameliorate biological events responsible for hyperglycemia. These polyphenols thus provide an alternative to conventional medicines toward prevention and management of diabetes and related complications either alone or in combination with other therapies.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
The work is supported by the Council of Scientific and Industrial Research (CSIR), New Delhi, India, in the form of a research associateship to KBP.
References
- 1.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34:62–69. [Google Scholar]
- 2.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 3.Kaneko M, Bucciarelli L, Hwang YC, Lee L, Yan SF, Schmidt AM. Aldose reductase and AGE–RAGE pathways: key players in myocardial ischemic injury. Ann N Y Acad Sci. 2005;1043:702–709. doi: 10.1196/annals.1333.081. [DOI] [PubMed] [Google Scholar]
- 4.Pandey KB, Mishra N, Rizvi SI. Protein oxidation biomarkers in plasma of type 2 diabetic patients. Clin Biochem. 2010;43:508–511. doi: 10.1016/j.clinbiochem.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Peyroux J, Sternberg M. Advanced glycation endproducts (AGEs): pharmacological inhibition in diabetes. Pathol Biol (Paris) 2006;54:405–419. doi: 10.1016/j.patbio.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steyn NP, Mann J, Bennett PH, Temple N, Zimmet P, Tuomilehto J. Diet, nutrition and the prevention of type 2 diabetes. Public Health Nutr. 2004;7:147–165. doi: 10.1079/phn2003586. [DOI] [PubMed] [Google Scholar]
- 8.Grundy SM. Pre-diabetes, metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol. 2012;59:635–643. doi: 10.1016/j.jacc.2011.08.080. [DOI] [PubMed] [Google Scholar]
- 9.Russo SB, Ross JS, Cowart LA. Sphingolipids in obesity, type 2 diabetes, and metabolic disease. Handb Exp Pharmacol. 2013;216:373–401. doi: 10.1007/978-3-7091-1511-4_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roper NA, Bilous RW, Kelly WF, Unwin NC, Connolly VM. Excess mortality in a population with diabetes and the impact of material deprivation: longitudinal, population based study. BMJ. 2001;322:1389–1393. doi: 10.1136/bmj.322.7299.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imam K. Management and treatment of diabetes mellitus. Adv Exp Med Biol. 2012;771:356–380. doi: 10.1007/978-1-4614-5441-0_26. [DOI] [PubMed] [Google Scholar]
- 12.Williamson G. Possible effects of dietary polyphenols on sugar absorption and digestion. Mol Nutr Food Res. 2013;57:48–57. doi: 10.1002/mnfr.201200511. [DOI] [PubMed] [Google Scholar]
- 13.Rochette L, Ghibu S, Richard C, Zeller M, Cottin Y, Vergely C. Direct and indirect antioxidant properties of α-lipoic acid and therapeutic potential. Mol Nutr Food Res. 2013;57:114–125. doi: 10.1002/mnfr.201200608. [DOI] [PubMed] [Google Scholar]
- 14.Pandey KB, Rizvi SI. Current understanding of dietary polyphenols and their role in health and disease. Curr Nutr Food Sci. 2009;5:249–263. [Google Scholar]
- 15.Pandey KB, Rizvi SI. Recent advances in health promoting effect of dietary polyphenols. Curr Nutr Food Sci. 2012;8:254–264. [Google Scholar]
- 16.Arts ICW, Hollman PCH. Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr. 2005;81 doi: 10.1093/ajcn/81.1.317S. 317S–25S. [DOI] [PubMed] [Google Scholar]
- 17.Anhea FF, Desjardinsb Y, Pilona G, Dudonneb S, Genovesec MI, Lajoloc FM. Polyphenols and type 2 diabetes: a prospective review. Pharma Nutr. 2013;1:105–114. [Google Scholar]
- 18.Terra X, Montagut G, Bustos M, Lopiz N, Ardevol A, Blade C. Grape-seed procyanidins prevent low grade inflammation by modulating cytokine expression in rats fed a high-fat diet. J Nutr Biochem. 2009;20:210–218. doi: 10.1016/j.jnutbio.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Pinent M, Blay M, Blade MC, Salvadó MJ, Arola L, Ardévol A. Grape seed-derived procyanidins have an antihyperglycemic effect in streptozotocin-induced diabetic rats and insulinomimetic activity in insulin-sensitive cell lines. Endocrinology. 2004;145:4985–4990. doi: 10.1210/en.2004-0764. [DOI] [PubMed] [Google Scholar]
- 20.Siwek J, Gourlay ML, Slawson DC, Shaughnessy AF. How to write an evidence-based clinical review article. Am Fam Physician. 2002;65:251–258. [PubMed] [Google Scholar]
- 21.Niedowicz DM, Daleke DL. The role of oxidative stress in diabetic complications. Cell Biochem Biophys. 2005;43:289–330. doi: 10.1385/CBB:43:2:289. [DOI] [PubMed] [Google Scholar]
- 22.Stadler K. Oxidative stress in diabetes. Adv Exp Med Biol. 2012;771:272–287. doi: 10.1007/978-1-4614-5441-0_21. [DOI] [PubMed] [Google Scholar]
- 23.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 24.Robertson RP. Oxidative stress and impaired insulin secretion in type 2 diabetes. Curr Opin Pharmacol. 2006;6:615–619. doi: 10.1016/j.coph.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Halliwell B, Gutteridge JMC. Cellular responses to oxidative stress: adaptation, damage, repair, senescence and death. In: Halliwell B., Gutteridge J.M.C., editors. Free radicals in biology and medicine. 4th ed. Oxford University Press; New York: 2007. pp. 187–267. [Google Scholar]
- 26.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- 27.Yamagishi S, Maeda S, Matsui T, Ueda S, Fukami K, Okuda S. Role of advanced glycation end products (AGEs) and oxidative stress in vascular complications in diabetes. Biochim Biophys Acta. 2012;1820:663–671. doi: 10.1016/j.bbagen.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 28.Greene DA, Stevens MJ, Obrosova I, Feldman EL. Glucose-induced oxidative stress and programmed cell death in diabetic neuropathy. Eur J Pharmacol. 1999;375:217–223. doi: 10.1016/s0014-2999(99)00356-8. [DOI] [PubMed] [Google Scholar]
- 29.Candiloros H, Muller S, Ziegler O, Donner M, Drouin P. Role of albumin glycation on the erythrocyte aggregation: an in vitro study. Diabet Med. 1996;13:646–650. doi: 10.1002/(SICI)1096-9136(199607)13:7<646::AID-DIA139>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 30.Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res. 2010;106:1319–1323. doi: 10.1161/CIRCRESAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Derubertis FR, Craven PA. Activation of protein kinase C in glomerular cells in diabetes: mechanism and potential links to the pathogenesis of diabetic glomerulopathy. Diabetes. 1994;43:1–8. doi: 10.2337/diab.43.1.1. [DOI] [PubMed] [Google Scholar]
- 32.Kuboki K, Jiang ZY, Takahara N, Ha SW, Igarashi M, Yamauchi T. Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo: a specific vascular action of insulin. Circulation. 2000;101:676–681. doi: 10.1161/01.cir.101.6.676. [DOI] [PubMed] [Google Scholar]
- 33.Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci U S A. 2000;97:12222–12226. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel DK, Prasad SK, Kumar R, Hemalatha S. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac J Trop Biomed. 2012;2:320–330. doi: 10.1016/S2221-1691(12)60032-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sabu MC, Kuttan R. Anti-diabetic activity of medicinal plants and its relationship with their antioxidant property. J Ethnopharmacol. 2002;81:155–160. doi: 10.1016/s0378-8741(02)00034-x. [DOI] [PubMed] [Google Scholar]
- 36.Tag H, Kalita P, Dwivedi P, Das AK, Namsa ND. Herbal medicines used in the treatment of diabetes mellitus in Arunachal Himalaya, northeast, India. J Ethnopharmacol. 2012;141:786–795. doi: 10.1016/j.jep.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Bahadoran Z, Mirmiran P, Azizi F. Dietary polyphenols as potential nutraceuticals in management of diabetes: a review. J Diabetes Metab Disord. 2013;12:43–52. doi: 10.1186/2251-6581-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009;2:270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnston K, Sharp P, Clifford M, Morgan L. Dietary polyphenols decrease glucose uptake by human intestinal Caco-2 cells. FEBS Lett. 2005;579:1653–1657. doi: 10.1016/j.febslet.2004.12.099. [DOI] [PubMed] [Google Scholar]
- 40.Lecour S, Lamont KT. Natural polyphenols and cardioprotection. Mini Rev Med Chem. 2011;11:1191–1199. doi: 10.2174/13895575111091191. [DOI] [PubMed] [Google Scholar]
- 41.Pandey KB, Rizvi SI. Protection of protein carbonyl formation by quercetin in erythrocytes subjected to oxidative stress. Med Chem Res. 2010;19:186–192. [Google Scholar]
- 42.Aguirre L, Arias N, Macarulla MT, Gracia A, Portillo M. Beneficial effects of quercetin on obesity and diabetes. Open Nutraceuticals J. 2011;4:189–198. [Google Scholar]
- 43.Rizvi SI, Mishra N. Anti-oxidant effect of quercetin on type 2 diabetic erythrocytes. J Food Biochem. 2009;33:404–415. [Google Scholar]
- 44.Rivera L, Morón R, Sánchez M, Zarzuelo A, Galisteo M. Quercetin ameliorates metabolic syndrome and improves the inflammatory status in obese Zucker rats. Obesity (Silver Spring) 2008;16:2081–2087. doi: 10.1038/oby.2008.315. [DOI] [PubMed] [Google Scholar]
- 45.Wein S, Behm N, Petersen RK, Kristiansen K, Wolffram S. Quercetin enhances adiponectin secretion by a PPAR-gamma independent mechanism. Eur J Pharm Sci. 2010;41:16–22. doi: 10.1016/j.ejps.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 46.Kim JH, Kang MJ, Choi HN, Jeong SM, Lee YM, Kim JI. Quercetin attenuates fasting and postprandial hyperglycemia in animal models of diabetes mellitus. Nutr Res Pract. 2011;5:107–111. doi: 10.4162/nrp.2011.5.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coskun O, Kanter M, Korkmaz A, Oter S. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and β-cell damage in rat pancreas. Pharmacol Res. 2005;51:117–123. doi: 10.1016/j.phrs.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 48.Kobori M, Masumoto S, Akimoto Y, Takahashi Y. Dietary quercetin alleviates diabetic symptoms and reduces streptozotocin-induced disturbance of hepatic gene expression in mice. Mol Nutr Food Res. 2009;53:859–868. doi: 10.1002/mnfr.200800310. [DOI] [PubMed] [Google Scholar]
- 49.Kwon O, Eck P, Chen S, Corpe CP, Lee JH, Kruhlak M. Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. FASEB J. 2007;21:366–377. doi: 10.1096/fj.06-6620com. [DOI] [PubMed] [Google Scholar]
- 50.Pandey KB, Mishra N, Rizvi SI. Protective Role of myricetin on markers of oxidative stress in human erythrocytes subjected to oxidative stress. Nat Prod Commun. 2009;4:21–26. [PubMed] [Google Scholar]
- 51.Li Y, Ding Y. Therapeutic potential of myricetin in diabetes mellitus. Food Sci Hum Well. 2012;1:19–25. [Google Scholar]
- 52.Ding Y, Dai XQ, Zhang ZF, Li Y. Myricetin attenuates hyperinsulinemia-induced insulin resistance in skeletal muscle cells. Eur Food Res Technol. 2012;234:873–881. [Google Scholar]
- 53.Liu IM, Liou SS, Lan TW, Hsu FL, Cheng JT. Myricetin as the active principle of Abelmoschus moschatus to lower plasma glucose in streptozotocin-induced diabetic rats. Planta Med. 2005;71:617–621. doi: 10.1055/s-2005-871266. [DOI] [PubMed] [Google Scholar]
- 54.Ong KC, Khoo HE. Effects of myricetin on glycemia and glycogen metabolism in diabetic rats. Life Sci. 2005;67:1695–1705. doi: 10.1016/s0024-3205(00)00758-x. [DOI] [PubMed] [Google Scholar]
- 55.Liu IM, Tzeng TF, Liou SS, Lan TW. Myricetin, a naturally occurring flavonol, ameliorates insulin resistance induced by a high-fructose diet in rats. Life Sci. 2007;81:1479–1488. doi: 10.1016/j.lfs.2007.08.045. [DOI] [PubMed] [Google Scholar]
- 56.Pandey KB, Mishra N, Rizvi SI. Myricetin may provide protection against oxidative stress in type 2 diabetic erythrocytes. Z Naturforsch C. 2009;64:626–630. doi: 10.1515/znc-2009-9-1004. [DOI] [PubMed] [Google Scholar]
- 57.Pandey KB, Rizvi SI. Pleiotropic biological effects of resveratrol: implications for human health. Natl Acad Sci Lett. 2009;32:321–326. [Google Scholar]
- 58.Harikumar KB, Aggarwal BB. Resveratrol: a multi targeted agent for age-associated chronic diseases. Cell Cycle. 2008;7:1020–1035. doi: 10.4161/cc.7.8.5740. [DOI] [PubMed] [Google Scholar]
- 59.Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339:1523–1526. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- 60.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 61.Markus MA, Morris BJ. Resveratrol in prevention and treatment of common clinical conditions of aging. Clin Interv Aging. 2008;3:331–339. [PMC free article] [PubMed] [Google Scholar]
- 62.Szkudelski T, Szkudelska K. Anti-diabetic effects of resveratrol. Ann N Y Acad Sci. 2011;1215:34–39. doi: 10.1111/j.1749-6632.2010.05844.x. [DOI] [PubMed] [Google Scholar]
- 63.Timmers S, Hesselink MK, Schrauwen P. Therapeutic potential of resveratrol in obesity and type 2 diabetes: new avenues for health benefits? Ann N Y Acad Sci. 2013;1290:83–89. doi: 10.1111/nyas.12185. [DOI] [PubMed] [Google Scholar]
- 64.Orhan N, Aslan M, Orhan DD, Ergun F, Yeşilada E. In-vivo assessment of antidiabetic and antioxidant activities of grapevine leaves (Vitis vinifera) in diabetic rats. J Ethnopharmacol. 2006;108:280–286. doi: 10.1016/j.jep.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 65.Fujii H, Yokozawa T, Kim YA, Tohda C, Nonaka G. Protective effect of grape seed polyphenols against high glucose-induced oxidative stress. Biosci Biotechnol Biochem. 2006;70:2104–2111. doi: 10.1271/bbb.60053. [DOI] [PubMed] [Google Scholar]
- 66.Sharma S, Anjaneyulu M, Kulkarni SK, Chopra K. Resveratrol, a polyphenolic phytoalexin, attenuates diabetic nephropathy in rats. Pharmacology. 2006;76:69–75. doi: 10.1159/000089720. [DOI] [PubMed] [Google Scholar]
- 67.Pandey KB, Rizvi SI. Resveratrol upregulates erythrocyte plasma membrane redox system and mitigates oxidation induced alterations in erythrocytes during aging in humans. Rejuvenation Res. 2013;16:232–240. doi: 10.1089/rej.2013.1419. [DOI] [PubMed] [Google Scholar]
- 68.Rizvi SI, Pandey KB. Activation of erythrocyte plasma membrane redox system by resveratrol: a possible mechanism for antioxidant property. Pharmacol Rep. 2010;62:726–732. doi: 10.1016/s1734-1140(10)70330-3. [DOI] [PubMed] [Google Scholar]
- 69.Palsamy P, Subramanian S. Ameliorative potential of resveratrol on proinflammatory cytokines, hyperglycemia mediated oxidative stress, and pancreatic beta-cell dysfunction in streptozotocin-nicotinamide-induced diabetic rats. J Cell Physiol. 2010;224:423–432. doi: 10.1002/jcp.22138. [DOI] [PubMed] [Google Scholar]
- 70.Penumathsa SV, Thirunavukkarasu M, Zhan L, Maulik G, Menon VP, Bagchi D. Resveratrol enhances GLUT-4 translocation to the caveolar lipid raft fractions through AMPK/Akt/eNOS signalling pathway in diabetic myocardium. J Cell Mol Med. 2008;12:2350–2361. doi: 10.1111/j.1582-4934.2008.00251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chi TC, Chen WP, Chi TL, Kuo TF, Lee SS, Cheng JT. Phosphatidylinositol-3-kinase is involved in the antihyperglycemic effect induced by resveratrol in streptozotocin-induced diabetic rats. Life Sci. 2007;80:1713–1720. doi: 10.1016/j.lfs.2007.02.002. [DOI] [PubMed] [Google Scholar]