Abstract
Background
To screen methanolic extracts of 26 commonly used Indian spices against nine species of uropathogenic bacteria (Enterococcus faecalis, Staphylococcus aureus, Acinetobacter baumannii, Citrobacter freundii, Enterobacter aerogenes, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, and Pseudomonas aeruginosa), isolated from clinical samples of a tertiary care hospital for antibacterial activity.
Methods
Bacterial strains were subjected to antibiotic sensitivity testing by Kirby–Bauer's disc diffusion method. Monitoring antibacterial potentiality of spice extracts was done by the agar-well diffusion method with multidrug resistant (MDR) strains of nine uropathogens.
Results
The Gram-positive (GP) bacteria E. faecalis and S. aureus were resistant to 16 of the 21 antibiotics used. Among the Gram-negative (GN) bacteria, resistant patterns were A. baumannii and E. aerogenes to 12, C. freundii to 14, E. coli to 12, K. pneumoniae to 10, P. mirabilis to 11, and P. aeruginosa to 15 antibiotics of the 18 antibiotics used. The most effective 15 spices, having at least 25–29 mm as the size of the zone of inhibition, were Allium cepa, Brassica juncea, Cinnamomum tamala, Cinnamomum zeylanicum, Coriandrum sativum, Cuminum cyminum, Curcuma longa, Mentha spicata, Murraya koenigii, Nigella sativa, Papaver somniferum, Piper nigrum, S. aromaticum, Trachyspermum ammi, and Trigonella foenum for at least one of the GP or GN MDR bacterial strains used. Moderate control capacity was registered by nine spices, Curcuma amada, Foeniculum vulgare, Illicium verum, Mentha spicata, Papaver somniferum, Syzygium aromaticum, Trachyspermum ammi, Trigonella foenum, and Zingiber officinale. However, the best two spices for controlling all the pathogens used were C. zeylanicum and C. longa, with the highest value of 29 mm as the inhibition zone size.
Conclusion
The most effective and unique 16 spice plants recorded for the in vitro control of MDR uropathogens could further be pursued for the development of complementary and supplementary medicine against MDR bacteria.
Keywords: antibacterial activity, Indian spices, multidrug resistance, uropathogenic bacteria
1. Introduction
Several bacteria and fungi are causative organisms in urinary tract infections (UTIs), which may occur in more than 50% of the population and are more commonly reported in females. In medical practice, UTI is one of the few common laboratory-confirmed bacterial infections, with an inherent limitation of the requirement of at least 3–4 days for the results of antibiotic sensitivity patterns to yield the causative bacteria in a urine sample.1, 2, 3 Because of the lower abdominal radiating pain and the subsequent inability to carry out normal routine activities, empirical therapy is started according to the antibiotic stewardship program. The administered antibiotic often controls the disease; however, when there is an infection from a multidrug resistant (MDR) bacterium, more often than not the infection progresses to cystitis and then to pyelonephritis.4 Apprehensive of this difficulty, a clinician inadvertently prescribes a broad-spectrum antibiotic, possibly of a higher generation, which insidiously induces resistance to the same antibiotic in the intended pathogen and/or nontarget commensals.
The following organisms were reported at this hospital as UTI-causing bacteria: two Gram-positives (GPs), Staphylococcus aureus and Enterococcus faecalis, as well as nine Gram-negatives (GNs), Acinetobacter baumannii, Citrobacter freundii, Escherichia coli, Enterobacter aerogenes, Klebsiella oxytoca, Klebsiella pneumoniae, Proteus mirabilis, Proteus vulgaris, and Pseudomonas aeruginosa.1, 2, 3 Among these, two species, E. coli (19.5%) and S. aureus (15.3%), were the most prevalent bacteria in the hospital according to surveillance over the 18-month study period; the other species were present in remarkable but lesser proportions of prevalence compared to E. coli and S. aureus.2 In a recent survey on community acquired (CA) UTI cases, E. coli (19%) and S. aureus (15.1%) were also found to be the two major causative bacteria.5, 6 Indeed, these pathogens result in significant hospitalization costs—approximately 150 million UTI cases occur per annum on a global basis with an estimated cost of around 6 billion US dollars for hospitalization in the USA alone.7 Obviously, when several MDR bacterial species invade at one time, a complicated UTI episode can occur with the onset of multiple comorbidities.
Invariably in Indian food items, one or other spice or a selected mixture of spices is used. Furthermore, spices are used by elite, marginalized, and aborigine masses for ad hoc health care needs because they are readily available in the household. However, very few spices are in use specifically for an ailment with a systematic modality. Frankly, the use of most plants in medicine is not scientifically valid owing to the lack of pharmacological host toxicity testing of most drug preparations, particularly concoctions of nonedible plant extracts. However, Indian conventional spices, with several essential oils and/or aromatic compounds as unique secondary metabolites, do not cause host toxicity because they have been handed down through the generations.
Because the pharmacy world has been committed to introducing newer drugs for all sorts of health ailments from well-known, lesser-known, and unknown plant species, it was intuitive to monitor a group of commonly used spice plants for antibacterial activity using UTI-causing bacteria isolated from clinical samples in the hospital, and in view of the present commotion in health care as a result of MDR bacteria. This work is a continuation of our earlier report on the surveillance done for CA and nosocomial surveillance of UTI-causing bacteria.1, 2, 3 In particular, our school has screened a lot of plants from the Odishan subtropical forest, used by aborigines for primary health care needs,8 for antimicrobial efficacy with MDR pathogenic bacteria.9, 10, 11 The strive for locating suitable plants and their pure chemicals for use as antimicrobials or complementary medicine along with antibiotics for MDR bacteria in empiric therapy has been the impetus of this work. Because the UTI problem is now graver than imagined in societies of marginalized people in developing countries such as India, rapid and effective nonmicrobial antimicrobials must be located and used individually or in mixtures of individually pure compounds along with the routine chemoprophylaxis followed in mainstream medicine. Such a situation must be, a priori, prevailing in other developing nations. This work is a systematic study on the screening of 26 well-known Indian spices, used by most inhabitants of Odisha, for the in vitro control of MDR uropathogens. It is anticipated that the findings recorded here would benefit people all over.
2. Materials and methods
2.1. Preparation of plant extracts
Ten grams of a powdered spice sample was dissolved in a 100-mL aliquot of methanol and was incubated at 4 °C for 72 hours. The suspension was filtered and the methanolic filtrate was concentrated to a sticky mass in a rotary evaporator at 40 °C; the mass was weighed and dissolved in 1 mL 10% v/v dimethyl sulfoxide (DMSO); all spice extracts were stored at 4 °C for further use.12
2.2. Isolation, identification of bacterial strains, and antibiotic sensitivity test
Two GPs, S. aureus and E. faecalis, and seven GNs, A. baumannii, C. freundii, E. aerogenes, E. coli (Fig. 1), K. pneumoniae, P. mirabilis, and P. aeruginosa, were used in the study. These bacteria were directly collected from urine samples of UTI patients attending IMS & Sum Hospital, Bhubaneswar, India, using an appropriate medium specific for each bacterium.2, 3 Approval of the institutional ethical committee was duly obtained for the use of clinical samples in this work. For antibiotic sensitivity testing of the bacterial strains, Kirby–Bauer's disc diffusion method was used, using a 4-mm thick Mueller–Hinton (MH) agar medium (HiMedia, Mumbai, India), according to the antibiotic susceptibility testing standards of the Clinical and Laboratory Standards Institute guidelines, as described earlier.2, 13
Fig. 1.
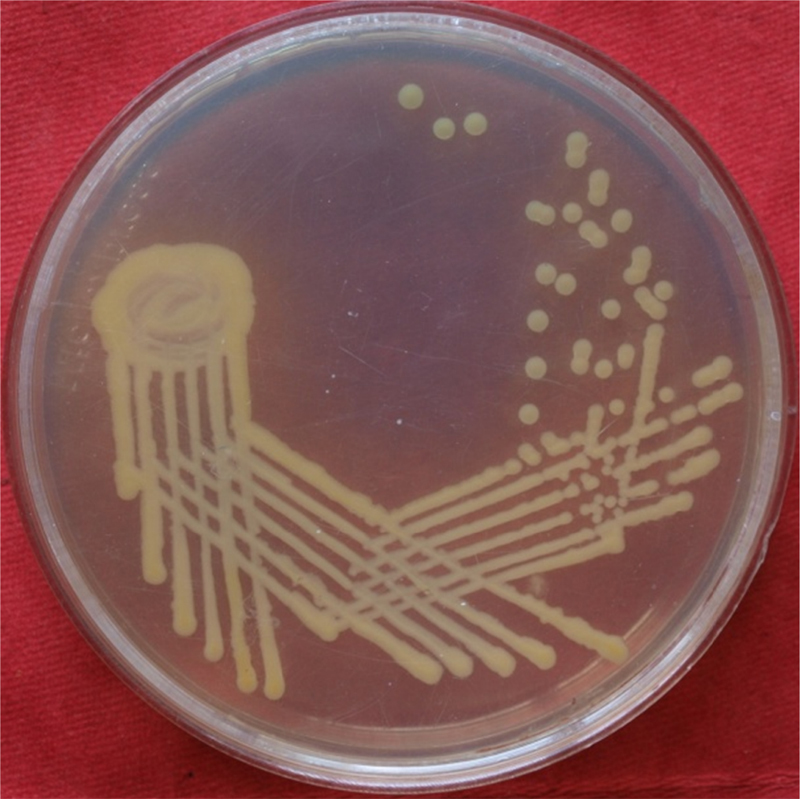
Escherichia coli on nutrient agar.
2.3. Antibacterial activity test
One strain from each bacterial species with resistance to a maximum number of antibiotics was further examined for antibacterial potentiality of the methanolic spice extracts using the agar-well diffusion method.11 Antibacterial activities were evaluated by measuring the diameter of zones of inhibition after the use of each spice extract; each experiment was conducted thrice and the results of the third repetition are presented. No inhibitory effect on any bacterium was observed with 10% DMSO.
2.4. Determinations of MIC and MBC
The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of each methanolic spice extract were determined. Original stock solutions of spice extracts were prepared with methanol, at the concentration 100 mg plant extract/mL in 10% DMSO (Merck, Mumbai, India) solution with distilled water. Each stock solution was diluted suitably for final concentrations of 0 mg/mL, 1.562 mg/mL, 3.125 mg/mL, 6.25 mg/mL, 12.5 mg/mL, 25 mg/mL, 50 mg/mL, and 100 mg/mL with the DMSO solution. A separate experiment was conducted for each spice extract. An 80-μL aliquot of each extract dilution was released to a well on a 96-well (12 × 8) micro-titer plate, along with a 100-μL aliquot of MH broth (HiMedia), a 20-μL aliquot of bacterial inocula [109 colon-forming units (CFU)/mL] and a 5-μL aliquot of 0.5% of 2,3,5-triphenyl tetrazolium chloride (TTC) (Loba Chemie, Mumbai, India). The micro-titer plate was then incubated at 37 °C for 18 hours. The development of pink coloration caused by TTC demonstrated bacterial growth, and the absence of the pink color indicated the inhibition of growth. The first well of the micro-titer was the control, which contained no spice extract; the MIC value was noted at this well, where no color manifested. Furthermore, bacteria from each well of the micro-titer plate were subcultured on a nutrient agar plate; the dilution level that caused no bacterial growth on the nutrient agar plate was considered the MBC value.14
2.5. Qualitative phytochemical analyses
Qualitative analyses using spices were performed for the following phytochemicals: reducing sugars, anthraquinones, saponins, flavonoids, steroids/terpenes, tannins, alkaloids, resins, and glycosides, as detailed previously.10, 14, 15
3. Results
Ethnomedicinal information on 26 spice plants was recorded (Table 1). Most of these spices are widely used in India for different infectious ailments, therefore they are considered traditional medicine (TM) in the Indian subcontinent. It is mostly the seeds that are used as food additives.
Table 1.
Ethnomedicinal information of 26 spice plants.
| Sample no. | Plant name | Family | Local name | Parts used | Ethnomedicinal uses |
|---|---|---|---|---|---|
| 1 | Amomum aromaticum | Zingiberaceae | Aleicha | Seed | Seed is used to treat high acidity, sickness |
| 2 | Allium sativum | Liliaceae | Rasuna | Stem | Stem is useful in fever, boils, wounds, and skin diseases |
| 3 | Allium cepa | Liliaceae | Piaja | Bark | Bark is used to treat diabetes and insect bite |
| 4 | Brassica juncea | Brassicaceae | Sorisha | Seed | Used for stomach diseases and piles |
| 5 | Capsicum annuum | Solanaceae | Lanka | Fruit | Fruits are used to cure urinal infection |
| 6 | Cinnamomum tamala | Lauraceae | Tejapatra | Leaf | Leaf is used as contraceptive and in treatment of sores, burns, and fever |
| 7 | Cinnamomum zeylanicum | Lauraceae | Dalchini | Bark | Bark is used to treat pimples and summer boils |
| 8 | Coriandrum sativum | Apiaceae | Dhania | Seed | Seeds are useful for stomach problems |
| 9 | Cucumis melo | Cucurbitaceae | Magaja manji | Seed | Seeds are used as an antiseptic, wound healing agent, and anti-acne treatment |
| 10 | Cuminum cyminum | Apiaceae | Jeera | Seed | Effective for diarrhea, indigestion, and morning sickness |
| 11 | Curcuma amada | Zingiberaceae | Ambakasi ada | Rhizome | Rhizome is used for burning sensations in genital organs, fever, piles, and urinary discharges |
| 12 | Curcuma longa | Zingiberaceae | Haladi | Rhizome | Rhizome is used for fever, skin diseases, high blood pressure |
| 13 | Elettaria cardamomum | Zingiberaceae | Gujrati | Seed | Seeds are useful in acidity and stomach disorders |
| 14 | Foeniculum vulgare | Apiaceae | Panmadhuri | Seed | Used against stomach pain, diarrhea, and indigestion |
| 15 | Ferula assafoetida | Apiaceae | Hingu | Seed | Used against indigestion and toothache |
| 16 | Illicium verum | Illiciaceae | Anasiphul | Flower | Flowers are used to treat diabetes |
| 17 | Mentha spicata | Lamiaceae | Podina | Leaf | Used in the treatment of toothache, joint pain, and muscle pain |
| 18 | Murraya koenigii | Rutaceae | Bhrusanga | Leaf | Used to treat fever and indigestion |
| 19 | Myristica fragrans | Myristicaceae | Jaiphala | Seed | Seeds are used to cure headache and constipation |
| 20 | Nigella sativa | Ranunculaceae | Kala jeera | Seed | Seeds are used to treat stomach pain |
| 21 | Papaver somniferum | Papaveraceae | Postak dana | Seed | Seeds are used to treat gastric and stomach problems |
| 22 | Piper nigrum | Piperaceae | Golmaricha | Seed | Seeds are used to cure stomach pain and throat infection |
| 23 | Syzygium aromaticum | Myrtaceae | Labanga | Flower bud | Used to cure toothache and oral diseases |
| 24 | Trachyspermum ammi | Apiaceae | Juani | Seed | Seeds are used to cure dysentery, bronchitis, and cough |
| 25 | Trigonella foenum | Fabaceae | Methi | Seed | Seeds are used for treatment of diabetes |
| 26 | Zingiber officinale | Zingiberaceae | Ada | Rhizome | Used to treat cough and asthma |
Antibiotic susceptibility tests of nine UTI-causing bacterial species were carried out using 21 antibiotics of nine different groups. The GP isolates E. faecalis and S. aureus were resistant to 16 of the 21 antibiotics used. Among the GN UTI-causing bacteria, the resistant patterns were A. baumannii and E. aerogenes to 12, C. freundii to 14, E. coli to 12, K. pneumoniae to 10, P. mirabilis to 11, and P. aeruginosa to 15 of the 18 antibiotics used. Antibiograms of nine MDR bacterial strains were recorded (Table 2). All of the isolated bacterial strains causing UTI were amply MDR.
Table 2.
Antibiotic susceptibility results of the selected multidrug resistant (MDR) organisms.
| Bacterium | Susceptibility to prescribed antibiotics* |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aminog lycosides |
Beta-lactams |
Cephalosporins |
Fluoroquinolones |
Glyco peptides |
Macro lides |
Linc osa mides |
Sulfonamides |
Synthetics |
|||||||||||||
| Ac | Ge | Ak | Am | Ox | P | Pit | Ctr | Cf | Of | Le | Nx | Gt | Tei | Va | E | Az | Cd | Cot | Ch | Lz | |
| E. faecalis | R | R | R | R | R | R | R | R | R | MS | R | MS | R | R | R | R | S | S | R | MS | R |
| S. aureus | R | R | R | R | R | R | R | R | R | R | R | R | R | MS | R | S | R | MS | MS | R | S |
| A. baumannii | R | R | R | MS | ND | R | R | R | R | R | R | MS | S | R | ND | R | R | ND | S | S | S |
| C. freundii | R | R | R | R | ND | R | R | R | R | R | R | R | MS | MS | ND | R | MS | ND | R | R | S |
| E. aerogenes | R | R | R | R | ND | R | R | MS | S | S | R | R | R | R | ND | R | MS | ND | S | R | S |
| E. coli | R | R | S | R | ND | R | S | S | R | R | R | R | R | S | ND | R | MS | ND | R | R | S |
| K. pneumoniae | S | R | R | R | ND | R | R | MS | MS | R | R | S | S | R | ND | MS | R | ND | S | S | R |
| P. mirabilis | R | R | R | S | ND | MS | S | MS | R | R | S | R | R | R | ND | R | R | ND | R | MS | S |
| P. aeruginosa | R | R | MS | R | ND | R | R | R | R | R | R | R | MS | R | ND | R | R | ND | R | R | S |
Antibiotics (μg/disc) = Ac, amikacin 30; Ak, amoxyclav 30; Am, ampicillin 10; Az, azithromycin 15; Cd, clindamycin 2; Cf, cefpodoxime 10; Ch, chloramphenicol 30; Cot, co-trimoxazole 25; Ctr, ceftriaxone 30; E, erythromycin 15; Ge, gentamicin 10; Gt, gatifloxacin 5; Le, levofloxacin 5; Lz, linezolid 30; Of, ofloxacin 5; Ox, oxacillin 1; P, penicillin 10; Pit, piperacillin/tazobactam 100/10; Nx, norfloxacin 10; Tei, teichoplanin 5; Va, vancomycin 30.
MS, moderately sensitive; R, resistant; S, sensitive.
Methanolic extracts of 26 spices were tested against nine MDR bacterial species for antibacterial properties by the agar-well diffusion method, and the sizes of the zones of inhibition were recorded (Table 3). The methanolic extract of Allium cepa had a zone of inhibition of 29 mm against S. aureus and the methanolic extract of Syzygium aromaticum exhibited an inhibition zone size of 29 mm against E. aerogenes. Furthermore, the most effective 15 spices having at least 25–29 mm as the size of the zone of inhibition were A. cepa, Brassica juncea, Cinnamomum tamala, Cinnamomum zeylanicum, Coriandrum sativum, Cuminum cyminum, Curcuma longa, Mentha spicata, Murraya koenigii (Fig. 2), Nigella sativa, Papaver somniferum, Piper nigrum, Syzygium aromaticum, Trachyspermum ammi, and Trigonella foenum for at least one GP or GN MDR bacterial strain. Notably, the three spices that controlled all of the pathogens used were C. zeylanicum, C. longa, and M. koenigii, with the highest value of 29 mm as the inhibition zone size. Extracts of the three spices A. cepa, B. juncea, and C. cyminum controlled one species each, namely P. aeruginosa, E. faecalis, and P. mirabilis, respectively. Similarly, extracts of the seven spices Cinnamomum tamala, Cucumis melo, Curcuma amada, Foeniculum vulgare, Syzygium aromaticum, Trachyspermum ammi, and Zingiber officinale exhibited control of two pathogens each. Extracts of six spices, Allium sativum, Capsicum annuum, Illicium verum, Mentha spicata, Myristica fragrans, and Piper nigrum, exhibited control of three bacterial species each. Thus, a total of 3 + 6 + 7 = 16 spices, as listed above, could be taken as having moderate control capacity. The detailed data for the sizes of zones of inhibition for all 26 methanolic spice extracts were recorded (Table 3).
Table 3.
Antibacterial activities of selected spices by the agar-well diffusion method.
| Bacterium | Size of inhibition zones by extracts of 26 spices (mm)* |
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | |
| E. faecalis | 20 | — | 28 | — | 19 | 23 | 26 | — | 19 | 25 | 23 | 24 | — | 22 | — | 22 | — | 26 | 22 | — | 25 | 17 | 27 | 24 | — | 23 |
| S. aureus | 19 | 17 | 29 | 26 | 21 | 26 | 27 | 27 | — | 26 | 23 | 25 | 21 | 24 | — | 21 | 17 | 27 | — | — | — | 19 | 25 | 26 | 25 | 18 |
| A. baumannii | — | 17 | 23 | 24 | 22 | — | 26 | 26 | — | 26 | 23 | 22 | — | — | 19 | 19 | 15 | 26 | — | — | 19 | — | 24 | — | — | 18 |
| C. freundii | — | 18 | 26 | 24 | 22 | 27 | 23 | — | 17 | 25 | 21 | 19 | 21 | 19 | — | — | — | 23 | 19 | 21 | — | 17 | — | 24 | 24 | — |
| E. aerogenes | 17 | 22 | 21 | 16 | — | — | 17 | 21 | 20 | 19 | — | 21 | 12 | 13 | 19 | 18 | 28 | 17 | 22 | 23 | — | 25 | 29 | 21 | 23 | 23 |
| E. coli | — | — | 22 | 19 | — | 17 | 21 | — | 19 | 21 | 22 | 26 | — | — | — | — | — | 21 | — | — | — | — | 21 | — | — | 21 |
| K. pneumoniae | — | 17 | 20 | 22 | — | 17 | 26 | 21 | 17 | 22 | 17 | 22 | 12 | 17 | 18 | 17 | 22 | 26 | 12 | 15 | 28 | 22 | 21 | 21 | 21 | 20 |
| P. mirabilis | 17 | 21 | 17 | 22 | 15 | 17 | 17 | 16 | 13 | — | — | 19 | 16 | 12 | 21 | — | 23 | 17 | 17 | 18 | 19 | 18 | — | 18 | — | — |
| P. aeruginosa | 19 | — | — | 22 | 23 | 19 | 25 | — | 21 | 26 | 23 | 23 | — | 15 | — | 20 | 19 | 25 | 17 | 26 | 26 | — | 20 | 25 | 24 | 19 |
Numbers 1–26 are serial numbers of the spice plants given in Table 1. Values are measurements of zone of inhibition due to methanol extracts.
—, no activity.
Fig. 2.

The Murraya koenigii plant.
MDR strains of E. faecalis were not controlled by eight spices: A. sativum, B. juncea, C. sativum, E. cardamomum, F. assafoetida, M. spicata, N. sativa, and T. foenum; S. aureus was not controlled by five spices: C. melo, F. assafoetida, M. fragrans, N. sativa, and P. somniferum; A. baumannii was not controlled by 10 spices: A. aromaticum, C. tamala, C. melo, E. cardamomum, F. vulgare, M. fragrans, N. sativa, P. nigrum, T. ammi, and T. foenum; C. freundii was not controlled by eight spices: A. aromaticum, C. sativum, F. assafoetida, I. verum, M. spicata, P. somniferum, S. aromaticum, and Z. officinale; E. aerogenes was not controlled by four spices: C. annuum, C. tamala, C. amada, and P. somniferum; E. coli was not controlled by 15 spices: A. aromaticum, A. sativum, C. annuum, C. sativum, E. cardamomum, F. vulgare, F. assafoetida, I. verum, M. spicata, M. fragrans, N. sativa, P. somniferum, P. nigrum, T. ammi, and T. foenum; MDR K. pneumoniae was not controlled by A. aromaticum and C. annuum; P. mirabilis was not controlled by six spices: C. cyminum, C. amada, I. verum, S. aromaticum, T. foenum, and Z. officinale; P. aeruginosa was not controlled by extracts of six spices: A. sativum, A. cepa, C. sativum, E. cardamomum, F. assafoetida, and P. nigrum (Table 3).
MIC and MBC values of methanolic extracts of spices were evaluated. C. zeylanicum had 1.51 mg/mL as the lowest MIC value and 3.41 mg/mL as the lowest MBC value against E. faecalis, S. aureus, A. baumannii, C. freundii, K. pneumonia, and P. aeruginosa, but it had the highest MIC value of 9.63 mg/mL and the highest MBC value of 21.67 mg/mL for P. mirabilis. A lower MIC/MBC value signifies that a minimum amount (lower level) of spice extract is used, whereas a higher value signifies the use of a higher amount of spice extract for the control of a bacterium. Based on MIC and MBC values, the spices could be arranged in decreasing order of efficacy: C. zeylanicum > M. koenigii > C. cyminum > C. longa > B. juncea > A. cepa. Among the bacteria, E. coli, followed by A. baumannii, C. freundii, E. aerogenes, K. pneumoniae, P. mirabilis, and P. aeruginosa, were controlled by higher amounts/levels of spice extracts, as evident from MIC and MBC values. The MIC and MBC values as a result of the spices for all isolated UTI bacteria are presented in Table 4.
Table 4.
MIC and MBC values of selected spices (mg/mL).
| Bacterium | 1* |
2 |
3 |
4 |
5. |
6 |
7 |
8 |
9 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| E. faecalis | 3.41 | 4.27 | — | — | 1.51 | 3.41 | — | — | 4.27 | 9.63 | 3.41 | 4.27 | 1.51 | 3.41 | — | — | 4.27 | 21.67 |
| S. aureus | 4.27 | 9.63 | 9.63 | 21.67 | 1.51 | 3.41 | 1.51 | 3.41 | 3.41 | 4.27 | 1.51 | 3.41 | 1.51 | 3.41 | 1.51 | 3.41 | — | — |
| A. baumannii | — | — | 9.63 | 21.67 | 3.41 | 4.27 | 1.51 | 3.41 | 3.41 | 4.27 | — | — | 1.51 | 3.41 | 1.51 | 3.41 | — | — |
| C. freundii | — | — | 4.27 | 9.63 | 1.51 | 3.41 | 1.51 | 3.41 | 1.51 | 3.41 | — | — | 1.51 | 3.41 | — | — | — | — |
| E. aerogenes | 9.63 | 21.67 | 3.41 | 4.27 | 3.41 | 4.27 | 4.27 | 9.63 | — | — | 9.63 | 21.67 | 3.41 | 4.27 | 3.41 | 4.27 | 3.41 | 4.27 |
| E. coli | — | — | — | — | 3.41 | 4.27 | 3.41 | 4.27 | — | — | — | — | 3.41 | 4.27 | — | — | — | — |
| K. pneumoniae | — | — | 9.63 | 21.67 | 3.41 | 4.27 | 3.41 | 4.27 | — | — | 9.63 | 21.67 | 1.51 | 3.41 | 3.41 | 4.27 | 9.63 | 21.67 |
| P. mirabilis | 9.63 | 21.67 | 3.41 | 4.27 | 9.63 | 21.67 | 3.41 | 4.27 | 9.63 | 21.67 | 9.63 | 21.67 | 9.63 | 21.67 | 9.63 | 21.67 | 9.63 | 21.67 |
| P. aeruginosa | 4.27 | 9.63 | — | — | — | — | 3.41 | 4.27 | 3.41 | 4.27 | 4.27 | 9.63 | 1.51 | 3.41 | — | — | — | — |
| Bacterium | 10 |
11 |
12 |
13 |
14 |
15 |
16 |
17 |
18 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| E. faecalis | 1.51 | 3.41 | 3.41 | 4.27 | 1.51 | 3.41 | — | — | 3.41 | 4.27 | — | — | 3.41 | 4.27 | — | — | 1.51 | 3.41 |
| S. aureus | 1.51 | 3.41 | 3.41 | 4.27 | 1.51 | 3.41 | 3.41 | 4.27 | 1.51 | 3.41 | — | — | 3.41 | 4.27 | 9.63 | 21.67 | 1.51 | 3.41 |
| A. baumannii | 1.51 | 3.41 | 3.41 | 4.27 | 3.41 | 4.27 | — | — | — | — | 4.27 | 9.63 | 4.27 | 9.63 | 9.63 | 21.67 | 1.51 | 3.41 |
| C. freundii | 1.51 | 3.41 | 3.41 | 4.27 | 4.27 | 9.63 | 3.41 | 4.27 | 4.27 | 9.63 | — | — | — | — | — | — | 1.51 | 3.41 |
| E. aerogenes | 4.27 | 9.63 | — | — | 3.41 | 4.27 | — | — | — | — | 4.27 | 9.63 | 9.63 | 21.67 | 1.51 | 3.41 | 3.41 | 4.27 |
| E. coli | 3.41 | 4.27 | 3.41 | 4.27 | 1.51 | 3.41 | — | — | — | — | — | — | — | — | — | — | 3.41 | 4.27 |
| K. pneumoniae | 3.41 | 4.27 | 9.63 | 21.67 | 3.41 | 4.27 | — | — | 9.63 | 21.67 | 9.63 | 21.67 | 9.63 | 21.67 | 3.41 | 4.27 | 1.51 | 3.41 |
| P. mirabilis | — | — | — | — | 4.27 | 9.63 | — | — | — | — | 3.41 | 4.27 | — | — | 3.41 | 4.27 | 9.63 | 21.67 |
| P. aeruginosa | 1.51 | 3.41 | 3.41 | 4.27 | — | — | — | — | 9.63 | 21.67 | — | — | 3.41 | 4.27 | 4.27 | 9.63 | 1.51 | 3.41 |
| Bacterium | 19 |
20 |
21 |
22 |
23 |
24 |
25 |
26 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| E. faecalis | 3.41 | 4.27 | — | — | 1.51 | 3.41 | 4.27 | 9.63 | 1.51 | 3.41 | 1.51 | 3.41 | — | — | 3.41 | 4.27 |
| S. aureus | — | — | — | — | — | — | 9.63 | 21.67 | 1.51 | 3.41 | 1.51 | 3.41 | 1.51 | 3.41 | 9.63 | 21.67 |
| A. baumannii | — | — | — | — | 4.27 | 9.63 | — | — | 1.51 | 3.41 | — | — | — | — | 9.63 | 21.67 |
| C. freundii | 4.27 | 9.63 | 3.41 | 9.63 | — | — | 9.63 | 21.67 | — | — | 1.51 | 3.41 | 1.51 | 3.41 | — | — |
| E. aerogenes | 3.41 | 4.27 | 3.41 | 9.63 | — | — | 1.51 | 3.41 | 1.51 | 3.41 | 3.41 | 4.27 | 1.51 | 3.41 | 3.41 | 4.27 |
| E. coli | — | — | — | — | — | — | — | — | 3.41 | 4.27 | — | — | — | — | 3.41 | 4.27 |
| K. pneumoniae | 9.63 | 21.67 | 9.63 | 21.67 | 1.51 | 3.41 | 3.41 | 4.27 | 3.41 | 4.27 | 3.41 | 4.27 | 3.41 | 4.27 | 3.41 | 4.27 |
| P. mirabilis | 9.63 | 21.67 | 4.27 | 9.63 | 4.27 | 9.63 | 4.27 | 9.63 | — | — | 9.63 | 21.67 | — | — | — | — |
| P. aeruginosa | 9.63 | 21.67 | 1.51 | 3.41 | 1.51 | 3.41 | — | — | 9.63 | 21.67 | 1.51 | 3.41 | 1.51 | 3.41 | 3.41 | 4.27 |
Numbers 1–26 are the serial numbers of spice plants given in Table 1.
Values are measurements of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) due to methanol extracts.
—, no activity.
Qualitative phytochemical analysis was carried out for all of the plants. The spices C. zeylanicum, M. koenigii, C. cyminum, C. longa, B. juncea, and A. cepa contained alkaloids, flavonoids, carbohydrates, terpenoids, steroids, tannins, resins, and saponins, which could be attributed to the significant antibacterial activities that were recorded. The spices A. aromaticum, A. sativum, C. annuum, C. sativum, C. melo, E. cardamomum, F. assafoetida, M. fragrans, N. sativa, and P. nigrum contained limited phytochemicals, and did not register as having considerable antibacterial activity. The results of phytochemical analysis of all these spices was recorded (Table 5).
Table 5.
Qualitative phytochemical analysis of methanolic extracts of spices.
| Sample no. | Spice | Alkaloids | Resins | Glycosides | Terpenoids | Carbohydrates | Tannins | Flavonoids | Steroids |
|---|---|---|---|---|---|---|---|---|---|
| 1 | A. aromaticum | — | + | + | — | + | — | — | — |
| 2 | A. sativum | + | + | — | + | + | — | — | + |
| 3 | A. cepa | + | — | + | + | + | + | + | + |
| 4 | B. juncea | + | + | + | — | + | + | + | + |
| 5 | C. annuum | — | + | — | + | + | + | + | + |
| 6 | C. tamala | — | + | + | + | + | — | — | + |
| 7 | C. zeylanicum | — | + | + | — | + | + | + | + |
| 8 | C. sativum | — | + | + | — | + | — | — | + |
| 9 | C. melo | — | + | — | + | + | + | — | + |
| 10 | C. cyminum | — | — | — | + | — | + | + | — |
| 11 | C. amada | + | + | + | — | + | + | + | + |
| 12 | C. longa | — | + | — | + | + | + | — | + |
| 13 | E. cardamomum | + | + | — | — | + | — | — | + |
| 14 | F. vulgare | + | + | — | — | + | — | — | + |
| 15 | F. assafoetida | + | + | — | — | + | — | — | + |
| 16 | I. verum | + | + | — | — | — | — | + | — |
| 17 | M. spicata | — | + | + | + | + | + | — | + |
| 18 | M. koenigii | + | + | — | + | + | + | + | + |
| 19 | M. fragrans | + | + | — | — | + | + | + | — |
| 20 | N. sativa | — | + | + | + | + | — | — | + |
| 21 | P. somniferum | + | — | — | — | + | + | + | — |
| 22 | P. nigrum | — | — | + | + | + | + | — | + |
| 23 | S. aromaticum | + | + | — | + | — | + | — | + |
| 24 | T. ammi | + | + | + | — | + | — | + | + |
| 25 | T. foenum | + | + | — | + | — | + | — | + |
| 26 | Z. officinale | — | + | — | + | + | + | + | + |
+, Present; —, absent.
4. Discussion
This work substantiates that all isolated uropathogens were floridly MDR. The highest effective spice plants for all MDR UTI strains were A. cepa, B. juncea, C. tamala, C. zeylanicum, Cuminum cyrainum, Cuminum ambada, C. longa, S. aromaticum, T. ammi, and Z. officinale.
The vast majority of medicinal plants cannot yet be manufactured economically and are still obtained from the wild or cultivated. Furthermore, compounds (muscarine, physostigmine, cannabinoids, yohimbine, forskolin, colchicines, and phorbol esters) acquired from plants are important tools used in pharmacological, physiological, and biochemical studies.16 Several phytochemicals have been developed and evaluated and find their own places as pharmaceutical agents. Phytochemicals are generally considered safe because they have traditional uses in ethnic medicine. Nevertheless, certain phytochemicals also have both known and unknown toxic effects on the human body.17 Obviously, the drug-targeting endeavor of certain phytochemicals obtained from nonedible plants needs due host toxicity testing in animal systems and mammalian cell lines prior to using in humans. Moreover, plant-based antimicrobials have enormous therapeutic potentials, as seen in the literature published to date.18 Furthermore, phytochemicals usually have multiple beneficial effects, often acting beyond symptomatic treatment of diseases. For example, Hydrastis canadensis (orange root) has not only antimicrobial activity but also increases blood supply to the spleen to release curative compounds.19
Antimicrobial herbal drugs show promising marketing potential because bacterial resistance to phyto-antimicrobials would never be as fast as that witnessed with antibiotics and pure-chemical drugs, owing to the fact that each crude plant extract is a combination of diverse types of phytochemicals. Secondly, bacterial resistance is achieved for 70S ribosome or blocking at the cell membrane level in bacteria, and the bacterial cell producing the antibiotic is resistant to its own antibiotic in a mechanism that could be easily achieved by the sensitive target bacterium. The antimicrobial activity of plants involves diverse molecular mechanisms because diverse phytochemicals in crude form would have different mechanisms of toxicity to bacteria; prokaryotes (bacteria) may have limited mechanisms for breaking down the panoply of phytocompounds encountered in vivo. By contrast, plant extracts, tantamount to a large armamentarium, would provide a holistic approach towards toxicity to pathogens.20 An example of a top-selling antimicrobial in the US herbal market is H. canadensis, which has been used by Native Americans and is in cultivation to supply the demands for its herbal products.21 Furthermore, leaves of Cassia fistula (Indian laburnum) are gaining importance in the market as an ingredient in the preparation of purgatives—the information originated from Indian Vedic and folklore literature. In the 1840s, German researchers examined the role of European Oxycoccus palustris (cranberry) on UTIs. The urine of people who consumed cranberries had the chemical hippuric acid, which acidifies urine, thereby preventing infection. However, the chemical failed to show that it increased urine acidity enough to prevent infection. Today, studies are again being undertaken in addressing the relationship between cranberries and a healthy urinary tract, focusing on a different action: the potentiality of cranberry leaves to keep bacteria from attaching to urinary tract walls.
Patients of diverse ethnic groups without the minimum level of education, as in certain regions of developing countries, often neglect UTIs and take them to be curable by deliberate cleanliness. A UTI may appear as a trivial problem initially, but unbeknown to one it has a retinue of comorbidities that could lead to fatality from secondary infections. However, the traditional clandestine medicinal system of ethnic masses does not have any curative drug for the problem. The myriad of phytodrugs are often known for their preventive roles in health care, for which the elite in society use them. Consequently, many a concoction is sold on market shelves, notwithstanding the approval of those preparations by pharmacists. Traditional knowledge on the use of plants is the basis for their use as traditional medicine; the concept of complementary and alternative medicine has therefore emerged. After all, many safe and efficacious drugs in crude plant preparations languish without proper attention being focused on them as microbial control agents; at the same time, the development of suitable antimicrobials is central to the endeavor for the control of MDR bacterial pathogens.22 For a litany of preventive medicinal plant extracts, curative drugs are to be harnessed. For this purpose, fractional isolation with the available range of organic solvents should be done, and then fractions of plant extracts should be pursued for microbial bioassay work with the frequently isolated MDR bacteria. The most effective phytocompounds, with their repertoire of pharmacognosy, could be isolated and purified with great finesse. The preventive characteristics of medicinal plants could be prudently exploited for the development of antimicrobials along with other drugs in trials for possible use in curative medicine. Indeed, the partially poisonous plants Lantana camara,14 Argemone Mexicana,18 and timber-leaf extracts23 had remarkable antimicrobial activities in controlling MDR bacterial pathogens, especially those isolated from urine samples of UTI patients.
Methanolic extracts of the two spice plants C. zeylanicum and C. longa were seen to be highly effective in this study in controlling most MDR strains of bacterial isolates. The volatile oil of C. zeylanicum was reported to contain 91.5% cinnamic aldehyde,24 which specifically could be used for the development of adjuvant/complimentary medicine after due verification of the synergistic interaction of the chemical and a moribund antibiotic (resistant to most bacteria), similar to the synergy work that had been reported for a MDR P. aeruginosa strain resistant to ceftriaxone and the extract of the lesser-known plant Combretum albidum, whose absence of host toxicity was also verified.25 Furthermore, C. longa has several cytotoxic and antimicrobial phytocompounds,21 but the antibacterial properties of leading/individual compounds of this plant for any MDR bacteria have not yet been systematically pursued. However, the chemical structures, molecular formulae, and molecular weights of the three leading compounds, which have probable antimicrobial activity, from these two plants were retrieved from the PubChem database (Table 6).
Table 6.
Antibacterial compounds of two spices, cinnamon and turmeric.
| Plant source | Chemical name | Structure |
|---|---|---|
|
Cinnamomum zeylanicum (cinnamon) |
1. Cinnamic aldehyde MF: C9H8O MW: 132.15922 [g/mol] PubChem ID: CID 637511 |
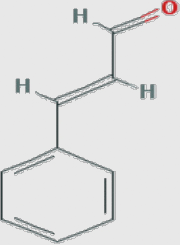 |
|
Curcuma longa (turmeric) |
1. α-Phellandrene MF: C10H16 MW: 136.23404 [g/mol] PubChem ID: CID 7460 2. 4-Hydroxybenz aldehyde MF: C7H6O2 MW: 122.12134 [g/mol] PubChem ID: CID 126 |
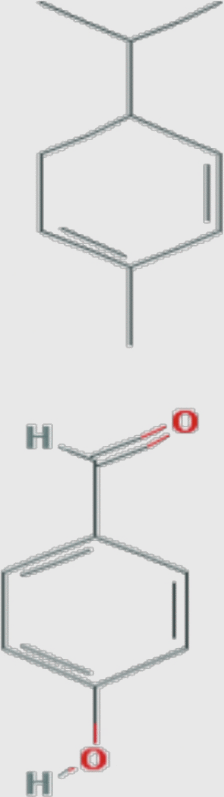 |
MF, molecular formula; MW, molecular weight.
In conclusion, it can be stated that 16 of 26 Indian spice plants have been seen in this study as effective control agents for pathogenic bacteria in vitro. These could serve as potential sources of nonmicrobial antimicrobials in the crusade against MDR bacteria if we lean towards herbal drugs. The disregard of phyto-drugs by some researchers should now be considered an unreasonable and misguided notion given the present alarm in public health regarding bacterial infections and multidrug resistance.
Conflicts of interest
The authors declare that they have no conflict of interests.
Acknowledgments
This work is supported by the major research project “Development of standardized herbal extracts against urinary tract bacterial infections” (grant no. BT/PR8214/PBD/17/863/2013), from the Department of Biotechnology, Government of India.
References
- 1.Rath S, Dubey D, Sahu MC, Debata NK, Padhy RN. Antibacterial activity of 25 medicinal plants used by aborigines of India against 6 uropathogens with surveillance of multidrug resistance. Asian Pacific J Trop Biomed. 2012;2:S846–S854. [Google Scholar]
- 2.Mishra MP, Debata NK, Padhy RN. Surveillance of multidrug resistant uropathogenic bacteria in hospitalized patients—an Indian study. Asian Pacif J Trop Biomed. 2013;3:315–324. doi: 10.1016/S2221-1691(13)60071-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rath SN, Padhy RN. Surveillance of multidrug resistance in community acquired urinary tract bacterial infections in an Indian teaching hospital. J Acute Dis. 2014;3 (in press) [Google Scholar]
- 4.Baishya RK, Mathew A, Dhawan DR, Desai MR. Urinary tract infection in diabetic patients. Indian J Nephrol. 2010;20:222. doi: 10.4103/0971-4065.73429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rath S, Dubey D, Sahu MC, Padhy RN. Surveillance of ESBL producing multidrug resistant Escherichia coli in a teaching hospital in India. Asian Pacific J Trop Dis. 2014;4:140–149. [Google Scholar]
- 6.Dubey D, Rath S, Sahu MC, Pattnaik L, Debata NK, Padhy RN. Surveillance of infection status of drug resistant Staphylococcus aureus in an Indian teaching hospital. Asian Pacif J Trop Dis. 2013;3:133–142. [Google Scholar]
- 7.Lau HS, Reddy S, Cheesbrough J, Bolton FJ, Willshaw G, Cheasty T. Major uropathogenic Escherichia coli strain isolated in the northwest of England identified by multilocus sequence typing. J Clin Microbiol. 2008;46:1076–1080. doi: 10.1128/JCM.02065-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mallik BK, Panda T, Padhy RN. Traditional herbal practices by the ethnic people of Kalahandi district of Odisha, India. Asian Pacif J Trop Biomed. 2012;2:S988–S994. [Google Scholar]
- 9.Dubey D, Padhy RN. Surveillance of multidrug resistance of two Gram-positive pathogenic bacteria in a teaching hospital and in vitro efficacy of 30 ethnomedicinal plants used by an aborigine of India. Asian Pacific J Trop Dis. 2012;2:273–281. [Google Scholar]
- 10.Sahu MC, Padhy RN. In vitro efficacy of Butea monosperma Lam. against 12 clinically isolated multidrug resistant bacteria. Asian Pacif J Trop Dis. 2013;3:217–226. [Google Scholar]
- 11.Rath S, Padhy RN. Monitoring in vitro antibacterial efficacy of Terminalia alata Heyne ex. Roth, against multidrug resistant enteropathogenic bacteria. J Acut Med. 2013;3:93–102. [Google Scholar]
- 12.Rath S, Padhy RN. Surveillance of multidrug resistance of 10 enteropathogens in a teaching hospital and in vitro efficacy of 25 ethnomedicinal plants used by an Indian aborigine. Asian Pacif J Trop Dis. 2012;2:S336–S346. [Google Scholar]
- 13.Sahu MC, Rath S, Dubey D, Debata NK, Padhy RN. Multidrug resistance of Pseudomonas aeruginosa as known from surveillance of nosocomial and community infections in an Indian teaching hospital. J Publ Health. 2012;20:413–423. [Google Scholar]
- 14.Dubey D, Padhy RN. Antibacterial activity of Lantana camara L. against multidrug resistant pathogens from ICU patients of a teaching hospital. J Herb Med. 2013;3:65–75. [Google Scholar]
- 15.Nayak N, Rath SN, Mishra MP, Ghosh G, Padhy RN. Antibacterial potency of the terrestrial fern Lygodium flexuosum (L.) Sw. against multidrug resistant enteric- and uro-pathogenic bacteria. J Acute Dis. 2013;2:270–276. [Google Scholar]
- 16.Rates SMK. Plants as source of drugs. Toxicon. 2001;39:603–613. doi: 10.1016/s0041-0101(00)00154-9. [DOI] [PubMed] [Google Scholar]
- 17.Gilbert B, Ferreira JLP, Almeida MBS, Carvalho ES, Cascon V, Rocha LM. The official use of medicinal plants in public health, Cienciae Cultura. J Brazil Assoc Adv Sci. 1997;49:339–344. [Google Scholar]
- 18.Sahu MC, Debata NK, Padhy RN. Antibacterial activity of Argemone mexicana L. against multidrug resistant Pseudomonas aeruginosa. Asian Pacif J Trop Biomed. 2012;2:S800–S807. [Google Scholar]
- 19.Chan EWC, Lim YY, Omar M. Antioxidant and antibacterial activity of leaves of Etlingera sp. (Zingiberaceae) in peninsular Malaysia. Food Chem. 2007;41:586–593. [Google Scholar]
- 20.Dubey D, Rath S, Sahu MC, Debata NK, Padhy RN. Antimicrobials of plant origin against multi-drug resistant bacteria including the TB bacterium and economics of plant-drugs—introspection. Indian J Trad Knowl. 2012;11:225–233. [Google Scholar]
- 21.Khan MGU, Nahar K, Rahman MS, Hasan CM, Rashid MA. Phytochemical and biological investigations of Curcuma longa. Dhaka Univ J Pharm Sci. 2008;8:39–45. [Google Scholar]
- 22.Dubey D, Rath S, Sahu MC, Debata NK, Padhy RN. Phytochemicals of plant origin against multidrug resistant bacteria including the TB bacterium and economics of plant-drugs. J Publ Health. 2013;21:115–119. [Google Scholar]
- 23.Mishra MP, Padhy RN. In vitro antibacterial efficacy of 21 Indian timber-yielding plants against multidrug resistant bacteria causing urinary tract infection. Osong Publ Health Res Persp. 2013;4:347–357. doi: 10.1016/j.phrp.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Bayati FA, Mohammed MJ. Isolation, identification, and purification of cinnamic aldehyde from Cinnamomum zeylanicum bark oil. An antibacterial study. Pharmaceut Biol. 2009;47:61–66. [Google Scholar]
- 25.Sahu MC, Patnaik R, Padhy RN. In vitro combination-efficacy of ceftriaxone and leaf-extract of Combretum albidum G. Don against multidrug resistant Pseudomonas aeruginosa and host-toxicity testing with lymphocytes from human cord blood. J Acut Med. 2014;4:26–37. [Google Scholar]


