Abstract
Skeletal muscle has been emerging as a research field since the past 2 decades. Contraction of a muscle, which acts as a secretory organ, stimulates production, secretion, and expression of cytokines or other muscle fiber-derived peptides, i.e., myokines. Exercise-induced myokines influence crosstalk between different organs in an autocrine, endocrine, or paracrine fashion. Myokines are recently recognized as potential candidates for treating metabolic diseases through their ability to stimulate AMP-activated protein kinase signaling, increase glucose uptake, and improve lipolysis. Myokines may have positive effects on metabolic disorders, type 2 diabetes, or obesity. Numerous studies on myokines suggested that myokines offer a potential treatment option for preventing metabolic diseases. This review summarizes the current understanding of the positive effects of exercise-induced myokines, such as interleukin-15, brain-derived neurotrophic factor, leukemia inhibitory factor, irisin, fibroblast growth factor 21, and secreted protein acidic and rich in cysteine, on metabolic diseases.
Keywords: exercise, health, metabolic diseases, myokines
1. Introduction
Many studies have demonstrated the benefits of exercise in preventing all-cause mortality, including cardiovascular disease, metabolic disease, and cancer.1, 2, 3 Exercise reduces the risk of death by preventing metabolic diseases and protects against chronic diseases. Organ-to-organ crosstalk involving muscle contraction at the molecular level is emerging as a field related to exercise.4 Additionally, adipokines are identified as hormones that mediate crosstalk between adipose tissue and brain, as well as metabolic functions during the activation of tissues.5 The proinflammatory role of various adipocyte-produced adipokines has also been identified. Tumor necrosis factor, chemokine C–C motif ligand 2, and plasminogen activator inhibitor-1 are proinflammatory adipokines that are overly secreted in obesity, leading to metabolic and cardiovascular diseases.6 Proinflammatory effects of these adipokines have now been clearly recognized to be counterbalanced by the protective effects of skeletal muscle-secreted peptides.4
Exercise-induced benefits are well known to prevent harmful effects of proinflammatory adipokines through skeletal muscle-secreted proteins.4 A recent study by Pedersen et al.7 demonstrated the endocrine effects of muscle fiber-derived cytokines or peptides produced and secreted during skeletal muscle contraction. The classified these cytokine and peptides as myokines. Furthermore, numerous studies demonstrated that myokines are exercise induced.8, 9, 10 The most well-known exercise-induced myokine interleukin (IL)-6 was the first myokine to be identified in the bloodstream in response to muscle contractions.11 IL-6 is a peptide that plays an anti-inflammatory role by inhibiting tumor necrosis factor-α; it also improves glucose uptake by stimulating AMP-activated protein kinase (AMPK) signaling.12, 13, 14 Surprisingly, circulating IL-6 levels are increased during exercise without any sign of muscle damage.15
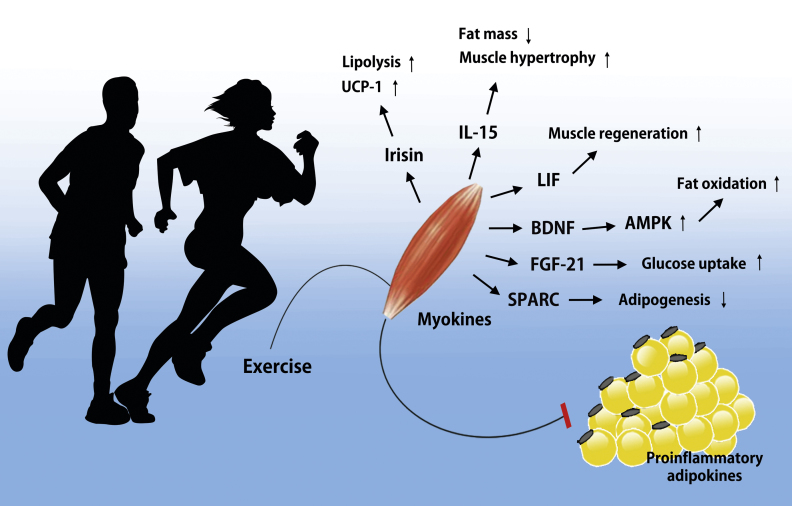
Myokines likely provide beneficial metabolic effects during crosstalk between skeletal muscle and liver, and skeletal muscle and adipose tissue.16, 17, 18 Additionally, several studies demonstrated that exercise-induced myokines have positive effects on glucose uptake,19, 20 glucose tolerance,21 regulation of fat oxidation,11, 21 and satellite cell proliferation.22, 23 Myokines, as one of multiple health factors, constitute an important area of research in metabolic diseases.24, 25 This review summarizes the potential positive effects of exercise-induced myokines, such as IL-15, brain-derived neurotrophic factor (BDNF), leukemia inhibitory factor (LIF), irisin, fibroblast growth factor 21 (FGF-21), and secreted protein acidic and rich in cysteine (SPARC), on metabolic diseases (Fig. 1 and Table 1).
Fig. 1.
Potential role of exercise-induced myokines. Skeletal muscle expresses and releases myokines into the circulation. Especially under conditions of metabolic diseases including obesity and diabetes, adipose tissue secretes proinflammatory adipokines that promote pathological processes such as atherosclerosis and insulin resistance. However, exercise-induced myokines may balance and counteract the effect of adipokines. In response to muscle contraction following exercise, muscle fibers express myokines such as irisin, IL-15, LIF, BDNF, FGF-21, and SPARC, which subsequently exert their effects locally within the muscle or their target organs.
AMPK, AMP-activated protein kinase; BDNF, brain-derived neurotrophic factor; FGF-21, fibroblast growth factor 21; IL, interleukin; LIF, leukemia inhibitory factor; SPARC, secreted protein acidic and rich in cysteine; UCP-1, uncoupling protein 1.
Table 1.
Studies of exercise-induced myokines
| Myokine | Subjects | Exercise | Results | Refs |
|---|---|---|---|---|
| IL-15 | Human | Treadmill running (70% maximum heart rate, 30 min) |
Serum IL-15 level ↑ | 34 |
| Rat | Treadmill running (26 m/min, 60 min each, 5 d/wk for 8 wk) |
IL-15 mRNA (in muscle) ↑ IL-15 Receptor ↑ IL-15 immunoreactivity (in muscle, plasma) ↑ |
35 | |
| Human | Resistance exercise (4 sets, 10 repetitions, 2–3 min rest intervals) |
IL-6, IL-10, IL-1 receptor antagonist (IL-1r), IL-8, cortisol ↑ | 36 | |
| Human | Resistance exercise (3 d/wk, 75% of one repetition maximum, 6–10 repetitions) |
Plasma IL-15 level ↑ IL-15 receptor-α gene ↑ |
32 | |
| Rat | Treadmill exercise (5 d/wk, 60 min, 12 wk) |
IL-15 level ↑ (in soleus muscle) |
38 | |
| Rat | Resistance exercise (acute ladder climbing) |
IL-15 level ↑ (in soleus and tibialis anterior muscles) |
39 | |
| Rat | Resistance exercise (chronic ladder climbing) |
IL-15 level ↑ (in soleus muscle) |
||
| BDNF | Rat | Treadmill exercise (1 d or 5 consecutive days) |
BDNF mRNA ↑ BDNF protein ↑ NT-3 ↑ |
48 |
| Rat | Motor-driven bicycle (2–30 d acute exercise) |
BDNF mRNA ↑ | 49 | |
| Human | Bicycle exercise ( max 60%, 120 min) |
BDNF mRNA ↑ BDNF protein ↑ |
42 | |
| C2C12 cell | Electrically stimulated | BDNF mRNA ↑ BDNF protein ↑ AMPK ↑ ACCβ ↑ |
||
| LIF | Human | Cycle ergometer exercise (3 h, max 60%) |
LIF mRNA↑ (increased 4.5-fold after exercise) |
58 |
| Human | Resistance exercise (a bout of heavy resistance exercise) |
LIF mRNA↑ (increased 9-fold after exercise) |
59 | |
| MDX mice | Voluntary wheel running (2 wk) |
LIF mRNA ↓ LIF receptor ↓ |
61 | |
| Irisin | Mice | Voluntary wheel running (free wheel running, 3 wk) |
Plasma irisin level ↑ | 63 |
| Human | Endurance exercise (cycle, max 65%, 4–5 sessions/10 wk) |
Plasma irisin level ↑ | ||
| Human | Acute exercise (80 m spirit runs, 3 d/1 wk) |
Serum irisin level ↑ | 67 | |
| FGF-21 | Human | Treadmill exercise (the Bruce's protocol 5 d/2 wk, 21 min) |
Serum FGF-21 level ↑ | 75 |
| Human | Acute exercise (a single bout of treadmill running, until exhaustion) |
76 | ||
| Mice | Acute exercise (a single bout of treadmill exercise, 30 min or until exhaustion) |
|||
| SPARC | Human | Acute exercise (a single bout of cycling, max 70%, 30 min) |
Serum SPARC level ↑ (gradually decreased) | 83 |
| Cycling ( max 70%; 2, 4 weeks) |
Serum SPARC level ↑ |
ACCβ, acetyl-CoA carboxylase-beta; AMPK, AMP-activated protein kinase; BDNF, brain-derived neurotropic factor; FGF-21, fibroblast growth factor 21; IL, interleukin; LIF, leukemia inhibitory factor; NT-3, neurotrophin-3; SPARC, secreted protein acidic and rich in cysteine.
2. Interleukin-15
IL-15 may play a role not only in muscle–fat interaction, but also in skeletal muscle fiber growth.26 IL-15, a member of the IL-2 superfamily, activates its functions through the β and γ chains of the IL-2 receptor.27, 28 Quinn et al29 showed that IL-15 can stimulate differentiated myocytes and muscle fibers to accumulate increased amounts of contractile proteins in skeletal muscle. Additionally, IL-15 stimulates muscle-specific myosin heavy-chain accumulation in differentiated myocytes and muscle fibers in culture.29, 30 Among its various roles, IL-15 has been demonstrated to regulate metabolic diseases, such as obesity and diabetes.21 IL-15 modulates glucose uptake in incubated skeletal muscle and muscle cell cultures.31 These results suggested that IL-15 may prevent the development of diabetes.26 Busquets et al19 showed that in vivo administration of IL-15 increased glucose uptake in skeletal muscle and in vitro IL-15 treatment increased glucose transporter type 4 mRNA content in C2C12 cells. These findings indicate that IL-15 may be an important mediator (regulator) of skeletal muscle fiber growth, hypertrophy, and glucose uptake.
Numerous studies demonstrated that exercise alters IL-15 concentration in serum or at the mRNA level. The most noticeable change in serum IL-15 was observed after moderate-intensity resistance training.32, 33 Other studies showed that aerobic training increased IL-15 in human and rodent serum and at the mRNA level.34, 35 Given that IL-15 controls glucose and lipid metabolism, it may have an important role in controlling metabolic diseases, including obesity and type 2 diabetes. However, studies on IL-15 expression in the skeletal muscle and plasma after exercise showed inconsistent results. It is evident that after resistance exercise, IL-15 protein level increases in the plasma32, 33; however, mRNA levels of IL-15 decrease after 2 hours of intensive strength training.36 In addition, circulating levels of IL-15 were increased in healthy young men, but remained unchanged after a single bout of treadmill running.37 Treadmill exercise increased IL-15 expression in the skeletal muscle of high-fat-induced obese rats.35 Similar treadmill exercise conducted in our laboratory showed an increase of IL-15 expression in the soleus muscle of a transgenic diabetic Zucker rat. Our data also demonstrated that treadmill exercise improved glucose tolerance in the Zucker rat.38 In addition, 1 hour of acute exercise increased IL-15 expression in Sprague–Dawley rats.39 These data collectively suggest that exercise-induced IL-15 may be important for the modulation of glucose uptake and improved glucose tolerance. While the role of IL-15 in treating metabolic diseases, such as obesity and type 2 diabetes, has now been revealed, further investigation on changes in IL-15 and its potential role is required.
3. Brain-derived neurotrophic factor
Neurotrophins are well-known regulators of various neuronal processes and act primarily through tropomyosin-related kinase receptor tyrosine kinases. The mammalian family of neurotrophins consists of nerve growth factor, neurotrophin-3, neurotrophin-4/5, and BDNF. Among these neurotrophins, BDNF and its receptor tropomyosin-related kinase B are most widely and abundantly expressed in the brain.40 To date, numerous studies suggested that BDNF may play a role not only in central metabolic pathways, but also as a metabolic regulator of skeletal muscle. Wisse and Schwartz41 reported that BDNF is a key modulator of the hypothalamic pathway that controls body composition and energy homeostasis. BDNF is a regulator of metabolism in skeletal muscle42 and an enhancer of glucose utilization in diabetic skeletal muscle.43 Current studies demonstrated that BDNF reduces food intake and lowers blood glucose level in genetically modified (db/db) obese mice, suggesting that BDNF plays a role in energy balance and insulin signaling.44, 45, 46 In addition, a recent study suggested that gene transfer of BDNF has therapeutic efficacy in mouse models of obesity and diabetes.47 It is now known that BDNF is expressed in non-neurogenic tissues, including skeletal muscle. Animal studies demonstrated that BDNF mRNA increases in skeletal muscle in response to contraction.48, 49 Numerous studies showed that BDNF mRNA and protein expression were increased in human skeletal muscle after exercise; however, skeletal muscle-derived BDNF was not released into circulation.42 Additionally, BDNF increased phosphorylation of AMPK and acetyl-CoA carboxylase-beta, and enhanced fat oxidation. Based on recent research evidence, BDNF appears to be a myokine that acts in an autocrine or paracrine fashion with strong effects on peripheral metabolism, including fat oxidation, and a subsequent effect on the size of adipose tissue.50
Skeletal muscle BDNF was reported as a key modulator in metabolic diseases, including type 2 diabetes mellitus. In previous studies about diabetes and BDNF, increased BDNF mRNA in the soleus muscle of diabetic rats compared to age-matched controls indicated that elevated BDNF may protect the distal nerve from the denervated muscle of diabetic rat.51, 52 Our unpublished data also showed an increased BDNF expression in the soleus muscle of type 2 diabetes mellitus-induced rats as compared to the lean controls. We also found significant decreases in BDNF expression following resistance exercise in the soleus, tibialis anterior, and extensor digitorum longus muscles. BDNF in skeletal muscle may be expressed as a compensatory neurotrophic factor against diabetic neuropathy or myopathy.
Although the important role of BDNF in peripheral metabolism including skeletal muscle metabolism is well known, the role of BDNF expression in diabetic skeletal muscle with severe glucose tolerance is not fully established. Moreover, considering the role of BDNF in skeletal muscle, further research is required to investigate the effect of physical exercise and nutritional treatment on BDNF expression and its potential to improved glucose metabolism in diabetic skeletal muscle.
4. Leukemia inhibitory factor
LIF is known as an important player in skeletal muscle hypertrophy and regeneration by enhancing cell proliferation though the common signaling mediators janus kinase (JAK), signal transducer and activator of transcription-3 (STAT3), and phosphoinositide-3-kinase.53, 54, 55, 56 Previous studies showed that LIF mRNA is upregulated exogenously after muscle injury, including muscle crush and contusion injury.57 LIF is a newly discovered myokine23; it is produced by skeletal muscle and affects intact muscles, as well as isolated muscle cells.58, 59 Among its various roles, the most important role of LIF in muscle satellite cell is proliferation for proper muscle hypertrophy and regeneration.23
Although studies on exercise-related LIF expression remained controversial, LIF mRNA levels increased after a single bout of both cycle ergometer exercise58 and heavy resistance exercise59 in the human vastus lateralis muscle, whereas LIF protein levels were not significantly changed. Since LIF has a very short half-life of 6–8 minutes in serum, it is difficult to detect circulating levels of LIF protein.60 Moreover, LIF promotes survival of myoblasts in dystrophic muscle; however, LIF mRNA levels were found to decrease after 2 weeks of voluntary wheel running in mdx mice.61 Numerous studies demonstrated that LIF clearly has the potential to regulate skeletal muscle disease, including muscular dystrophy, and it furthermore promotes myoblast survival in dystrophic muscle.61 These studies suggested that LIF provides alternative therapeutic ways to stimulate skeletal muscle regeneration in an autocrine or paracrine fashion after injury. However, the duration of increased LIF mRNA and protein levels after exercise is not well known. Further investigation is needed to describe the relationships between alteration of LIF mRNA, and protein levels and exercise duration or type.
5. Irisin
Irisin is a peroxisome proliferator-activated receptor-γ coactivator-1α (PPAR-γ) coactivator-1 α (PGC1-α)-dependent myokine, and seems to be capable of inducing white adipose tissue browning and uncoupling protein 1 expression.62 PGC-1α is best known to regulate energy metabolism; however, it has other beneficial actions, such as control of mitochondrial biogenesis and oxidative metabolism in many cell types.63 PGC-1α is induced in muscles by exercise and stimulates mitochondrial biogenesis, angiogenesis, and fiber-type switching.64 PGC-1α stimulates the expression of fibronectin type III domain-containing protein 5 (FNDC5) gene that encodes irisin, a type I membrane protein secreted into the blood. Irisin activates profound changes in the subcutaneous adipose tissue by stimulating browning and uncoupling protein 1 expression.63
Recent research indicated that, in an obese rodent model, FNDC5 treatment improved glucose tolerance and elevated expression of mitochondrial genes that increase oxygen consumption.63, 65 Exercise may increase plasma irisin levels. Results from several studies suggested that plasma irisin levels were elevated in rodents and humans following exercise.63, 66, 67 Bostrom et al63 demonstrated an increase of plasma irisin level in mice after 3 weeks of voluntary wheel running, and a twofold increase of circulating plasma irisin level in healthy adults compared to the nontraining group after 10 weeks of endurance exercise. In addition, irisin levels increased in response to acute exercise.63 Our recent laboratory data on 8 weeks of aerobic exercise and resistance exercise in obese humans demonstrated a positive correlation between the circulating irisin level and changes of muscle mass, and a negative correlation between the circulating irisin level and changes of fat mass and body fat percentage.67 Despite differences in results, both previous studies and our study consistently demonstrated an increased level of irisin following exercise.66 Therefore, irisin has potential as a therapeutic agent. Further study is required to investigate the changes in irisin levels and its protective role in obesity and diabetes following exercise.
6. Fibroblast growth factor 21
FGF-21, an endocrine hormone, plays an important role in the progression of metabolic regulation by controlling glucose and lipid metabolism.68, 69, 70 FGF-21 is expressed in peripheral tissues, such as white adipose tissue, liver, pancreas, and skeletal muscle.71 FGF-21 levels increased during starvation and the ketogenic state, and decreased after refeeding.72 Administration of FGF-21 reduced plasma glucose and triglycerides, and increased insulin sensitivity in diabetic rodents.73 Walsh and coworkers74 also demonstrated that FGF-21 is produced by skeletal muscle. Several studies determine FGF-21 as a key factor for downstream target of PPAR-α. FGF-21 may thus play an important role in metabolism. Recent studies suggested that FGF-21 levels were elevated in metabolic diseases, including insulin resistance, metabolic syndrome, and type 2 diabetes. Hojman et al's70 study demonstrated an increased FGF-21 expression in muscle and plasma in response to insulin stimulation. Additionally, FGF-21-null mice not only failed to express PGC-1α, but also had impaired gluconeogenesis and ketogenesis.
Recent studies showed that FGF-21 is elevated after exercise. In healthy humans, serum FGF-21 levels increased after 2 weeks of treadmill exercise.75 Serum FGF-21 levels also increased in mice and humans by a single bout of acute exercise.76 The increase in FGF-21 was accompanied by an increase in lipolytic response, leading to the release of free fatty acid and glycerol. Data of these studies might have suggested that increased serum FGF-21 levels lead to increased lipolysis and decreased glucose levels after a high-intensity exercise.75, 76 According to our unpublished data, 8 weeks of ladder climbing exercise increased FGF-21 level in the soleus and extensor digitorum longus muscles in type 2 diabetes rats. FGF-21 might be elevated in response to exercise, greatly affecting metabolic mechanisms. Therefore, further study is required to investigate changes in the FGF-21 level in response to the duration or type of exercise, for effective treatment of diabetes.
7. Secreted protein acidic and rich in cysteine
SPARC, also known as BM-40 was initially identified in bone and hence was named osteonectin initially.77 For a decade, intense studies on the effect of SPARC on various pathological conditions of organs such as the liver and kidney were conducted.78 SPARC acts as a counteradhesive and a modulator of cell–surface interaction during tissue renewal and remodeling, which requires regulation of cell–matrix or cell–cell contact.79 Most studies on regulation of SPARC expression and function are related to bone. During osteogenesis, SPARC links the organic and mineral phases of the bone extracellular matrix.80 However, recent studies revealed that SPARC is also found in myoblasts, myotubes, and muscle fibers, where its levels increase during muscle development and regeneration.81 Importantly, SPARC secretion was found to increase in muscles not only during regeneration, but also following resistance exercise and muscle hypertrophy.82 A single bout of exercise increases secretion of SPARC in mice. Levels of SPARC in the gastrocnemius muscle were significantly increased after a single bout of exercise, specifically around the plasma membrane. However, SPARC levels decreased immediately after exercise. In addition, SPARC was increased in the serum of young healthy men immediately after a single bout of cycling at 70% max for 30 minutes, but gradually decreased to the resting level. However, 4 weeks of training at 70% max resulted in a significant increase in serum SPARC, with no changes in the resting state.83
SPARC is currently identified as one of the myokines that reduces fat accumulation and is involved in glucose metabolism.84, 85 First, SPARC is involved in the regulation of adipocyte differentiation and adipose tissue turnover84 by activating the Wnt/β-caternin pathway that inhibits adipogenesis and enhances osteoblastogenesis.86, 87, 88 In addition, SPARC, by having a dose-dependent inhibitory effect on adipogenesis and formation of osteoblast, was also enhanced in a dose-dependent fashion. Primary culture of SPARC-null mice cells showed increased adipocyte and fat accumulation as compared to wild-type mice.84 SPARC decreased adipogenesis by regulating expression, deposition, and organization of extracellular matrix protein rather than by interfering adipogenesis directly. It modulates monoclonal anticellular fibronectin and antimouse laminin production in the extracellular matrix during adipogenesis, where fibronectin inhibits adipogenesis and laminin enhances adipogenesis.84 These changes inhibit the rearrangement of F-actin and stress fibers form the fiber-like preadipocyte cell structure, which weakens during adipogenesis to allow fat accumulation.84 SPARC is also involved in glucose metabolism via the AMPK signaling pathway. The AMPK signaling pathway has been identified as an energy-sensing pathway that plays a critical role in regulating glucose and fat metabolism in skeletal muscle during exercise.89, 90 It stimulates glucose uptake by increasing glucose transporter translocation.91, 92 Further study is needed to investigate the change in SPARC level according to the exercise duration, to effectively regulate glucose and fat metabolism.
8. Conclusion
Small changes induced by exercise can create a ripple effect of benefits to the entire body. It is well known that exercise satisfies essential requirements for a healthy life. Regular physical activity or exercise can prevent chronic diseases, such as cardiovascular diseases, metabolic diseases, and cognitive disorders. Findings from previous studies indicated that exercise-induced myokines might be the potential candidates to provide beneficial effects by stimulating metabolic pathways, improving glucose uptake, improving fat oxidation, and regulating skeletal muscle regeneration. Based on a review of the literature, we provide new insights into the role of exercise-induced myokines as important potential therapeutic factors for the prevention of metabolic diseases. Further research is clearly required to identify how exercise-induced myokines relate to chronic or metabolic diseases, including type 2 diabetes, obesity, and muscle atrophy.
Conflicts of interest
There are no conflicts of interest to declare.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology, MEST (2011-0030135), (NRF-2013M3A9B6046417) and also supported by the grants from KMPC (2013M3A9D5072550).
References
- 1.Pedersen B.K., Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Sports. 2006;16(Suppl 1):3–63. doi: 10.1111/j.1600-0838.2006.00520.x. [DOI] [PubMed] [Google Scholar]
- 2.Tanasescu M., Leitzmann M.F., Rimm E.B., Willett W.C., Stampfer M.J., Hu F.B. Exercise type and intensity in relation to coronary heart disease in men. JAMA. 2002;288:1994–2000. doi: 10.1001/jama.288.16.1994. [DOI] [PubMed] [Google Scholar]
- 3.Motl R.W., Pilutti L.A. The benefits of exercise training in multiple sclerosis. Nat Rev Neurol. 2012;8:487–497. doi: 10.1038/nrneurol.2012.136. [DOI] [PubMed] [Google Scholar]
- 4.Pedersen B.K., Febbraio M.A. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8:457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 5.Scherer P.E. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–1545. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- 6.Shetty S., Kusminski C.M., Scherer P.E. Adiponectin in health and disease: evaluation of adiponectin-targeted drug development strategies. Trends Pharmacol Sci. 2009;30:234–239. doi: 10.1016/j.tips.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Pedersen B.K., Steensberg A., Fischer C., Keller C., Keller P., Plomgaard P. Searching for the exercise factor: is IL-6 a candidate? J Muscle Res Cell Motil. 2003;24:113–119. doi: 10.1023/a:1026070911202. [DOI] [PubMed] [Google Scholar]
- 8.Bortoluzzi S., Scannapieco P., Cestaro A., Danieli G.A., Schiaffino S. Computational reconstruction of the human skeletal muscle secretome. Proteins. 2006;62:776–792. doi: 10.1002/prot.20803. [DOI] [PubMed] [Google Scholar]
- 9.Yoon J.H., Yea K., Kim J., Choi Y.S., Park S., Lee H. Comparative proteomic analysis of the insulin-induced L6 myotube secretome. Proteomics. 2009;9:51–60. doi: 10.1002/pmic.200800187. [DOI] [PubMed] [Google Scholar]
- 10.Henningsen J., Rigbolt K.T., Blagoev B., Pedersen B.K., Kratchmarova I. Dynamics of the skeletal muscle secretome during myoblast differentiation. Mol Cell Proteomics. 2010;9:2482–2496. doi: 10.1074/mcp.M110.002113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pedersen B.K., Febbraio M.A. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88:1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- 12.Keller C., Hellsten Y., Steensberg A., Pedersen B.K. Differential regulation of IL-6 and TNF-alpha via calcineurin in human skeletal muscle cells. Cytokine. 2006;36:141–147. doi: 10.1016/j.cyto.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Carey A.L., Steinberg G.R., Macaulay S.L., Thomas W.G., Holmes A.G., Ramm G. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes. 2006;55:2688–2697. doi: 10.2337/db05-1404. [DOI] [PubMed] [Google Scholar]
- 14.Kahn B.B., Alquier T., Carling D., Hardie D.G. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Fischer C.P. Interleukin-6 in acute exercise and training: what is the biological relevance? Exerc Immunol Rev. 2006;12:6–33. [PubMed] [Google Scholar]
- 16.Pedersen B.K., Akerstrom T.C., Nielsen A.R., Fischer C.P. Role of myokines in exercise and metabolism. J Appl Physiol. 2007;103:1093–1098. doi: 10.1152/japplphysiol.00080.2007. [DOI] [PubMed] [Google Scholar]
- 17.Pedersen B.K. The diseasome of physical inactivity—and the role of myokines in muscle–fat cross talk. J Physiol. 2009;587:5559–5568. doi: 10.1113/jphysiol.2009.179515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pedersen B.K. Muscles and their myokines. J Exp Biol. 2011;214:337–346. doi: 10.1242/jeb.048074. [DOI] [PubMed] [Google Scholar]
- 19.Busquets S., Figueras M., Almendro V., Lopez-Soriano F.J., Argiles J.M. Interleukin-15 increases glucose uptake in skeletal muscle. An antidiabetogenic effect of the cytokine. Biochim Biophys Acta. 2006;1760:1613–1617. doi: 10.1016/j.bbagen.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Barra N.G., Chew M.V., Holloway A.C., Ashkar A.A. Interleukin-15 treatment improves glucose homeostasis and insulin sensitivity in obese mice. Diabetes Obes Metab. 2012;14:190–193. doi: 10.1111/j.1463-1326.2011.01495.x. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez B., Carbo N., Lopez-Soriano J., Drivdahl R.H., Busquets S., Lopez-Soriano F.J. Effects of interleukin-15 (IL-15) on adipose tissue mass in rodent obesity models: evidence for direct IL-15 action on adipose tissue. Biochim Biophys Acta. 2002;1570:33–37. doi: 10.1016/s0304-4165(02)00148-4. [DOI] [PubMed] [Google Scholar]
- 22.Serrano A.L., Baeza-Raja B., Perdiguero E., Jardi M., Munoz-Canoves P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab. 2008;7:33–44. doi: 10.1016/j.cmet.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Broholm C., Pedersen B.K. Leukaemia inhibitory factor—an exercise-induced myokine. Exerc Immunol Rev. 2010;16:77–85. [PubMed] [Google Scholar]
- 24.Pedersen B.K. Exercise-induced myokines and their role in chronic diseases. Brain Behav Immun. 2011;25:811–816. doi: 10.1016/j.bbi.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Henriksen T., Green C., Pedersen B.K. Myokines in myogenesis and health. Recent Pat Biotechnol. 2012;6:167–171. doi: 10.2174/1872208311206030167. [DOI] [PubMed] [Google Scholar]
- 26.Argiles J.M., Lopez-Soriano F.J., Busquets S. Therapeutic potential of interleukin-15: a myokine involved in muscle wasting and adiposity. Drug Discov Today. 2009;14:208–213. doi: 10.1016/j.drudis.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 27.Giri J.G., Ahdieh M., Eisenman J., Shanebeck K., Grabstein K., Kumaki S. Utilization of the beta and gamma chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J. 1994;13:2822–2830. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Argiles J.M., Lopez-Soriano J., Almendro V., Busquets S., Lopez-Soriano F.J. Cross-talk between skeletal muscle and adipose tissue: a link with obesity? Med Res Rev. 2005;25:49–65. doi: 10.1002/med.20010. [DOI] [PubMed] [Google Scholar]
- 29.Quinn L.S., Haugk K.L., Grabstein K.H. Interleukin-15: a novel anabolic cytokine for skeletal muscle. Endocrinology. 1995;136:3669–3672. doi: 10.1210/endo.136.8.7628408. [DOI] [PubMed] [Google Scholar]
- 30.Quinn L.S., Anderson B.G., Drivdahl R.H., Alvarez B., Argiles J.M. Overexpression of interleukin-15 induces skeletal muscle hypertrophy in vitro: implications for treatment of muscle wasting disorders. Exp Cell Res. 2002;280:55–63. doi: 10.1006/excr.2002.5624. [DOI] [PubMed] [Google Scholar]
- 31.Busquets S., Figueras M.T., Meijsing S., Carbo N., Quinn L.S., Almendro V. Interleukin-15 decreases proteolysis in skeletal muscle: a direct effect. Int J Mol Med. 2005;16:471–476. [PubMed] [Google Scholar]
- 32.Riechman S.E., Balasekaran G., Roth S.M., Ferrell R.E. Association of interleukin-15 protein and interleukin-15 receptor genetic variation with resistance exercise training responses. J Appl Physiol. 2004;97:2214–2219. doi: 10.1152/japplphysiol.00491.2004. [DOI] [PubMed] [Google Scholar]
- 33.Lambert C.P., Flynn M.G., Sullivan D.H., Evans W.J. Effects of megestrol acetate on circulating interleukin-15 and interleukin-18 concentrations in healthy elderly men. J Gerontol A Biol Sci Med Sci. 2004;59:855–858. doi: 10.1093/gerona/59.8.m855. [DOI] [PubMed] [Google Scholar]
- 34.Tamura Y., Watanabe K., Kantani Y., Hayashi J., Ishida N., Kaneki M. Upregulation of circulating IL-15 by treadmill running in healthy individuals: is IL-15 an endocrine mediator of the beneficial effects of endurance exercise? Endocr J. 2011;58:211–215. doi: 10.1507/endocrj.k10e-400. [DOI] [PubMed] [Google Scholar]
- 35.Yang H., Chang J., Chen W., Zhao L., Qu B., Tang C. Treadmill exercise promotes interleukin 15 expression in skeletal muscle and interleukin 15 receptor alpha expression in adipose tissue of high-fat diet rats. Endocrine. 2013;43:579–585. doi: 10.1007/s12020-012-9809-6. [DOI] [PubMed] [Google Scholar]
- 36.Nieman D.C., Davis J.M., Brown V.A., Henson D.A., Dumke C.L., Utter A.C. Influence of carbohydrate ingestion on immune changes after 2 h of intensive resistance training. J Appl Physiol. 2004;96:1292–1298. doi: 10.1152/japplphysiol.01064.2003. [DOI] [PubMed] [Google Scholar]
- 37.Ostrowski K., Hermann C., Bangash A., Schjerling P., Nielsen J.N., Pedersen B.K. A trauma-like elevation of plasma cytokines in humans in response to treadmill running. J Physiol. 1998;513(Pt 3):889–894. doi: 10.1111/j.1469-7793.1998.889ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim H.J., Park J.Y., Oh S.L., Kim Y.A., So B., Seong J.K. Effect of treadmill exercise on interleukin-15 expression and glucose tolerance in Zucker diabetic fatty rats. Diabetes Metab J. 2013;37:358–364. doi: 10.4093/dmj.2013.37.5.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oh S., Kim H., Kim Y.A., Song W. Effects of acute and chronic resistance exercise on IL-15 expression in rat skeletal muscle. Int J Appl Sports Sci. 2013;25:85–90. [Google Scholar]
- 40.Huang E.J., Reichardt L.F. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wisse B.E., Schwartz M.W. The skinny on neurotrophins. Nat Neurosci. 2003;6:655–656. doi: 10.1038/nn0703-655. [DOI] [PubMed] [Google Scholar]
- 42.Matthews V.B., Astrom M.B., Chan M.H., Bruce C.R., Krabbe K.S., Prelovsek O. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia. 2009;52:1409–1418. doi: 10.1007/s00125-009-1364-1. [DOI] [PubMed] [Google Scholar]
- 43.Yamanaka M., Tsuchida A., Nakagawa T., Nonomura T., Ono-Kishino M., Sugaru E. Brain-derived neurotrophic factor enhances glucose utilization in peripheral tissues of diabetic mice. Diabetes Obes Metab. 2007;9:59–64. doi: 10.1111/j.1463-1326.2006.00572.x. [DOI] [PubMed] [Google Scholar]
- 44.Zuccato C., Cattaneo E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat Rev Neurol. 2009;5:311–322. doi: 10.1038/nrneurol.2009.54. [DOI] [PubMed] [Google Scholar]
- 45.Krabbe K.S., Nielsen A.R., Krogh-Madsen R., Plomgaard P., Rasmussen P., Erikstrup C. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia. 2007;50:431–438. doi: 10.1007/s00125-006-0537-4. [DOI] [PubMed] [Google Scholar]
- 46.Strachan M.W., Deary I.J., Ewing F.M., Frier B.M. Is type II diabetes associated with an increased risk of cognitive dysfunction? A critical review of published studies. Diabetes Care. 1997;20:438–445. doi: 10.2337/diacare.20.3.438. [DOI] [PubMed] [Google Scholar]
- 47.Ono M., Ichihara J., Nonomura T., Itakura Y., Taiji M., Nakayama C. Brain-derived neurotrophic factor reduces blood glucose level in obese diabetic mice but not in normal mice. Biochem Biophys Res Commun. 1997;238:633–637. doi: 10.1006/bbrc.1997.7220. [DOI] [PubMed] [Google Scholar]
- 48.Gomez-Pinilla F., Ying Z., Opazo P., Roy R.R., Edgerton V.R. Differential regulation by exercise of BDNF and NT-3 in rat spinal cord and skeletal muscle. Eur J Neurosci. 2001;13:1078–1084. doi: 10.1046/j.0953-816x.2001.01484.x. [DOI] [PubMed] [Google Scholar]
- 49.Dupont-Versteegden E.E., Houle J.D., Dennis R.A., Zhang J., Knox M., Wagoner G. Exercise-induced gene expression in soleus muscle is dependent on time after spinal cord injury in rats. Muscle Nerve. 2004;29:73–81. doi: 10.1002/mus.10511. [DOI] [PubMed] [Google Scholar]
- 50.Pedersen B.K., Pedersen M., Krabbe K.S., Bruunsgaard H., Matthews V.B., Febbraio M.A. Role of exercise-induced brain-derived neurotrophic factor production in the regulation of energy homeostasis in mammals. Exp Physiol. 2009;94:1153–1160. doi: 10.1113/expphysiol.2009.048561. [DOI] [PubMed] [Google Scholar]
- 51.Funakoshi H., Frisen J., Barbany G., Timmusk T., Zachrisson O., Verge V.M. Differential expression of mRNAs for neurotrophins and their receptors after axotomy of the sciatic nerve. J Cell Biol. 1993;123:455–465. doi: 10.1083/jcb.123.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koliatsos V.E., Clatterbuck R.E., Winslow J.W., Cayouette M.H., Price D.L. Evidence that brain-derived neurotrophic factor is a trophic factor for motor neurons in vivo. Neuron. 1993;10:359–367. doi: 10.1016/0896-6273(93)90326-m. [DOI] [PubMed] [Google Scholar]
- 53.Alter J., Rozentzweig D., Bengal E. Inhibition of myoblast differentiation by tumor necrosis factor alpha is mediated by c-Jun N-terminal kinase 1 and leukemia inhibitory factor. J Biol Chem. 2008;283:23224–23234. doi: 10.1074/jbc.M801379200. [DOI] [PubMed] [Google Scholar]
- 54.Diao Y., Wang X., Wu Z. SOCS1, SOCS3, and PIAS1 promote myogenic differentiation by inhibiting the leukemia inhibitory factor-induced JAK1/STAT1/STAT3 pathway. Mol Cell Biol. 2009;29:5084–5093. doi: 10.1128/MCB.00267-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spangenburg E.E., Booth F.W. Multiple signaling pathways mediate LIF-induced skeletal muscle satellite cell proliferation. Am J Physiol Cell Physiol. 2002;283:C204–C211. doi: 10.1152/ajpcell.00574.2001. [DOI] [PubMed] [Google Scholar]
- 56.Sun L., Ma K., Wang H., Xiao F., Gao Y., Zhang W. JAK1–STAT1–STAT3, a key pathway promoting proliferation and preventing premature differentiation of myoblasts. J Cell Biol. 2007;179:129–138. doi: 10.1083/jcb.200703184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kurek J.B., Bower J.J., Romanella M., Koentgen F., Murphy M., Austin L. The role of leukemia inhibitory factor in skeletal muscle regeneration. Muscle Nerve. 1997;20:815–822. doi: 10.1002/(sici)1097-4598(199707)20:7<815::aid-mus5>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 58.Broholm C., Mortensen O.H., Nielsen S., Akerstrom T., Zankari A., Dahl B. Exercise induces expression of leukaemia inhibitory factor in human skeletal muscle. J Physiol. 2008;586:2195–2201. doi: 10.1113/jphysiol.2007.149781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Broholm C., Laye M.J., Brandt C., Vadalasetty R., Pilegaard H., Pedersen B.K. LIF is a contraction-induced myokine stimulating human myocyte proliferation. J Appl Physiol. 2011;111:251–259. doi: 10.1152/japplphysiol.01399.2010. [DOI] [PubMed] [Google Scholar]
- 60.Hilton D.J., Nicola N.A., Waring P.M., Metcalf D. Clearance and fate of leukemia-inhibitory factor (LIF) after injection into mice. J Cell Physiol. 1991;148:430–439. doi: 10.1002/jcp.1041480315. [DOI] [PubMed] [Google Scholar]
- 61.Hunt L.C., Anthea Coles C., Gorman C.M., Tudor E.M., Smythe G.M., White J.D. Alterations in the expression of leukemia inhibitory factor following exercise: comparisons between wild-type and mdx muscles. PLoS Curr. 2011;3:RRN1277. doi: 10.1371/currents.RRN1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cannon B., Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 63.Bostrom P., Wu J., Jedrychowski M.P., Korde A., Ye L., Lo J.C. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Handschin C., Spiegelman B.M. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454:463–469. doi: 10.1038/nature07206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Castillo-Quan J.I. From white to brown fat through the PGC-1alpha-dependent myokine irisin: implications for diabetes and obesity. Dis Model Mech. 2012;5:293–295. doi: 10.1242/dmm.009894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Timmons J.A., Baar K., Davidsen P.K., Atherton P.J. Is irisin a human exercise gene? Nature. 2012;488:E9–E10. doi: 10.1038/nature11364. discussion E10–1. [DOI] [PubMed] [Google Scholar]
- 67.Huh J.Y., Panagiotou G., Mougios V., Brinkoetter M., Vamvini M.T., Schneider B.E. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism. 2012;61:1725–1738. doi: 10.1016/j.metabol.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Izumiya Y., Bina H.A., Ouchi N., Akasaki Y., Kharitonenkov A., Walsh K. FGF21 is an Akt-regulated myokine. FEBS Lett. 2008;582:3805–3810. doi: 10.1016/j.febslet.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang X., Yeung D.C., Karpisek M., Stejskal D., Zhou Z.G., Liu F. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. 2008;57:1246–1253. doi: 10.2337/db07-1476. [DOI] [PubMed] [Google Scholar]
- 70.Hojman P., Pedersen M., Nielsen A.R., Krogh-Madsen R., Yfanti C., Akerstrom T. Fibroblast growth factor-21 is induced in human skeletal muscles by hyperinsulinemia. Diabetes. 2009;58:2797–2801. doi: 10.2337/db09-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Canto C., Auwerx J. Cell biology. FGF21 takes a fat bite. Science. 2012;336:675–676. doi: 10.1126/science.1222646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Badman M.K., Pissios P., Kennedy A.R., Koukos G., Flier J.S., Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 73.Kharitonenkov A., Shiyanova T.L., Koester A., Ford A.M., Micanovic R., Galbreath E.J. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Walsh K. Adipokines, myokines and cardiovascular disease. Circ J. 2009;73:13–18. doi: 10.1253/circj.cj-08-0961. [DOI] [PubMed] [Google Scholar]
- 75.Cuevas-Ramos D., Almeda-Valdes P., Meza-Arana C.E., Brito-Cordova G., Gomez-Perez F.J., Mehta R. Exercise increases serum fibroblast growth factor 21 (FGF21) levels. PLoS One. 2012;7:e38022. doi: 10.1371/journal.pone.0038022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim K.H., Kim S.H., Min Y.K., Yang H.M., Lee J.B., Lee M.S. Acute exercise induces FGF21 expression in mice and in healthy humans. PLoS One. 2013;8:e63517. doi: 10.1371/journal.pone.0063517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Termine J.D., Kleinman H.K., Whitson S.W., Conn K.M., McGarvey M.L., Martin G.R. Osteonectin, a bone-specific protein linking mineral to collagen. Cell. 1981;26:99–105. doi: 10.1016/0092-8674(81)90037-4. [DOI] [PubMed] [Google Scholar]
- 78.Schulz A., Jundt G., Berghauser K.H., Gehron-Robey P., Termine J.D. Immunohistochemical study of osteonectin in various types of osteosarcoma. Am J Pathol. 1988;132:233–238. [PMC free article] [PubMed] [Google Scholar]
- 79.Bornstein P. Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J Cell Biol. 1995;130:503–506. doi: 10.1083/jcb.130.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brekken R.A., Sage E.H. SPARC, a matricellular protein: at the crossroads of cell-matrix communication. Matrix Biol. 2001;19:816–827. doi: 10.1016/s0945-053x(00)00133-5. [DOI] [PubMed] [Google Scholar]
- 81.Kupprion C., Motamed K., Sage E.H. SPARC (BM-40, osteonectin) inhibits the mitogenic effect of vascular endothelial growth factor on microvascular endothelial cells. J Biol Chem. 1998;273:29635–29640. doi: 10.1074/jbc.273.45.29635. [DOI] [PubMed] [Google Scholar]
- 82.Norheim F., Raastad T., Thiede B., Rustan A.C., Drevon C.A., Haugen F. Proteomic identification of secreted proteins from human skeletal muscle cells and expression in response to strength training. Am J Physiol Endocrinol Metab. 2011;301:E1013–E1021. doi: 10.1152/ajpendo.00326.2011. [DOI] [PubMed] [Google Scholar]
- 83.Aoi W., Naito Y., Takagi T., Tanimura Y., Takanami Y., Kawai Y. A novel myokine, secreted protein acidic and rich in cysteine (SPARC), suppresses colon tumorigenesis via regular exercise. Gut. 2013;62:882–889. doi: 10.1136/gutjnl-2011-300776. [DOI] [PubMed] [Google Scholar]
- 84.Nie J., Sage E.H. SPARC inhibits adipogenesis by its enhancement of beta-catenin signaling. J Biol Chem. 2009;284:1279–1290. doi: 10.1074/jbc.M808285200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Song H., Guan Y., Zhang L., Li K., Dong C. SPARC interacts with AMPK and regulates GLUT4 expression. Biochem Biophys Res Commun. 2010;396:961–966. doi: 10.1016/j.bbrc.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 86.Gregoire F.M., Smas C.M., Sul H.S. Understanding adipocyte differentiation. Physiol Rev. 1998;78:783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- 87.Nuttall M.E., Gimble J.M. Controlling the balance between osteoblastogenesis and adipogenesis and the consequent therapeutic implications. Curr Opin Pharmacol. 2004;4:290–294. doi: 10.1016/j.coph.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 88.Farmer S.R. Regulation of PPARgamma activity during adipogenesis. Int J Obes (Lond) 2005;29(Suppl 1):S13–S16. doi: 10.1038/sj.ijo.0802907. [DOI] [PubMed] [Google Scholar]
- 89.Wadley G.D., Lee-Young R.S., Canny B.J., Wasuntarawat C., Chen Z.P., Hargreaves M. Effect of exercise intensity and hypoxia on skeletal muscle AMPK signaling and substrate metabolism in humans. Am J Physiol Endocrinol Metab. 2006;290:E694–E702. doi: 10.1152/ajpendo.00464.2005. [DOI] [PubMed] [Google Scholar]
- 90.Shaw R.J., Lamia K.A., Vasquez D., Koo S.H., Bardeesy N., Depinho R.A. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fryer L.G., Foufelle F., Barnes K., Baldwin S.A., Woods A., Carling D. Characterization of the role of the AMP-activated protein kinase in the stimulation of glucose transport in skeletal muscle cells. Biochem J. 2002;363:167–174. doi: 10.1042/0264-6021:3630167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kurth-Kraczek E.J., Hirshman M.F., Goodyear L.J., Winder W.W. 5′ AMP-activated protein kinase activation causes GLUT4 translocation in skeletal muscle. Diabetes. 1999;48:1667–1671. doi: 10.2337/diabetes.48.8.1667. [DOI] [PubMed] [Google Scholar]