Abstract
The productivity decline in drug discovery and development is mainly caused by two factors; higher regulatory hurdles and low-hanging fruits being all picked. In addition, the recent target-based approach is thought to be increasing the price of innovation. Although target-based approach had many successes, a postreductionism method, which is systems biology, is on the rise. In this review, we discuss the foundations of two distinct approaches in finding a new drug.
Keywords: drug development, integrative, reductionism, systems biology, target-based approach
1. Introduction
The pharmaceutical industry is currently facing unparalleled challenges to develop innovative new drugs. Although the annual number of new drugs approved by the Food and Drug Administration (FDA) has not changed much, research and development (R&D) investment per drug is escalating at a marked rate. The estimated cost of developing a new drug is approximately $1 billion.1, 2, 3 This phenomenon, the increase in R&D investment without the corresponding increase in the number of new drug approval, is known as the “innovation gap.”4 After the Thalidomide and Vioxx incidents, regulatory bodies throughout the world are demanding more safety data, which in turn increases the development costs. Lack of efficacy is another important factor that contributes to the high attrition rate. Nowadays, even me-too drugs must provide more benefit than the conventional therapeutics to be approved. Both safety and efficacy hurdles are responsible for the rising cost in drug discovery and development. To minimize the risk in internal R&D, pharmaceutical companies began to rely more on outside innovation. The effectiveness of big pharmaceutical companies’ (big pharmas) R&D externalization strategies are being questioned as more and more assets are put into early-stage pipelines. Many state-of-the-art technologies such as high-throughput screening are speeding experimental procedures that are required by today's drug discovery and development. However, applying new technologies and devices also means increased costs. From 1950 to 2008, the FDA approved 1222 new drugs new molecular entities or new biological entities (NMEs or NBEs). Even though the amount of investment per drug has increased exponentially, the annual number of approvals has remained unaffected.5 There are > 4000 companies undergoing some forms of drug discovery and development. However, only 261 companies have succeeded in registering a new drug since 1950. In the United States alone, > 50,000 doctoral and postdoctoral researchers are conducting basic, translational, and clinical research. These research ventures spend > $90 billion annually. The National Institute of Health alone provides $33 billion into life sciences. Lazonick and Tulum6 explained that the strength of the U.S. biopharmaceutical industry originated on three factors: large National Institute of Health funding, strong appetite for biotechnology initial public offering, and vibrant venture capital investment. Indisputably, the pharmaceutical industry contributed greatly to improve health conditions and longevity. Nevertheless, the time is ripe to discuss the mounting problems in drug discovery and development approaches to push the pharmaceutical industry into the next level. So, two questions arise. Is target-based approach the reason behind the current fall in productivity? Should the concept of systems biology replace the reductionists’ view to succeed in drug discovery and development?
2. Target-based approach and systems biology
The purpose of drug design is to find the optimal structure that possesses high specificity around the target and interferes less with other sites to decrease the likelihood of side effects. Screening is very expensive, thus contributing heavily to drug development cost. In the past few decades, knowledge in science has leaped forward dramatically. With the help of reductionist methodologies, our understanding of the human body and diseases has increased enormously. Reductionism, as preached by Ernest Nagel, considers that all higher-level theories can be reduced to some basal-level theories.7, 8 This is in agreement with Marshall Nirenberg's dictum that science progress best when there are simple assays capable of generating large data sets rapidly.9 In short, gene to protein to function is the central tenet in modern biology. Because most drug action sites are proteins, targeting protein became the foundation of modern drug discovery and development. Meanwhile, the so-called low-hanging fruit is now picked, which suggests that more effort, whether financial or scientific, is needed to develop a new drug. Therefore, redefining the drug discovery and development is a grand challenge for the pharmaceutical industry. In order to endure the upcoming challenges, for example, blockbuster patent cliff and price containment pressures from the payers, a more integrative approach must be implemented.
Until the 1990s, drug discovery and development was largely based on a phenotypic approach or observation-based approach. However, the accumulation of knowledge in biochemistry and molecular biology led to a shift toward the target-based approach. The appearance of recombinant DNA and low-cost fast protein liquid chromatography facilitated this change.10 At that time, the phenotypic approach was challenged by many scientists just as target-based approach is being scrutinized at present. Even in Phase 1 of the clinical trial, the phenotypic approach was unable to provide the mechanisms of the action of a drug. Lack of knowledge was particularly risky when tested on human volunteers for various reasons (e.g., toxicity). Therefore, drug-developing chemists and biologists in the 1990s mostly welcomed the transformation into a target-based approach, which was thought to be more predictable and science-driven. Two decades of experience shows that the target-based approach is failing to boost the productivity in drug discovery and development. Selected targets were often not druggable and with poor disease linkage, leading to either high toxicity or poor efficacy. The off-target effect of a drug was much more difficult to predict in comparison to the phenotypic approach. Because the whole industry was using similar compound libraries for druggable targets, the diversity of pharmaceutical companies’ portfolio has been damaged. This led to intense competition, where speed of clinical trials and marketing were the main attributes in determining the first-in-class or best-in-class.
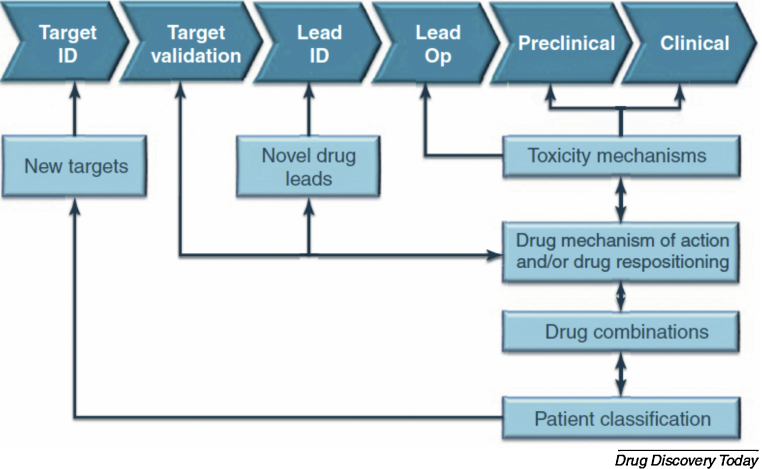
The decline in productivity in the past two decades coincided with the introduction of target-based approaches.11 However, the target-based approach is not the only explanation for this decline in productivity because innovative therapeutics such as monoclonal antibodies, antibody-drug conjugates (ADCs), and Gleevec had appeared. However, once the target-based approach has become a standard in all disease areas, it may lead to a predicament. The debate on physiology-based approach and target-based approach is still ongoing. But both physiology-based and target-based approaches should be taken into account to have a better chance of controlling the so-called difficult diseases. A holistic view or integrative approach is therefore the key to blend the two contradictory, yet complementary, methods (Fig. 1).12
Fig. 1.
Impact of systems biology in drug discovery and development. Various –omics can be useful to improve target-based drug discovery pathways, especially in finding novel leads and targets.
Note. From “Systems biology in drug discovery and development,” by E. Berg, 2014, Drug Discov Today, 19, p. 113–125. Copyright 2014, Korea Institute of Oriental Medicine. Published by Elsevier. All rights reserved, Reproduced with permission.
Consequently, no one pharmaceutical company can handle the entire spectrum of science, not to mention the vast disease areas. This is why collaborations between the industry and universities are becoming a prerequisite. To keep going in this challenging era, pharmaceutical companies need to innovate constantly with the outside world. Open innovation, which was first coined by Henry Chesbrough,13 is the most talked-about term when discussing future research and development (R&D). More and more pharmaceutical companies are implementing the concept of open innovation in their business model. Although the limits of target-based approach are well established, it still remains as the gold standard to push the candidate molecule all the way to Phase 3. This is also the case in government grants or when submitting a research paper. Prior to the rise of molecular biology, phenotypic screening was the norm in finding a new candidate molecule. At that time, the mode of action was not fully elucidated. Thus, the majority of drugs entered clinical trials without the understanding right down to a molecular level. A single target drug is very desirable theoretically in terms of both safety and efficacy. It will be straightforward to predict and control the strength of action. Alas, each drug on average acts on at least five different targets, causing mild to severe side effects. In reality, a drug that acts via a single target is very difficult to find. With the advent of information technology, the concept of big data is infused into the early stage screening process. Even with the today's gigantic computing power, it is not feasible to examine drug interactions at all levels. Unknown targets remain to be discovered, and the accuracy of screening process is far from satisfactory. In terms of cost, screening, although widely used, become inefficient at certain points.
3. Interdisciplinary research becoming the key issue in collaboration
Until recently, pharmaceutical companies preferred developing drugs in a closed system. Information and knowledge generated within the company was tightly concealed. This tendency began to change since the early 1980s when complex biologicals started to appear in the market. Biologicals had a significant impact on the way things were done in the pharmaceutical industry. After Genentech, numerous biotech ventures emerged with the latest technologies. Recombinant DNA, at that time was very new to traditional pharmaceutical companies. Simultaneously, codevelopment between academia and industry became a trend in diversifying the pipeline. Numerous new molecular entities (and new biological entities) came from universities. If a promising candidate (or technology) was discovered, building a venture was a common path to take. Big pharmas monitor and work with universities and small biotechnology companies for licensing opportunities. In academia–industry collaborations, conflicts of interests may occur owing to their disparate priorities. Academic scientists’ main role in the university is to disseminate knowledge through publications. Quality (and sometimes quantity) publication puts them in a good position to apply for a grant. Conversely, industry players want to keep interesting findings away from their competitors. That is, filing a patent instead of publication to maximize the chance of a commercial breakthrough.
There is a huge chasm between universities and pharmaceutical companies, but they are learning to work together. From pharmaceutical companies’ point of view, merger and acquisitions (M&A), licensing, and academic collaboration are vital in strengthening the pipelines. As mentioned previously, low-hanging fruits are no longer available; this is why the industry keeps on tapping universities for novel ideas. Academia, by contrast, is always short on research grants, so funding from the industry is considered a good alternative. Although publication is the number one choice for academics, they are beginning to accept the slight delay (6 months or after Phase 2 proof-of-concept stage) in publication that is requested by pharmaceutical companies. For startups, geographical proximity with world-renowned universities is very important in nurturing their innovative capabilities. The transfer of tacit knowledge between organizations within bioclusters is possibly the most notable benefit of being grouped together.14 In the United States, there are many successful bioclusters such as San Diego, CA, and Boston, MA. Many tried to reproduce the innovative environment in those bioclusters, but only the hardware (buildings, research facilities, etc.) was implemented with the absence of software (scientists, collaborative atmosphere, etc.). Bioclusters will no doubt continue to be the birthplace of innovative products in the pharmaceutical industry. Thus, policy makers should concentrate on the innovative ecosystem, especially the software segment, to build a sustainable pharmaceutical industry. Furthermore, clustering of various participants in industry is also suitable for practicing the integrative approach in drug discovery and development.
4. A new hype needed?
The Human Genome Project at the turn of the century resulted in a hype where newly formed biotechnology ventures benefitted from the constant inflow of investments. In the end, high expectations led to a bubble burst. Most industry observers consider hype as detrimental for both investors and companies. But in terms of focusing resources, for example, policy makers, analysts, and the industry itself, hype has some merit in advancing the technological development forward.15 In addition to this genomic revolution, combinatory chemistry and ultrahigh-throughput technologies increased the expectations of the public.16 About 8000 potential drug targets had been identified, of which only 218 are currently used as drug targets. And 100 of those are undergoing clinical trials, meaning that there are plenty of druggable targets to lessen the unmet medical needs. Unfortunately, drug discovery and development is not just driven by scientific or philanthropic motives. In order to attract pharmaceutical companies’ attention, corresponding targets or diseases must have strong financial incentives. The pharmaceutical industry has been criticized for investing rather heavily on me-too drugs. Rare diseases and tropical diseases hitherto have been largely neglected by major pharmaceutical companies owing to their low market potential. In the case of rare diseases, the government and payers are willing to accept high prices, giving pharmaceutical companies a good reason to develop a drug.
Big pharma is currently under heavy pressure from their shareholders to innovate with much less resources and time. With the recent blockbuster patent expiry, major pharmaceutical companies are under a restructuring process. Many R&D centers are closed and research staff are being laid off. From small biotechnology firms to big pharma, rising costs in drug development is impacting their business model. The open innovation that is disseminated throughout the industry will no doubt enhance the effectiveness of the drug discovery and development process.17. It can be argued that the aforementioned reductionists’ method, although very pragmatic and scientifically strong, will not be sufficient to confront the mounting problems. Application of big data technologies in screening processes seems inevitable but when applied to a target-based approach, it might further increase the drug development cost with little advantage. There are many examples where a single-target drug failed to generate the required safety and efficacy in the late phase. Late failures significantly affect the future prospects of a company. The situation gets worse when the exact mechanism of a disease is far from complete. Alzheimer's disease is a classic example where the wrong biomarker resulted in a staggering cost, only to fail in the late clinical stage. Our understandings of Alzheimer's disease is at an infant stage, hence the typical target-based approach can be misguiding. Yet, the combination of the right target and highly specific drug candidate can be a very powerful tool. To have any chance of developing a novel therapy in a difficult disease area, all insights from both the reductionist and nonreductionist approaches are required. The importance of interdisciplinary research is stressed at all times, but never between molecular biologists and systems biologists. If that were to occur, a great synergy is expected.
5. Postreductionism era
…each part of Nature agrees with the whole, and the manner in which it is associated with the remaining parts…. By the association of parts, then, I merely mean that the laws or nature of one part adapt themselves to the laws or nature of another part, so as to cause the least possible inconsistency….
B. Spinoza
Although the reductionist approach — identifying drugs that activate or inhibit specific targets — dominated the way drugs were developed, the complexity of biology is calling for more action. It is clear that the biological function or malfunction cannot be manipulated by a single protein or gene.18 Moreover, as Frank Sams-Dodd put it,19 by reducing something to its components, we lose understanding of how the components interact to produce function and why they change as they do. Systems biology, by contrast, is providing a more holistic view in medicine. Interdisciplinary research is the key aspect of systems biology where mathematicians, engineers, physicists, computer scientists, and biologists are brought together.20 In experiments, simplicity helps, but it may not directly represent the physiology in real-life situations. Many clinical trials fail even with positive in vitro and in vivo studies, because the complexity and variables in clinical trials are much greater. Therefore, the dynamic picture of the disease, mechanisms, and drug interactions is a prerequisite to decrease the dire attrition rates. Various technologies that were developed in areas of next-generation sequencing, transcriptomics, metabolomics, and proteomics can be of value to systems biology. Quite often, systems biology is perceived as managing Big Data obtained from combinations of “–omics.” Understanding life is not just about adding its components together. Noble's computational model of the heart gives a perfect example of a multiscale approach that includes biochemistry, anatomy, and functional level parameters. Systems biology entails investigating phenomena in terms of how the objects are related, rather than what their compositions are.21
The most noticeable movement in modern pharmaceutical industry is opening up large databases in order to facilitate drug development in areas of unmet medical needs. Universities, big pharmas, the FDA, and many others are disclosing their own database to be assessed for research use. As a result, a tremendous amount of data is available, but without the advanced computational tools, interpretation of these data would be impractical. The database on the bioavailability of chemicals is increasing at a substantial rate. The number of bioassay data on PubChem has increased from 800 records to 500,000 records in a period of 3 years. Currently, data from in vitro target binding assays and chemical perturbation experiments with associated gene expression profiles is routinely deposited in public databases.22 There were many efforts from the commercial and academic institutions to apply systems biology into drug discovery and development platforms (Table 1).23
Table 1.
Examples of organizations that incorporate systems biology approaches for drug discovery
| Name | Approach |
|---|---|
| Bioseek (http://www.bioseekinc.com) |
Uses systems biology approach to study primary human cell disease models |
| Beyond Genomics (http://www.beyondgenomics.com) |
Technology platform facilitates analysis of clinically relevant samples and integrates data from the gene, protein, metabolite, and clinic for biomarker and target identification |
| Cellnomica (http://www.cellnomica.com) |
Conducts novel multicellular modeling in drug discovery and development |
| Cellzome (http://www.cellzome.com) |
Proprietary functional proteomics technology for therapeutic target discovery, validation, and drug development |
| Department of Energy's Genomes to Life initiative (http://doegenomestolife.org/overview/pdf) |
The Genomes to Life roadmap (plans to design and exploit new high-throughput strategies to obtain a blueprint of how living systems function) |
| Eli Lilly Center for Systems Biology (http://www.lilly.com) |
Focuses on integration of proteomic and genomic technologies to support drug discovery efforts |
| Entelos (http://www.entelos.com) |
Biosimulation company that develops computer models of human disease using novel PhysioLab technology |
| Institute for Systems Biology (http://www.systemsbiology.org) |
Broad based program. Uses systems biology to investigate the complex interaction of biological elements that form hierarchical networks that define systems |
| Kitano Symbiotic Systems Project (http://www.symbio.jst.go.jp) |
The project aims to understand and design biological systems, thus creating a new paradigm in biology focuses on model organisms including fruit fly, yeast, and bacteria |
| Physiome Sciences (http://www.physiome.com) |
Biosimulation company that has created and develops integrated software platform for computer-based biological models applicable to drug discovery |
| SurroMed (http://www.surromed.com) |
Develops and implements biological marker discovery platform to profile biochemical components in blood and other biological samples |
Note. From “Advancing drug discovery through systems biology,” by E. Davidov et al., 2003, Drug Discov Today, 8, p. 175–183. Copyright 2014, Korea Institute of Oriental Medicine. Published by Elsevier. All rights reserved, Reproduced with permission.
Another interesting development in the science of drug discovery and development is the rise of chemical biology. Instead of one-protein–one-ligand model, chemical biology tries to define a drug promiscuity24 and look for a possible indication. This multitarget approach is thought to be more sensible because, as Denis Noble25 expounded, no simple, one-to-one correspondence between genes and phenotypes can be made. Throughout human history, herbal medicines were developed by long experience. Korean traditional medicine aims to understand the whole, which shares the similar concept of systems biology. There are many attempts to turn herbal medicine products into a Western-style drug, where multitarget approach also became the preferred tactic.
In the past century, reductionists have divided medicine into small bits. In this coming era, it is an opportune time to integrate the scattered blocks of our knowledge. The mechanisms and organizations are the key to understanding living things and consequently to develop a new drug. Although very sensible in theory, systems biology is very difficult to apply in clinical trials and even in actual medical practice. In heart failure, for example, reductionists will view the problem in a single gene, a single target, or a single drug molecule, which illustrates the current inertia in medicine. For systems biologist, the problem gets more complicated and subjective. There would be tons of data to explore and connect the puzzle pieces together to find the right treatment. The multitarget approach is the proposed choice at this point, but reductionist will argue that this approach is simply adding two or more target-based approaches in the hopes of attaining beneficial outcomes. Accordingly, systems biology holds a difficult task in itself to embrace the disciplines of reductionism and add some philosophy to become the next driving force in science and industry.
6. Conclusion
Biological science in the 20th century focused on breaking things down into microscopic or more manageable pieces in order to unlock the complexity of bodily function. Just as the environment influences the characteristics of organisms, an innovative ecosystem also has a critical role in drug discovery and development. The limitation of target-based approaches has been addressed, but the most commercially successful drugs are, in fact, still developed using such methodologies. Therefore, a shift from reductionism to nonreductionism is difficult to envisage within the industry in the near future. Such scientific endeavors require vision, and patience, which counterbalances the shareholders’ interests: a short-term gain in stock markets. To begin with, the paradigm shift to a more holistic perspective must come from university research groups. However, for this development to occur, science-funding organizations should be well informed about the pros and cons of systems biology. Interdisciplinary research is happening most frequently between university departments. For innovative ecosystem to be implemented, collaboration should be formed between all sorts of stakeholders. It is essential to note that universities, public organizations, venture capitals, consulting firms, and other groups are all contributing to bioclusters, where most licensing deals, investments, and collaborations are occurring. For academics, the integrative approach is synonymously used as systems biology. On the industry level, the integrative approach could also represent the blending of the elements of various stakeholders’ view together for better results. Systems biology is regarded as a postgenome technology, together with bioinformatics that will provide new solutions to various impediments in the pharmaceutical industry. Whether the recent rise in systems biology could be the next driving force in drug discovery and development remains to be seen. Unlike the Human Genome Project, systems biology is less likely to result in an investment hype; rather, it is apt to initiate a change at the very starting point: the basic science. Therefore, the change will be slower but in the long run, the impact on drug discovery and development would be substantial. Urgent agenda is not about speeding things up but rather, seeing things in an integrative manner to unravel the mystery of life. Nonetheless, advances in technologies that reduce cost and time for screening procedures, although insufficient to reverse the current slowdown of drug discovery and development, should not be undervalued. Tomorrow's drug discovery and development is more in need of a philosophy instead of incrementally improved technologies. Paul Janssen26 often described drug discovery and development as an orchestra. For a successful paradigm shift, what we need most is probably the conductor of science bringing together both reductionists and systemic biologists.
Conflicts of interest
The author declares no conflicts of interest.
References
- 1.DiMasi J., Hansen R., Grabowski H. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22:151–185. doi: 10.1016/S0167-6296(02)00126-1. [DOI] [PubMed] [Google Scholar]
- 2.Morgan S., Grootendorst P., Lexchin J., Cunningham C., Greyson D. The cost of drug development: a systematic review. Health Policy. 2011;100:4–17. doi: 10.1016/j.healthpol.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Paul S., Mytelka D., Dunwiddie C., Persinger C., Munos B., Lindborg S. How to improve R&D productivity: the pharmaceutical industry's grand challenge. Nat Rev Drug Discov. 2010;9:203–214. doi: 10.1038/nrd3078. [DOI] [PubMed] [Google Scholar]
- 4.Burrill & Company . Burrill & Company; San Francisco, CA: 2010. Biotech 2010 Life Sciences: adapting for success. [Google Scholar]
- 5.Munos B. Lessons from 60 years of pharmaceutical innovation. Nat Rev Drug Discov. 2009;8:959–968. doi: 10.1038/nrd2961. [DOI] [PubMed] [Google Scholar]
- 6.Lazonick W., Tulum O. US biopharmaceutical finance and the sustainability of the biotech business model. Res Policy. 2011;40:1170–1187. [Google Scholar]
- 7.Ney A. Reductionism. In: The Internet encyclopedia of philosophy. Available from: http://www.iep.utm.edu/red-ism/. Accessed 7th June 2014.
- 8.Bose B. Systems biology: a biologist's viewpoint. Prog Biophys Mol Biol. 2013;113:358–368. doi: 10.1016/j.pbiomolbio.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Hurko O. Target-based drug discovery, genetic diseases, and biologics. Neurochem Int. 2012;61:892–898. doi: 10.1016/j.neuint.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Brown D. Unfinished business: target-based drug discovery. Drug Discov Today. 2007;12:1007–1012. doi: 10.1016/j.drudis.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Sams-Dodd F. Target-based drug discovery: is something wrong? Drug Discov Today. 2005;10:139–147. doi: 10.1016/S1359-6446(04)03316-1. [DOI] [PubMed] [Google Scholar]
- 12.Berg E. Systems biology in drug discovery and development. Drug Discov Today. 2014;19:113–125. doi: 10.1016/j.drudis.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Chesbrough H.W. Harvard Business School Press; Boston, MA: 2006. Open innovation: the new imperative for creating and profiting from technology. [Google Scholar]
- 14.Claysse B., Wright M., Bruneel J., Mahajan A. Creating value in ecosystem: crossing the chasm between knowledge and business ecosystems. Res Policy. 2014;43:1164–1176. [Google Scholar]
- 15.van Lente H., Spitters C., Peine A. Comparing technological hype cycles: towards a theory. Technol Forecast Soc Change. 2013;80:1615–1628. [Google Scholar]
- 16.Macarron R. Critical review of the role of HTS in drug discovery. Drug Discov Today. 2006;11:277–279. doi: 10.1016/j.drudis.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Lo Nigro G., Morreale A., Enea G. Open innovation: a real option to restore value to the biopharmaceutical R&D. Int J Prod Econ. 2014;149:183–193. [Google Scholar]
- 18.Schneider H.C., Klabunde T. Understanding drugs and diseases by systems biology? Bioorg Med Chem Lett. 2013;23:1168–1176. doi: 10.1016/j.bmcl.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 19.Sams-Dodd F. Is poor research the cause of the declining productivity of the pharmaceutical industry? An industry in need of a paradigm shift. Drug Discov Today 18:211–7. [DOI] [PubMed]
- 20.Noble D. Why integration? Integr Med Res. 2012;1:2–4. doi: 10.1016/j.imr.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bizzarri M., Palombo A., Cucina A. Theoretical aspects of systems biology. Prog Biophys Mol Biol. 2013;112:33–43. doi: 10.1016/j.pbiomolbio.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 22.Iskar M., Zeller G., Zhao X.M., van Noort V., Bork P. Drug discovery in the age of systems biology: the rise of computational approaches for data integration. Curr Opin Biotechnol. 2012;23:609–616. doi: 10.1016/j.copbio.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 23.Davidov E., Holland J., Marple E., Naylor S. A dancing drug discovery through systems biology. Drug Discov Today. 2003;8:175–183. doi: 10.1016/s1359-6446(03)02600-x. [DOI] [PubMed] [Google Scholar]
- 24.Brown J.B., Okuno Y. Systems biology and systems chemistry: new directions for drug discovery. Chem Biol. 2012;19:23–28. doi: 10.1016/j.chembiol.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 25.Noble D. Genes and causation. Philos Trans R Soc. 2008;366:3001–3015. doi: 10.1098/rsta.2008.0086. [DOI] [PubMed] [Google Scholar]
- 26.Lewi P. The conductor and his orchestra. Drug Discov Today. 2008;13:281–284. doi: 10.1016/j.drudis.2008.02.008. [DOI] [PubMed] [Google Scholar]