Abstract
Current studies have indicated the association of chronic sterile inflammation (inflammation in the absence of pathogens) with the pathogenesis of age-related and metabolic diseases. The inflammation is under the control of transcription factor NF-κB through an antagonistic crosstalk between SIRT1, PARP-1, and -2 signaling pathways. The transcriptional activity of NF-κB is increased in various tissues with aging and metabolic abnormalities and is related with various aging and metabolic diseases such as Alzheimer's disease, diabetes, and osteoporosis. Furthermore, NF-κB activation with chronic inflammation is connected with many known life span and metabolic regulators including DNA damage, obesity, SIRT, and PARP. Thus, the crossroads between PARP and SIRT signaling pathways represent efficient therapeutic targets for extending health span without metabolic diseases.
Keywords: inflammation, metabolic diseases, PARP-1, PARP-2, SIRT1
1. Introduction
There are many reports that demonstrate the molecular regulation between inflammatory response, metabolic and age-related diseases.1, 2, 3 As an innate host defense mechanism, inflammation engages specific stages, such as acute and resolution stages of acute infection, and chronic sterile conditions of metabolic and age-associated degenerative diseases. The transcription factor NF-κB plays an important role in modulating innate immunity-mediated inflammation.4, 5, 6 There is abundant evidence indicating that NF-κB activity can be controlled by sirtuin (SIRT)1 and poly(adenosine diphosphate-ribose) polymerases (PARPs). SIRTs are a family of deacetylases with homology to Saccharomyces cerevisiae silent information regulator 2 (Sir2), which require nicotinamide adenine dinucleotide (NAD)+ as a cofactor for their enzymatic activity. In mammals, seven sirtuins are known, and the SIRTs contain a conserved central core deacetylase domain flanked by diverse length N- and C-termini. Interestingly, as SIRTs need NAD+ cofactor for their enzymatic activity, it is speculated that these deacetylases react to changes in environment, oxidative stress, and metabolism.5
SIRT1 regulates the diverse cellular targets and functions, so it became the most widely studied SIRT in mammals.
The expression of seven mammalian SIRTs is quite common and everywhere, and they share a conserved catalytic domain of 275 amino acids. However, each of the SIRTs shows distinct features with specific functions. Initially, their difference can be found in their subcellular localization. That is, SIRT1 can shuttle between the nucleus and the cytosol, and its predominant localization depends on various cell types and environmental causes.7, 8 SIRT2 is mostly cytosolic.7 By contrast, SIRT3, SIRT4, and SIRT5 are regarded as mitochondrial proteins, whereas SIRT6 and SIRT7 are located in the nucleus. However, while SIRT6 is placed in the heterochromatin, SIRT7 is predominantly found in the nucleolus.7
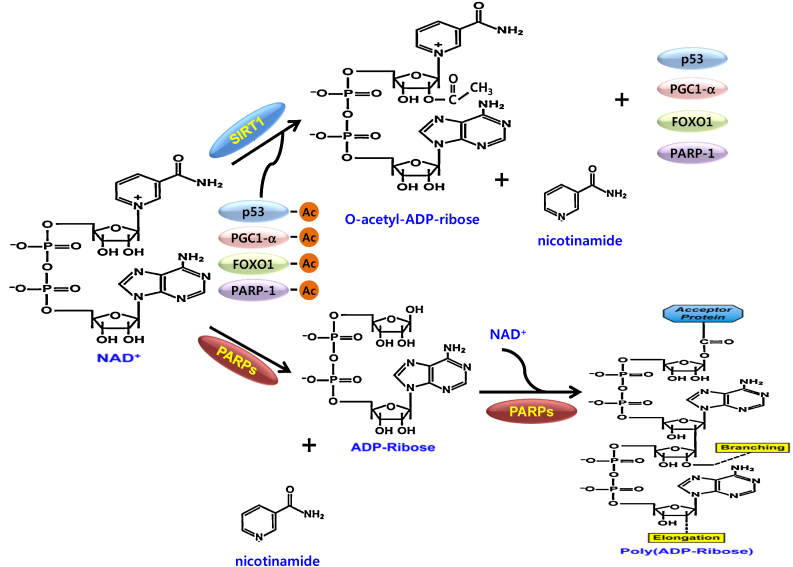
PARPs consist of enzymes with a conserved catalytic domain. PARPs have mono- or poly(ADP-ribosyl)transferase activity using NAD+ as a donor of ADP-ribose units. PARP-1 and PARP-2 have various functions in the maintenance of genome stability, regulation of chromatin structure and transcription, cell proliferation, and apoptosis.9 Because Poly(ADP-ribose)(PAR) is negatively charged and noncovalently couples with nuclear proteins, (PARylation), 9 PAR can act as a scaffolding for chromatin remodeling and DNA repair processes (Fig. 1). PARP1 is activated by oxidative and genotoxic stresses. The activated PARP1 causes the conversion of NAD+ into PAR chains, which noncovalently convert nuclear proteins.10
Fig. 1.
Two typical NAD+ consuming enzymes, PARP and SIRT1, uses NAD+ for PARylation and deacetylation, respectively.
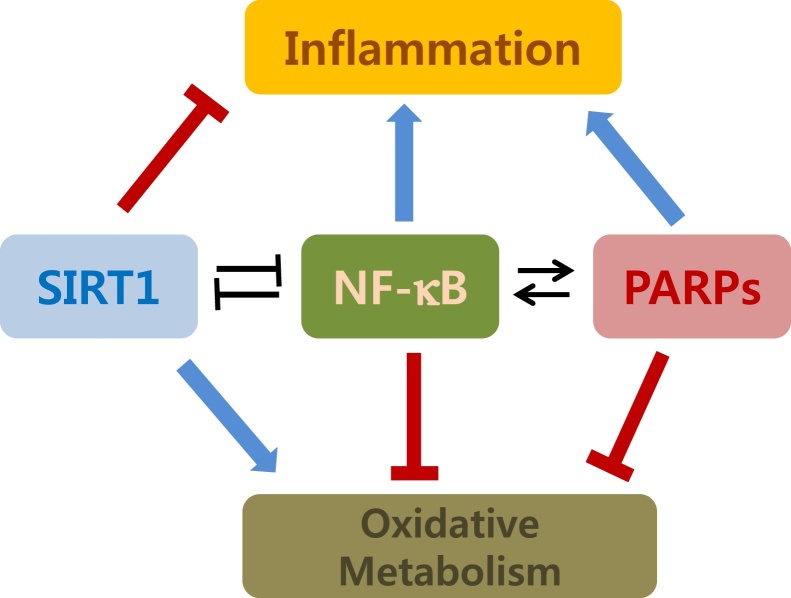
Thus, we review the current knowledge of the antagonistic interactions considering the biochemical reaction of the crosstalk and the pathophysiological consequences. In addition, we will overview the pharmacological involvement to regulate PARP and SIRT enzymes for the attenuation of the inflammatory metabolic and age-related diseases (Fig. 2).
Fig. 2.
A schematic presentation on the antagonistic interactions between NF-kB, SIRT1, and PARP in the regulation of inflammation and energetic homeostasis.
2. Antagonistic crosstalk between PARP and SIRT1
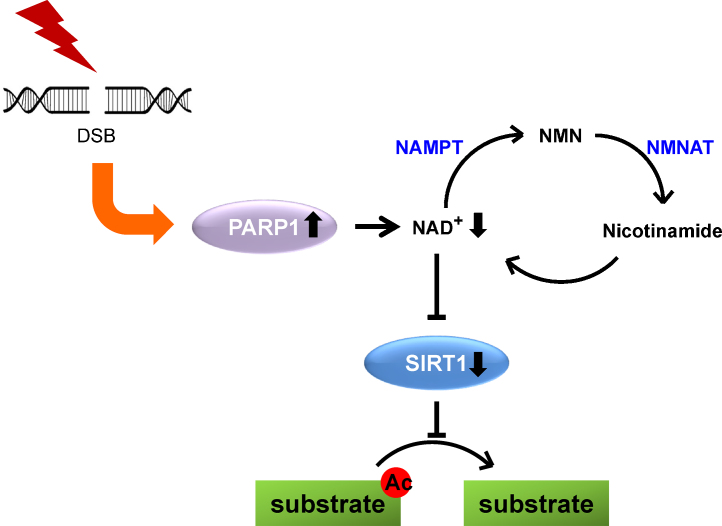
Oxidized and reduced Nicotinamide Adenine Dinucleotide (NAD+ and NADH respectively) are crucial metabolites in metabolic reactions. PARP-1 and PARP-2 use NAD+ as a cofactor in their catalytic activity. Continuous PARP activation can reduce intracellular levels of NAD+ by 80% and raises nicotinamide (NAM).11 SIRT1 is a NAD+-dependent protein deactelyase.12 It has been known that the decrease of NAD+ and the increase of NAM ensured by enhanced PARP activity correlates with a downregulation of SIRT1 activity.13, 14 Similarly, the activation of SIRT1 reduced PARP activity.10 These observations raised four possible crosstalks between SIRT1, PARP-1, and -2 (Fig. 3).
Fig. 3.
An antagonistic crosstalk between SIRT1 and PARP in the double-strand break-induced DNA damage response. DNA double-strand break on oxidative stress activates PARP, leading to the depletion of NAD+ and subsequent inactivation of SIRT1 deacetylase activity. By contrast, SIRT1 regulates PARP activity by deacetylating PARP1.
NAM, nicotinamide mononucleotide; NAMPT, nicotinamide phosphoribosyltransferase; NMNAT, nicotinamide mononucleotide adenyltransferase.
2.1. Competition for NAD+
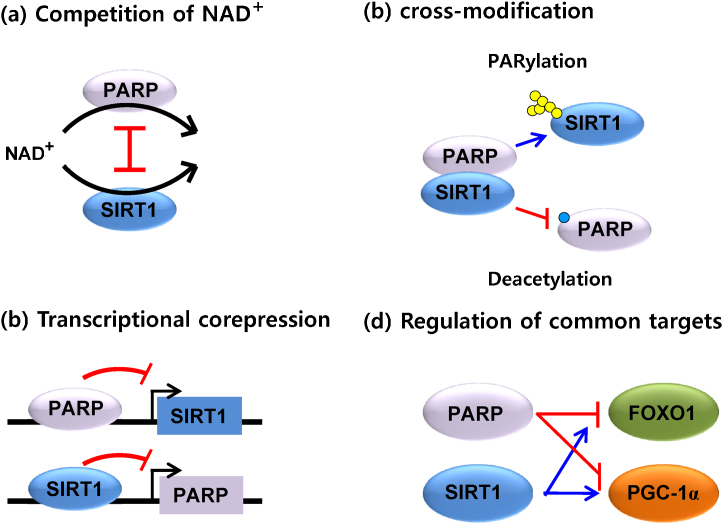
PARPs and SIRT1 may compete for the limiting NAD+ substrate because they are NAD+ dependent enzymes.15 The SIRT1 activity induced the fluctuations in NAD+ levels as the Km of SIRT1 falls in the bounds of physiological cellular NAD+ change.15 It is well known that NAD+ levels upon excessive DNA damage is decreased due to PARP activation. In the excessive DNA damage, NAD+ levels may decrease to 20–30% of the control that is likely to be rate limiting of SIRT enzymes.15 SIRT1 activity is dramatically declined under these conditions which might consequently lead to decreased SIRT1 expression (Fig. 4-a).16
Fig. 4.
Antagonistic crosstalks between PARP-1 or -2 and SIRT in the various cellular situations. See the details in the text.
2.2. Cross-modification
The cross-action of both activities of PARRs and SIRT is that PARPS could PARylate SIRT1 and that SIRT1 could deacetylate PARPs.17 It has not been demonstrated that SIRT1 was PARylated by PARPs. However, it was reported that PARP-1 could be deacetylated by SIRT1.18, 19 Overexpression of SIRT1 or resveratrol treatment induced the deacetylation of PARP-1 in cell culture. It is also reported that SIRT1-mediated deacetylation obstructs PARP-1 activity.20 Thus, the increase of SIRT1 activity would decrease PARP-1 activity through direct deacetylation (Fig. 4-b).
2.3. Transcriptional corepression
The SIRT1 promoter activity has been shown to be regulated by several transcription factors including PARPs.17, 21 It was demonstrated that the SIRT1 promoter has the binding site of PARP-2 and suggested that PARP-2 operates as a suppressor of SIRT1 transcription.13, 21 Thus, under the condition of PARP-2 deletion, the activity of the SIRT-1 promoter that translated into higher SIRT1 mRNA and protein levels in skeletal muscle, liver, and pancreas was enhanced.13, 21 Since the depletion of PARP-1 had no effect on the activity of the SIRT1 promoter, PARP-2 appears specific for it (Fig. 4-c).
2.4. Regulation of common targets
According to Bai et al,17 the increase of energy expenditure drives the metabolic phenotype of the PARP-1 knockout mice. This effect is likely induced by a potentiation in SIRT1 activity and the activation of key transcriptional factors including the transcriptional coactivator PGC-1α.19 PGC-1α regulates the enhanced mitochondrial biogenesis in skeletal muscle fibers.22 The forkhead box O (FOXO) family of transcriptional factors is also a major subsequent effector of SIRT1 in the regulation of oxidative metabolism.23 SIRT1 deacetylates FOXOs, prompting their activation of genes related to lipid oxidation and stress resistance.24 In coincide with SIRT1 activation, mice with genetically or pharmacologically impaired PARP activity demonstrate a clear deacetylation of PGC-1α and FOXO1 in a metabolic tissue such as skeletal muscle.17 Corresponding to the activation of genes related to mitochondrial biogenesis, the muscles of PARP-1 deficient mice showed a huge increase of mitochondrial content and an improved oxidative profile in their muscle fibers.17 The brown adipose tissue (BAT) is well known to have a vital role in thermogenesis. The BAT from the PARP-1 deficient mice is characterized by increased NAD+ substance and SIRT1 activity as well as the deacetylation and activation of PGC-1α.17 This causes the mitochondrial matter to increase in the BAT of the mice. All of these phenomena shown in the PARP-1 deficient mice represent that the transcriptional coactivators including PGC-1α and FOXO1 are cross-regulated by PARP and SIRT1, respectively (Fig. 4-d).
3. Signaling crosstalk between PARP and SIRT in the diseases
3.1. PARP-SIRT interaction in age-related diseases
Muiras et al25 found increased PARP activities in cells from centenarians, suggesting larger capacity of PARP activation is related with successful aging. The larger PARylation ability is postulated to be reliable DNA repair that prevents the DNA damage-related diseases including neoplasms.26, 27 Recently, many data demonstrate that the hPARP-1 mouse overexpressed PARP-1 are prevented against neoplastic disease.28 However, other age-related pathologies such as inflammatory diseases is increasingly occurred by PARP-1 overexpression.28 Aging is strongly related with enhanced oxidative stress. This oxidative stress and increased PARP activation29 in old age place a considerable strain on NAD+ homeostasis. As a result, NAD+ levels and accompanied SIRT1 activities are decreasing with aging. The decrease in SIRT1 activity induces mitochondrial dysfunction, which represents a characteristic of aging and is probably a leading cause of various age-related chronic inflammatory and metabolic diseases.
3.2. PARP-SIRT interaction in metabolic diseases
Two articles by Bai et al13, 17 expanded the physiological roles of PARP-1 and -2 to the area of metabolism of energy homeostasis. The authors observed that PARP-1 or PARP-2 deficient mice display enhanced energy consumption and protection against diet-induced obesity. This metabolic phenotype is partly gained via SIRT1 activation, either by increasing NAD+ levels by PARP-1 deficiency or by promoting SIRT1 expression by PARP-2 deficiency.13 They characterized the metabolic phenotypes of PARP-1−/− mice to know the role of PARP-1 in metabolic homeostasis. PARP−/− mice showed the following characteristics.
Although food intake was increased, the mice were more lean with less fat accumulation, increased energy expenditure, and enhanced glucose oxidation. PARP-1−/− mice also demonstrated higher mitochondrial content, along with the increase of oxidation- and respiration-associated gene expression in metabolic tissues, such as muscle and BAT.30 PARP-1−/− mice have similar results in which SIRT1 is overexpressed or chemically activated.31
Interaction between PARP-1 and SIRT1 is suggested by the results since they are intensively regulated by cellular NAD+ levels.30 In a second study, the authors investigated that RNA interference-mediated depletion of PARP-2 did not alter cellular NAD+ levels, but increased the levels of SIRT1 messenger RNA (mRNA), protein, and activity.17 This was represented by the reduction of acetylation of SIRT1 targets, as well as enhanced mitochondrial biogenesis and oxidation. The authors demonstrated that PARP-2 localizes to the SIRT1 promoter, where it operates as a suppressive transcription regulator.
To sum it up, the capacity of PARP enzymes to regulate SIRT1 activity and the resulting PAPR-NAD+-SIRT1 pathway additionally intensifies the encouraging potential of modulating PARP and SIRT1 activities in the control of meta-inflammation for metabolic diseases.
4. Pharmacological modulation of PARP-SIRT1 axis
PARP-SIRT1 axis can be modulated pharmacologically,32, 33, 34, 35 allowing the regulation of both ends of the axis. Especially, there is important data that genetical and pharmacological regulation of PARP activity influences the other associate,17, 20, 36, 37, 38 suggesting that it is possible to appropriately regulate the PARP-SIRT balance through pharmacological agents. Indeed, various small molecules could effectively inhibit the ability of PARP-1 and -2. The therapeutic application of PARP inhibitors has lately attracted a lot of attention due to their capacity in the treatment of cancers. However, the possibility of therapeutic applications of PARP inhibitors stretch beyond cancer to various chronic inflammation, such as cardiovascular diseases, metabolic disorders, diabetes, and autoimmunity, and any disease with sterile inflammation.7, 39, 40, 41, 42, 43 PARP-1 makes a contribution as a regulator of cell adaptation to a changing circumstance. In various disease beyond cancers, enhanced cellular stress generates unregulated PARP-1 activation and subsequent inflammation, cell death, and tissue damage. In this respect, PARP inhibitors have been investigated as treatment channels to protect cell death, tissue damage, and aging or oxidative damage-related pathologies.7, 39, 40, 43 The recent finding of the metabolic functions of PARP-1 and PARP-2, in accordance with SIRT1 by using knockout mouse models indicates the possibility of therapeutics of PARP inhibitors in metabolic disorders.13, 17 The common context to various aforementioned diseases is a fundamental inflammatory response. Therefore, the effects of PARP inhibitors as well as SIRT activators in these diseases may share the inhibition of inflammatory pathways as a common mechanism. Inflammation is nowadays regarded as an indication of cancer.44 In other words, the effects of PARP inhibitors on inflammatory responses may play a role in the therapeutic effects of cancers. Therefore, study for the modulation and function of PARP-1 and -2, as well as SIRT1 in chronic inflammatory pathways is critical for the development of PARP inhibitors and SIRT1 activators as therapeutic agents.
5. Conclusion
Cellular responses act against various extrinsic and intrinsic stress signals caused by metabolic, oxidative, and genotoxic stresses. The PARP-NAD+-SIRT1 pathway plays an important role in cellular stress responses, where NAD+ and its redox counterpart, NADH, are essential metabolites in the metabolic reactions.12, 15 Major functions of PARP are regulated by using NAD+ as a substrate. SIRT1 is NAD+-dependent deacetylase associated in the same stress responses as PARP. In order to get homeostasis, PARP and SIRT enzymes may interact with each other. In this short review, the crosstalk between SIRT1 and PARP-1 and -2 in consideration of the biochemical nature of the interaction (competition for NAD+, cross-modification, transcription corepression, and regulation of common targets) is discussed, along with the resulting consequences of the crosstalks (chronic low-grade inflammatory metabolic and age-related diseases). Finally, the pharmacological modulation of PARP and SIRT1 axis is reviewed. Our current knowledge on the crosstalk between SIRT1, PARP-1 and -2 is rapidly expanding into further research in the field.
Conflicts of interest
There is no conflict of interest.
Acknowledgments
This study was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Ministry of Science, ICT & Future Planning (2012M3A9C3048687) and NRF-2014R1A6A1030318.
References
- 1.Kominsky D.J., Campbell E.L., Colgan S.P. Metabolic shifts in immunity and inflammation. J Immunol. 2010;184:4062–4068. doi: 10.4049/jimmunol.0903002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shapiro H., Lutaty A., Ariel A. Macrophages, meta-inflammation, and immuno-metabolism. Scientific World Journal. 2011;11:2509–2529. doi: 10.1100/2011/397971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu T.F., Brown C.M., El Gazzar M., McPhail L., Millet P., Rao A. Fueling the flame: bioenergy couples metabolism and inflammation. J Leukoc Biol. 2012;92:499–507. doi: 10.1189/jlb.0212078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker R.G., Hayden M.S., Ghosh S. NF-kappaB, inflammation, and metabolic disease. Cell Metab. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houtkooper R.H., Pirinen E., Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012;13:225–238. doi: 10.1038/nrm3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tornatore L., Thotakura A.K., Bennett J., Moretti M., Franzoso G. The nuclear factor kappa B signaling pathway: integrating metabolism with inflammation. Trends Cell Biol. 2012;22:557–566. doi: 10.1016/j.tcb.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Masutani M., Nakagama H., Sugimura T. Poly(ADP-ribosyl)ation in relation to cancer and autoimmune disease. Cell Mol Life Sci. 2005;62:769–783. doi: 10.1007/s00018-004-4509-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanno M., Sakamoto J., Miura T., Shimamoto K., Horio Y. Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J Biol Chem. 2007;282:6823–6832. doi: 10.1074/jbc.M609554200. [DOI] [PubMed] [Google Scholar]
- 9.Krishnakumar R., Kraus W.L. The PARP side of the nucleus: molecular actions, physiological outcomes, and clinical targets. Mol Cell. 2010;39:8–24. doi: 10.1016/j.molcel.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolthur-Seetharam U., Dantzer F., McBurney M.W., de Murcia G., Sassone-Corsi P. Control of AIF-mediated cell death by the functional interplay of SIRT1 and PARP-1 in response to DNA damage. Cell Cycle. 2006;5:873–877. doi: 10.4161/cc.5.8.2690. [DOI] [PubMed] [Google Scholar]
- 11.Bai P., Canto C. The role of PARP-1 and PARP-2 enzymes in metabolic regulation and disease. Cell Metab. 2012;16:290–295. doi: 10.1016/j.cmet.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 12.Houtkooper R.H., Auwerx J. Exploring the therapeutic space around NAD+ J Cell Biol. 2012;199(2):205–209. doi: 10.1083/jcb.201207019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bai P., Canto C., Brunyanszki A., Huber A., Szanto M., Cen Y. PARP-2 regulates SIRT1 expression and whole-body energy expenditure. Cell Metab. 2011;13:450–460. doi: 10.1016/j.cmet.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pillai J.B., Isbatan A., Imai S., Gupta M.P. Poly(ADP-ribose) polymerase-1-dependent cardiac myocyte cell death during heart failure is mediated by NAD+ depletion and reduced Sir2alpha deacetylase activity. J Biol Chem. 2005;280:43121–43130. doi: 10.1074/jbc.M506162200. [DOI] [PubMed] [Google Scholar]
- 15.Houtkooper R.H., Canto C., Wanders R.J., Auwerx J. The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocrine Rev. 2010;31:194–223. doi: 10.1210/er.2009-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin W.D., Wei S.J., Wang X.P., Wang J., Wang W.K., Liu F. Poly(ADP-ribose) polymerase 1 inhibition protects against low shear stress induced inflammation. Biochim Biophys Acta. 2013;1833:59–68. doi: 10.1016/j.bbamcr.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Bai P., Canto C., Oudart H., Brunyanszki A., Cen Y., Thomas C. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 2011;13:461–468. doi: 10.1016/j.cmet.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canto C., Sauve A.A., Bai P. Crosstalk between poly(ADP-ribose) polymerase and sirtuin enzymes. Mol Aspects Med. 2013;34:1168–1201. doi: 10.1016/j.mam.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodgers J.T., Lerin C., Haas W., Gygi S.P., Spiegelman B.M., Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 20.Rajamohan S.B., Pillai V.B., Gupta M., Sundaresan N.R., Birukov K.G., Samant S. SIRT1 promotes cell survival under stress by deacetylation-dependent deactivation of poly(ADP-ribose) polymerase 1. Mol Cell Biol. 2009;29:4116–4129. doi: 10.1128/MCB.00121-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szanto M., Rutkai I., Hegedus C., Czikora A., Rozsahegyi M., Kiss B. Poly(ADP-ribose) polymerase-2 depletion reduces doxorubicin-induced damage through SIRT1 induction. Cardiovasc Res. 2011;92:430–438. doi: 10.1093/cvr/cvr246. [DOI] [PubMed] [Google Scholar]
- 22.Lin J., Wu H., Tarr P.T., Zhang C.Y., Wu Z., Boss O. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 23.Brunet A., Sweeney L.B., Sturgill J.F., Chua K.F., Greer P.L., Lin Y. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 24.Gross D.N., van den Heuvel A.P., Birnbaum M.J. The role of FoxO in the regulation of metabolism. Oncogene. 2008;27:2320–2336. doi: 10.1038/onc.2008.25. [DOI] [PubMed] [Google Scholar]
- 25.Muiras M.L., Muller M., Schachter F., Burkle A. Increased poly(ADP-ribose) polymerase activity in lymphoblastoid cell lines from centenarians. J Mol Med. 1998;76:346–354. doi: 10.1007/s001090050226. [DOI] [PubMed] [Google Scholar]
- 26.Nicolas L., Martinez C., Baro C., Rodriguez M., Baroja-Mazo A., Sole F. Loss of poly(ADP-ribose) polymerase-2 leads to rapid development of spontaneous T-cell lymphomas in p53-deficient mice. Oncogene. 2010;29:2877–2883. doi: 10.1038/onc.2010.11. [DOI] [PubMed] [Google Scholar]
- 27.Tong W.M., Hande M.P., Lansdorp P.M., Wang Z.Q. DNA strand break-sensing molecule poly(ADP-Ribose) polymerase cooperates with p53 in telomere function, chromosome stability, and tumor suppression. Mol Cell Biol. 2001;21:4046–4054. doi: 10.1128/MCB.21.12.4046-4054.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mangerich A., Herbach N., Hanf B., Fischbach A., Popp O., Moreno-Villanueva M. Inflammatory and age-related pathologies in mice with ectopic expression of human PARP-1. Mech Ageing Devel. 2010;131:389–404. doi: 10.1016/j.mad.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Massudi H., Grant R., Braidy N., Guest J., Farnsworth B., Guillemin G.J. Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PloS One. 2012;7:e42357. doi: 10.1371/journal.pone.0042357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo X., Kraus W.L. A one and a two expanding roles for poly(ADP-ribose) polymerases in metabolism. Cell Metab. 2011;13:353–355. doi: 10.1016/j.cmet.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herranz D., Serrano M. SIRT1: recent lessons from mouse models. Nature Rev. Cancer. 2010;10:819–823. doi: 10.1038/nrc2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feige J.N., Lagouge M., Canto C., Strehle A., Houten S.M., Milne J.C. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008;8:347–358. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 33.Fong P.C., Boss D.S., Yap T.A., Tutt A., Wu P., Mergui-Roelvink M. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 34.Jagtap P., Szabo C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov. 2005;4:421–440. doi: 10.1038/nrd1718. [DOI] [PubMed] [Google Scholar]
- 35.Lagouge M., Argmann C., Gerhart-Hines Z., Meziane H., Lerin C., Daussin F. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 36.Liu D., Pitta M., Mattson M.P. Preventing NAD(+) depletion protects neurons against excitotoxicity: bioenergetic effects of mild mitochondrial uncoupling and caloric restriction. Ann N Y Acad Sci. 2008;1147:275–282. doi: 10.1196/annals.1427.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pang J., Gong H., Xi C., Fan W., Dai Y., Zhang T.M. Poly(ADP-ribose) polymerase 1 is involved in glucose toxicity through SIRT1 modulation in HepG2 hepatocytes. J Cell Biochem. 2011;112:299–306. doi: 10.1002/jcb.22919. [DOI] [PubMed] [Google Scholar]
- 38.Pillai J.B., Gupta M., Rajamohan S.B., Lang R., Raman J., Gupta M.P. Poly(ADP-ribose) polymerase-1-deficient mice are protected from angiotensin II-induced cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2006;291:H1545–H1553. doi: 10.1152/ajpheart.01124.2005. [DOI] [PubMed] [Google Scholar]
- 39.Mota R.A., Sanchez-Bueno F., Saenz L., Hernandez-Espinosa D., Jimeno J., Tornel P.L. Inhibition of poly(ADP-ribose) polymerase attenuates the severity of acute pancreatitis and associated lung injury. Lab Invest. 2005;85:1250–1262. doi: 10.1038/labinvest.3700326. [DOI] [PubMed] [Google Scholar]
- 40.Pacher P., Szabo C. Role of poly(ADP-ribose) polymerase 1 (PARP-1) in cardiovascular diseases: the therapeutic potential of PARP inhibitors. Cardiovasc Drug Rev. 2007;25:235–260. doi: 10.1111/j.1527-3466.2007.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shevalye H., Stavniichuk R., Xu W., Zhang J., Lupachyk S., Maksimchyk Y. Poly(ADP-ribose) polymerase (PARP) inhibition counteracts multiple manifestations of kidney disease in long-term streptozotocin-diabetic rat model. Biochem Pharmacol. 2010;79:1007–1014. doi: 10.1016/j.bcp.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ford A.L., Lee J.M. Climbing STAIRs towards clinical trials with a novel PARP-1 inhibitor for the treatment of ischemic stroke. Brain Res. 2011;1410:120–121. doi: 10.1016/j.brainres.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Underhill C., Toulmonde M., Bonnefoi H. A review of PARP inhibitors: from bench to bedside. Ann Oncol. 2011;22:268–279. doi: 10.1093/annonc/mdq322. [DOI] [PubMed] [Google Scholar]
- 44.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]