Abstract
Deficits in sensory functioning, such as poor vision and hearing, take a significant toll on quality of life. Little is known, however, about their relation with personality development across adulthood. This study examined whether baseline and change in vision and hearing were associated with personality change over a four-year period. Participants (N= 7471; Mage= 66.89; 59% women) were drawn from the Health and Retirement Study. They provided data on vision, hearing, and personality both at baseline and four years later. Poor vision and hearing at baseline and declines in vision and hearing over time were independently related to steeper declines in extraversion, agreeableness, openness and conscientiousness, and less decline in neuroticism, controlling for demographic factors, disease burden and depressive symptoms. Sensory functioning was generally a stronger predictor of personality change than disease burden or depressive symptoms. Consistent with evidence that poor and worsening sensory functions compromise individuals’ interactions with the social and physical environment, this study found deficits in hearing and vision were also associated with maladaptive personality trajectories in older adults.
Keywords: Vision, hearing, sensory impairment, personality development
Sensory impairment is associated with quality of life across the lifespan. Poor vision and hearing impair daily interactions with the external world, undermine social communication and engagement, and may lead to social isolation (Viljanen, Törmäkangas, Vestergaard, & Andersen-Ranberg, 2014; Wahl & Tesch-Römer, 2001). Self-reported impairment in sensory functioning is also associated with difficulties in independent activities of daily living (Brennan, Horowitz, & Su, 2005), higher disease burden and lower self-rated health (Crews & Campbell, 2004). In addition, objective and self-reported measures of sensory impairment are related to depression (Capella-McDonall, 2005, 2009; Kiely, Anstey, & Luszcz, 2013) and with both cognitive impairment (Lin et al., 2013; Rogers & Langa, 2010) and mortality (Gopinath et al., 2013; Lam, Lee, Gómez-Marín, Zheng, & Caban, 2006). Despite strong evidence for the pervasive negative association between sensory impairment and nearly all aspects of individuals’ lives, little is known about the extent to which it is associated with changes in their characteristic patterns of thoughts, feelings, and behaviors, that is their personality traits.
Personality development follows a normative pattern across most of adulthood (Donnellan & Lucas, 2008; Lucas & Donnellan, 2011; McCrae et al., 2005; Soto, John, Gosling, & Potter, 2011; Terracciano, McCrae, & Costa, 2006). For example, neuroticism, openness, and extraversion tend to decline, whereas conscientiousness and agreeableness generally increase with age during the middle part of adulthood. There are also individual deviations from these normative trajectories (Specht, Egloff, & Schmukle, 2011; Sutin, Stephan, & Terracciano, 2016). Non-normative personality change in neuroticism and conscientiousness deserve particular attention because these traits are associated with an increased risk for a range of negative outcomes, such as dementia (Terracciano et al., 2014). The association between personality traits and health is unlikely to be unidirectional. Indeed, poor health has attracted significant interest for its associations with maladaptive personality trajectories (Jokela, Hakulinen, Singh-Manoux, & Kivimaki 2014; Stephan, Sutin, Luchetti, & Terracciano, 2016; Sutin, Zonderman, Ferrucci, & Terracciano, 2013). Specifically, dysregulation of physiological systems and the presence of chronic disease have been associated with accelerated declines in extraversion, openness, agreeableness, conscientiousness and higher neuroticism over time (Jokela et al., 2014; Stephan et al., 2016; Sutin et al., 2013).
The present study examines whether deficits in sensory functions are associated with change in personality. Vision and hearing have implications for a range of health, cognitive, and behavioral factors that are associated with personality change. Both self-reported and objective sensory deficits often restrict social and physical activity (Brown & Barrett, 2011; Crews & Campbell, 2004; Willis, Jefferys, Vitale, Ramulu, 2012). Such lifestyle changes are related to higher neuroticism and lower extraversion, openness, agreeableness, and conscientiousness over time (Stephan, Sutin, & Terracciano, 2014). Worse self-reported and objective visual and hearing functions are associated with lower mental, cognitive and physical health (Crews & Campbell, 2004; Kiely et al., 2013; Lin et al., 2013). Prior research has shown that depressive symptoms, cognitive impairment and poor physical health are associated with declines in conscientiousness, extraversion, openness to experience, agreeableness and increases neuroticism over time (Hakulinen et al., 2015; Jokela et al., 2014; Pocnet, Rossier, Antonietti, & Von Gunten, 2013; Sutin et al., 2013).
Further theoretical support for an association between sensory impairment and personality change comes from lifespan developmental theories, such as the socioemotional selectivity theory (SST, Carstensen, 2006) and assimilation-accommodation theory (Brandtstädter & Renner, 1990). These theoretical frameworks have been used to examine the association between vision and hearing impairment and indicators of successful aging (Boerner, 2004; Wahl et al., 2013). The SST suggests that when time is perceived as limited, older adults invest less in exploratory behavior and developing new relationships and more in familiar activities and closer, intimate relationships (Carstensen, 2006). Consistent with this theory, Wahl et al. (2013) found lower engagement in marginal social relationships among sensory impaired individuals. This process may result in a decline of socially-oriented personality traits, such as extraversion and agreeableness. In addition, the restriction of interest and exploratory behaviors that follows from sensory impairment may be reflected in a decline in openness. In addition, individuals with sensory deficits may use assimilative and/or accommodative coping strategies. Assimilation refers to an effort to modify the actual situation to one’s personal goals, whereas the accommodative mode refers to flexible goal adjustment to situational constraints (Brandtstädter & Renner, 1990). Individuals with sensory impairment tend to pursue goals less tenaciously than unimpaired individuals (Wahl et al., 2013). This lower persistence and effort toward achieving a given goal may be reflected in lower conscientiousness over time.
The relation between sensory impairment and personality development, however, has received limited attention. To our knowledge, only one study by Berg and Johansson (2014) reported that individuals with self-rated hearing impairment had a steeper decline in extraversion over a 6-year period. We build on this foundational study in several ways. First, we examine whether sensory impairment is associated with change in all five traits, rather than the two (extraversion and neuroticism) assessed by Berg and Johansson. Second, we examine this association across a broader age range (50–99 years) rather than only among older adults (i.e., 80–98). Third, we also include a sensory impairment score that includes both hearing and vision deficits. Such dual sensory loss deserves attention given its detrimental effect over time, beyond any single contribution (Gopinath et al., 2013).
Using data from a large national sample, the purpose of the present study is to examine whether vision and hearing are associated with personality development across the latter part of adulthood. Based on the rational described above, we hypothesized that both baseline and greater impairment in vision and hearing over time would be associated with a steeper decline in extraversion, agreeableness, conscientiousness and openness, and an increase in neuroticism over a four-year period. This hypothesis was also tested using a sensory impairment score that combined both vision and hearing functioning. The study further tested whether age moderated the association between sensory functioning and personality development. Finally, we also tested whether baseline personality predicted change in sensory functioning. Higher neuroticism and lower extraversion, openness, agreeableness and conscientiousness have been found to predict several health-related outcomes in prospective studies of middle-aged and older adults (e.g., Weston, Hill, & Jackson, 2015). Therefore, these traits were expected to be associated with worsening sensory function over time.
Method
Participants
Participants were drawn from the Health and Retirement Study (HRS), a national longitudinal study of Americans ages 50 and older sponsored by the National Institute of Aging (grant number NIA U01AG009740) and conducted by the University of Michigan. The Health and Retirement Study is conducted under Institutional Review Board approval by the relevant committees at the University of Michigan and the National Institute on Aging. Since its inception, participants reported on their eyesight and hearing. Starting in 2006, HRS implemented an enhanced face-to-face interview that included a psychosocial questionnaire with a measure of personality traits. Half of the HRS participants completed the enhanced interview in 2006; the other half completed it in 2008. We used the combined 2006–2008 samples as our baseline. Only participants who had available data on all measures of interest were included, leaving a sample of 11,383 participants at baseline. Follow-up personality, eyesight and hearing measures were available in 2010 and 2012 for the 2006 and the 2008 samples, respectively, and were also combined. Of the baseline sample, 7471 participants (59% women, Mean age = 66.89, SD = 8.97) provided complete data at follow-up and corresponded to the final sample. Descriptive statistics are presented in Table 1.
Table 1.
Means and Standard Deviations for the Variables Under Study at Time 1 and Time 2 (N= 7471)
| Variables | Time 1 | Time 2 | Correlated Change between Sensory Functioning and Personality c | Correlated Change between Eyesight and Personalityd | Correlated Change between Hearing and Personalityd |
|---|---|---|---|---|---|
| Sex (% Female) | 59% | ||||
| Ethnicity (% White) | 89% | ||||
| Education | 13.19(2.70) | ||||
| Disease Burden | 1.86(1.26) | ||||
| Depressive symptoms | 1.16(1.80) | ||||
| Age | 66.89(8.97) | - | |||
| Sensory functioning | 2.62(0.80) | 2.70(0.82)a | |||
| Self-reported eyesight | 2.66(0.93) | 2.74(0.95)a | |||
| Self-reported hearing | 2.58(1.06) | 2.67(1.09)a | |||
| Neuroticism | 2.02(0.59) | 1.97(0.59)b | 0.06*** | 0.05*** | 0.03** |
| Extraversion | 3.22(0.54) | 3.17(0.56)b | −0.06*** | −0.06*** | −0.03* |
| Openness | 2.97(0.53) | 2.91(0.56)b | −0.07*** | −0.06*** | −0.03** |
| Agreeableness | 3.54(0.46) | 3.52(0.49)b | −0.06*** | −0.06*** | −0.02* |
| Conscientiousness | 3.41(0.45) | 3.39(0.48)b | −0.08*** | −0.06*** | −0.05*** |
Note.
p < .05,
p < .01,
p < .001
Significantly higher than the baseline value
Significantly lower than the baseline value
Partial correlations controlling for age, age squared, sex, education, race, disease burden and depressive symptoms
Partial correlations controlling for age, age squared, sex, education, race, disease burden, depressive symptoms and changes in eyesight or hearing
Participants with follow-up data were younger (d= 0.43), more educated (d= 0.32), more likely to be white, had fewer diseases (d=0.29), fewer depressive symptoms (d= 0.26), and better self-rated eyesight (d= 0.29), and hearing (d= 0.14) than those with incomplete data at follow-up (N= 3912). There were no sex differences. Further, participants in the longitudinal sample had lower neuroticism (d= 0.08) and were more extraverted (d= 0.09), open (d= 0.13), agreeable (d= 0.04), and conscientious (d= 0.25) at baseline than participants without follow-up data.
Measures
Personality
Personality was assessed at both waves using the Midlife Development Inventory (MIDI; Lachman & Weaver, 1997). Participants were asked how much 26 adjectives that assessed Neuroticism, Conscientiousness, Extraversion, Openness, and Agreeableness described them on a scale ranging from 1 (not at all) to 4 (a lot). The adjectives were moody, worrying, nervous, and calm (Neuroticism); outgoing, friendly, lively, active, and talkative (Extraversion); creative, imaginative, intelligent, curious, broad minded, sophisticated, and adventurous (Openness); organized, responsible, hardworking, thorough, and careless (Conscientiousness); and helpful, warm, caring, softhearted, and sympathetic (Agreeableness). Cronbach’s alphas for each trait at the first and second wave, respectively, were as follows: .72 and .71 for Neuroticism, .76 and .76 for Extraversion, .79 and .79 for Openness, .66 and .68 for Conscientiousness, and .79 and .79 for Agreeableness. Supplementary analysis indicated measurement invariance both across time and between the two subsamples in the present study (See supplementary Table S1).
Sensory functioning
Participants were asked to rate their eyesight while using glasses or corrective lenses as usual and their hearing while using a hearing aid as usual on two separate items. For both items, the response scale ranged from 1 (excellent) to 5 (poor). Higher scores on the two items represented poor vision and hearing. Vision and hearing were moderately correlated at baseline (r= .29, p<.001) and follow-up (r=.31, p<.001). The two items were averaged to give a sensory functioning score, with higher values representing higher impairment.
Covariates
Age (in years), sex (coded as 1 for men and 0 for women), race (coded as 1 for white and 0 for black and others), and educational level (in years) were included as covariates. Depressive symptoms were measured using an 8-item version of the Center for Epidemiologic Studies Depression (CES-D) (Wallace et al., 2000). Participants were asked to report whether they had experienced eight specific symptoms for much of the past week. The total number of endorsed symptoms was summed to create a total depressive symptom score ranging from 0 to 8 (α= .80). Disease burden was measured as the sum of seven diseases and conditions reported by the participants on a pre-established list, including high blood pressure, diabetes, cancer of any kind excluding skin, lung disease, heart condition, stroke, and arthritis.
Data analysis
To test whether baseline sensory functioning was associated with change in personality traits, we used multiple regression to predict each personality trait at follow-up from baseline self-rated eyesight and hearing, controlling for age, age squared, sex, ethnicity, education, depression, disease burden, and baseline personality. For each trait, both self-rated eyesight and hearing were included simultaneously to examine their independent association with personality change. The same analysis was repeated with the sensory functioning score as a predictor. In a supplemental analysis, we examined whether age moderated the relation between self-reported eyesight, vision, and global sensory functioning and personality change. To test the relation between change in sensory functioning and change in personality traits, we examined partial correlations between residual change scores for vision, hearing, and overall sensory functioning and each personality trait, controlling for the covariates. To ensure that results were not dependent on analytic method, we also examined correlated change using a Latent Change Score (LCS) framework (McArdle, 2009). LCS is a type of structural equation model used to estimate the latent change in a construct over time. In this model, both the level of the construct and change in the construct over the two assessments are modeled as latent variables. In the present research, the correlation between latent change in personality and latent change in sensory functioning reflect correlated change.
Results
Baseline sensory functioning and personality development
Baseline correlations between sensory functioning variables and demographic and personality are presented in supplemental Table S2. Consistent with our hypothesis, the main analysis revealed that both lower self-rated vision and hearing at baseline were independently related to declines in extraversion, openness, agreeableness, conscientiousness, and higher neuroticism over the four years period, controlling for the covariates (See Table 2). For most of the observed associations, the effect of sensory functioning on trait change was comparable to or even stronger than those of demographic factors, disease burden, and depression. The combined hearing and vision sensory functioning score at baseline was likewise related to steeper declines in extraversion, openness, conscientiousness, agreeableness, and higher neuroticism over the four-year period, controlling for the covariates (See Table 3). The latent change analyses confirmed this overall pattern of results (see Table 4). In addition, higher neuroticism and lower extraversion, openness, agreeableness, and conscientiousness at baseline were associated with worsening eyesight, hearing, and overall sensory functioning over time.
Table 2.
Regression Analysis Predicting Follow-up Personality Traits from Baseline Eyesight and Hearing (N= 7471)
| Variables | Neuroticism | Extraversion | Openness | Agreeableness | Conscientiousness | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| β (p) | 95% CI | β (p) | 95% CI | β (p) | 95% CI | β (p) | 95% CI | β (p) | 95% CI | |
| Sex | −0.03(.001) | [−0.047; −0.011] | −0.03(.003) | [−0.042; −0.009] | −0.01(.19) | [−0.028; 0.005] | −0.09(<.001) | [−0.110; −0.073] | −0.04(<.001) | [−0.056; −0.021] |
| Age | −0.01(.44) | [−0.027; 0.012] | −0.01(.09) | [−0.033; 0.002] | −0.05(<.001) | [−0.063; −0.028] | −0.03(.006) | [−0.045; −0.007] | −0.04(<.001) | [−0.055; −0.017] |
| Age Squared | 0.00(.61) | [−0.013; 0.022] | −0.03(<.001) | [−0.050; −0.018] | −0.01(.46) | [−0.023; 0.010] | −0.03(.003) | [−0.044; −0.009] | −0.01(.13) | [−0.031 ; 0.004] |
| Educational level | −0.02(.01) | [−0.041; −0.005] | 0.02(.02) | [0.003; 0.036] | 0.07(<.001) | [0.053; 0.088] | 0.03(.002) | [0.010; 0.046] | 0.03(<.001) | [0.016; 0.052] |
| Race | 0.04(<.001) | [0.021; 0.056] | −0.01(.12) | [−0.029; 0.003] | −0.02(.004) | [−0.040; −0.008] | 0.02(.05) | [−0.000; 0.035] | −0.01(.15) | [−0.030; 0.004] |
| Depressive symptoms | 0.10(<.001) | [0.076; 0.115] | −0.02(.04) | [−0.035; −0.001] | −0.00(.82) | [−0.019; 0.015] | −0.03(.004) | [−0.045; −0.009] | −0.04(<.001) | [−0.062; −0.026] |
| Disease Burden | 0.02(.01) | [0.005; 0.043]] | −0.02(.006) | [−0.041; −0.007] | −0.01(.29) | [−0.027; 0.008] | −0.00(.78) | [−0.021; 0.016] | −0.03(.002) | [−0.047; −0.010] |
| Baseline Personality Trait | 0.59(<.001) | [0.574; 0.612 | 0.69(<.001) | [0.675; 0.708] | 0.67(<.001) | [0.649; 0.683] | 0.61(<.001) | [0.595;0.631] | 0.62(<.001) | [0.604; 0.640] |
| Baseline eyesight | 0.02(.04) | [0.000; 0.038] | −0.03(.003) | [−0.043; −0.009] | −0.04(<.001) | [−0.055; −0.019] | −0.03(.005) | [−0.046; −0.008] | −0.03(<.001) | [−0.051; −0.013] |
| Baseline hearing | 0.03(.005) | [0.008; 0.046] | −0.04(<.001) | [−0.057; −0.023] | −0.03(<.001) | [−0.047; −0.012] | −0.02(.03) | [−0.039; −0.002] | −0.04(<.001) | [−0.055; −0.018] |
| Adjusted R2 | .43 | .51 | .50 | .43 | .45 | |||||
Note. β = Standardized Coefficient; p = p-value; 95%CI= 95 % Confidence Intervals
Table 3.
Regression Analysis Predicting Follow-up Personality Traits from Baseline Sensory Functioning Score (N= 7471)
| Variables | Neuroticism | Extraversion | Openness | Agreeableness | Conscientiousness | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| β (p) | 95% CI | β (p) | 95% CI | β (p) | 95% CI | β (p) | 95% CI | β (p) | 95% CI | |
| Sex | −0.03(.001) | [−0.046; −0.011] | −0.03(.002) | [−0.042; −0.010] | −0.01(.23) | [−0.026; 0.006] | −0.09(<.001) | [−0.109; −0.073] | −0.04(<.001) | [−0.056 ;−0.021] |
| Age | −0.01(.45) | [−0.026; 0.012] | −0.01(.08) | [−0.033 ; 0.002] | −0.05(<.001) | [−0.063; −0.027] | −0.03(.007) | [−0.045 ; −0.007] | −0.04(<.001) | [−0.055 ; −0.017] |
| Age Squared | 0.00(.61) | [−0.013 ; 0.022] | −0.03(<.001) | [−0.050; −0.018] | −0.01(.45) | [−0.023 ; 0.010] | −0.03(.003) | [−0.045 ; −0.009] | −0.01(.13) | [−0.031 ; 0.004] |
| Educational level | −0.02(.01) | [−0.041; −0.005] | 0.02(.03) | [0.002; 0.036] | 0.07(<.001) | [0.054 ; 0.088] | 0.03(.002) | [0.010; 0.047] | 0.03(<.001) | [0.016 ; 0.052] |
| Race | 0.04(<.001) | [0.021; 0.056] | −0.01(.11) | [−0.029 ; 0.003] | −0.02(.005) | [−0.040 ; −0.007] | 0.02(.04) | [0.000 ; 0.035] | −0.01(.14) | [−0.030 ; 0.004] |
| Depressive symptoms | 0.10(<.001) | [0.076; 0.115] | −0.02(.04) | [−0.034, −0.000] | −0.00(.78) | [−0.019 ; 0.015] | −0.03(.003) | [−0.046; −0.009] | −0.04(<.001) | [−0.062 ;−0.026] |
| Disease Burden | 0.02(.01) | [0.005; 0.042] | −0.02(.006) | [−0.041; −0.007] | −0.01(.28) | [−0.027 ; 0.008] | −0.00(.77) | [−0.021 ; 0.016] | −0.03(.002) | [−0.047; −0.010] |
| Baseline Personality Trait | 0.59(<.001) | [0.574; 0.612] | 0.69(<.001) | [0.675; 0.708] | 0.67(<.001) | [0.649 ; 0.683] | 0.61(<.001) | [0.595 ; 0.631] | 0.62(<.001) | [0.604 ; 0.640] |
| Baseline Sensory Functioning | 0.04(<.001) | [0.019; 0.056] | −0.05(<.001) | [−0.071; −0.036] | −0.05(<.001) | [−0.070; −0.035] | −0.04(<.001) | [−0.057; −0.020] | −0.06(<.001) | [−0.074 ; −0.037] |
| Adjusted R2 | .43 | .51 | .50 | .43 | .45 | |||||
Note. β = Standardized Coefficient; p = p-value; 95%CI= 95 % Confidence Intervals
Table 4.
Estimates from Latent Change Models
| Trait | Trait→Δ Sensory Functioning | Sensory Functioning→ ΔTrait | Correlated Change |
|---|---|---|---|
| Sensory Functioning Score | |||
| Neuroticism | .06** | .04** | .07** |
| Extraversion | −.06** | −.07** | −.07** |
| Openness | −.06** | −.07** | −.08** |
| Agreeableness | −.04** | −.05** | −.06** |
| Conscientiousness | −.06** | −.07** | −.09** |
| Eyesight | |||
| Neuroticism | .05** | .03** | .06** |
| Extraversion | −.05** | −.05** | −.07** |
| Openness | −.06** | −.06** | −.08** |
| Agreeableness | −.04** | −.04** | −.07** |
| Conscientiousness | −.06** | −.05** | −.07** |
| Hearing | |||
| Neuroticism | .05** | .04** | .04** |
| Extraversion | −.05** | −.06** | −.04** |
| Openness | −.04** | −.05** | −.05** |
| Agreeableness | −.03** | −.03** | −.04** |
| Conscientiousness | −.04** | −.06** | −.07** |
Note:
p < .01;
Trait→Δ Sensory Functioning : Relationship between baseline personality and changes in sensory functioning; Sensory Functioning → ΔTrait: Relation between the baseline sensory functioning variables and change in the five personality traits; Correlated change: Relation between change in the sensory functioning variables and change in the five personality traits
Age, age squared, sex, education, race, disease burden and depressive symptoms were controlled
There was limited support for age as a moderator of these relations; only the relation between poor eyesight and decline in openness was slightly stronger among younger than older individuals in the sample (βinteraction=.02, p = .01; 95% CI: 0.005; 0.039).
Changes in sensory functioning and personality development
We next examined whether changes in sensory functioning were correlated with changes in personality traits. Partial correlations between residuals revealed that worse self-rated eyesight, hearing, and overall sensory functioning over time were related to declines in extraversion, openness, agreeableness, conscientiousness and higher neuroticism over time (Table 1). Again, the latent change analyses confirmed this pattern of relations (see Table 4). In both analyses, the association between change in eyesight and change in personality was stronger than the association between change in hearing and change in personality.
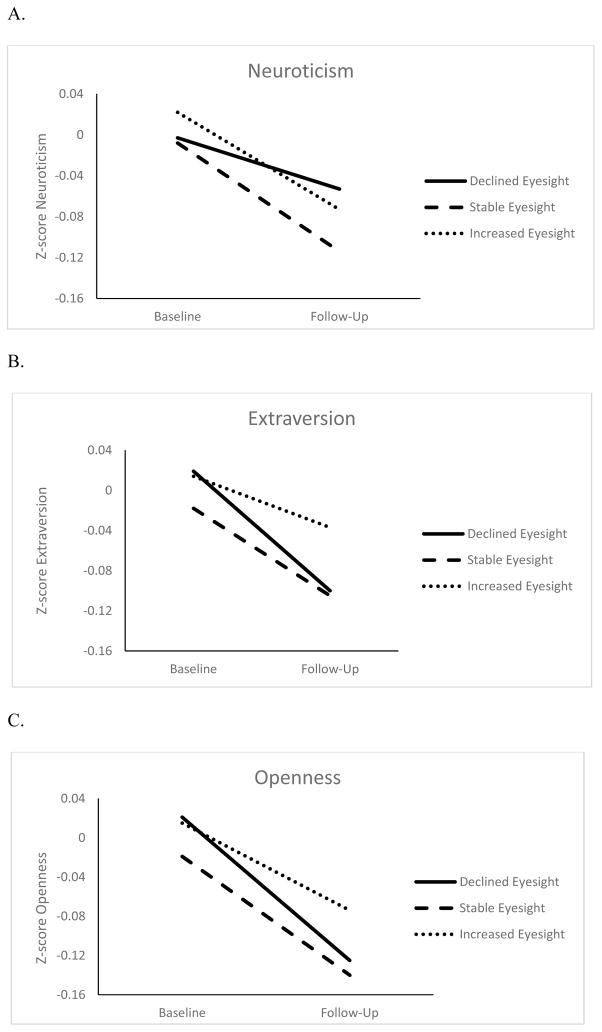
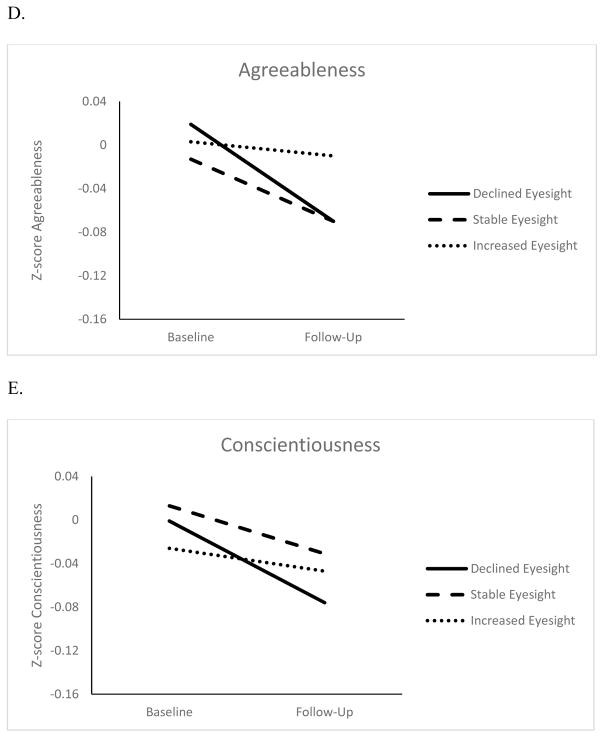
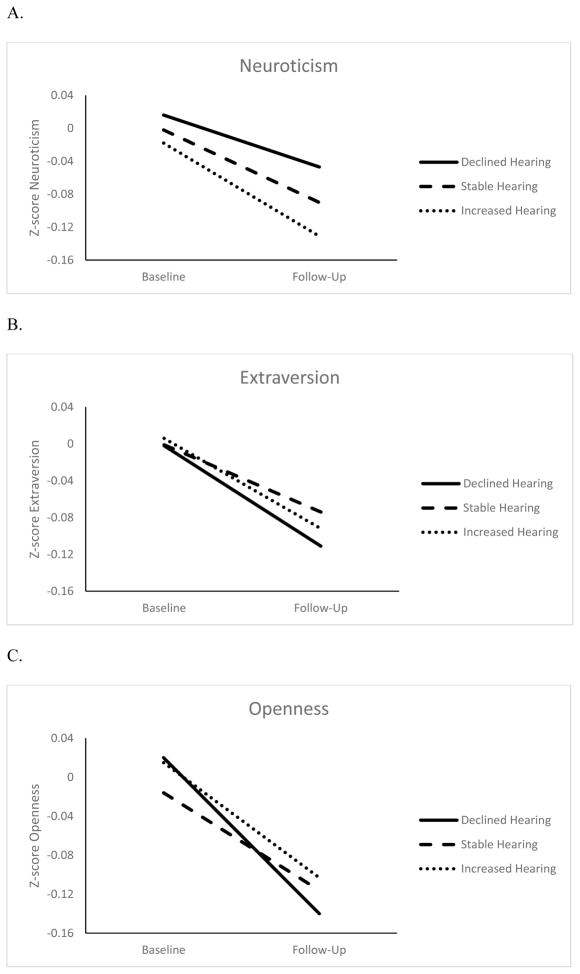
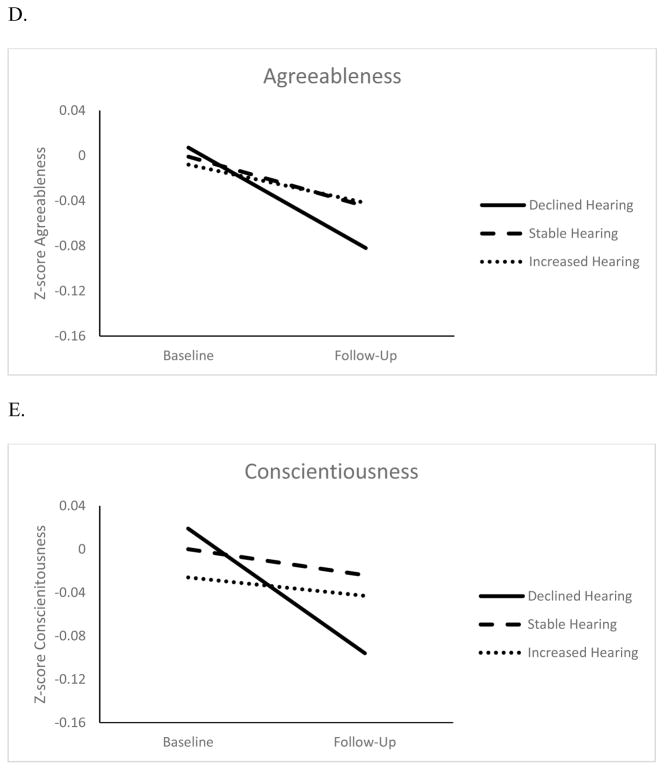
Figures 1 and 2 show the changes in personality for three eyesight and hearing groups: Participants with changes at least 1 SD beyond the baseline mean (increase or decrease) and those who remained within 1 SD. Overall, all groups had a decline in self-reported eyesight and hearing. However, participants with the largest change in sensory functioning also had the largest change in personality. The difference in personality change was around .05 SD between participants with worse eyesight over time and those who remained relatively stable (d= .06 for neuroticism, d= .02 for extraversion, d= .05 for openness and agreeableness, d= .03 for conscientiousness), and those with worse hearing compared to those with relatively stable function (d= .02 for neuroticism, d= .05 for extraversion, d= .07 for openness, d= .05 for agreeableness, and d= .10 for conscientiousness). This difference between the declining and stable groups was more pronounced when examining personality change associated with the sensory functioning score (d= .10 for neuroticism, d= .09 for extraversion, d= .07 for openness, d= .07 for agreeableness, and d= .15 conscientiousness). 1
Figure 1.
Changes in Neuroticism (Panel A), Extraversion (Panel B) Openness to Experience (Panel C), Agreeableness (Panel D), and Conscientiousness (Panel E) for Declined, Stable and Increased Eyesight Groups
Note. Z-scores adjusted for sex, age, age squared, educational level, and race
Figure 2.
Changes in Neuroticism (Panel A), Extraversion (Panel B) Openness to Experience (Panel C), Agreeableness (Panel D), and Conscientiousness (Panel E) for Declined, Stable and Increased Hearing Groups
Note. Z-scores adjusted for sex, age, age squared, educational level, and race
Discussion
Using a large longitudinal sample, this study provides evidence that sensory functioning is associated with personality development across the latter part of adulthood. Consistent with our hypothesis, worse vision and hearing at baseline and declines in vision and hearing over a four year period were independently related to a steeper decline in extraversion, openness to experience, agreeableness, conscientiousness, and the maintenance of neuroticism. In most cases, the size of the association between sensory functioning and personality change was similar or stronger than the effect of demographic variables, depressive symptoms, and disease burden on personality change.
Sensory impairment has profound implications for an individual’s quality of life (Brown & Barrett, 2011). Studies based on SST (Carstensen, 2006) suggest that individuals invest more in close relationships when faced with sensory impairment (Wahl et al., 2013). Indeed, impaired eyesight and hearing restrict social interactions and communication (Viljanen et al., 2014; Wahl & Tesch-Römer, 2001). Such deficits may lead individuals to refrain from engaging in basic daily conversations, going on social outings, and engaging in other social activities. As a result, they may be less sociable (extraversion) and less prosocially oriented and more angry (agreeableness) over time. In the same vein, higher sensory impairment and lower receptivity to visual and auditory cues may lead individuals to restrict their interest toward the familiar, to be less exploratory and less inclined to search for novelty, which manifests in lower openness over time. Furthermore, sensory impairment leads to lower assimilative coping, illustrated by less tenacious and persistent goals pursuits (Wahl et al., 2013), which is likely to be reflected in lower conscientiousness over time. Individuals with vision and/or hearing deficits may feel more isolated and rejected, which are experiences associated with higher neuroticism (Sutin et al., 2016). Individuals with sensory difficulties are also more likely to experience depressive symptoms (Heine & Browning, 2014), which may translate in a broader propensity to experience negative emotions. Behavioral pathways may also operate in this relation. Specifically, older individuals with sensory impairments experience more limitations in their daily activities (Brown & Barrett, 2011) and are less physically active (Gispen, Chen, Genther, & Lin, 2014). Over time, physical inactivity is likely to alter the energetic capacities required to behave in extraverted and conscientious ways and may also substantially limit the exposure to a range of experiences and social interactions, resulting in lower openness and agreeableness, respectively, over time (Stephan et al., 2014).
The present study thus provides new evidence on factors associated with personality change that are implicated in a range of negative outcomes in adulthood and old age. It supports a prior report of an association between hearing impairment and declines in extraversion (Berg & Johansson, 2014). Using a larger sample of older adults, this study extended these findings by showing that both self-reported hearing and eyesight are independently related to a generalized change across the five traits. These results add to previous research on health-related personality changes, which have been primarily focused on global measures of health, such as self-rated health or disease burden. In particular, the present study shows that changes in specific functions that drive individuals’ interactions with the social and physical environment play a role in non-normative change in personality traits. Furthermore, this contribution is independent of and even stronger than disease burden and depressive symptoms in most cases. This pattern suggests that personality is particularly sensitive to sensory functioning. Even if individuals are free from major disease, sensory deficits may lead to lifestyle changes and emotional reactions that are reflected in their personality over time. In addition, complementary analysis revealed that personality predicted change in sensory functioning. Consistent with existing knowledge on the link between personality and health among middle aged and older adults (Weston et al., 2015), higher neuroticism and lower extraversion, openness, agreeableness and conscientiousness were related to steeper declines in self-reported eyesight, hearing, and overall sensory functioning.
The present study has several strengths, including a large longitudinal sample of older individuals, repeated measures of personality and sensory functions, and the control of several covariates. There are also limitations that should be considered. First, this study used self-reported measures of eyesight and hearing. These measures correlated strongly with actual performance (Chasteen, Pichora-Fuller, Dupuis, Smith, & Singh, 2015; El-Gasim, Munoz, West, & Scott, 2013) but may also underestimate the degree of sensory loss (Kamil, Genther, & Lin, 2015). Future research could examine whether the pattern of personality change observed in the present study replicates using objective measures of hearing and vision. In addition, the association between sensory functioning and personality change was tested over a relatively short period of four years and only two waves. Future research must examine this link over a longer time frame with more waves of measurement. Another limitation is the positive selection of participants in the HRS, especially the attrition over the follow-up period. This selection may limit somewhat the generalizability of our findings. Future research could also use a more detailed measure of personality to examine which specific facets are most vulnerable to sensory impairment. Although comparable or larger than the effect size of other factors related to personality change in older adults, such as disease burden (Jokela et al., 2014; Sutin et al., 2013), the size of the direct association of sensory functioning at baseline and personality change over time was relatively small. This finding suggests that deficits in vision and hearing are distal predictors of personality changes that may drive processes that have a more proximal influence on personality. Despite these limitations, this study provides new evidence on factors associated with personality development. Deficits in sensory functioning, such as poor vision and hearing, are related to maladaptive personality trajectories across adulthood and old age.
Supplementary Material
Footnotes
Several alternative measures of sensory function from the HRS battery were examined, including distal and proximal vision, cataract surgery, and use of hearing aids. Controlling for covariates and self-reported hearing, regression analysis revealed that poor self-rated distal vision at baseline was associated with decline in extraversion, openness, agreeableness and the maintenance of neuroticism, whereas no association was observed with self-rated proximal vision. In addition, partial correlations between residuals revealed an association between worsening distal vision and decline in openness and conscientiousness, whereas worsening proximal vision was related to lower extraversion, openness, agreeableness, conscientiousness and higher neuroticism over time. We found no association between cataract surgery at baseline and over time and personality change, controlling for covariates. Finally, we found no association between baseline and increased use of hearing aids and personality change.
References
- Berg AI, Johansson B. Personality change in the oldest-old: Is it a matter of compromised health and functioning? Journal of Personality. 2014;82:25–31. doi: 10.1111/jopy.12030. [DOI] [PubMed] [Google Scholar]
- Boerner K. Adaptation to disability among middle-aged and older adults: The role of assimilative and accommodative coping. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2004;59:35–42. doi: 10.1093/geronb/59.1.P35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandtstädter J, Renner G. Tenacious goal pursuit and flexible goal adjustment: explication and age-related analysis of assimilative and accommodative strategies of coping. Psychology and Aging. 1990;5:58–67. doi: 10.1037/0882-7974.5.1.58. [DOI] [PubMed] [Google Scholar]
- Brennan M, Horowitz A, Su YP. Dual sensory loss and its impact on everyday competence. The Gerontologist. 2005;45:337–346. doi: 10.1093/geront/45.3.337. [DOI] [PubMed] [Google Scholar]
- Brown RL, Barrett AE. Visual impairment and quality of life among older adults: An examination of explanations for the relationship. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2011;66:364–373. doi: 10.1093/geronb/gbr015. [DOI] [PubMed] [Google Scholar]
- Capella-McDonnall ME. The effects of single and dual sensory loss on symptoms of depression in the elderly. International Journal of Geriatric Psychiatry. 2005;20:855–861. doi: 10.1002/gps.1368. [DOI] [PubMed] [Google Scholar]
- Capella-McDonnall ME. The effects of developing a dual sensory loss on depression in older adults: A longitudinal study. Journal of Aging and Health. 2009;21:1179–1199. doi: 10.1177/0898264309350077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen LL. The influence of a sense of time on human development. Science. 2006;312:1913–1915. doi: 10.1126/science.1127488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasteen AL, Pichora-Fuller MK, Dupuis K, Smith S, Singh G. Do negative views of aging influence memory and auditory performance through self-perceived abilities? Psychology and Aging. 2015;30:881–893. doi: 10.1037/a0039723. [DOI] [PubMed] [Google Scholar]
- Crews JE, Campbell VA. Vision impairment and hearing loss among community-dwelling older americans: Implications for health and functioning. American Journal of Public Health. 2004;94:823–829. doi: 10.2105/ajph.94.5.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnellan MB, Lucas RE. Age differences in the Big Five across the life span: Evidence from two national samples. Psychology and Aging. 2008;23:558–566. doi: 10.1037/a0012897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Gasim M, Munoz B, West SK, Scott AW. Associations between self-rated vision score, vision tests, and self-reported visual function in the Salisbury Eye Evaluation Study. Investigative Ophthalmology & Visual Science. 2013;54:6439–6445. doi: 10.1167/iovs.12-11461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gispen FE, Chen DS, Genther DJ, Lin FR. Association of hearing impairment with lower levels of physical activity in older adults. Journal of the American Geriatrics Society. 2014;62:1427–1433. doi: 10.1111/jgs.12938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath B, Schneider J, McMahon CM, Burlutsky G, Leeder SR, Mitchell P. Dual sensory impairment in older adults increases the risk of mortality: A population-based study. PLoS ONE. 2013;8:e55054. doi: 10.1371/journal.pone.0055054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakulinen C, Elovainio M, Pulkki-Raback L, Virtanen M, Kivimäki M, Jokela M. Personality and depressive symptoms: Individual participant meta-analysis of 10 cohort studies. Depression and Anxiety. 2015;7:461–470. doi: 10.1002/da.22376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine C, Browning CJ. Mental health and dual sensory loss in older adults: A systematic review. Frontiers in Aging Neuroscience. 2014;6:83. doi: 10.3389/fnagi.2014.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokela M, Hakulinen C, Singh-Manoux A, Kivimaki M. Personality change associated with chronic diseases: Pooled analysis of four prospective cohort studies. Psychological Medicine. 2014;44:2629–2640. doi: 10.1017/S0033291714000257. [DOI] [PubMed] [Google Scholar]
- Kamil RJ, Genther DJ, Lin FR. Factors associated with the accuracy of subjective assessments of hearing impairment. Ear and Hearing. 2015;36:164–167. doi: 10.1097/AUD.0000000000000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiely KM, Anstey KJ, Luszcz MA. Dual sensory loss and depressive symptoms: The importance of hearing, daily functioning, and activity engagement. Frontiers in Human Neuroscience. 2013;7:837. doi: 10.3389/fnhum.2013.00837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman ME, Weaver SL. Technical report. 1997. The Midlife Development Inventory (MIDI) Personality Scales: Scale construction and scoring. [Google Scholar]
- Lam BL, Lee DJ, Gómez-Marín O, Zheng DD, Caban AJ. Concurrent visual and hearing impairment and risk of mortality: The National Health Interview Survey. Archives of Ophthalmology. 2006;124:95–101. doi: 10.1001/archopht.124.1.95. [DOI] [PubMed] [Google Scholar]
- Lin FR, Yaffe K, Xia J, Xue QL, Harris TB, Purchase-Helzner E … Health ABC Study Group. Hearing loss and cognitive decline in older adults. JAMA Internal Medicine. 2013;173:293–299. doi: 10.1001/jamainternmed.2013.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas RE, Donnellan MB. Personality development across the life span: Longitudinal analyses with a national sample from Germany. Journal of Personality and Social Psychology. 2011;101:847–861. doi: 10.1037/a0024298. [DOI] [PubMed] [Google Scholar]
- McArdle JJ. Latent variable modeling of differences and changes with longitudinal data. Annual Review of Psychology. 2009;60:577–605. doi: 10.1146/annurev.psych.60.110707.163612. [DOI] [PubMed] [Google Scholar]
- McCrae RR, Terracciano A Members of the Personality Profiles of Cultures Project. Universal features of personality traits from the observer’s perspective: Data from 50 cultures. Journal of Personality and Social Psychology. 2005;88:547–561. doi: 10.1037/0022-3514.88.3.547. [DOI] [PubMed] [Google Scholar]
- Pocnet C, Rossier J, Antonietti JP, Von Gunten A. Personality features and cognitive level in patients at an early stage of Alzheimer’s disease. Personality and Individual Differences. 2013;54:174–179. doi: 10.1016/j.paid.2012.08.035. [DOI] [Google Scholar]
- Rogers MA, Langa KM. Untreated poor vision: A contributing factor to late-life dementia. American Journal of Epidemiology. 2010;171:728–735. doi: 10.1093/aje/kwp453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto CJ, John OP, Gosling SD, Potter J. Age differences in personality traits from 10 to 65: Big-Five domains and facets in a large cross-sectional sample. Journal of Personality and Social Psychology. 2011;100:330–348. doi: 10.1037/a0021717. [DOI] [PubMed] [Google Scholar]
- Specht J, Egloff B, Schmukle SC. Stability and change of personality across the life course: The impact of age and major life events on mean-level and rank-order stability of the Big Five. Journal of Personality and Social Psychology. 2011;101:862–882. doi: 10.1037/a0024950. [DOI] [PubMed] [Google Scholar]
- Stephan Y, Sutin AR, Luchetti M, Terracciano A. Allostatic load and personality: A 4-year longitudinal study. Psychosomatic Medicine. 2016;78:302–310. doi: 10.1097/PSY.0000000000000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan Y, Sutin AR, Terracciano A. Physical activity and personality development across adulthood and old age: Evidence from two longitudinal studies. Journal of Research in Personality. 2014;49:1–7. doi: 10.1016/j.jrp.2013.12.003. [DOI] [Google Scholar]
- Sutin AR, Stephan Y, Terracciano A. Perceived discrimination and personality development in adulthood. Developmental Psychology. 2016;52:155–163. doi: 10.1037/dev0000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutin AR, Zonderman AB, Ferrucci L, Terracciano A. Personality traits and chronic disease: Implications for adult personality development. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2013;68:912–920. doi: 10.1093/geronb/gbt036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano A, McCrae RR, Costa PT., Jr Longitudinal trajectories in Guilford-Zimmerman Temperament Survey data: Results from the Baltimore Longitudinal Study of Aging. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2006;61:108–116. doi: 10.1093/geronb/61.2.P108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano A, Sutin AR, An Y, O’Brien RJ, Ferrucci L, Zonderman AB, Resnick SM. Personality and risk of Alzheimer’s disease: New data and meta-analysis. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association. 2014;10:179–186. doi: 10.1016/j.jalz.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viljanen A, Törmäkangas T, Vestergaard S, Andersen-Ranberg K. Dual sensory loss and social participation in older Europeans. European Journal of Ageing. 2014;11:155–167. doi: 10.1007/s10433-013-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl HW, Heyl V, Drapaniotis PM, Hörmann K, Jonas JB, Plinkert PK, Rohrschneider K. Severe vision and hearing impairment and successful aging: A multidimensional view. The Gerontologist. 2013;53:950–962. doi: 10.1093/geront/gnt013. [DOI] [PubMed] [Google Scholar]
- Wahl H-W, Tesch-Römer C. Aging, sensory loss, and social functioning. In: Charness N, Parks DC, Sabel BA, editors. Communication, Technology and Aging. Opportunities and Challenges for the Future. New York: Springer; 2001. pp. 108–126. [Google Scholar]
- Wallace R, Herzog AR, Ofstedal MB, Steffick D, Fonda S, Langa K. Documentation of affective functioning measures in the health and retirement study. Ann Arbor, MI: Survey Research Center, University of Michigan; 2000. [Google Scholar]
- Weston SJ, Hill PL, Jackson JJ. Personality traits predict the onset of disease. Social Psychological and Personality Science. 2015;6:309–317. doi: 10.1177/1948550614553248. [DOI] [Google Scholar]
- Willis JR, Jefferys JL, Vitale S, Ramulu PY. Visual impairment, uncorrected refractive error, and accelerometer-defined physical activity in the United States. Archives of Ophthalmology. 2012;130:329–335. doi: 10.1001/archopthalmol.2011.1773. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.