Abstract
Rationale
Child abuse and neglect are universal risk factors for delinquency, violence, and aggression; this phenomenon is known as the cycle of violence. Additional factors—psychopathy, impulsiveness, and disruptions in the hypothalamic–pituitary–adrenal (HPA) axis—play a role in aggressive behavior but have rarely been examined in the same conceptual and experimental framework.
Objectives
We sought to examine the above-mentioned risk factors for aggression in a prospective study employing psychopharmacologic and psychometric techniques.
Methods
Sixty-seven adult participants were given an acute dose of 20 mg cortisol in a placebo-controlled, within-subject, counter-balanced dosing design. Salivary cortisol was measured at baseline and at regular intervals across a 5 h testing period. Following dosing, state-aggressive behavior was measured by a laboratory task, the Point-Subtraction Aggression Paradigm. History of child abuse/−neglect, psychopathy, impulsivity, and a trait measure of aggression were assessed through self-report questionnaires.
Results
Using multiple regression, a model including abuse/neglect, psychopathy, impulsivity, and baseline cortisol explained 58 % of the variance in trait aggression and 26 % of the variance in state aggression. Abuse/neglect predicted diminished HPA-axis reactivity and HPA-axis reactivity showed a trend toward predicting state and trait aggression, although it was not a significant mediating variable between abuse/neglect and aggression.
Conclusions
The results indicate that child maltreatment, psychopathy and HPA-axis reactivity interact to provide a confluence over aggressive behavior, and intervention efforts need to consider all these factors.
Keywords: Aggression, HPA-axis, Stress, Child abuse and neglect, Impulsivity
Introduction
Child abuse and neglect present both immediate risks—over five US children die from abuse each day (U.S. Department of Health and Human Services 2010)—and a long-lasting burden on society. In the USA, the lifetime cost of abuse and neglect, including health care, foster programs and criminal investigations, is estimated at US$210,012 per abuse victim (Fang et al. 2012). Further, children who emerge from abusive and neglectful homes face an increased risk for a wide range of physical (Widom et al. 2012) and mental health issues (Cohen et al. 2001), including higher rates of delinquency, aggression and violent crime—a phenomenon known as the cycle of violence (Caspi et al. 2002; Widom 1989). In a seminal study, Widom (1989) notes, “the scientific issue should not be the magnitude of the association between [victimization and later criminal behavior], but rather the goal should be further knowledge of the processes involved” (p.165). Despite an extensive literature demonstrating the relationship between abuse and violence, there exists a limited understanding of the key mediating factors behind this relationship.
Both human (Gunnar et al. 2001) and nonhuman studies (Veenema 2009) have shown that early life stress can disrupt the hypothalamic–pituitary–adrenal (HPA) axis, leading to altered cortisol levels. Important to physical and mental health, a number of disorders are characterized by HPA-axis disruptions (Thorn et al. 2011), notably depression and posttraumatic stress disorder, common outcomes of abuse and neglect (Cohen et al. 2001). In humans, maltreated children had increased free cortisol excretion (the cortisol system was not directly manipulated) over a 24-h period compared to non-maltreated comparison children (De Bellis et al. 1999). In comparison with a matched, non-abused group, females with a history of sexual abuse had a blunted cortisol response to a psychological stressor as adolescents, and this blunted cortisol response predicted antisocial behavior 7 years later (Shenk et al. 2010). Changes in HPA axis function are hypothesized to play a role in the development of psychopathology associated with child abuse and neglect (De Bellis 2005).
While much of the early life maltreatment literature has focused on biological outcomes that may lead to later life aggression, converging evidence from human research indicate that multiple pathways, including psychosocial and dispositional outcomes, may be key linkages (De Bellis 2005; Graham et al. 2012). For instance, physical maltreatment of children has been causally linked to antisocial behavior (Jaffee et al. 2004) and is associated with higher levels of psychopathy (Graham et al. 2012). Psychopathy is identified by a constellation of traits including callousness, interpersonal manipulation, erratic lifestyle, and criminal tendencies (Paulhus et al. 2009). Since psychopathy is also associated with antisocial and aggressive behavior (Hare and Neumann 2008), it is a strong candidate for a mediating variable between early life maltreatment and aggression. Psychopathy is also marked by differences in resting HPA-axis activity (Cima et al. 2008; Glenn et al. 2011) and decreased cortisol release in response to a stressor compared to control populations (O’Leary et al. 2010), providing further evidence that it is related to biological outcomes associated with child maltreatment.
Similarly, impulsivity may be associated with maltreatment (Corstorphine et al. 2007), but it has seldom been examined in conjunction with biological outcomes. Impulsivity is the tendency toward rapid, unplanned actions without regard to negative consequences (Moeller et al. 2001) and higher levels of impulsivity have been found in individuals with higher scores on a measure of psychopathy (Mathieu et al. 2012). Further, individuals with higher measures of impulsivity have shown higher levels of aggressive behavior in laboratory tasks (Cherek and Lane 2001; Coccaro et al. 1997). Given the relationship between impulsivity and both maltreatment and aggression, it is plausible that impulsivity may play linking role between child abuse/neglect and later life aggression in conjunction with psychopathy and HPA-axis disruptions.
Aggression is commonly studied in humans using state (e.g., laboratory tasks) or trait (e.g., psychometric questionnaires) measurements, and both may be useful in determining the relationship between maltreatment, psychopathy, impulsivity, and HPA-axis function. Aggression may be operationally defined as any behavior intended to cause harm to another person that the other person finds aversive and would act to avoid (Baron and Richardson 1994). Human laboratory experiments have shown that oral administration of 20 mg cortisol leads to increases in aggression, but only for females (Bohnke et al. 2010b). Further, participants with lower salivary cortisol levels measured upon awakening in the morning had higher levels of aggressive responding in a laboratory task (Bohnke et al. 2010a). As cortisol is released from the adrenal gland and serves as the end-point of HPA-axis activity, cortisol administration prior to laboratory aggression tasks offers a tool to examine how HPA-axis function may affect aggression. Also, because cortisol serves as a negative feedback signal for the HPA-axis, acute cortisol dosing can modulate the HPA-axis (Schlosser et al. 2010). Blunted or exaggerated levels of endogenous cortisol in response to acute cortisol dosing may be a marker for psychopathology and heightened aggression.
It is well documented that child maltreatment increases the chance for later aggression, delinquency and violence, but it is less clear why. Specifically, the key variables and their interactions are not fully understood. While the constellation of factors described above—child maltreatment, psychopathic traits, impulsivity, and HPA-axis dysfunction—appear to present shared risk for violent and aggressive behavior, to our knowledge they have not been previously examined in a collective manner. Here we explored the cycle of the violence and potential linkages between abuse and aggression using a novel approach featuring manipulation of cortisol levels (HPA-axis reactivity) via acute cortisol administration and both laboratory (state) and psychometric (trait) measures of aggression. We hypothesized that a history of child abuse and neglect, psychopathic traits, and impulsivity would predict heightened state and trait aggression in a multiple regression model. We further hypothesized that abuse and neglect would predict altered HPA-axis reactivity, and this effect would serve as a mediator between abuse/neglect and aggression.
Methods and materials
All experimental procedures were approved by the Institutional Review Board for the University of Texas Health Science Center–Houston.
General experimental design
Adults with a range of histories of both childhood maltreatment and later life aggression, delinquency and violence were recruited from the community. Estimates suggest that 14 % of male American prisoners have a history of abuse or neglect (Harlow 1999), whereas a representative survey of over 10,000 individuals in the San Diego health care system suggests that roughly 10 % of the general population experience episodes of physical abuse (Felitti et al. 1998). We therefore recruited parolees and probationers as well as participants without a criminal record from the same zip code to increase the range of aggression and abuse/neglect in the sample.
To measure HPA-axis reactivity, a pharmacological challenge was employed. Each subject provided a surrogate measure for free circulating cortisol via saliva samples. Samples were collected on three separate days: a placebo dose day, a 20 mg hydrocortisone dose day, and a day where no capsules were administered. HPA-axis reactivity was measured as the difference between cortisol levels on the placebo and cortisol dosing days. State and trait measures of aggression were obtained. A laboratory behavioral paradigm (Point-Subtraction Aggression Paradigm (PSAP)) provided state-dependent measures and a questionnaire (Buss Perry Aggression Questionnaire (BPAQ)) provided trait measures of aggression. Impulsivity (Barratt Impulsiveness Scale 11 (BIS-11)), and psychopathy (SPR-III) were both assessed via questionnaire.
Participants
Potential volunteers responded to newspaper advertisements seeking either (1) individuals on parole or probation or (2) individuals over the age of 18 who drank two to 12 alcoholic beverages per week. Advertisements were placed in target zip codes in the Houston area to control for socioeconomic status and education level. Before participation, participants signed an informed consent document and underwent a standard physical examination (including blood work, ECG, and a complete medical history). Assessment of axis-I and II psychiatric disorders was conducted using the Structured Clinical Interview for DSM-IV or SCID-I (First et al. 2002) and SCID-II (First et al. 1997).
Inclusion criteria
Eligible participants included males and females between the ages of 18 and 55. All psychiatric and health diagnoses were reached through consensus meetings between a licensed nurse practitioner, trained counselors, and a psychiatrist (FGM). Participants had no medical (e.g., high blood pressure) or psychiatric (e.g., mood disorder) contraindications for study procedures. Past criminal offenses included drug charges, theft, forgery, burglary, assault, domestic violence, and attempted murder. Eligible participants had no psychiatric diagnoses other than substance use disorders (SUD) in remission, anxiety disorders, or personality disorders. Anxiety disorders were included because the literature suggested that victims of child abuse have a high risk for posttraumatic stress disorder (De Bellis 2005). Participants with substance use disorders were included because they represent a significant proportion of the violent criminal population (Arseneault et al. 2000).
Exclusion criteria
The following conditions were exclusionary due to the possibility of complications with cortisol dosing: a history of cancer, tuberculosis, HIV, AIDS, diabetes, heart problems, abnormal liver function tests, current use of CNS-active medications, seizures and loss of consciousness lasting longer than 20 min. Participants diagnosed with mood disorders, psychotic disorders, or current SUDs were excluded. Participants who smoked more than ten cigarettes per day were excluded to avoid acute nicotine withdrawal on testing days. Drug (amphetamines, cocaine, benzodiazepines, marijuana, and opiates) and alcohol use were monitored using the One Step Drug Screen Test Card (Inovacon, San Diego, CA) and Alcosensor III Breathalyzer (AlcoPro Inc., Knoxville, TN), respectively—a positive sample on testing days was exclusionary.
Demographics
Of the 74 participants initially enrolled, 67 completed the study. Seven participants did not finish because they (a) dropped out (N=4), (b) refused to comply with study procedures (N=2), or (c) were removed after they reported that the laboratory task involved deception, invalidating the results (N=1). The sample was 31.5±9.6 years old with 13.1±1.9 years of education. Forty-five (seven females) were on parole or probation, 41 (five females) had a SUD in remission, and 13 (two females) were diagnosed with antisocial personality disorder. One male met the PTSD criteria. No other participants met the anxiety disorder criteria. The mean Shipley II score of intellectual aptitude was 200.4±24.7. A score of 200 is the mean for the general population, indicating the sample was of normal intelligence (Shipley et al. 2009).
Procedures
Participants came to the laboratory on 4 days during the study and were paid daily, commensurate with time and earnings during laboratory tasks. On day 1, participants completed the Childhood Trauma Questionnaire (CTQ) (Bernstein and Fink 1998), were introduced to the PSAP (described below), and completed two PSAP sessions. On dosing days (2 and 3), participants came to the laboratory at 8:00 a.m. and provided clean breath and urine samples prior to testing to ensure that no drug or alcohol use would confound behavioral results. At six time points throughout the day (8:30, 9:00, 9:50, 10:30, 11:30, and 12:30), participants provided saliva samples to measure cortisol levels and had their blood pressure and heart rate measured. All dosing was conducted in an unoccupied room. At 9:30 (between the second and third saliva measurement), participants received a small envelope enclosing a dark blue, #00 capsule prepared by a licensed pharmacist that contained either 20 mg of cortisol and corn starch or corn starch only (placebo). A research assistant watched the subject take the capsule and then verified it had been swallowed by checking the cheeks and tongue. Following dosing, participants completed three sessions on the PSAP (10:00, 11:00, and 12:00) and were then released at 1:00 p.m.
On day 4, participants filled out psychometric questionnaires and continued to provide heart rate, blood pressure, and saliva samples at the same time intervals as on dosing days in order to provide control measurements in the absence of both an external stressor (PSAP) and capsule administration.
Dosing design and capsule administration
A dose of 20 mg was selected based on previous research which found significant effects on laboratory-measured aggression at 20 mg (Bohnke et al. 2010b) and because FDA and manufacturer recommendations suggested this dose would be safe.
Dosing was counter-balanced and double-blind. Participants were randomized to receive either placebo or cortisol first using urn-randomization software, obtained from the University of Connecticut Health Center’s Project MATCH (Project MATCH Research Group 1993), that controlled for gender and history of child maltreatment.
Point-subtraction aggression paradigm
The PSAP, based on the operational definition of aggression noted previously (e.g., Baron and Richardson 1994), has been well-validated and used in a number of laboratories for over 20 years as a measure of state aggression (Cherek et al. 2006). Extensive description of the PSAP can be found in Cherek et al. (2003). A brief description is provided below.
The PSAP took place in a small room with a computer and four-button response device. The participants were told, via instructional deception, that their computer was connected through a network to another participant’s computer. Three letters appeared on the screen (A, B, and C), corresponding to three buttons on the subject’s response device. The stated object of the task was to earn money by pressing the A button 100 times (Fixed Ratio [FR], 100), which added 15 cents to the subject’s counter. Participants were provoked at random intervals during the session (90±25 s apart) by a subtraction of 15 cents from their counter, which was attributed to the other person, as established in the instructions. The subject could retaliate by pressing the B button ten times (FR10), which ostensibly subtracted 15 cents from the fictitious person but did not add any money to the subject’s counter. B-responding meets the operational definition of aggression (Cherek et al. 2003; Gowin et al. 2010). The subject could also prevent subtractions for a variable period of time by pressing the C button ten times (FR10). At the end of the day, participants were given a brief questionnaire about their interaction with the other (fictitious) individuals to ensure the instructional deception was effective. Any answer indicating suspicion about the validity of the other person resulted in the subject’s removal from the study. One subject was removed for this reason.
Physiological measures
Cortisol
Salivary cortisol samples were obtained at six time points (8:30, 9:00, 9:50, 10:30, 11:30, and 12:30) for a surrogate measurement of endogenous free circulating cortisol. Salivary cortisol was measured by enzyme-linked immunosorbent assay (Raff et al. 2003). A single missing data point was interpolated by regression-based imputation (single data points were imputed for four participants). Cortisol levels less than 0.3 nmol/L were assigned the result 0.3 nmol/L for statistical analysis. From the cortisol measures at each time point, a curve was drawn to represent changing cortisol levels across time. The area under the curve (AUCG) with respect to ground, or zero, was calculated using Pruessner et al.’s technique (2003).
Dose preparation errors resulted in the contamination of 22 participants’ salivary cortisol. Capsules were prepared by breaking hydrocortisone tablets in two and placing both halves in a capsule. For 22 capsules, cortisol from the tablet remained on the external portion of the capsule, evident by spikes in salivary cortisol 20 min after dosing (extremely rapid) that were outside the range of human endogenous cortisol levels (≥85 nmol/L) (Raff and Singh 2012), with some measurements in excess of 200 nmol/L. The cutoff of 85 nmol/L has been employed by past studies with contamination problems to discriminate contaminated from uncontaminated samples (Raff and Singh 2012). Salivary cortisol levels above 85 nmol/L reflected cortisol deposited in the mouth during dosing, not endogenous levels of cortisol, making it inaccurate to use these samples in analyses. These participants’ salivary cortisol data were excluded from analysis on the cortisol dosing day to prevent bias or confound in the data. Thus, only 45 participants were analyzed with regard to HPA-axis reactivity to cortisol dosing. However, placebo and baseline (no dose) salivary cortisol data from all 67 participants were retained in the sample, as there was no evidence of contamination of these samples in the way of rapid spiking or extreme values.
Heart rate/blood pressure
Heart rate and blood pressure were measured using a sphygmomanometer (BpTru Vital Signs Monitor, Coquitlam, Canada) at six time points throughout the day immediately after saliva collection. The sphygmomanometer cycled through six times per reading and computed the mean of the last five cycles. This information was obtained to complement salivary cortisol measurements of HPA-axis reactivity and to assess subject vitals for safety.
Questionnaires
The Childhood Trauma Questionnaire (CTQ; Bernstein and Fink 1998) is a self-report, 28-item, likert-rated scale of history of maltreatment during childhood. With five subscales (physical abuse, emotional abuse, sexual abuse, physical neglect, and emotional neglect), it asks questions such as “People in my family hit me so hard that it left me with bruises or marks,” or “I didn’t have enough to eat.” Based on therapists’ ratings of patients’ history, the CTQ has high convergent validity and sensitivity (Bernstein et al. 1994).
Buss Perry Aggression Questionnaire (BPAQ; Buss and Perry 1992) is a 29-item true–false rating scale of trait aggression. It provides scores on four subscales: physical aggression, verbal aggression, anger, and hostility. The total score from this instrument has been correlated with laboratory measures of aggression (Cherek et al. 1997).
The 65-item Self-Report Psychopathy Scale III (SRP-III; Paulhus et al. 2009) was used to measure psychopathy. Participants rate their level of agreement with statements such as “I think I could beat a lie detector” on a 1–5 likert scale. The SRP-III subcategorizes into callous affect, erratic lifestyle, criminal tendencies, and interpersonal manipulation. Either the subscales or the total score can be used as measures of psychopathic traits.
The Barratt Impulsiveness Scale 11 (BIS-11; Patton et al. 1995) is a well-validated 30-item self-report measure of impulsivity used extensively in the psychiatry and social sciences (Moeller et al. 2001). It provides a measure of impulsive traits and can be used as a total score or broken down into three subscales: non-planning, attentional, and motor impulsivity.
The Shipley II (Shipley et al. 2009) is a test of general intellectual aptitude that includes a 40-item vocabulary test and a 20-item abstraction test. Shipley score estimates of WAIS IQ correlate highly (0.76–0.87) with actual WAIS IQ scores (Zachary et al. 1985).
Data analyses
Salivary cortisol and cardiovascular data
All analyses were conducted using STATA statistical software, version 11 (College Station, TX). The effects of day (20 mg cortisol vs. placebo vs. non-dose/baseline) on salivary cortisol (AUCg), blood pressure, and heart rate (mean across each day) were analyzed using repeated-measures analysis of variance (ANOVA). A day by time analysis for cortisol levels was conducted on placebo and no-dose to determine if PSAP sessions altered cortisol levels. Post hoc tests were conducted using the Sidak method.
Psychometric and behavioral data
A repeated-measures ANOVA was used for PSAP data to test for main effect of dose, session, and a dose by session interaction. Two separate multiple linear regression analyses were used to test the predictive value of the model and the influence of each variable (child abuse/neglect, psychopathy, impulsivity, and baseline cortisol) on aggression as measured by (a) aggressive responses per provocation during PSAP sessions on the placebo day and (b) the total score on the BPAQ. Since aggressive responding per provocation on the PSAP was positively skewed, data were natural-log transformed. For child abuse/neglect, psychopathy, and impulsivity, the total scores on the CTQ, SRP-III and BIS-11, respectively, were used. To estimate baseline cortisol, the AUCg for the first two time points (8:30 and 9:00) was calculated for all 3 days (cortisol, placebo, no dose). The average AUCg of these 3 days was used, as Hellhammer et al. (2007) demonstrated that data from a single day are more reflective of situational factors that stable traits.
Two types of analyses were conducted to assess the influence of five potentially confounding variables: SUD diagnosis, parole/probation status, race, age, and gender. Assman et al. (2000) and Pocock et al. (2002) argue that it is necessary to adjust for potential confounders if they are shown to be strong predictors of the outcome variable or if they moderate the effect of predictor variables. Accordingly, the first analysis examined the correlation between each of the five potential confounders and the two outcome variables (i.e., BPAQ score and natural-log adjusted PSAP data) using pairwise Pearson correlation (see Supplemental Materials for correlation matrix). SUD diagnosis and parole/probation status were significantly correlated with BPAQ score, but not PSAP data. Neither age, nor race, nor gender significantly correlated with the two outcome variables. For the second analysis, the two potential confounders that were significantly correlated with BPAQ (i.e., SUD diagnosis and parole/probation status) were added one at a time to the linear regression model with the four originally hypothesized predictor variables (i.e., abuse/neglect, psychopathy, impulsivity, and baseline cortisol) with BPAQ as the dependent variable. The purpose of this analysis was to determine if the addition of either potential confounder (a) increased the variance in BPAQ explained or (b) changed the predictive value of any of the four originally hypothesized variables. Neither SUD diagnosis nor parole/probation status altered the model as described above (see Supplemental Materials). Likewise, while neither variable was correlated with PSAP responding, regression models with SUD diagnosis and parole/probation status did not significantly change the regression outcomes with PSAP responding as the dependent measure. As neither variable was part of the original hypothesis, both were excluded from the final models reported in the results. Additionally, removing potential (non-significant) confounders from the model protected against running underpowered statistical analyses (Assmann et al. 2000; Pocock et al. 2002).
HPA-axis reactivity was examined as both an outcome of abuse/neglect and a predictor of aggression. HPA-axis reactivity was calculated as the difference between AUCg on the cortisol vs. the placebo day, representing a single score for each subject. It was not included in the multiple linear regression model with the other predictor variables due to the high number of participants (22) lost to salivary cortisol contamination on the cortisol dosing day. However, CTQ was independently tested as a predictor of HPA-axis reactivity using linear regression, and HPA-axis reactivity was tested as a predictor of each metric of aggression (PSAP and BPAQ). Using the approach of Baron and Kenny (1986), regression models were used to test the likelihood that HPA-axis (for 45 participants) reactivity to cortisol mediated the relationship between the abuse/neglect and each metric of aggression. A Sobel test was used to test significance of mediation and to evaluate the percent of the total effect that was mediated (Sobel 1982).
Results
Descriptive statistics
There were a total of 67 observations for both metrics of aggression (i.e., BPAQ, PSAP) impulsivity, psychopathy and baseline cortisol, and 45 observations for HPA-axis reactivity. Measures of central tendency and variance are shown in Table 1.
Table 1.
Measures of central tendency and variance for primary study variables
| Variable | Mean | S.E. |
|---|---|---|
| HPA reactivitya | 85.56 | 4.59 |
| Baseline cortisolb | 3.47 | 0.16 |
| SRP-III total score | 157.66 | 3.71 |
| BIS-11 total score | 57.37 | 1.24 |
| CTQ total score | 38.58 | 1.37 |
| PSAP responsesc,d | 30.68 | 3.83 |
| BPAQ total scored | 65.12 | 2.27 |
Nanomole/liter/4 h (AUCg for 4 h)
Nanomole/liter/0.5 h (AUCg for 0.5 h)
Aggressive responses/provocation
PSAP and BPAQ were standardized and summed to create the total aggression score
Salivary cortisol
In the 45 participants with valid salivary cortisol data on all 3 days, one-way repeated-measures ANOVA of AUCg for cortisol level across dosing conditions (cortisol, placebo, no dose) revealed a significant main effect of dosing condition on AUCg (F2, 43=307.4, p<.001). Sidak post hoc tests revealed that the cortisol day was significantly different than both the placebo (p<.001) and no dose (p<.001) days, providing a manipulation check to ensure effective cortisol dosing. There was no difference between placebo and no dose days (p=.99). Measures of central tendency and variance for the cortisol data are provided in Table 2.
Table 2.
Mean and standard deviation for cortisol (nanomole/liter/4 h or AUCg for 4 h), heart rate (beats/minute), systolic blood pressure (millimeter mercury) and diastolic blood pressure (millimeter mercury) across three study days (placebo, cortisol, no dose)
| Cortisol (S.D.) |
Heart rate (S.D.) |
Systolic BP (S.D.) |
Diastolic BP (S.D.) |
|
|---|---|---|---|---|
| Placebo | 18.9 (9.4) | 63.9 (8.6) | 112.9 (9.4) | 72.6 (7.2) |
| Cortisol | 104.4 (32.0) |
64.8 (8.7) | 112.4 (9.1) | 72.5 (7.2) |
| No dose |
18.0 (7.3) | 64.6 (8.4) | 112.0 (9.2) | 72.5 (7.5) |
In all 67 participants, a follow up two-way repeated-measures ANOVA for time and day (placebo and no dose) revealed no main effect of day (F1, 66 =.08, p=.775), but an effect of time (F5, 330 =45.4, p<.001) and a day by time interaction (F5, 330 =3.31, p=.006). Sidak post hoc tests revealed that the first and third time points were significantly different between placebo and no-dose day (p<.05), but none of the other time points were significantly different (p>.05), and thus no differences in salivary cortisol were evident following PSAP sessions (i.e., time points 4–6).
Cardiovascular
One-way repeated-measures ANOVA revealed no effect of cortisol dosing condition for heart rate (F2, 65=.98, p=.379), systolic (F2, 65=.83, p=.441), or diastolic blood pressure (F2, 65=.03, p=.974). Measures of central tendency and variance are provided in Table 2.
Behavioral
Two-way repeated-measures ANOVA revealed no main effect of dosing condition (cortisol versus placebo) on aggressive responding per provocation on the PSAP, no main effect of session, and no dosing condition by session interaction (all Fs<1).
Multiple linear regression analysis
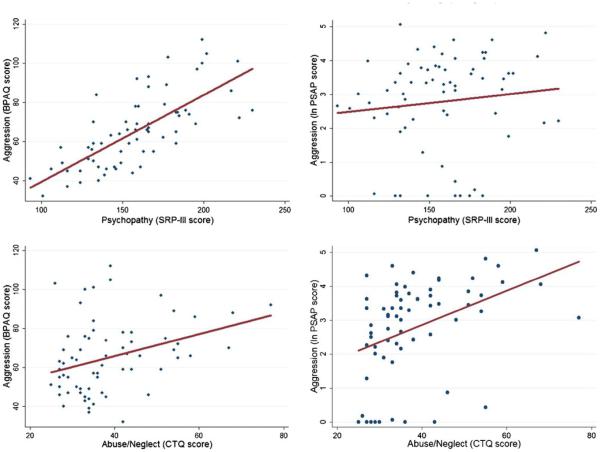
Based on the multiple linear regression analysis, the model of abuse/neglect, psychopathy, baseline cortisol, and impulsivity significantly predicted aggression as measured by PSAP (F4, 62=5.53, p<.001) and BPAQ (F4, 62 =21.3, p<.001). The model accounted for 26 % of the variance in aggressive responding per provocation on the PSAP and 58 % of the variance in BPAQ score. For the BPAQ, both psychopathy (t =7.8, p<.001) and abuse/neglect (t =2.2, p = .03) were significant predictors of aggression scores, where individuals with higher levels of psychopathy (b = .41 ± .05; 95 % CI, .31, .52) and abuse/neglect (b = .31 ± .14; 95 % CI, .03, .60) had higher scores on the BPAQ. Neither baseline cortisol nor impulsivity had significant predictive value of BPAQ score when adjusting for other predictors (p > .05). For the PSAP, only abuse/neglect was a significant predictor of aggressive responses per provocation (t =3.1, p<.01), where individuals with higher levels of abuse/neglect used the aggressive response more frequently (b = .05 ± .02; 95 % CI, .02, .08). Figure 1 shows the relative impact of each individual variable on total aggression score, holding the other variables constant at their means. Table 3 presents the correlation matrix for all variables included in the model.
Fig. 1.
The four panels depict the relationship between aggression and a psychopathy and b abuse/neglect. The top two graphs show a scatter plot of aggression scores on the BPAQ and PSAP versus psychopathy along with a linear fit to the data. The bottom two graphs show a scatter plot of aggression scores on the BPAQ and PSAP versus abuse/neglect along with a linear fit to the data
Table 3.
Correlation matrix for primary variables in regression models
| Variable | Abusea | Impulsivityb | Psychopathyc | Baseline cortisold |
BPAQe | PSAPf | HPA-axis reactivityg |
|---|---|---|---|---|---|---|---|
| Abuse (N=67) |
1.00 | ||||||
| Impulsivity (N=67) | .20 | 1.00 | |||||
| Psychopathy (N=67) | .19 | .19 | 1.00 | ||||
| Baseline cortisol (N=67) |
−.11 | −.03 | .17 | 1.00 | |||
| BPAQ (N=67) |
.34* | .26** | .73*** | .07 | 1.00 | ||
| PSAP (N=67) |
.40*** | .14 | .12 | −.17 | .17 | 1.00 | |
| HPA-axis reactivity (N=45) |
−.31** | .07 | −.28 | −.03 | −.26**** | −.28**** | 1.00 |
p<.01;
p<.05;
p<.001;
p<.10
Total score from CTQ
Total score from BIS-11
Total score from SRP-III
AUCg for first half hour of saliva measurements (nanomole/liter/0.5 h)
Total score from BPAQ
Natural-log transformed aggressive responses per provocation on the PSAP averaged across the placebo dosing day
AUCg from cortisol dosing day–AUCg from placebo dosing day (nanomole/liter/4 h)
Abuse, HPA-axis reactivity, and aggression
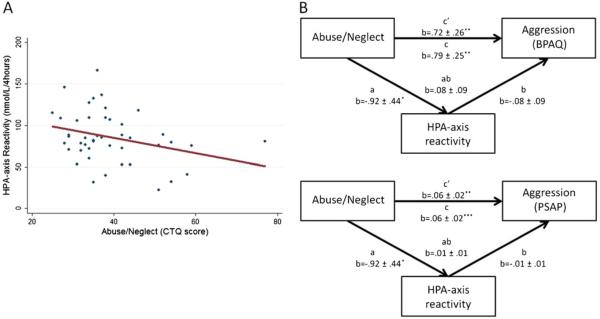
To test whether individuals with greater levels of abuse/neglect had diminished HPA-axis reactivity, linear regression analysis was performed with total CTQ score as the predictor variable and HPA-axis reactivity as the dependent measure. The linear regression model was significant (F1, 43 =4.4, p=.04), explaining 9 % of the variance of HPA-axis reactivity. Abuse/neglect was a significant predictor of HPA-axis reactivity (t=−2.1, p=.04), where individuals with higher levels of abuse/neglect had lower measures of HPA-axis reactivity (b=−.92±.44, 95 % CI, −1.8, −.04). The relationship between abuse/neglect and HPA-axis reactivity is presented in Fig. 2. To test whether individuals with diminished HPA-axis reactivity had higher levels of aggression, two linear regression analyses were performed with HPA-axis reactivity as the predictor and (a) PSAP responding and (b) BPAQ scores as the dependent measures. Each model showed a trend toward significance: for PSAP (F1, 43 =3.8, p=.06; 8 % VAF); for BPAQ (F1, 43 =3.1, p=.08; 7 % VAF). In both cases, lower HPA-axis reactivity was related to higher aggression scores. Since abuse/neglect predicted HPA-axis reactivity and HPA-axis reactivity showed a trend toward predicting both PSAP responding and BPAQ score, HPA-axis reactivity could serve as a possible mediator between abuse/neglect and aggression, warranting mediation analysis.
Fig. 2.
a Depicts the relationship between abuse/neglect and HPA-axis reactivity. b Abuse/neglect has a significant total affect on aggression as measured by BPAQ (top) or PSAP (bottom). Although HPA-axis reactivity does not significantly mediate the relationship between abuse/neglect and either measure of aggression, it does carry 10 % of the total effect of abuse/neglect on both measures of aggression. *p<.05; **p< .01; ***p<.001
Mediation analysis
To test for a mediating effect of HPA-axis reactivity between abuse/neglect and aggression, a Sobel test of mediation was employed. Based on the Sobel test, HPA-axis reactivity was not a significant mediator between abuse/neglect and PSAP responding (z=.98, p=.33) or BPAQ score (z=.88, p=.38). However, 10 % of the total effect of abuse/neglect on PSAP was mediated by HPA-axis reactivity, and 10 % of the effect of abuse/neglect on BPAQ was mediated by HPA-axis reactivity, indicating a possible albeit modest role as a mediator. A display of the mediation steps of Baron and Kenny (1986) is provided in Fig. 2.
Discussion
This study examined factors that contribute to the cycle of violence, with the hypothesis that child abuse and neglect lead to heightened aggression through the combination of heightened psychopathy and impulsivity and altered HPA-axis reactivity and baseline cortisol levels. A multiple regression model with four predictors (abuse/neglect, psychopathy, impulsivity, and baseline cortisol) explained 58 % of the variance in aggression as measured by BPAQ and 26 % of the variance in aggression as measured by PSAP. The present report features three novel contributions. First, this study examined the relative contributions of multiple pathways (child maltreatment, psychopathy, impulsivity, and changes to HPA-axis function) and represents the first combined examination of these variables and their predictive value with regards to aggression. Second, while other studies have used acute cortisol dosing in humans to examine aggression, none have looked at the interaction between abuse and acute cortisol effects on aggression. Third, we examined both state and trait metrics of aggression in the same sample to provide complementary data to test the present hypotheses. The results from his study provide evidence that alterations to HPA-axis reactivity contribute to the cycle of violence and that multiple pathways contribute to aggression, including child maltreatment and psychopathy.
Based on linear regression models, child maltreatment served as a significant predictor of both state and trait measures of aggression. Abuse/neglect as measured by the CTQ had a positive correlation with BPAQ score and aggressive responses per provocation on the PSAP. The BPAQ measures trait aggression and is stable across time, whereas the PSAP measures state aggression that is sensitive to situational factors (e.g., degree of provocation; Cherek et al. 2003). Thus, these metrics provide unique and complementary evidence that abuse/neglect is related to outcomes of aggression. While this cross-sectional study does not allow examination of causality, it does suggest a sequential relationship as abuse/neglect occurred prior to age 18, but aggression was not measured after age 18. Further, as many studies of the cycle of violence rely on epidemiological data (Caspi et al. 2002; Herrera and McCloskey 2001; Widom 1989), the use of laboratory measurements of aggression offers an opportunity not only to test the relationship between child maltreatment and aggression but also to examine factors which might regulate aggression in individuals who have been exposed to maltreatment. The present results support the cycle of violence hypothesis with two metrics of aggression.
In addition to a history of maltreatment, psychopathy was also a significant predictor of aggression, but only when BPAQ served as the dependent measure. Considering the strong predictive value of SRP-III scores of psychopathy for aggression as measured by the BPAQ, it is somewhat surprising that SRP-III did not predict PSAP responding. Previous studies using laboratory measures of aggression, including the PSAP, have shown that individuals with higher levels of psychopathy have higher levels of unprovoked aggressive behavior (Nouvion et al. 2007; Reidy et al. 2011). Since the present study did not examine an unprovoked task condition to measure aggression, and instead only employed a condition with provocations, the lack of a predictive relationship between psychopathy and PSAP score may reflect task conditions or variability in aggressive responding across the somewhat heterogeneous sample. Psychopathic traits had significant predictive value on BPAQ score. The strong correlation may reflect the measurement modality, as both BPAQ and SRP-III are trait-based self-report questionnaires. The individuals most likely to self-report aggression may also be most likely to self-report psychopathic traits.
Measurements of child maltreatment in the present study predicted altered HPA-axis reactivity such that greater childhood maltreatment corresponded to diminished HPA-axis reactivity. This complements a study by Shenk et al., which showed that maltreated females had a blunted cortisol response to a psychological stressor as adolescents, and this cortisol effect led to increased risk of crime and antisocial behavior as adults (Shenk et al. 2010). The present study expanded on Shenk et al. (2010) by examining the abuse–HPA-axis interaction in both genders and by using a pharmacological rather than a psychological challenge; however, the results are comparable. Maltreatment predicted a blunted HPA-axis reactivity, which predicted aggression. This finding is contrary to the conclusion Hawes et al. (2009) draw, suggesting that the relationship between HPA-axis hyporeactivity and aggression is independent of early-life adversity. Although HPA-axis reactivity did not mediate a statistically significant portion of the effect of child abuse on aggression, it did carry some of the effect, which may have reached significance with a larger sample size.
Cortisol dosing represents a tool to study the effects of abuse and neglect on the HPA-axis and the stress response. A previous study found that a single dose of 20 mg cortisol was sufficient to induce changes in aggressive responding (Bohnke et al. 2010b). Unfortunately, this result was not replicated in the present study. The original study, however, found that cortisol only increased aggressive responding in females, not in males, thus the current results may be complicated by the small number of females in the sample. The effects of cortisol dosing may well be moderated by gender (see limitations below). Additionally, the use of a single 20-mg dose may have limited our ability to detect an effect. Successful exploration of the effects of cortisol may require multiple dose levels to examine a full a dose–response curve. Future studies should look at a wider range of cortisol doses to better understand both the effect of cortisol on aggression and the relationship between HPA-axis function, child maltreatment, and aggression.
Although other studies have found that baseline cortisol (Bohnke et al. 2010a) and impulsivity (Cherek et al. 1997; Martin et al. 1994) were both predictors of aggressive behavior, these relationships were not significant in the present sample. The lack of a relationship between aggression and baseline cortisol may have been due to measurement time, as the first saliva sample was taken at 8:30 a.m. Peak levels of cortisol are typically present immediately after waking (Hellhammer et al. 2007), and by measuring at 8:30 a.m., subjects likely were at different phases in their diurnal cycle. This added variability limited predictive value. A meta-analysis suggested that basal cortisol has a small effect size for aggressive behavior (Alink et al. 2008). Accordingly, our sample size may not have been large enough to detect an effect. Similarly, the lack of a relationship between impulsivity and aggression could be a result of the tool used to measure impulsivity (i.e., BIS-11). A recent study examining the impulsivity in a group diagnosed with antisocial personality disorder similarly reported no significant relationship between BIS-11 and aggression but did find a relationship between aggression and behavioral tasks that measured impulsivity (Swann et al. 2011). As Swann et al. (2011) reported that inconsistencies between the relationship between impulsivity and aggression may depend on the type of measurement used (e.g., psychometric or behavioral), it is plausible that each measurement only captures a portion of the variability in the underlying impulsiveness in the sample. The lack of a relationship between impulsivity and aggression in the present sample may have resulted from the measurement tools employed.
Limitations of this study include contamination of cortisol doses, use of a relatively small sample composed mostly of males, and measurement time for baseline cortisol. Cortisol dose contamination stemmed from participants ingesting cortisol orally and also providing samples orally, via saliva. If the capsules had any cortisol on them when being swallowed, it could remain in the saliva. As the contamination was due to methodological problems (Raff and Singh 2012), not due to characteristics of participants with contaminated samples, contamination can be considered a random event that does not bias the sample. Therefore, the participants who had contaminated samples were removed from measures of HPA-axis reactivity but were retained in the overall sample. The low number of female participants prevented a valid gender analysis. The effect of gender is important, as females exposed to maltreatment are more likely to develop antisocial externalizing behavior problems than males (Herrera and McCloskey 2001). Future research utilizing larger samples of females will be important, as abuse and neglect may have unique or more robust effects on female development, including risk for aggressive behavior (De Bellis 2005; Herrera and McCloskey 2001). Gender by HPA-axis interactions may reveal differential outcomes in maltreatment. Lastly, baseline cortisol was measured beginning at 8:30 a.m., and we were unable to experimentally regulate time of awakening. Obtaining cortisol samples in the morning may have also introduced more variance into the measurements, as morning cortisol release is relatively more volatile than afternoon cortisol release. Future studies may obtain more stable measurements of cortisol by having subjects provide samples at home upon awakening or conducting study procedures in the afternoon.
This study is novel in investigating the cycle of violence and the mediating effects of HPA-axis in child abuse and adult aggression in humans. The results suggest that abuse and neglect present a significant risk factor for aggressive behavior and that a (perhaps modest) portion of this risk may be conferred by changes in HPA-axis reactivity. Additionally, psychopathic traits present a significant risk factor that may operate in concert with childhood maltreatment to confer additional risk for aggressive and violent behavior. Overall, the four-factor model predicted a significant portion of the variance in both state and trait total aggression and suggested that the pathways represented in the model have a moderate effect size. The outcomes should encourage replication and extension of research on the processes involved in the cycle of violence, especially as it relates to psychopathic traits and HPA-axis function. Research using different or complementary methodologies may provide further understanding of key factors in the cycle of violence and their interactions. Ultimately, better understanding may translate into successful prevention and treatment strategies for abused and neglected youth.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the contributions of Nuvan Rathnayaka, Ellen Desmarais, Tara Watts, and Sarly Vasquez to this research project. Additional thanks are due to Hershel Raff of Aurora St. Luke’s Medical Center, Milwaukee, WI, who analyzed the saliva samples for the cortisol data, Martin Paulus who looked at an earlier version of this manuscript and Don R. Cherek for his lasting influence over the work in our laboratory. This project was supported by NIH/NIDA grants DA 003166 and P50 09262.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00213-013-2992-1) contains supplementary material, which is available to authorized users.
Contributor Information
Joshua L. Gowin, Program in Neuroscience, Graduate School of Biomedical Sciences, University of Texas Health Science Center, Houston, TX, USA University of California San Diego, 8939 Villa La Jolla Dr., Suite 263, La Jolla, CA 92037, USA.
Charles E. Green, Department of Psychiatry & Behavioral Sciences, School of Medicine, University of Texas Health Science Center, Houston, TX, USA Center for Clinical Research & Evidence Based Medicine, University of Texas Health Science Center, Houston, TX, USA.
Joseph L. Alcorn, III, Program in Neuroscience, Graduate School of Biomedical Sciences, University of Texas Health Science Center, Houston, TX, USA.
Alan C. Swann, Program in Neuroscience, Graduate School of Biomedical Sciences, University of Texas Health Science Center, Houston, TX, USA Department of Psychiatry & Behavioral Sciences, School of Medicine, University of Texas Health Science Center, Houston, TX, USA.
F. Gerard Moeller, Program in Neuroscience, Graduate School of Biomedical Sciences, University of Texas Health Science Center, Houston, TX, USA; Department of Psychiatry & Behavioral Sciences, School of Medicine, University of Texas Health Science Center, Houston, TX, USA.
Scott D. Lane, Program in Neuroscience, Graduate School of Biomedical Sciences, University of Texas Health Science Center, Houston, TX, USA Department of Psychiatry & Behavioral Sciences, School of Medicine, University of Texas Health Science Center, Houston, TX, USA.
References
- Alink LR, van Ijzendoorn MH, Bakermans-Kranenburg MJ, Mesman J, Juffer F, Koot HM. Cortisol and externalizing behavior in children and adolescents: mixed meta-analytic evidence for the inverse relation of basal cortisol and cortisol reactivity with externalizing behavior. Dev Psychobiol. 2008;50:427–450. doi: 10.1002/dev.20300. [DOI] [PubMed] [Google Scholar]
- Arseneault L, Moffitt TE, Caspi A, Taylor PJ, Silva PA. Mental disorders and violence in a total birth cohort: results from the Dunedin Study. Arch Gen Psychiatry. 2000;57:979–986. doi: 10.1001/archpsyc.57.10.979. [DOI] [PubMed] [Google Scholar]
- Assmann SF, Pocock SJ, Enos LE, Kasten LE. Subgroup analysis and other (mis)uses of baseline data in clinical trials. Lancet. 2000;355:1064–1069. doi: 10.1016/S0140-6736(00)02039-0. [DOI] [PubMed] [Google Scholar]
- Baron RA, Richardson DR. Human aggression. Plenum Press; New York: 1994. [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable dis-tinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bernstein D, Fink L. Childhood Trauma Questionnaire. Pearson; San Antonio: 1998. [Google Scholar]
- Bernstein D, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. 1994;151:1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Bohnke R, Bertsch K, Kruk MR, Naumann E. The relationship between basal and acute HPA axis activity and aggressive behavior in adults. J Neural Transm. 2010a;117:629–637. doi: 10.1007/s00702-010-0391-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnke R, Bertsch K, Kruk MR, Richter S, Naumann E. Exogenous cortisol enhances aggressive behavior in females, but not in males. Psychoneuroendocrinology. 2010b;35:1034–1044. doi: 10.1016/j.psyneuen.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Buss AH, Perry M. The aggression questionnaire. J Pers Soc Psychol. 1992;63:452–459. doi: 10.1037//0022-3514.63.3.452. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Cherek DR, Lane SD. Acute effects of D-fenfluramine on simultaneous measures of aggressive escape and impulsive responses of adult males with and without a history of conduct disorder. Psychopharmacology (Berl) 2001;157:221–227. doi: 10.1007/s002130100812. [DOI] [PubMed] [Google Scholar]
- Cherek DR, Pietras CJ, Lane SD. Laboratory measures: point subtraction aggression paradigm (PSAP) In: Coccaro EF, editor. Aggression: assessment and treatment. Marcel Dekker; New York: 2003. pp. 215–228. [Google Scholar]
- Cherek DR, Tcheremissine OV, Lane SD. Psychopharmacology of aggression. In: Nelson RJ, editor. Biology of Aggression. Oxford University Press; Oxford: 2006. pp. 424–446. [Google Scholar]
- Cherek DR, Moeller FG, Schnapp W, Dougherty DM. Studies of violent and nonviolent male parolees: I. Laboratory and psychometric measurements of aggression. Biol Psychiatry. 1997;41:514–522. doi: 10.1016/s0006-3223(96)00059-5. [DOI] [PubMed] [Google Scholar]
- Cima M, Smeets T, Jelicic M. Self-reported trauma, cortisol levels, and aggression in psychopathic and non-psychopathic prison inmates. Biol Psychol. 2008;78:75–86. doi: 10.1016/j.biopsycho.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Kavoussi RJ, Sheline YI, Berman ME, Csernansky JG. Impulsive aggression in personality disorder correlates with platelet 5-HT2A receptor binding. Neuropsychopharmacology. 1997;16:211–216. doi: 10.1016/S0893-133X(96)00194-7. [DOI] [PubMed] [Google Scholar]
- Cohen P, Brown J, Smaile E. Child abuse and neglect and the development of mental disorders in the general population. Dev Psychopathol. 2001;13:981–999. [PubMed] [Google Scholar]
- Corstorphine E, Waller G, Lawson R, Ganis C. Trauma and multi-impulsivity in the eating disorders. Eat Behav. 2007;8:23–30. doi: 10.1016/j.eatbeh.2004.08.009. [DOI] [PubMed] [Google Scholar]
- De Bellis MD. The psychobiology of neglect. Child Maltreat. 2005;10:150–172. doi: 10.1177/1077559505275116. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Baum AS, Birmaher B, Keshavan MS, Eccard CH, Boring AM, Jenkins FJ, Ryan ND. A.E. Bennett Research Award. Developmental traumatology. Part I: Biological stress systems. Biol Psychiatry. 1999;45:1259–1270. doi: 10.1016/s0006-3223(99)00044-x. [DOI] [PubMed] [Google Scholar]
- Fang X, Brown DS, Florence CS, Mercy JA. The economic burden of child maltreatment in the United States and implications for prevention. Child Abuse Negl. 2012;36:156–165. doi: 10.1016/j.chiabu.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. SCID-II Personality Questionnaire. American Psychiatric Press; Washington, DC: 1997. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Biometrics Research. New York State Psychiatric Institute; New York: 2002. Structured clinical interview for DSM-IV-TR axis I disorders. [Google Scholar]
- Glenn AL, Raine A, Schug RA, Gao Y, Granger DA. Increased testosterone-to-cortisol ratio in psychopathy. J Abnorm Psychol. 2011;120:389–399. doi: 10.1037/a0021407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowin JL, Swann AC, Moeller FG, Lane SD. Zolmitriptan and human aggression: interaction with alcohol. Psychopharmacology (Berl) 2010;210:521–531. doi: 10.1007/s00213-010-1851-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham N, Kimonis ER, Wasserman AL, Kline SM. Associations among childhood abuse and psychopathy facets in male sexual offenders. Personal Disord. 2012;3:66–75. doi: 10.1037/a0025605. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Morison SJ, Chisholm K, Schuder M. Salivary cortisol levels in children adopted from Romanian orphanages. Dev Psychopathol. 2001;13:611–628. doi: 10.1017/s095457940100311x. [DOI] [PubMed] [Google Scholar]
- Hare RD, Neumann CS. Psychopathy as a clinical and empirical construct. Annu Rev Clin Psychol. 2008;4:217–246. doi: 10.1146/annurev.clinpsy.3.022806.091452. [DOI] [PubMed] [Google Scholar]
- Harlow C. Prior abuse reported by inmates and probationers. U.S. Department of Justice, Office of Justice Programs; Washington DC: 1999. [Google Scholar]
- Hawes DJ, Brennan J, Dadds MR. Cortisol, callous-unemotional traits, and pathways to antisocial behavior. Curr Opin Psychiatry. 2009;22:357–362. doi: 10.1097/YCO.0b013e32832bfa6d. [DOI] [PubMed] [Google Scholar]
- Hellhammer J, Fries E, Schweisthal OW, Schlotz W, Stone AA, Hagemann D. Several daily measurements are necessary to reliably assess the cortisol rise after awakening: state- and trait components. Psychoneuroendocrinology. 2007;32:80–86. doi: 10.1016/j.psyneuen.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Herrera VM, McCloskey LA. Gender differences in the risk for delinquency among youth exposed to family violence. Child Abuse Negl. 2001;25:1037–1051. doi: 10.1016/s0145-2134(01)00255-1. [DOI] [PubMed] [Google Scholar]
- Jaffee SR, Caspi A, Moffitt TE, Taylor A. Physical maltreatment victim to antisocial child: evidence of an environmentally mediated process. J Abnorm Psychol. 2004;113:44–55. doi: 10.1037/0021-843X.113.1.44. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Blackson TC, Vanyukov MM, Moss HB, Tarter RE. Aggressivity, inattention, hyperactivity, and impulsivity in boys at high and low risk for substance abuse. J Abnorm Child Psychol. 1994;22:177–203. doi: 10.1007/BF02167899. [DOI] [PubMed] [Google Scholar]
- Mathieu C, Hare RD, Jones DN, Babiak P, Neumann CS. Factor structure of the B-scan 360: a measure of corporate psychopathy. Psychol Assess (in press) 2012 doi: 10.1037/a0029262. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. Am J Psychiatry. 2001;158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Nouvion SO, Cherek DR, Lane SD, Tcheremissine OV, Lieving LM. Human proactive aggression: association with personality disorders and psychopathy. Aggress Behav. 2007;33:552–562. doi: 10.1002/ab.20220. [DOI] [PubMed] [Google Scholar]
- O'Leary MM, Taylor J, Eckel L. Psychopathic personality traits and cortisol response to stress: the role of sex, type of stressor, and menstrual phase. Horm Behav. 2010;58:250–256. doi: 10.1016/j.yhbeh.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Paulhus DL, Hemphill JF, Hare RD. Scoring manual for the Hare self-report psychopathy scale-III. 2009 [Google Scholar]
- Pocock SJ, Assmann SE, Enos LE, Kasten LE. Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: current practice and problems. Stat Med. 2002;21:2917–2930. doi: 10.1002/sim.1296. [DOI] [PubMed] [Google Scholar]
- Project MATCH Research Group Project MATCH: rationale and methods for a multisite clinical trial matching patients to alcoholism treatment. Alcohol Clin Exp Res. 1993;17:1130–1145. doi: 10.1111/j.1530-0277.1993.tb05219.x. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Raff H, Singh RJ. Measurement of late-night salivary cortisol and cortisone by LC-MS/MS to assess preanalytical sample contamination with topical hydrocortisone. Clin Chem. 2012;58:947–948. doi: 10.1373/clinchem.2012.182717. [DOI] [PubMed] [Google Scholar]
- Raff H, Homar PJ, Skoner DP. New enzyme immunoassay for salivary cortisol. Clin Chem. 2003;49:203–204. doi: 10.1373/49.1.203. [DOI] [PubMed] [Google Scholar]
- Reidy DE, Zeichner A, Seibert LA. Unprovoked aggression: effects of psychopathic traits and sadism. J Pers. 2011;79:75–100. doi: 10.1111/j.1467-6494.2010.00691.x. [DOI] [PubMed] [Google Scholar]
- Schlosser N, Wolf OT, Fernando SC, Riedesel K, Otte C, Muhtz C, Beblo T, Driessen M, Lowe B, Wingenfeld K. Effects of acute cortisol administration on autobiographical memory in patients with major depression and healthy controls. Psychoneuroendocrinology. 2010;35:316–320. doi: 10.1016/j.psyneuen.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Shenk CE, Noll JG, Putnam FW, Trickett PK. A prospective examination of the role of childhood sexual abuse and physiological asymmetry in the development of psychopathology. Child Abuse Negl. 2010;34:752–761. doi: 10.1016/j.chiabu.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley WC, Gruber CP, Martin TA, Klein AM. Western Psychological Services. Los Angeles; 2009. Shipley-II: manual. [Google Scholar]
- Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. In: Leinhardt S, editor. Sociological methodology. American Sociological Association; Washington, DC: 1982. pp. 290–312. [Google Scholar]
- Swann AC, Lijffijt M, Lane SD, Steinberg JL, Moeller FG. Interacting mechanisms of impulsivity in bipolar disorder and antisocial personality disorder. J Psychiatr Res. 2011;45:1477–1482. doi: 10.1016/j.jpsychires.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn L, Evans P, Cannon A, Hucklebridge F, Clow A. Seasonal differences in the diurnal pattern of cortisol secretion in healthy participants and those with self-assessed seasonal affective disorder. Psychoneuroendocrinology. 2011;36:816–823. doi: 10.1016/j.psyneuen.2010.11.003. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services . Child maltreatment in administration for children YaF, Children's Bureau edn. U.S. Department of Health and Human Services; Washington DC: 2010. [Google Scholar]
- Veenema AH. Early life stress, the development of aggression and neuroendocrine and neurobiological correlates: what can we learn from animal models? Front Neuroendocrinol. 2009;30:497–518. doi: 10.1016/j.yfrne.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Widom CS. The cycle of violence. Science. 1989;244:160–166. doi: 10.1126/science.2704995. [DOI] [PubMed] [Google Scholar]
- Widom CS, Czaja SJ, Bentley T, Johnson MS. A prospective investigation of physical health outcomes in abused and neglected children: new findings from a 30-year follow-up. Am J Public Health. 2012;102:1135–1144. doi: 10.2105/AJPH.2011.300636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary RA, Paulson MJ, Gorsuch RL. Estimating WAIS IQ from the Shipley Institute of Living Scale using continuously adjusted age norms. J Clin Psychol. 1985;41:820–831. doi: 10.1002/1097-4679(198511)41:6<820::aid-jclp2270410616>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.