Abstract
Background
The Candida species are the most important factors of fungal infections in humans and animals. It is necessary to prepare antifungal or antimicrobial drugs because of increasing drug resistance. The natural treatment of diseases of bacterial origin using medicinal plants is important. In this study the effect of antimicrobial medicinal herbal essential oils and conventional antifungal drugs were evaluated on Candida albicans in vitro.
Methods
Disc diffusion assay and the microbroth dilution method were used to investigate the anticandidal effects of Foeniculum vulgare Mill, Satureja hortensis L, Cuminum cyminum, and Zataria multiflora Boiss essential oils. The anticandidal effect of these essential oils was compared with that of amphotricin B and ketoconazole in vitro. We then measured the chemical composition of the studied essential oils using gas chromatography–mass spectroscopy.
Results
Z. multiflora Boiss essential oil at the minimum inhibitory concentration (MIC) of 34 μg/mL and minimal lethal concentration [i.e., minimal fungicidal concentration (MFC)] of 64 μg/mL had more powerful anti-Candida activity than the other essential oils. C. cyminum essential oil showed the least effect on the tested fungus. A comparison of the effect of the studied essential oils and antifungal drugs showed that the antifungal effect on the C. albicans fungus was better with the fungicides than with the essential oils.
Conclusion
In the present study, essential oils with different components showed antifungal activity (especially Z. multiflora Boiss essential oil). They can therefore be used as new antifungal substances.
Keywords: Candida albicans, disc diffusion assay, gas chromatograph–mass spectroscopy, herbal essential oil, microbroth dilution method
1. Introduction
Medicinal plants are valuable in providing health care and preventing diseases. These natural resources have been a very important source of human food and medicine throughout the generations.1, 2 Clinical microbiologists are interested in using these drugs to treat infections because the adverse effects of these drugs are remarkably low, compared to chemical drugs. In recent decades, an essential factor of fungal infections in humans and animals has been infections due to opportunistic Candida fungi (e.g., Candida albicans); and these diseases are occurring at an extremely increasing rate.3, 4 These infections are more common in people who have underlying risk factors such as cancer, leukemia, diabetes mellitus, long-term antibiotic and corticosteroid treatment, human immunodeficiency virus (HIV), pregnancy, scorch, and transplant. The range of these infections varies from colonization of the mucosa to invasive deadly infections. Among the different clinical forms of Candida infections, cutaneous candidiasis and mucosal candidiasis are most prevalent.5 One of the most important organisms is C. albicans, which causes infections such as oral thrush, vaginal candidiasis, and Candida onychomycosis infections of the nails.6
Limitations in the treatment of fungal diseases such as expense, few available antifungal drugs, adverse effects, and drug resistance have led to the search for new antifungal drugs, especially medicinal plants.7, 8 Foeniculum vulgare Mill is a native plant in Iran that is a biennial herbaceous plant from the Umbelliferae family. In traditional medicine, the seed of this plant is a carminative; it is also consumed as flavoring in candy, liquor, medicines, and food.9 To date, various studies have been performed on the antimicrobial effect of essential oil and anise extract.10 Another native plant in Iran is Satureja hortensis L. This annual herbaceous plant is in the family Labiatae.11 This plant has many applications in traditional medicine, and it has antimicrobial activity on some fungal strains because of its phenolic, thymol, and carvacrol compounds.12, 13
Another native plant in Iran is Zataria multiflora Boiss. It has been used in traditional medicine as a healing plant to treat digestive diseases and different infections. Its essence comprises compounds such as thymol, carvacrol, and 1,8-cineole. The essence has a higher antimicrobial property than each of its compounds, which shows the synergistic effect of the compounds. Because of its antimicrobial property, this essence has also been used in food materials.2, 12, 14
Cuminum cyminum, a fragrant annual herb from the Umbelliferae family, is another plant used in this project. This herb has been used as a medicine with antibacterial and antispasm effects.15, 16 In this study, the antibacterial activity of S. hortensis L, F. vulgare Mill, C. cyminum, and Z. multiflora Boiss were evaluated against C. albicans.
2. Methods
2.1. Origin and isolation of the essential oils
For this study, fresh aerial parts of the herbs F. vulgare Mill, S. hortensis L, C. cyminum, and Z. multiflora Boiss were collected from the Mazandaran, Lorestan, and Chaharmahal provinces in Iran in 2012. The herbs were dried at room temperature for 3 days. Dried herb samples (500 g) were ground and subjected to hydrodistillation by a Clevenger-type apparatus. The essential oils were dried over anhydrous sodium sulfate (Na2SO4) and stored at 4 °C in sealed amber vials, until the time of use.
2.2. Gas chromatography–mass spectrometry
Analysis was performed by gas chromatography–mass chromatography using a HP-5MS column (United States, Technology Agilent) (30 m × 0.25 mm, film thickness 0.25 m). Helium was used as the carrier gas at a flow rate of 0.8 mL/min. The column temperature was maintained at 50 °C for 2 minutes. It was programmed to 200 °C at a rate of 3 °C/min and remained constant at 200 °C for 10 minutes. The injection was performed in split mode at a ratio of 50:1 at 250 °C. The compounds were identified by a comparison of their relative retention indices (RRI) with those reported in the literature, and identified by a comparison of their mass spectra with published mass spectra.17, 18 The retention indices for all components were determined by the van Den Dool method using n-alkanes as the standards.19
2.3. Antifungal activity assays
2.3.1. Standard bacterial strains
The standard strain of Candida albicans (ATCC 10231) was used in this study. Lyophilized strains were prepared from the Traditional Medicine and Herbal Research Institute of Iran (Isfahan, Iran). They were then cultured on Sabouraud dextrose agar at 25 °C and incubated for 2 days.
2.3.2. Anti-Candida activity of herbal essential oils using agar diffusion methods
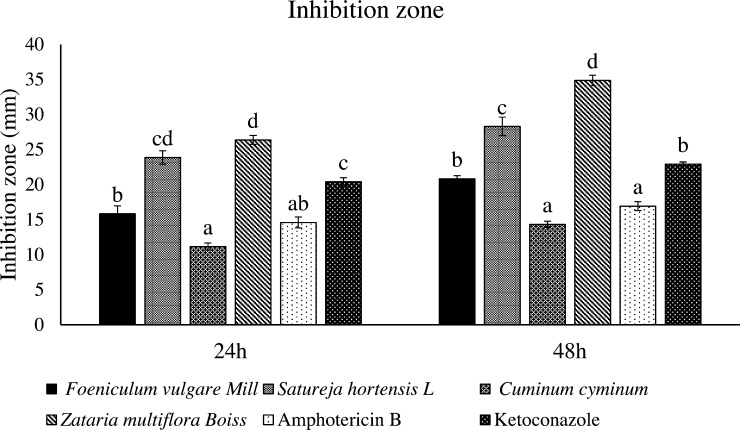
Sabouraud dextrose agar was used to examine the antimicrobial effects of the herbal essential oils of F. vulgare Mill, S. hortensis L, C. cyminum, and Z. multiflora Boiss on C. albicans. C. albicans was cultured for 48 hours prior to testing, and then two to three colonies were added to sterile saline. The turbidity was set to 0.5 McFarland [1 × 106 colony-forming units (CFU)/mL]. Using a sterile swab, the desired suspension was cultured on dextrose agar medium. It was used from a disc (6/4 mm) that contained essential oil concentrations of 0.625 μg/mL, 1.25 μg/mL, 2.5 μg/mL, 5 μg/mL, 10 μg/mL, 20 μg/mL, 40 μg/mL, 60 μg/mL, 80 μg/mL, 100 μg/mL, 200 μg/mL, 300 μg/mL, 400 μg/mL, and 500 μg/mL dissolved in dimethyl sulfoxide solvent. This disc was used to compare the effect of the herbal essential oils versus the effect of the amphotericin B (10 μg) discs and ketoconazole (15 μg) discs as the positive controls and the effect of a dimethyl sulfoxide-containing disc as the negative control (Fig. 1). The plates were incubated for 72 hours at 37 °C and the diameter of the inhibition zone was measured in millimeters at 24 hours, 48 hours, and 72 hours.20
Fig. 1.
Comparison of the effects of the herbal essential oils and antibiotics on the Candida albicans fungus using the disc method. The data are expressed as the mean ± the standard error. Different letters in each column indicate significant differences (p < 0.0001). The data are separately compared for 24 hours and 48 hours.
2.3.3. Detection of the minimum inhibitory concentration and minimal lethal concentration using the microbroth dilution method
Using the microbroth dilution method, the minimum inhibitory concentration (MIC) and minimal lethal concentration [i.e., minimal fungicidal concentration (MFC)] of amphotericin B and ketoconazole herbal essential oils were determined against Candida albicans. Herbal essential oils were diluted in dimethyl sulphoxide. Herbal essential oil concentrations of 1–400 μg/mL were prepared for each well. Sabouraud dextrose agar medium was used as the liquid medium. One hundred microliters of each dilution was added to each well of 96-well plate and microbial suspension (prepared as in the previous step) was diluted to a concentration of 104–105 CFU/mL; 100 mL was then added to each well. The plates were incubated at 35 °C for 24 hours.20 The first well in which there was no growth was the MIC. The MIC dilution and dilutions higher than the MIC were cultured (10 μL). The first dilution in which no growth had occurred in the environment was the MFC. Data analysis was performed with SPSS (version 20; SPSS Inc., Chicago, IL, USA) using one way analysis of variance (ANOVA) and Tukey's statistical comparison method.
3. Results
3.1. Antimicrobial efficiency
Because the results were similar at 48 hours and 72 hours, they were ignored and not entered into the Table 1. For each of the four essential oils, the concentration of 500 μg/mL was more effective than the lower concentrations (p < 0.0001). All essential oils had no effect on the fungi up to the concentration of 2.5 μg/mL. For the Z. multiflora Boiss essential oil, the least effective concentration on the fungi was the 5 μg/mL concentration. This concentration also had a better effect on the Candida fungi inhibition zone (IZ), compared to similar concentrations of all other tested essential oils. The 40 μg/mL concentration of C. cyminum essential oil had no effect on the Candida fungi IZ.
Table 1.
Antifungal activity of different concentrations of herbal essential oils using the disc diffusion method (i.e., zone of inhibition)
|
Foeniculum vulgare Mill |
Satureja hortensis L |
Cuminum cyminum |
Zataria multiflora Boiss |
|||||
|---|---|---|---|---|---|---|---|---|
| Concentration (μg/mL) | 24 | 48 | 24 | 48 | 24 | 48 | 24 | 48 |
| 0.63 | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| 1.25 | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| 2.50 | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| 5 | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.17 ± 0.17 a | 0.17 ± 0.17 a |
| 10 | 0.07 ± 0.07 a | 0.07 ± 0.07 a | 0.17 ± 0.17 a | 0.40 ± 0.21 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 1.23 ± 0.20 a | 2.47 ± 0.44 a,b |
| 20 | 0.70 ± 0.21 a,b | 1.40 ± 0.23 a,b | 0.93 ± 0.18 a | 1.63 ± 0.32 a,b | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 3.17 ± 0.44 a,b | 4.43 ± 0.42 b |
| 40 | 1.10 ± 0.10 a,b | 1.93 ± 0.12 b | 1.77 ± 0.23 a | 3.67 ± 0.48 b | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 4.87 ± 0.13 b | 8.20 ± 0.61 c |
| 60 | 2.23 ± 0.28 b,c | 3.60 ± 0.32 c | 4.70 ± 0.70 b | 6.70 ± 0.30 c | 0.23 ± 0.12 a | 0.23 ± 0.12 a | 9.83 ± 0.44 c | 11.80 ± 0.76 d |
| 80 | 4.20 ± 0.20 cd | 6.40 ± 0.23 d | 6.90 ± 0.38 b,c | 10.57 ± 0.38 d | 1.07 ± 0.12 a | 1.50 ± 0.29 a,b | 14.00 ± 0.29 d | 16.40 ± 0.38 e |
| 100 | 5.60 ± 0.38 d | 8.13 ± 0.47 e | 8.67 ± 0.42 c | 12.37 ± 0.35 d | 2.40 ± 0.21 b | 3.77 ± 0.28 c | 20.23 ± 1.48 e | 23.37 ± 0.33 f |
| 200 | 8.00 ± 0.21 e | 12.30 ± 0.47 f | 13.50 ± 0.81 d | 16.20 ± 0.61 e | 3.67 ± 0.49 c | 6.73 ± 0.27 d | 20.20 ± 0.64 e | 25.87 ± 0.58 f,g |
| 300 | 11.33 ± 0.70 f | 14.57 ± 0.2 g | 16.80 ± 0.15 e | 20.43 ± 0.73 f | 7.20 ± 0.25 d | 8.80 ± 0.42 e | 22.57 ± 1.33 ef | 27.70 ± 1.8 g,h |
| 400 | 12.80 ± 0.40 f | 17.27 ± 0.50 h | 20.20 ± 0.40 f | 24.43 ± 0.6 g | 10.50 ± 0.29 e | 12.40 ± 0.61f | 25.60 ± 0.7 f,g | 29.80 ± 0.40 h |
| 500 | 15.83 ± 1.1 g | 20.83 ± 0.44 i | 23.87 ± 0.9 g | 28.30 ± 1.33 h | 11.17 ± 0.49 e | 14.33 ± 0.4 g | 26.37 ± 0.6 g | 34.87 ± 0.73 i |
Data are presented as mean ± SE.
a-i Different letters on every column represent meaningful difference (p < 0.0001).
Based on Table 1, the best effective concentration, 500 μg/mL, was selected for each herbal essential oil IZ. This IZ was compared with two synthetic fungicides at concentrations of 10 μg/mL and 15 μg/mL at 24 hours and 48 hours. For all testing hours, the 500 μg/mL concentration of the S. hortensis L. and Z. multiflora Boiss essential oils had a better effect on the IZ, compared to the other essential oils and antibiotics. Among the antibiotics, ketoconazole performed better than other antibiotics, based on the IZ, at all time intervals. As a result, Z. multiflora Boiss essential oil had the best performance and C. cyminum herbal essential oil had the lowest performance.
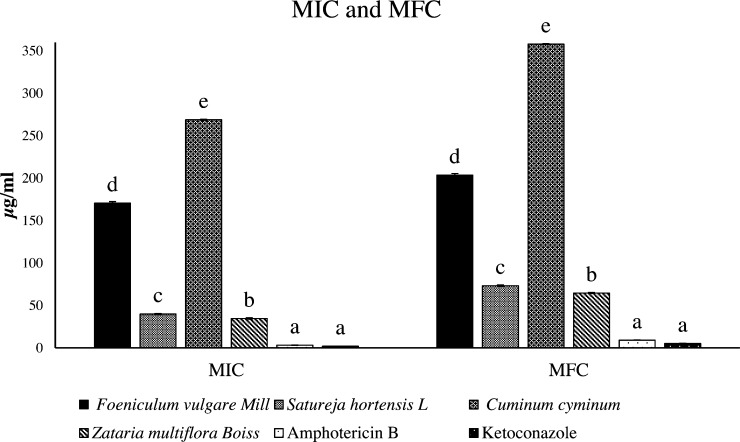
The evaluation of MIC and MFC of the tested essential oils and fungicides showed that all four essential oils (which were at a higher dosage rate than the fungicides) had an effect on C. albicans (Fig. 2). This indicates that the tested fungicides had a better MIC and MFC on C. albicans. The two traits (i.e., MIC and MFC) of the two fungicides showed no significant difference. Among the essential oils, Z. multiflora Boiss had the best MIC and MFC, and C. cyminum essential oil had the lowest effect on MIC and MFC.
Fig. 2.
Comparison of minimum inhibitory concentration (MIC) and minimum lethal concentration (MFC) of herbal essential oils and antibiotics on the fungus Candida. The data are expressed as the mean ± the standard error. Different letters in each column indicate significant differences (p < 0.0001). The data are separately compared for 24 hours and 48 hours.
3.2. Analysis of the effective compositions of herbal plants
Results from gas chromatography–mass chromatography revealed that Z. multiflora Boiss possesses 34 compounds, the greatest proportion of which are thymol (33.05%), carvacrol (25.88%), and p-cymene (11.34%). C. cyminum contains 24 recognized compounds, which include cuminic alcohol (30.32%), γ-terpinene (25.32%), β-pinene (15.94%), cuminic alcohol (11.15%), and p-cymene (6.22%) (Table 2). S. hortensis L has 20 recognized compounds, which include carvacrol (32.38%), γ-terpinene (31.96%), and p-cymene (6.62%). F. vulgare Mill has 17 recognized compounds, which include anethole (68.62%), fenchone (12.08%), and limonene (6.30%) (Table 3).
Table 2.
The composition of Cuminum cyminum and Zataria multiflora Boiss
|
Cuminum cyminum |
Zataria multiflora Boiss |
||||||
|---|---|---|---|---|---|---|---|
| No. | Composition | % | RI | No | Composition | % | RI |
| 1 | α-Thujene | 0.39 | 929 | 1 | α-Thujene | 0.34 | 931 |
| 2 | α-Pinene | 1.04 | 941 | 2 | α-Pinene | 3.88 | 937 |
| 3 | Sabinene | 1.13 | 974 | 3 | Camphene | 0.18 | 951 |
| 4 | β-Pinene | 15.94 | 978 | 4 | Verbenene | 0.02 | 956 |
| 5 | β-Myrcene | 1.11 | 988 | 5 | Sabinene | 0.02 | 974 |
| 6 | α-Phellandrene | 0.96 | 1006 | 6 | β-Pinene | 0.68 | 979 |
| 7 | Δ-3-Carene | 0.06 | 1011 | 7 | β-Myrcene | 0.68 | 993 |
| 8 | α-Terpinene | 0.23 | 1016 | 8 | α-Phellandrene | 0.11 | 1007 |
| 9 | p-Cymene | 6.22 | 1028 | 9 | Δ-3-Carene | 0.04 | 1012 |
| 10 | 1,8-Cineole | 0.2 | 1030 | 10 | α-Terpinene | 1.32 | 1016 |
| 11 | β-Phellandrene | 0.84 | 1032 | 11 | p-Cymene | 11.34 | 1025 |
| 12 | γ-Terpinene | 25.32 | 1056 | 12 | Limonene | 0.67 | 1032 |
| 13 | α-Terpinolene | 0.08 | 1082 | 13 | 1,8-Cineole | 0.55 | 1030 |
| 14 | Linalool | 0.11 | 1098 | 14 | γ-Terpinene | 4.73 | 1057 |
| 15 | cis-Sabinene hydrate | 0.06 | 1100 | 15 | trans-Sabinene hydrate | 0.27 | 1087 |
| 16 | Terpin-4-ol | 0.22 | 1173 | 16 | Linalool | 1.46 | 1098 |
| 17 | α-Terpienol | 0.05 | 1186 | 17 | Borneol | 0.37 | 1162 |
| 18 | Cuminic aldehyde | 11.15 | 1225 | 18 | Terpinen-4-ol | 0.82 | 1186 |
| 19 | Safranal | 2.91 | 1274 | 19 | α-Terpineol | 0.67 | 1191 |
| 20 | Cuminic alcohol | 30.32 | 1282 | 20 | Carvacrol methyl ether | 0.77 | 1239 |
| 21 | γ-Elemene | 0.09 | 1394 | 21 | Carvol | 0.77 | 1239 |
| 22 | Myrtenol | 0.14 | 1402 | 22 | trans-Anethole | 2.46 | 1281 |
| 23 | β-Caryophyllene | 0.08 | 1412 | 23 | Thymol | 33.05 | 1285 |
| 24 | trans-β-Farnesene | 0.1 | 1427 | 24 | Carvacrol | 25.88 | 1297 |
| 25 | Thymyl acetate | 1.03 | 1311 | ||||
| 26 | Carvacryl acetate | 0.69 | 1371 | ||||
| 27 | β-Caryophyllene | 1.83 | 1412 | ||||
| 28 | Aromadendrene | 0.84 | 1437 | ||||
| 29 | α-Humulene | 0.09 | 1443 | ||||
| 30 | Germacrene-d | 0.13 | 1473 | ||||
| 31 | Ledene | 0.77 | 1491 | ||||
| 32 | cis-α-Bisabolene | 0.09 | 1537 | ||||
| 33 | (+) spathulenol | 0.24 | 1579 | ||||
| 34 | Caryophyllene oxide | 0.15 | 1589 | ||||
| Total 98.75 | Total 96.94 | ||||||
RI, Retention Index.
Table 3.
The composition of Foeniculum vulgare Mill and Satureja hortensis L
|
Foeniculum vulgare Mill |
Satureja hortensis L |
||||||
|---|---|---|---|---|---|---|---|
| No. | Composition | % | RI | No. | Composition | % | RI |
| 1 | α-Thujene | 0.08 | 931 | 1 | α-Thujene | 0.88 | 931 |
| 2 | Camphene | 0.15 | 951 | 2 | α-Pinene | 1.32 | 937 |
| 3 | Sabinene | 0.33 | 974 | 3 | Camphene | 0.14 | 951 |
| 4 | β-Pinene | 0.09 | 979 | 4 | Sabinene | 0.07 | 974 |
| 5 | β-Myrcene | 0.53 | 993 | 5 | β-Pinene | 0.57 | 979 |
| 6 | α-Phellandrene | 0.23 | 1007 | 6 | β-Myrcene | 1.45 | 993 |
| 7 | α-Terpinene | 0.14 | 1016 | 7 | α-Phellandrene | 0.39 | 1007 |
| 8 | p-Cymene | 0.28 | 1025 | 8 | Δ-3-Carene | 0.1 | 1012 |
| 9 | Limonene | 6.3 | 10.32 | 9 | α-Terpinene | 4.31 | 1016 |
| 10 | β-Ocimene Z | 0.91 | 10.38 | 10 | p-Cymene | 6.62 | 1025 |
| 11 | γ-Terpinene | 1.35 | 10.57 | 11 | Limonene | 1.63 | 1032 |
| 12 | Fenchone | 12.08 | 1089 | 12 | 1,8-Cineole | 0.25 | 1030 |
| 13 | Camphor | 0.27 | 1143 | 13 | β-Ocimene Z | 0.15 | 1038 |
| 14 | Anisole, p-allyl or methyl chavicol | 3.76 | 1200 | 14 | γ-Terpinene | 31.96 | 1057 |
| 15 | Fenchyl acetate | 0.15 | 1216 | 15 | α-Thujone | 2.17 | 1087 |
| 16 | Anethole | 68.62 | 1251 | 16 | Borneol | 0.3 | 1062 |
| 17 | Thymol | 1.81 | 1285 | 17 | α-Terpineol | 0.22 | 1086 |
| 18 | Thymol | 9.96 | 1285 | ||||
| 19 | Carvacrol | 32.38 | 1285 | ||||
| 20 | β-Caryophyllene | 0.26 | 1412 | ||||
| Total 97.94 | Total 94.25 | ||||||
RI, Retention Index.
4. Discussion
For the past few decades, immunosuppressive diseases (e.g., acquired immunodeficiency syndrome and various hematologic malignancies) and the excessive consumption of antibiotics and corticosteroids are two of the most important causes of mortality, particularly for patients admitted to hospitals.21, 22 The incidence of fungal infections has led to the increased use of antifungal drugs and to a significant increase in the acquired resistance of the Candida species to the available compounds. Because of the increasing resistance to antifungal drugs, researchers are finding new compounds of natural origin with microorganism-inhibitory properties. In recent years many researchers have reported the antimicrobial effects of various plants.23, 24 This study showed that Z. multiflora Boiss and S. hortensis L herbal essential oils had the highest inhibitory effect on C. albicans strains. The MIC and MFC of Z. multiflora Boiss herbal essential oil were 34 μg/mL and 64 μg/mL, respectively. In 2008, Maksimovic and colleagues25 reported that the MIC rate was 50 μg/mL in Pannonicus thymus. In 2011, Al-Maqtari et al26 studied the effects of Thymus vulgaris essential oil on C. albicans and Candida vaginalis; the rates of MIC were reportedly 80 μg/mL and 97 μg/mL, respectively. The amount of thymol and carvacrol was 51.34% and 2.03%, respectively.26 A comparison indicated that Z. multiflora Boiss essential oil in Al-Maqtari's study had a better effect than the Thymus vulgaris essential oil used in this study. The inhibitory concentrations and antimicrobial effect of this essential oil may be because of a difference in the percentage of thymol and carvacrol—the carvacrol percentage used in our study was higher than the used percentage in Al- Maqtari et al's26 study.
Our study revealed that Z. multiflora exhibits a stronger inhibitory property on C. albicans in comparison to the other extracts. This higher inhibitory property is because of the effective compounds in the plant. In 2012, Shokri et al27 examined the effect of Z. multiflora extract on Candida zeylanoides; they reported an IZ of 40.8 mm by thymol (25.05%), carvacrol (61%), and p-cymene (2%).27 In our study, the IZ for Z. multiflora at the 500 μg/mL concentration was 26.37 mm at 24 hours and 34.87 mm at 48 hours. Our results were thus compatible with those of the Shokri et al27 study. In 2004, Shahidi Bonjar28 examined the effect of Thymus vulgaris extract on C. albicans. The MIC was reportedly 640 μg/mL. The comparison of our results with those of Shahidi Bonjar28 showed that the Z. multiflora extract, compared with the other tested extracts, possessed a better effect in controlling C. albicans. This was because Z. multiflora had higher amounts of the effective compounds, compared to T. vulgaris. In our study, the MIC and MFC of S. hortensis L herbal essential oil were 40 μg/mL and 73 μg/mL, respectively. Studies that analyzed the Thyme and Satureja species essential oil chemical compounds show many similarities between the two groups.29 Determination of the antibacterial and antifungal effect of thyme and savory variations could help in better understanding these plants and lead to better productivity and selection of valuable plant species as sources of natural antimicrobial substances.12 In 2003, Sahin et al30 determined that S. hortensis L herbal essential oil has antifungal activity. In that study, the effect of S. hortensis herbal essential oil was examined on C. albicans; its MIC was 300 μg/mL. In a 2010 study, Zarrin et al31 examined the effect of Satureja khuzestanica essential oil on C. albicans; its rate of MIC was 100 μg/mL. A comparison of our results with those of Zarrin et al31 showed that our tested essential oil had an inhibitory effect on C. albicans at a lower concentration. This is because of the resistance by the microorganisms or because of differences in the effective components in these herbal essential oils. Another herb used in our research was F. vulgare Mill, which had an inhibitory effect on C. albicans strains, but had a weaker effect than that of Z. multiflora Boiss and S. hortensis L essential oils. In our study, the rate of MIC and MFC of F. vulgare Mill herbal essential oil was 170 μg/mL and 203 μg/mL, respectively. In 2008, Khosravi et al32 studied the effect of fennel seed essential oil on candidiasis; the MIC of this essential oil was 300 μg/mL. In a 2009 survey by Naeini et al,33 the effect of F. vulgare Mill essential oil was assessed on C. albicans; the MIC and MFC were obtained at 300 μg/mL and 308 μg/mL, respectively. A comparison of our results with those of Naeini indicate that the inhibitory effect of our tested essential oil was nearly at the same level, but our essential oil had a better inhibitory effect on C. albicans, compared to that reported in the Naeini et al33 study. In a 2013 study by Skrobonja et al34 on C. albicans (ATCC 10231), the IZ was 17.50 mm (5000 μg/mL). In our study, the IZ for F. vulgare at the concentration of 500 μg/mL was 15.83 ± 1.11 mm at 24 hours and 20.83 mm at 48 hours. A comparison of our results with those of Skrobonja et al34 shows that the F. vulgare extract in our study was more effective in controlling C. albicans. This could be because of the higher amounts of effective compounds such as thymol and limonene and other effective compounds in the plant. In this study, the lowest inhibitory effect was associated with C. cyminum herbal essential oil. The MIC and MFC were obtained at 269 μg/mL and 358 μg/mL, respectively. In 2014, Naeini et al35 examined the effect of C. cyminum on C. albicans (ATCC 14053) and Candida dubliniensis (ATCC CD60). In their study, the MIC was 289 mg/L A comparison between our results and those of Naeini et al35 demonstrated that C. cyminum herbal essential oil in our study possessed a higher inhibitory effect on C. albicans. This result could be because of the microbial resistance of C. albicans and C. dubliniensis and the presence of effective compounds in the plants. Pai and co worker36 in 2010 examined the effect of C. cyminum herbal essential oil on C. albicans. He observed an inhibitory effect on the fungi, but the effect was not significant. Similar to Mithum et al's36 study, C. cyminum herbal essential oil in our study had a weak inhibitory property on C. albicans. Antimicrobial properties of plants is generally because they contain phenolic compounds, saponins, and flavonoids; some of these factors are effective on the plasma membrane or inhibit cell membrane structural enzymes of microorganisms with antimicrobial properties.37, 38
5. Conclusion
There are increasing restrictions on the use of chemical antimicrobial substances such as adverse effects and drug resistance. Therefore, these ingredients need to be replaced with natural ingredients such as essential oils. Treating candidiasis that is resistant to common antifungal ingredients is a complex issue, based on some investigations. The common antifungal ingredients are limited in number, and they are toxic and expensive. Thus, the need to improve new antifungal ingredients and expand the range of activity against strains that are resistant to antifungal ingredients is significantly desirable. This can increase the use of herbal derivations.
The disk and MIC results of this study showed that the essential oils have antifungal activities. The results of this study showed that ketoconazole and amphotricin B have a better effect on pathogenic bacteria in comparison to the studied essential oils. However, further experiments in the future can be performed to discover and purify better compositions in the plants and study each of them in isolation. Because the herbal plants have long been used as natural ingredients with fewer adverse effects and because Iran is rich in Z. multiflora, further investigations on anticandidal properties of the plant are expected to be performed. Based on the acceptability of these plants, these essential oils can be used after undergoing toxicological tests and clinical trials for the treatment of Candida albicans infections.
Conflicts of interest
All authors declare no conflicts of interest.
References
- 1.Gavanji S., Larki B., Mohammadi E., Bakhtari A. Antimicrobial and cytotoxic evaluation of some herbal essential oils in comparison with common antibiotics in bioassay condition. Integr Med Res. 2014;3:142–152. doi: 10.1016/j.imr.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gavanji S., Larki B., Bakhtari A. The effect of extract of Punicagranatum var. pleniflora for treatment of minor recurrent aphthous stomatitis. Integr Med Res. 2014;3:83–90. doi: 10.1016/j.imr.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eggimann P., Garbino J., Pittet D. Management of Candida species infections in critically ill patients. Lancet Infect Dis. 2003;3:772–785. doi: 10.1016/s1473-3099(03)00831-4. [DOI] [PubMed] [Google Scholar]
- 4.Khan Z.U., Chandy R., Metwali K.E. Candida albicans strain carriage in patients and nursing staff of an intensive care unit: a study of morphotypes and resistotypes. Mycoses. 2003;46:476–486. doi: 10.1046/j.0933-7407.2003.00929.x. [DOI] [PubMed] [Google Scholar]
- 5.Anaissie E.J., McGinnis M.R., Pfaller M.A. 2nd ed. Elsevier Science; Churchill Livingstone, USA: 2003. Clinical Mycology; pp. 69–75. [Google Scholar]
- 6.Khosravi A.R., Eslami A., Shokri H., Kashanian M. Zataria multiflora cream for the treatment of acute vaginal candidiasis. Int J Gynaecol Obstet. 2008;101:201–202. doi: 10.1016/j.ijgo.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Klepser M.E. Antifungal resistance among Candida species. Pharmacother. 2001;21:124–132. doi: 10.1592/phco.21.12.124s.34511. [DOI] [PubMed] [Google Scholar]
- 8.Tavanti A., Campa D., Bertozzi A., Pardini G., Naglik J.R., Barale R. Candida albicans isolates with different genomic backgrounds display a differential response to macrophage infection. Microbes Infect. 2006;8:791–800. doi: 10.1016/j.micinf.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 9.Saeedi M., Ebrahimzadeh M.A., Morteza-Semnani K., Akha A., Rabiei K. Evaluation of antibacterial effect of ethanolic extract of Foeniculum vulgare Mill. J Mazand Univ Med Sci. 2010;20:88–91. [Google Scholar]
- 10.Soylu S., Soylu E.M., Evrendilek G.A. Chemical composition and antibacterial activity of essential oils of bitter fennel (Foeniculum vulgare) and dill (Anethum graveolens) against the growth of food-borne and seed-born pathogenic bacteria. Italian J Food Sci. 2009;21:347–355. [Google Scholar]
- 11.Mohammadpour M., Ghasemnejad A., Lebaschy M.H., Abbaszadeh B., Azadbakht M. Effects of sowing date and plant density on morphological characteristics and yield of summer savory (Satureja hortensis L.) Iranian J Med Arom Plants. 2013;29:621–634. [Google Scholar]
- 12.Mehrorosh H., Gavanji S., Larki B., Mohammadi M.D., Karbasiun A., Bakhtari A. Essential oil composition and antimicrobial screening of some Iranian herbal plants on Pectobacterium carotovorum. Global NEST J. 2014;16:240–250. [Google Scholar]
- 13.Gavanji S., Asgari M.J., Vaezi R., Larki B. Antifungal effect of the extract of propolis on the growth of three species of Epidermophyton flucosum, Trichophyton violaseum and Trichophyton tonsorans in laboratory environment. Afr J Pharm Pharmacol. 2011;5:2642–2646. [Google Scholar]
- 14.Simbar M., Azarba Z., Mojab F. A comparative study of the therapeutic effects of Zataria multiflora vaginal cream and metronidazole vaginal gel on bacterial vaginosis. Phytomedicine. 2008;15:1025–1031. doi: 10.1016/j.phymed.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Gachkar L., Yadegari D., Rezaei M.D., Rasooli I. Chemical and biological characteristics of Cuminum cyminum and Rosmarinus officinalis essential oils. Food Chemistry. 2007;102:898–904. [Google Scholar]
- 16.Moghtader M., Mansori A.I., Salari H., Farahmand A. Chemical composition and antimicrobial activity of the essential oil of Bunium persicum Boiss seed. J Med Arom Plants. 2009;25:20–28. [Google Scholar]
- 17.Adams R.P. Identification of essential oil components by gas chromatography-quadropole mass spectroscopy. J Am Soc Mass Spectrom. 2005;16:1902–1903. [Google Scholar]
- 18.Sparkman O.D. Identification of essential oil components by gas chromatography/quadrupole mass spectroscopy. J Am Soc Mass Spectrom. 2005;16:1902–1903. [Google Scholar]
- 19.van Den Dool H., Kratz P.D. A generalization of the retention index system including linear temperature programmed gas–liquid partition chromatography. J Chromatogr. 1963;11:463–471. doi: 10.1016/s0021-9673(01)80947-x. [DOI] [PubMed] [Google Scholar]
- 20.Griggs J.K., Manandhar N.P., Towers G.H., Taylor R.S. The effects of storage on the biological activity of medicinal plants from Nepal. J Ethnopharmacol. 2001;77:47–52. doi: 10.1016/s0378-8741(01)00291-4. [DOI] [PubMed] [Google Scholar]
- 21.Al-Fattani M.A., Douglas L.J. Biofilm matrix of Candida albicans and Candida tropicalis: chemical composition and role in drug resistance. J Med Microbiol. 2006;55:999–1008. doi: 10.1099/jmm.0.46569-0. [DOI] [PubMed] [Google Scholar]
- 22.Patterson T. Vol. 23. Applied Clinical Education; New York: 2007. pp. 7–80. (Treatment and prevention of fungal infections. Focus on candidemia). [Google Scholar]
- 23.Pfaller M.A., Diekema D.J. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Worth L.J., Blyth C.C., Booth D.L., Kong D.C., Marriott D., Cassumbhoy M. Optimizing antifungal drug dosing and monitoring to avoid toxicity and improveoutcomes in patients with haematological disorders. Intern Med J. 2008;38(6):521–537. doi: 10.1111/j.1445-5994.2008.01726.x. [DOI] [PubMed] [Google Scholar]
- 25.Maksimovic Z., Milenkovic M., Vucicevic D., Ristic M. Chemical composition and antimicrobial activity of Thymus pannonicus All. (Lamiaceae) essential oil. Cent Europ J Biol. 2008;3:149–154. [Google Scholar]
- 26.Al-Maqtari M.A.A., Alghalibi S.M., Alhamzy E.H. Chemical composition and antimicrobial activity of essential oil of Thymus vulgaris from Yemen. Turk J Biochem. 2011;36:342–349. [Google Scholar]
- 27.Shokri H., Sharifzadeh A., Ashrafi Tamai I. Anti-Candida zeylanoides activity of some Iranian plants used in traditional medicine. J Mycol Med. 2012;22:211–216. doi: 10.1016/j.mycmed.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Bonjar G.H. Inhibition of clotrimazole-resistant Candida albicans by plants used in Iranian folkloric medicine. Fitoterapia. 2004;75:74–76. doi: 10.1016/j.fitote.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Cruz T., Cabo M.M., Castillo M.J., Jimenez J., Ruiz C., Ramos-Cormenzana A. Chemical composition and antimicrobial activity of the essential oils of different samples of Thymus baeticus Boiss. Phytotherapy Res. 2006;7:92–94. [Google Scholar]
- 30.Sahin F., Karaman I., Gulluce M. Evaluation of antimicrobial activities of Saturega hortensis L. J Ethnopharmacol. 2003;87:61–65. doi: 10.1016/s0378-8741(03)00110-7. [DOI] [PubMed] [Google Scholar]
- 31.Zarrin M., Amirrajab N., Sadeghi-Nejad B. In vitro antifungal activity of Satureja khuzestanica Jamzad against Cryptococcus neoformans. Pak J Med Sci. 2010;26:880–882. [Google Scholar]
- 32.Khosravi A.R., Katiraee F., Eidi S., Bahonar A.R., Zarrinfar H. Comparison of MICs of some Iranian herbal essences against azole resistance and azole susceptible of Candida albicans. J Med Plants. 2008;7:37–44. [Google Scholar]
- 33.Naeini A., Khosravi A., Tajbakhsh H., Ghazanfari T., Yaraei R. Anti-Candida and immunomodulatory effects of Foeniculum vulgare Mill in vitro. Daneshvar. 2009;16:7–20. [Google Scholar]
- 34.Skrobonja J.R., Delić D.N., Karaman M.A., Matavulj M.N., Bogavac M.A. Antifungal properties of Foeniculum vulgare, Carum carvi and Eucalyptus sp. essential oils against Candida albicans strains. J Nat Sci. 2013;124:195–202. [Google Scholar]
- 35.Naeini A., Naderi N.J., Shokri H. Analysis and in vitro anti-Candida antifungal activity of Cuminum cyminum and Salvadora persica herbs extracts against pathogenic Candida strains. J Mycol Med. 2014;24:13–18. doi: 10.1016/j.mycmed.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Pai M.B., Prashant G.M., Murlikrishna K.S., Shivakumar K.M., Chandu G.N. Antigungal efficacy of Punica grantum, Acacia nilotica, Cuminum cyminum and Foeniculum vulgare on Candida albicans: an invitro study. Indian J Den Res. 2010;21:334–336. doi: 10.4103/0970-9290.70792. [DOI] [PubMed] [Google Scholar]
- 37.Runyoro D.K.B., Ngassapa O.D., Matee M.I.N., Joseph C.C., Moshi M.J. Medicinal plants used by Tanzanian traditional healers in the management of Candida Infections. J Ethnopharmacol. 2006;106:158–165. doi: 10.1016/j.jep.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 38.Pina-Vaz C., Rodrigues A.G., Pinto E., Costa-de-Oliveira S., Tavares C., Salgueiro L. Antifungal activity of Thymus oils and their major compounds. J Eur Acad Dermatol Venereol. 2004;18:73–78. doi: 10.1111/j.1468-3083.2004.00886.x. [DOI] [PubMed] [Google Scholar]