Graphical abstract
Keywords: Caryophyllaceae, Dianthus, pharmacological properties, Silene, traditional medicinal systems
Abstract
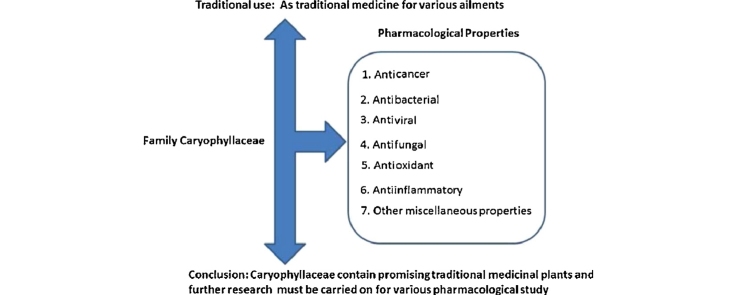
Several species of the family Caryophyllaceae are widely used by many ethnic communities as traditional medicine throughout the world. The highest number of plants of the family are used in Chinese traditional medicine. The ethnopharmacologial studies of this family indicate that plants of the family possess anticancer, antibacterial, antifungal, antiviral, antioxidant, and anti-inflammatory properties. Other miscellaneous properties reported are ribosome inactivation properties, inhibition of prostatic enlargement in rats, and inhibition of intestinal enzyme carboxyelasterase in rats, cerebro-protective activity, and antiobesity in rats. Few reviews have been published yet, providing information regarding medicinal plants of the family and their biomedical properties. All published reviews have focused either on a particular taxa or a few species. The present review is focused on the traditional medicinal uses of the plants of the family Caryophyllaceae along with phytochemical and pharmacological studies of the family. A study of the literature revealed significant traditional medicinal importance of the family. Major chemical constituents of Caryophyllceae are saponins, Phytoecdysteroids, benzenoids, phenyl propanoids, and nitrogen containing compounds. The most important property of plants of the family is anticancer activity and is shown by the large number of plant species studied. This review of traditional medicinal and pharmacological uses of plants of the family, provide a ground for future research in the family.
1. Introduction
The Caryophyllaceae Juss. is one of the major dicot family of angiosperms and is globally represented by 85 genera and 2,630 species.1 This family is popularly known as the pink family or carnation family. Plants of the family are present worldwide particularly in the Northern Hemisphere with the exception of most of the wet tropics. The Caryophyllaceae family is mainly centered in the Mediterranean area and exhibits great diversity in the habitat and growth form there. Plants of the family are erect, prostrate, annual or perennial herbs or small shrubs, and few species (Sanctambrosia spp.) are larger shrubs or small trees. The family is characterized by swollen nodes, with simple opposite leaves, solitary flowers or dichasial cymes inflorescence, actinomorphic pentamerous or tetramerous flowers, clawed petals, ten stamens or less in obdiplostemonous condition, ovary superior with free-central placentation, fruit capsule opening by teeth or valve and presence of anthocyanin pigments.2 The family Caryophyllaceae is well known for ornamental flowering plants such as Dianthus chinensis (Pink), Dianthus barbatus (Sweet William), Gypsophila spp. (Baby's Breath), Agrostemma spp. (Corn Cockle), Saponaria spp. (Soapwort), Lychnis spp. (Fire Pink), and Silene spp. (Campions) which form a major fraction of world's cut flower trade. Some species of Caryophyllaceae as Stellaria media (Chickweed), Cerastium cerastoides (Mouse-ear Chickweed) and other Stellaria spp. Cearstium spp., Silene spp., etc. are noxious weeds of agricultural lands.3
The family Cayophyllaceae is widely known for gardening herbs but medicinal importance of its members is sparsely known. In the present work we have tried to compile information regarding the medicinal plants of the family, their ethnomedicinal uses, and pharmacological significance of these plants in different diseases. Only a few reviews provide information regarding medicinal plants of the family and their biomedical properties have been published yet. All of these reviews are either focused on particular taxa4, 5, 6 or a few species.7 This review is unique for this family and fills the knowledge gap related to the medicinal importance of this family. This review will also help others in future for compilation of such information of this family.
2. Methods
For the present review, information regarding medicinal properties and biochemical properties of plants was gathered via searching books and scientific databases including PubMed, Elsevier, GoogleScholar, Springer, etc.
3. Phytochemistry of the family
The family is characterized by the presence of anthocyanin pigments instead of the betalain. Proanthocyanidin pigments are rarely detected from the seed coats and C-glycosylflavonoids pigment are rather common in the family.2 The unusual characteristic of the family is appearance of stable and endurable foam when parts of the plants are put into water and shaken. This behavior is due to the occurrence of high amount of saponins in the family. The saponins are found in various organs of the plants, especially in roots of Saponaria spp. Silene spp. Gypsophila spp., etc. and seeds of Agrostemma githago.8 The phytoecdysteroids mimics insect molting hormone and strongly interferes with metamorphosis of the insects. Phytoecdysteroids are synthesized mainly in the tribe Lychnideae of the subfamily Caryophylloideae of Caryophyllaceae, whereas Silene, Lychnis, Petrocoptis, Sagina, and Saponaria are main phytoecdysteroid synthesizing genera of the family.9, 10 A number of other compounds such as fatty acid derivatives, benzenoids, phenyl propanoids, isoprenoids, and nitrogen containing compounds are also isolated from the plants belonging to the family.11, 12, 13
4. Medicinal properties of plants
4.1. Plants used by ethnic communities for health care
Ethnobotany is the study of how people of a particular culture and region use indigenous plants in their lives for their daily health management and other needs. The American biologist R.E. Schultes14 described ethnobotany as “the study of the relationship which exists between people of primitive societies and their environment”. In more simple words, it is an anthropological approach to botany. The father of Indian Ethnobotany S.K. Jain15 described it as “the study of the direct relationship between plants and man is an interdisciplinary science and called Ethnobotany”. A total of 422,000 plant species are present on the earth, among which 52,885–72,000 plant species are used as medicinal plants around the world.16, 17 Thus, ∼17.1% of the total world flora comprises medicinally important plants. These plants are used to cure many diseases in different medicinal systems around the world. It is estimated that 70–80% of people worldwide rely chiefly on traditional herbal medicine to meet their primary health care needs.18
Among 2,630 species of the family1, only a small fraction (∼50–90 species) is known to have medicinal properties. The majority of the plants are used for some common ailments as cold, cough, fever, diarrhea, throat infection, and gastrointestinal infection etc. Table 1 summarizes ethnomedicinal uses of different plants of the family, their parts used, and references from different part of the world. Though, Table 1 makes no claim to be really complete, it represents the most up to date published account of medicinally important plants of the family.
Table 1.
List of ethnomedicinal uses of plants of the family Caryophyllaceae
| Plant name | Part used | Ethnomedicinal use | Refs |
|---|---|---|---|
| Acanthophyllum squarrosum Boiss | Root | Inhibit urease activity & thus prevent gastric upsets | 19 |
| Arenaria bryophylla Fernald | Whole plant | Tablets used to control inflammation/pain of kidney & burning sensation of bladder/urine tract | 20, 21 |
| Arenaria festucoides Benth. | Aerial parts | In Tibetan system of medicine used to cure tsha-ba of lungs | 22 |
| Arenaria griffithii Boiss. | Aerial parts | Used in menstruation disorder & bile disorder | 23, 24 |
| Arenaria rubra L. = (Spergularia rubra J. et Prestl.) | Aerial parts | Decoction used as diuretic, antiseptic, for treating diseases related to the renal systems | 25, 26, 27 |
| Arenaria serpyllifolia L. | Aerial parts | Decoction used in bladder diseases, calculus troubles, chronic cystitis, & along with minerals & medicinal stones used to promote kidney functions | 28, 20 |
| Cerastium cerastoides (L.) Britton. | Leaf, aerial shoot parts | Used in headache, renal colic, body ache, & decoction used in cough | 23, 29 |
| Cerastium chlorifolium Fisch. & C.A. Mey | Aerial parts | Used as antiseptic in wounds | 30 |
| Cerastium fontanum Baumg | Whole plant | Used in fever, coughs, & as refrigerant | 31, 32 |
| Cerastium glomeratum Thuill. | Whole plant | Traditionally used as diuretic, galactofuge, & tonic | 33 |
| Corrigiola telephiifolia Pourr. | Root | Used to treat flu, dermatological diseases, inflammation, ulcer, coughs, jaundice, anaesthetic, & diuretic | 34 |
| Dianthus anatolicus Boiss | Whole plant | As an antipyretic in intermittent fever & general tonic | 35, 36 |
| Dianthus barbatu s L. | Whole plant | Used as substitute of Dianthus chinensis L. | 37 |
| Dianthus basuticus Burtt. Dav. | Root | Decoction for purification of blood, flatulency, & fertility in bulls | 38 |
| Dianthus caryophyllus L. | Flower buds | In the treatment of gum infections, gastro-intestinal disorder, wounds, throat infection, cardiotonic, diaphoretic, alexiteric, and used as vermifuge | 39, 40 |
| Dianthus chinensis L. | Whole plant | In the treatment of menostasis, gonorrhea, diuretic, emmenagogue, & coughs | 37, 41 |
| Drymaria cordata (L.) Willd. ex Schult. | Whole plant, fresh leaves | Paste applied on fore head to cure headache, to cure itching & ring warm, cure peptic ulcer & nephritis | 42, 43 |
| Drymaria villosa Schltdl. & Cham. | Whole plant | Juice used to treat gastric troubles, pneumonia, & sinusitis | 44, 45 |
| Gypsophila oldhamiana Miq. | Aerial parts | Used to treat lung diseases, typhoid, jaundice, rheumatism, fever, & infantile malnutrition syndrome | 46, 47 |
| Gypsophila paniculata L | Root | Used for washing hair & clothes | 35 |
| Herniaria erckertii F. Herm | Whole plant | Decoction used to cure sore throat | 38 |
| Lepyrodiclis holosteoides (C.A. Mey.) Fenzl ex Fisch. & C.A. Mey. | Aerial parts | Used as a vegetable, considered an appetizer & laxative | 48 |
| Lychnis coronaria (L.) Desr.= (Silene coronaria (Desr.) Clairv. ex Rchb) | Leaf, root | Used to treat leprosy, diarrhea, heal cuts & inflamed wounds; root show hepato-protactive function | 49, 50, 51 |
| Lychnis coronata Thunb. | Flower, leaf | Used to treat skin infection & inflammation, & applied in herpes | 47 |
| Melandrium firmum (Siebold & Zucc.) Rohrb. = (Silene firma Siebold & Zucc.) | Whole plant | Used for gonorrhoea, galactagogue, emmenagogue, & contraception | 52 |
| Pollichia campestris Aiton | Leaves, flower, roots | Leaves & flowers of plant used for sore throat & skin diseases; cooked roots to treat bronchitis & heavy coughs | 38, 53 |
| Polycarpaea corymbosa (L.) Lam. | Whole plant | Anti-inflammatory, astringent, demulcent; plant-spermicidal, applied as poultice, prescribed in jaundice in the form of pills with molasses | 54 |
| Polycarpon prostratum (Forssk.) Asch. & Schweinf | Leaves, whole plant | Infusion of roasted leaves is given for coughs following fever, particularly in measles; 1–2 cup of decoction in an empty stomach during suffering from malarial fever | 54, 55 |
| Pseudostellaria heterophylla (Miq.) Pax | Root | Used as paediatric, geriatric tonic, & to treat tuberculosis | 47, 56 |
| Sagina saginoides (L.) H. Karst. | Whole plant | Used to treat food poisoning, diarrhea, cold, & fever | 23 |
| Saponaria officinalis L. | Whole plant | Used for cough, bronchitis, stomach disorders, bone deformations, rheumatism, pimples, skin diseases, bile disorders, hepatic eruptions, venereal ulcers, respiratory system diseases, jaundice, & urine remover | 7, 54 |
| Saponaria vaccaria L. = (Vaccaria pyramidata Medik.) | Whole plant | The mucilaginous sap used as febrifugal, in chronic fevers, treatment of furuncles & scabies | 54 |
| Silene conoidea L. | Root | Used as emollient, to wash wounds & hair, also used as a fumigant, & juice used in opthalmia | 57, 58, 59 |
| Silene flos-cuculi (L.) Greuter & Burdet | Flower | Decoction (added to wine) used to treat headache, malaria, & stomach pains | 60 |
| Silene italica (L.) Pers. | Seed | To cure sore throat | 61 |
| Silene jenisseensis Willd. | Root | Used to treat fever in infant malnutrition & fever due to Yin-deficiency | 62 |
| Silene moorcroftiana Wall. ex Benth | Leaf, stem | Used to treat ear & nose problems, leaves warmed in mustard oil & applied on the swollen skin to release pus, juice of boiled leaves used as a mouthwash & gargle for inflammation of the mouth & throat; stem is chewed as an aphrodisiac agent | 23, 63 |
| Silene nigrescens (Edgew.) Majumdar | Root | Powder consumed with hot water to protect from cold, cures hair diseases, dandruff, & lice | 64 |
| Silene pilosellifolia Cham. & Schltdl. | Whole plant | Treat fever in delirious patients & for compounding of various other traditional medicines | 38 |
| Silene setisperma Majumdar | Leaves | Used as vegetable & considered as appetizer | 65 |
| Silene viridiflora L. | Aerial parts | Phytoecdysteroid cocktail, obtained from the aerial parts recommended as an effective adaptogene for use in sports, reduced functioning, medicine, & poor restoration after serious illnesses & heavy physical exertion | 8, 66 |
| Silene vulgaris (Moench) Garcke | Young shoots, leaves | Cooked as a vegetable, considered as good for bronchitis & asthma, used in traditional Spanish dishes | 57, 67, 68 |
| Stellaria aquatic (L.) Scop. | Leaves | Decoction used in galactorrhea | 54 |
| Stellaria dichotoma L. | Root | Used in the treatment of fever & infant's malnutrition with fever | 70, 79 |
| Stellaria media (L.) Vill. | Whole plant | Used as antirheumatic, anti-inflammatory, astringent, refrigerant, demulcent, emollient, vulnerary, antipruritic, infusion used to relieve in itching & to cure psoriasis; whole plant applied as a plaster for broken bones & swellings | 54, 59, 71 |
| Stellaria rubra Scop. | Whole plant | Juice rich in vitamin C & used in treatment of scurvy, weakness after illness, lung congestion, & tuberculosis | 69 |
| Stellaria vestita Kurz = (Stellaria saxatilis Buch.-Ham. ex D. Don) | Whole plant | Boiled in water & liquid obtained used to assuage aching bones, treat cough, hemorrhage, rheumatism & treat cut & wounds | 45, 47 |
| Stellaria yunnanensis Franch. | Root | Decoction used in appetite loss & dizziness | 72 |
4.2. Plants used in pharmacological studies
4.2.1. Anticancer properties
4.2.1.1. Dianthus caryophyllus L
A glycosylated flavonol Kaempferide triglycoside isolated from Dianthus caryophyllus exhibit inhibitory properties for human colon cancer cell line carrying induced to over express estrogen receptor β (ER-β). Kaempferide triglycoside suppresses the proliferation of colon cancer cells over expressing ER-β not through ligand binding to estrogen receptor. However, it affects progression of HCT8 cell cycle by enhancing the G0/G1 cell fraction and increased antioxidant enzymes production in ER-β over expressing cells.73 This flavonol is able to suppress DNA replication and cell growth in a dose-dependent manner and shows significant effects in cells over expressing ER-β. Kaempferide triglycoside is able to push cells into G0/G1 starvation and to over expression of two important antioxidant proteins metallothionein type 2 (MT2A) and proteins superoxide dismutase type 2 (SOD2).73
Cellular Zn metabolism regulates metallothionein by interacting with Zn ions through –SH groups. Accordingly, metallothionein controls Zn-containing enzymes, e.g., Cu Zn-superoxide dismutase (Cu Zn-SOD), proteins, proapoptotic proteins (e.g., p53), and transcription factors (TFIIA), these elements are essential for cellular signaling pathways. Zn-containing metallothioneins work as tumor inhibitory proteins through supplying Zn to p53 for its stability and optimum activity.74 Thus, activation of p53 cause cell cycle arrest at the G1 phase and prevents DNA replication. Superoxide dismutase (SOD) catalyze dismutation of superoxide into oxygen and hydrogen peroxide and hence, SOD are important antioxidant defense molecules in nearly all cells exposed to oxygen.75 SOD-2 acts as a downstream mediator of mac25/insulin-like growth factor binding-protein related protein-1 (IGFBP-rP1) in the inhibition of tumor formation and its proliferation in prostate epithelial cell lines and human breast cells.76 Furthermore, kaempferide triglycoside induced SOD probably inhibit cell growth by suppressing effects of some growth factor binding-proteins and growth factor binding-protein related proteins.
4.2.1.2. Dianthus chinensis L
Ethanol extract of Dianthus chinensis is used in the treatment of Human Hepatocellular Carcinoma HepG2 cells. The plant extract suppresses HepG2 cell viability and induces apoptosis. Proteins such as, bcl-2, bcl-xl, mcl-1, and bax belong to the Bcl-2 family of proteins and play an important role in controlling the release of cytochrome c and in mitochondria-mediated apoptosis.77 The ratio between the level of proapoptotic bax protein and the level of antiapoptotic bcl-2 protein in the cell determines the fate of cells either survival or death. The ethanol extract of D. chinensis did not alter the expression of bax protein in HepG2 cells but, it selectively downregulates the expression of bcl-2 and bcl-xl proteins and consequently increases the ratio of bax:bcl-2 and bax:bcl-xl. High bax:bcl-2 and bax:bcl-xl ratio in the cytosol causes a release of cytochrome c from mitochondria, which initiates apoptosis by activating caspase-3/7, -8, and -9.78
4.2.1.3. Drymaria cordata (L.) Willd. ex Schult
The leaf extract of Drymaria cordata shows cytotoxic activity against HeLa (cervical cancer), HT29 (colon cancer), and MCF-7 (breast cancer) cell lines.79, 80 by an unknown mechanism.
4.2.1.4. Melandrium firmum (Siebold & Zucc.) Rohrb
The root extract of Melandrium firmum shows apoptotic effects in Human SH-SY5Y neuroblastoma cells.81 The root extract exerts its anticancer effects by regulating expression of Bcl-2 protein family, same as of Dianthus chinensis.
4.2.1.5. Acanthophyllum squarrosum Boiss
The triterpenoid saponins isolated from the roots of Acanthophyllum squarrosum were tested in vitro for lymphocyte antiproliferation. The results revealed that they have cytotoxic effect on lymphocytes in culture. The saponins show moderate concentration-dependent cytotoxicity to lymphocytes, saponin concentration of 10 g/mL showed no cytotoxicity; although, higher concentrations showed strong cytotoxicity.82 The mechanism is not yet known.
4.2.1.6. Saponaria vaccaria L
The total methanolic extracts of Saponaria vaccaria seed were evaluated for their growth inhibitory activity in WiDr (colon), MDA-MB- 231 (breast), NCI-417 (lung), PC-3 (prostate) human cancer cells, and the nontumorigenic fibroblast BJ (CRL-2522) cell lines. Some compounds such as cyclopeptide segetalin A, monodesmosides, vaccarosides A, vaccarosides B, bisdesmosides, segetoside H, and segetoside I were present in the extract and evaluated for growth inhibitory activity of different cell lines. In the study it was found that compounds show apoptotic activity by activating caspase 9. Caspase 9 further brought many changes in the cell and cell apoptosis takes place.83
4.2.1.7. Gypsophila arrostii Guss
The water extract of Gypsophila arrostii roots was evaluated for human promyelocytic leukemia (HL 60) cells. The extract contains compounds such as, gypsogenin, gypsogenin thiosemicarbazone, gypsogenin thiosemicarbazone glyoxime, Cu(II), and Co(II). These compounds were evaluated for antiproliferation activities.84 When the water extract of the plant was mixed with ethanol and hydrolyzed, then a series of gypsogenin (3-Hydroxy-23-oxoolean-12-en-28-oic acid) and their derivatives (1a–i) were isolated, where 1a–i are 3-hydroxy-23-(hydroxyimino)olean-12-en-28-oic acid, 3-(acetyloxy)-23-oxoolean-12-en-28-oic acid, benzyl 3-hydroxy-23-oxoolean-12-en-28-oate, 3-(acetyloxy)-23-(hydroxyimino)olean-12-en-28-oic acid, 3-(acetyloxy)-23-[(aminocarbonothioyl) hydrazono]olean-12-en-28-oic acid, benzyl 3-hydroxy-23-(hydroxyimino)olean-12-en-28-oate, benzyl 23-[(aminocarbonothioyl)hydrazono]-3-hydroxyolean-12-en-28-oate, benzyl 3-(acetyloxy)-23-oxoolean-12-en-28-oate, and benzyl 3-(acetyloxy)-23-(hydroxyimino)olean-12-en-28-oate, respectively. These compounds tested for antiproliferation activity against HL-60 (acute promyelocytic leukemia), HT-29 (colorectal adenocarcinoma), Caco-2 (colorectal adenocarcinoma), Saos-2 (osteosarcoma), MCF-7 (breast cancer), and HeLa (cervical cancer) cell lines. The compounds 1a, 1c, and 1d are considered as possible anticancer agents as they were shown to causing cell cycle arrest and cell death.85
4.2.1.8. Gypsophila oldhamiana Miq
The root extract of Gypsophila oldhamiana was tested for apoptotic activity against human hepatoma cell lines (SMMC-7721) and normal human hepatic cell line (L02). Caspase-3 plays a very important role in apoptosis and is considered to be the terminal event preceding cell death. The extract induced apoptosis in SMMC-7721 cells, due to the fact that caspase-3 can be activated by proteolytic processing at internal aspartate residues when cells receive an apoptosis inducing signal. Mitogen activated protein kinases (MAPKs) as extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK), and protein p38 play an essential role in apoptosis of cells. The root extract induces apoptosis via activating ERK, JNK, and meanwhile inhibits phosphorylation of p38 in SMMC-7721 cells. Moreover, the antiproliferative activity of the root extract might be associated with apoptosis induction through MAPKs and caspase-3 signaling pathways.86
4.2.1.9. Silene viridiflora L
The methanol extract and pure phytoecdysteroids of Silene viridiflora were evaluated for antitumor activity against mice murine myeloma cells P3X. The studies have shown that the methanol extract and pure phytoecdysteroids suppressed growth of cells to different degrees. The methanol extract was found highest for an antitumor agent.66
4.2.1.10. Silene fortunei Vis
The root extracts of Silene fortunei were tested for apoptotic activities against human T-cell leukemia Jurkat cells. Saponins 1–3, jenisseensosides, and their derivatives were isolated from the extract. These compounds were found to stimulate the proliferation of the Jurkat tumor cell lines at low concentrations, whereas, at high concentrations they inhibit the proliferation of the cells and induce apoptosis.87
4.2.2. Antibacterial properties
Whole plant extracts of Dianthus caryophyllus show antibacterial activity against Klebsiella pneumonia, Bordetella bronchiseptica, and Staphylococcus epidermidis.88 Two antibacterial compounds thymol and eugenol extracted from dried buds, show activity against Gram-negative bacteria Proteus mirabilis and Escherichia coli with MIC (minimum inhibitory concentration) value of 7.8 μg/mL, whereas, for the three strains of Gram-positive bacteria Staphylococcus aureus, Bacillus cereus, and Listeria monocytogenes antibiotic activity was with MIC value of 15.6 μg/mL.40 Different aerial parts extracts of Drymaria cordata were tested for antibacterial efficacy against Escherichia coli ATCC 10536, Staphylococcus aureus ATCC 29737, Bacillus subtilis ATCC 6633, Bacillus pumilis ATCC 14884, and Pseudomonas aeruginosa ATCC 25619 and found effective.89
4.2.3. Antifungal properties
The plant powder of the Arenaria rubra was screened for antifungal activity against the principal postharvest fungal pathogens of citrus fruits such as, Penicillium digitatum, Penicillium italicum, and Geotrichum candidum. The plant powder inhibited mycelial growth of all tested fungi by > 50% and totally inhibited the growth of the P. digitatum fungus.90 Kaempferide triglycoside along with C- and O-flavonoid glycosides were isolated from carnation (Dianthus caryophyllus). The isolated compounds and other flavonoid glycoside analogues were tested against Fusarium wilt causative pathogenic fungi Fusarium oxysporum f.sp. dianthi pathotypes and exhibited antifungal activity against the same.91
4.2.4. Antiviral properties
The sap of the Dianthus caryophyllus suppressed local lesion development of tobacco mosaic virus (TMV) on Nicotiana glutinosa.92 The seed extract of the plant shows potent antiviral activity against herpes simplex virus-1 (HSV-1) and hepatitis A virus-27 (HAV-27).93 The seed extract of Dianthus barbatus inhibits the growth of tobacco mosaic virus ordinary strain (TMV- OM).94 The lipophilic extract of Silene guntensis were tested against herpes simplex virus (HSV) and parainfluenza virus (PIV) and showed substantial antiviral activity against both viruses.95
4.2.5. Antioxidant properties
The plants of the Caryophyllaceae family contain good amounts of phenolics and flavonoids; thus, showing a good amount of DPPH (2,2-diphenyl-2-picrylhydrazyl hydrate) radical scavenging activity. The plant extract of Arenaria rubra shows good DPPH radical scavenging activity and acts as an antioxidant.96 The methanol extract of Silene gynodioca, Silene spergulifolia, and Silene swertiifolia were screened for antioxidant activities by three complementary tests such as, DPPH activity, metal chelating activity, and β-carotene/linoleic acid oxidation. The result showed that the extract of S. swertiifolia contain the highest amount of flavonoid and phenolic compounds and also exhibited the greatest antioxidant activity among all species. In other species, radical scavenging activity of S. spergulifolia extract was high followed by S. gynodioca.97
4.2.6. Anti-inflammatory properties
Two saponins, barbatosides A and B isolated from aerial parts of Dianthus barbatus cultivar “China Doll” have shown analgesic and anti-inflammatory activities.98 The butanol fraction of the methanol extract of whole plants of Melandrium firmum inhibited COX-2 (prostaglandin-endoperoxide synthase 2) and 5-LOX (5-lipoxygenase) production of prostaglandin D2 (PGD2), and leukotriene C4 (LTC4) in mouse and thus, exhibit anti-inflammatory activity.99 The triterpene, trans-p-methoxycinnamoyl isolated from the roots of Silene jenisseensis in vitro exhibit weak inhibitory effects in the cyclooxygenase inhibition assay.100
4.2.7. Other miscellaneous properties
A single chain ribosome-inactivating protein with RNA N-glycosidase activity was isolated from leaves of Dianthus barbatus L. and named as Dianthin 29.101 This compound inhibits functioning of Escherichia coli ribosomes after incubation of intact Escherichia coli ribosomes with Dianthin 29. Dianthin 29 belongs to Type 1 ribosome inhibitor proteins (RIPs) category. RIPs generally induce apoptosis and subsequently necrosis both in organs of poisoned animals and in a variety of cultured cells,102 this property of D. barbatus is still to be evaluated.
Benign prostatic hyperplasia (BPH), a mammalian male age-related disease characterized by prostatic enlargement coincides with distinct alterations in tissue histomorphology. The methanol extract of Melandrium firmum effectively inhibits the development of BPH induced by testosterone in rats.103
Phenolic extracts from the aerial part of Arenaria serpyllifolia were found to inhibit rat intestinal enzyme carboxylesterase (CE) significantly, in a concentration-dependent manner. The CE inhibitory phenolic compounds present in A. serpyllifolia extracts, also regulate enterocyte cellular expression via biochemical mechanism.104
Extracts of Lychnis chalcedonica in a daily dose of 150 mg/kg for 5 days reduced the severity of hemorheological disorders and normalized EEG activity in mice. Hence, extracts of L. chalcedonica possess cerebroprotective activity and decrease the inhibitory effect of ischemia on electrical activity of the mouse brain.105
The aqueous ethanolic extract from the roots of Stellaria dichotoma in vivo showed antiallergic effects on ear passive cutaneous anaphylaxis (PCA) reaction in mice and in vitro inhibitory activity on the release of β-hexosaminidase in RBL-2H3 cells.106
The whole plant of Stellaria media has been tested for its antiobesity activity by using progesterone-induced obesity model in female albino mice.107 The leaves of S. media contain vitamin C, carotene, and mucilage and are rich in potassium and silicon.54
5. Conclusion
On the basis of data mentioned in Table 1, it is concluded that, plants of the family are used by different ethnic communities in different parts of the globe, such as D. caryophyllus, D. chinensis, D. anatolicus, G. oldhamiana, Pseudostellaria heterophylla, S. jenisseensis, Stellaria saxalis, S. dichotoma, and Stellaria yunnanensis are used in different parts of China; Arenaria festucoides used in Tibet; Silene firma used in Korea; Arenaria bryophylla, Arenaria griffithii, A. rubra, Cerastium cerastoides, Drymaria cordata, Polycarpon prostratum, Polycarpaea corymbosa, Sagina saginoides, Silene setisperma used in different parts of India; Drymaria villosa, Stellaria vestita used in Nepal; Cerastium fontanum, Silene conoidea, S. moorcroftiana, used in Pakistan; Lychnis coronata used in Cambodia; Acanthophyllum squarrosum, Gypsophila paniculata used in Iran; Cerastium chlorifolium, Lychnis coronaria used in Turkey; Cerastium glomeratum, Silene italica used in Italy; Silene vulgaris used in Spain; Stellaria rubra used in temperate America; A. serpyllifolia used in temperate Europe; Dianthus basuticus, Herniaria erckertii, Pollichia campestris, Silene pilosellifolia are used in Africa. In spite of diversity in culture and geography, some plants of the family such as D. cordata are used as a versatile remedy for many diseases by many tribal communities around the world. Thus, biomedical investigations must be carried out on such plants and their active principals for different activities should be identified. Moreover, Silene, Gypsophila, Dianthus, Stellaria, and Saponaria are the most studied genera for both ethanomedicinal and pharmaceutical studies so far, hence biomedical properties of the rest of the genera of the family must be carried out. Due to various promising biomedical activities, further studies must be carried out on drug development from different plant extracts and their constituents.
In the present review, we have tried to summarize ethanomedicinal and modern pharmaceutical studies on the plants of the Caryophyllaceae family. The plants of the family possess high amounts of secondary metabolites such as saponins, a number of compounds such as fatty acid derivatives, benzenoids, phenyl propanoids, isoprenoids, and nitrogen containing compounds. Indeed, due to the presence of these compounds, plants were used in different traditional medicine systems and show many biochemical activities. On the basis of data collected in this review, it is evident that the family Caryophyllaceae comprise a wide range of pharmaceutically important plants. Furthermore, a large number of plants were tested for biomedical activities and have shown anticancer activity either in vitro or in vivo studies. This property of the plants should be further evaluated and tested against many cancer cell lines. As cancer is one of the least curable disease of the present time and causes thousands of death per year, hence the study on these plants may be helpful in future.
Conflicts of interest
The authors have no conflicts of interest.
Acknowledgments
The authors are thankful to all researchers whose results are included in the present review. The authors are also thankful to anonymous reviewers for their valuable suggestions.
References
- 1.Mabberley D.J. Cambridge University Press; Cambridge: 2008. Mabberley's plant-book: a portable dictionary of plants, their classifications, and uses; pp. 1040–1041. [Google Scholar]
- 2.Bittrich V. Introduction to centrospermae. In: Kubitzki K., Rohwer J.G., Bittrich V., editors. The families and genera of vascular plants, Vol. II, Magnoliid, hamamelid, and caryophyllid families. Springer Verlag; Berlin: 1993. pp. 13–19. [Google Scholar]
- 3.Holm L.G., Plucknett D.L., Pancho J.V., Herberger J.P. University Press of Hawaii; The, Honolulu: 1977. The world's worst weeds; pp. 111–114. [Google Scholar]
- 4.Mamadalieva N.Z., Lafont R., Wink M. Diversity of secondary metabolites in the genus Silene L. (Caryophyllaceae)—structures distribution, and biological properties. Diversity. 2014;6:415–499. [Google Scholar]
- 5.Nono N.R., Nzowa K.L., Barboni L., Tapondjou A.L. Drymaria cordata (Linn.) Willd (Caryophyllaceae): ethnobotany, pharmacology, and phytochemistry. Adv Biol Chem. 2014;4:160. [Google Scholar]
- 6.Sharma A., Arora D. Phytochemical and pharmacological potential of genus Stellaria: a review. J Pharma Res. 2012;5:3591–3596. [Google Scholar]
- 7.Korkmaz M., Ozcelik H. Economic importance of Gypsophila L., Ankyropetalum Fenzl and Saponaria L. (Caryophyllaceae) taxa of Turkey. Afr J Biotechnol. 2013;10:9533–9541. [Google Scholar]
- 8.Bottger S., Melzig M.F. Triterpenoid saponins of the Caryophyllaceae and Illecebraceae family. Phytochem Lett. 2011;4:59–68. [Google Scholar]
- 9.Zibareva L., Volodin V., Saatov Z., Savchenko T., Whiting P., Lafont R. Distribution of phytoecdysteroids in the Caryophyllaceae. Phytochemistry. 2003;64:499–517. doi: 10.1016/s0031-9422(03)00376-5. [DOI] [PubMed] [Google Scholar]
- 10.Zibareva L.N. Phytoecdysteroids of Caryophyllaceae Juss. Contemp Probl Eol. 2009;2:476–488. [Google Scholar]
- 11.Jurgens A., Witt T., Gottsberger G. Flower scent composition in night-flowering Silene species (Caryophyllaceae) Biochem Syst Ecol. 2002;30:383–397. [Google Scholar]
- 12.Jurgens A., Witt T., Gottsberger G. Flower scent composition in Dianthus and Saponaria species (Caryophyllaceae) and its relevance for pollination biology and taxonomy. Biochem Syst Ecol. 2003;31:345–357. [Google Scholar]
- 13.Jurgens A. Flower scent composition in diurnal Silene species (Caryophyllaceae): phylogenetic constraints or adaption to flower visitors? Biochem Syst Ecol. 2004;32:841–859. [Google Scholar]
- 14.Schultes R.E. The role of ethnobotanist in search for new medicinal plants. Llyodia. 1962;25:57–266. [Google Scholar]
- 15.Jain S.K. Ethnobotany. Interdiscipl Sci Rev. 1986;11:285–292. [Google Scholar]
- 16.Schippmann U., Leaman D.J., Cunningham A.B. Impact of cultivation and gathering of medicinal plants on biodiversity: global trends and issues. (FAO), Biodiversity and the ecosystem approach in agriculture, forestry and fisheries. Satellite event on the occasion of the ninth regular session of the commission on genetic resources for food and agriculture; Rome; 2002. [Google Scholar]
- 17.Schippmann U., Leaman D.J., Cunningham A.B. A comparison of cultivation and wild collection of medicinal and aromatic plants under sustainability aspects. Frontis. 2006;17:75–95. [Google Scholar]
- 18.Hamilton A.C. Medicinal plants, conservation, and livelihoods. J Biodivers Conser. 2004;13:1477–1517. [Google Scholar]
- 19.Nabati F., Mojab F., Habibi-Rezaei M., Bagherzadeh K., Amanlou M., Yousefi B. Large scale screening of commonly used Iranian traditional medicinal plants against urease activity. DARU J Pharm Sci. 2012;20:72. doi: 10.1186/2008-2231-20-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ballabh B., Chaurasia O.P., Ahmed Z., Singh S.B. Traditional medicinal plants of cold desert Ladakh—used against kidney and urinary disorders. J Ethnopharmacol. 2008;118:331–339. doi: 10.1016/j.jep.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 21.Kala C.P. Medicinal plants of the high altitude cold desert in India: diversity, distribution, and traditional uses. Int J Biodiver Sci Manage. 2006;2:43–56. [Google Scholar]
- 22.Kletter C., Kriechbaum M. CRC Press; New York: 2001. Tibetan medicinal plants; pp. 228–229. [Google Scholar]
- 23.Angmo K., Adhikari B.S., Rawat G.S. Changing aspects of traditional healthcare system in Western Ladakh, India. J Ethnopharmacol. 2012;143:621–630. doi: 10.1016/j.jep.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 24.Ballabh B., Chaurasia O.P. Herbal formulations from cold desert plants used for gynecological disorders. Ethnobot Res App. 2011;9:59–66. [Google Scholar]
- 25.Al-Quran S. Taxonomical and pharmacological survey of therapeutic plants in Jordan. J Nat Prod. 2008;1:10–26. [Google Scholar]
- 26.Pullaiah T. Vol. 1. Daya books; New Delhi: 2006. p. 198. (Encyclopedia of world medicinal plants). [Google Scholar]
- 27.Gonzalez-Tejero M.R., Casares-Porcel M., Sanchez-Rojas C.P., Ramiro-Gutierrez J.M., Molero-Mesa J., Pieroni A. Medicinal plants in the Mediterranean area: synthesis of the results of the project Rubia. J Ethnopharmacol. 2008;116:341–357. doi: 10.1016/j.jep.2007.11.045. [DOI] [PubMed] [Google Scholar]
- 28.Vardhana R. Sarup and Sons; New Delhi: 2008. Direct uses of medicinal plants and their identification; p. 39. [Google Scholar]
- 29.Kumar P., Singhal V.K. Ethnobotany and ethnomedicinal uses, chromosomal status, and natural propagation of some plants of Lahaul-Spiti and adjoining hills. J Bot. 2013;2013:248943. doi:10.1155/2013/248943. [Google Scholar]
- 30.Tetik F., Civelek S., Cakilcioglu U. Traditional uses of some medicinal plants in Malatya (Turkey) J Ethnopharmacol. 2013;146:331–346. doi: 10.1016/j.jep.2012.12.054. [DOI] [PubMed] [Google Scholar]
- 31.Rana M.S., Samant S.S. Diversity, indigenous uses and conservation status of medicinal plants in Manali wildlife sanctuary, North western Himalaya. Indian J Tradit Know. 2011;10:439–459. [Google Scholar]
- 32.Sher Z., Khan Z., Hussain F. Ethnobotanical studies of some plants of Chagharzai valley, district Buner, Pakistan. Pak J Bot. 2011;43:1445–1452. [Google Scholar]
- 33.Dall’Acqua S., Cervellati R., Loi M.C., Innocenti G. Evaluation of in vitro antioxidant properties of some traditional Sardinian medicinal plants: investigation of the high antioxidant capacity of Rubus ulmifolius. Food Chem. 2008;106:745–749. [Google Scholar]
- 34.Lakmichi H., Bakhtaoui F.Z., Gadhi C.A., Ezoubeiri A., El Jahiri Y., El Mansouri A. Toxicity profile of the aqueous ethanol root extract of Corrigiola telephiifolia pourr. (Caryophyllaceae) in rodents. Evid Based Complement Alternat Med. 2011;2011:317090. doi: 10.1155/2011/317090. doi:10.1155/2011/317090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hooper D., Field H. Vol. 9. Field Museum Press; USA: 1937. p. 79. (Useful plants and drugs of Iran and Iraq). 123, 117. [Google Scholar]
- 36.Pullaiah T. Vol. 2. Daya books; New Delhi: 2006. p. 767. (Encyclopedia of world medicinal plants). 826. [Google Scholar]
- 37.Bown D. Dorling Kindersley; London: 1995. Encyclopedia of herbs and their uses; p. 424. [Google Scholar]
- 38.Moteetee A., Van Wyk B.E. The medical ethnobotany of Lesotho: a review. Bothalia. 2011;41:209–228. [Google Scholar]
- 39.Al-Rawi H.L. Chakravarty. Medicinal plants of Iraq, Ministry of Agriculture and irrigation, State Board for Agricultural and Water Resources Research. National Herbarium of Iraq, Baghdad. 1988:93. [Google Scholar]
- 40.Mohammed M.J., Al-Bayati F.A. Isolation and identification of antibacterial compounds from Thymus kotschyanus aerial parts and Dianthus caryophyllus flower buds. Phytomedicine. 2009;16:632–637. doi: 10.1016/j.phymed.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 41.WHO . Manila; Philippines: 1998. Medicinal plants in the Republic of Korea, World Health Organization, Regional Office for Western Pacific; p. 93. [Google Scholar]
- 42.Noumi E., Dibakto T.W. Medicinal plants used for peptic ulcer in the Bangangte region, western Cameroon. Fitoterapia. 2000;71:406–412. doi: 10.1016/s0367-326x(00)00144-1. [DOI] [PubMed] [Google Scholar]
- 43.Ramashankar, Rawat M.S. Ethnobotanical observations of Drymaria cordata Willd. Ex Roem & Schult. (Caryophyllaceae) Bull Arunachal For Res. 2008;24:22–24. [Google Scholar]
- 44.Lepcha S.R., Das A.P. Recent Studies in Biodiversity and Traditional Knowledge in India. Sarat Book House; Kolkata, India: 2011. Ethno-medicobotanical exploration along the international borders to Tibet Autonomous Region of China and the kingdom of Bhutan with special reference to the Pangolakha Wildlife Sanctuary, East Sikkim; pp. 257–270. [Google Scholar]
- 45.Manandhar N.P. Ethnobotanical note on folk lore remedies of Baglung district Nepal. Contrib Nepal Stud. 1993;20:183–196. [Google Scholar]
- 46.Luo J.G., Wang X.B., Ma L., Kong L.Y. Gypsophin: a novel α-glucosidase inhibitory cyclic peptide from the roots of Gypsophila oldhamiana. Bioorg Med Chem Lett. 2007;17:4460–4463. doi: 10.1016/j.bmcl.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 47.Wiart C. CRC Press; Boca Raton: 2012. Medicinal plants of China, Korea, and Japan: bioresources for tomorrow's drugs and sosmetics; pp. 271–291. [Google Scholar]
- 48.Noor A., Khatoon S., Ahmed M. Enumeration of the ethnobotaniacal uses of some herbs in Astore valley, Gilgit-Baltistan, Pakistan with particular reference to health cure purposes. FUUAST J Bio. 2012;2:31–48. [Google Scholar]
- 49.Govind P. Medicinal plants against liver diseases. IJPR. 2011;2:115–121. [Google Scholar]
- 50.Jeelani S.M., Wani M.P., Kumari S., Gupta R.C., Siddique M.A.A. Ethnobotany of some polypetalous plants from the Kashmir Himalaya. J Med Plants Res. 2013;7:2714–2721. [Google Scholar]
- 51.Kultur S. Medicinal plants used in Kuklareli province (Turkey) J Ethnopharmacol. 2007;111:341–364. doi: 10.1016/j.jep.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 52.National Product Research Institute . Western Pacific series No. 21; 1998. Medicinal plants in the Republic of Korea. Seoul National University, WHO regional publication; p. 177. [Google Scholar]
- 53.Mothana R.A.A., Gruenert R., Bednarski P.J., Lindequist U. Evaluation of the in vitro anticancer, antimicrobial, and antioxidant activities of some Yemeni plants used in folk medicine. Die Pharmazie. 2009;64:260–268. [PubMed] [Google Scholar]
- 54.Khare C.P. Springer-Verlag; Berlin/Heidelberg: 2007. Indian Medicinal Plants, An Illustrated Dictionary; p. 219. 508, 583. [Google Scholar]
- 55.Majumdar K., Datta B.K. A study on ethnomedicinal usage of plants among the folklore herbalists and Tripuri medical practitioners: part II. Nat Prod Radiance. 2007;6:66–73. [Google Scholar]
- 56.Reinecke M.G., Zhao Y.Y. Phytochemical studies of the Chinese herb tai-zi-shen, Pseudostellaria heterophylla. J Nat Prod. 1988;51:1236–1240. [Google Scholar]
- 57.Gaur R.D. Trans Media; Srinagar (Garhwal): 1999. Flora of the district Garhwal, North West Himalaya: with ethnobotanical notes; pp. 156–157. [Google Scholar]
- 58.Kunwar R.M., Adhikari N. Ethnomedicine of Dolpa district, Nepal: the plants, their vernacular names and uses. Lyonia. 2005;8:43–49. [Google Scholar]
- 59.Shinwari M.I., Khan M.A. Folk use of medicinal herbs of Margalla hills national park, Islamabad. J ethnopharmacol. 2000;69:45–56. doi: 10.1016/s0378-8741(99)00135-x. [DOI] [PubMed] [Google Scholar]
- 60.Leto C., Tuttolomondo T., La Bella S., Licata M. Ethnobotanical study in the Madonie Regional Park (Central Sicily, Italy)—medicinal use of wild shrub and herbaceous plant species. J ethnopharmacol. 2013;146:90–112. doi: 10.1016/j.jep.2012.11.042. [DOI] [PubMed] [Google Scholar]
- 61.Guarrera P.M., Lucia L.M. Ethnobotanical remarks on Central and Southern Italy. J Ethnobiol Ethnomed. 2007;3:1–11. doi: 10.1186/1746-4269-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lacaille-DuBois M.A., Hanquet B., Cui Z.H., Lou Z.C., Wagner H. Jenisseensosides C and D, biologically active acylated triterpene saponins from Silene jenisseensis. Phytochemistry. 1997;45:985–990. doi: 10.1016/s0031-9422(97)00087-3. [DOI] [PubMed] [Google Scholar]
- 63.Shuaib M., Khan I., Sharifullah R.K., Hashmatullah S.M., Naz R. Ethnobotanical studies of spring flora of Dir Lower, Khyber Pakhtunkhwa, Pakistan. Pak J Weed Sci Res. 2014;20:37–49. [Google Scholar]
- 64.Rokaya M.B., Munzbergova Z., Timsina B. Ethnobotanical study of medicinal plants from the Humla district of western Nepal. J Ethnopharmacol. 2010;130:485–504. doi: 10.1016/j.jep.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 65.Joshi P., Pande P.C. Indus Publishing Company; New Delhi: 1997. Ethnobotany of the Bhotia Tribe of Kumaun Himalaya. Studies on Kumaun Himalaya; p. 69. [Google Scholar]
- 66.Mamadalieva N.Z., Egamberdieva D., Zhanibekov A.A., Triggiani D., Tiezzi A. Chemical components of Silene viridiflora and their biological properties. Chem Nat Compd. 2009;45:589–591. [Google Scholar]
- 67.Rivera D., Obon C., Inocencio C., Heinrich M., Verde A., Fajardo J. The ethnobotanical study of local Mediterranean food plants as medicinal resources in Southern Spain. J Physiol Pharmacol. 2005;56:97–114. [PubMed] [Google Scholar]
- 68.Tardio J., Pascual H., Morales R. Wild food plants traditionally used in the province of Madrid, Central Spain. Econ Bot. 2005;59:122–136. [Google Scholar]
- 69.Pullaiah T. Vol. 4. Daya books; New Delhi: 2006. p. 1854. (Encyclopedia of world medicinal plants). [Google Scholar]
- 70.Zheng X., Chen H., Li R., Xing F.W. Medical ethnobotany of the Run dialect people of Li minority in Hainan. Acta Bot Yunna. 2008;30:195. [Google Scholar]
- 71.Hoffmann D. Inner Traditions/Bear & Co; Vermont: 2003. Medical herbalism: the science and practice of herbal medicine; p. 435. [Google Scholar]
- 72.Lee S., Xiao C., Pei S. Ethnobotanical survey of medicinal plants at periodic markets of Honghe Prefecture in Yunnan Province, SW China. J Ethnopharmacol. 2008;117:362–377. doi: 10.1016/j.jep.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 73.Martineti V., Tognarini I., Azzari C., Carbonell Sala S., Clematis F., Dolci M. Inhibition of in vitro growth and arrest in the G0/G1 phase of HCT8 line human colon cancer cells by kaempferide triglycoside from Dianthus caryophyllus. Phytother Res. 2010;24:1302–1308. doi: 10.1002/ptr.3105. [DOI] [PubMed] [Google Scholar]
- 74.Pedersen M.O., Larsen A., Stoltenberg M., Penkowa M. The role of metallothionein in oncogenesis and cancer prognosis. Prog Histochem Cytochem. 2009;44:29–64. doi: 10.1016/j.proghi.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 75.Khan M.A., Tania M., Zhang D.Z., Chen H.C. Antioxidant enzymes and cancer. Chin J Cancer Res. 2010;22:87–92. [Google Scholar]
- 76.Plymate S.R., Haugk K.H., Sprenger C.C., Nelson P.S., Tennant M.K., Zhang Y. Increased manganese superoxide dismutase (SOD-2) is part of the mechanism for prostate tumor suppression by Mac25/insulin-like growth factor binding-protein related protein-1[J] Oncogene. 2003;22:1024–1034. doi: 10.1038/sj.onc.1206210. [DOI] [PubMed] [Google Scholar]
- 77.Borner C. The Bcl-2 protein family: sensors and checkpoints for life-or-death decisions. Mol Immunol. 2003;39:615–647. doi: 10.1016/s0161-5890(02)00252-3. [DOI] [PubMed] [Google Scholar]
- 78.Nho K.J., Chun J.M., Kim H.K. Ethanol extract of Dianthus chinensis L. induces apoptosis in human hepatocellular carcinoma HepG2 Cells in vitro. Evid Based Complement Altern Med. 2012;2012:573527. doi: 10.1155/2012/573527. doi:10.1155/2012/573527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abimbola S., Maryna V.D.V., Lucinda B., Trevor K. Cytotoxicity evaluation of selected Nigerian plants used in traditional cancer treatment. J Med Plants Res. 2011;5:2442–2444. [Google Scholar]
- 80.Sowemimo A., Van de Venter M., Baatjies L., Koekemoer T. Cytotoxic activity of selected Nigerian plants. Afr J Tradit Complement Altern Med. 2009;6:526–528. doi: 10.4314/ajtcam.v6i4.57186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rahman M., Yang H., Lim S.S., Huh S.O. Apoptotic effects of Melandryum firmum root extracts in human SH-SY5Y neuroblastoma cells. Exp Neurol. 2013;22:208–213. doi: 10.5607/en.2013.22.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gaidi G., Miyamoto T., Rustaiyan A., Laurens V., Lacaille-Dubois M.A. Two new biologically active triterpene saponins from Acanthophyllum squarrosum. J Nat Prod. 2006;3:1497–1502. doi: 10.1021/np000212+. [DOI] [PubMed] [Google Scholar]
- 83.Balsevich J.J., Ramirez-Erosa I., Hickie R.A., Dunlop D.M., Bishop G.G., Deibert L.K. Antiproliferative activity of Saponaria vaccaria constituents and related compounds. Fitoterapia. 2012;83:170–181. doi: 10.1016/j.fitote.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 84.Emirdag-Ozturk S., Babahan I., Ozmen A. Synthesis, characterization, and in vitro antineoplastic activity of gypsogenin derivatives. Bioorg Chem. 2014;53:15–23. doi: 10.1016/j.bioorg.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 85.Emirdag-Ozturk S., Karayildirim T., Capci-Karagoz A., Alankus-Calıskan O., Ozmen A., Poyrazoglu-Coban E. Synthesis, antimicrobial and cytotoxic activities, and structure-activity relationships of gypsogenin derivatives against human cancer cells. Eur J Med Chem. 2014;82:565–573. doi: 10.1016/j.ejmech.2014.05.084. [DOI] [PubMed] [Google Scholar]
- 86.Zhang W., Luo J.G., Zhang C., Kong L.Y. Different apoptotic effects of triterpenoid saponin-rich Gypsophila oldhamiana root extract on human hepatoma SMMC-7721 and normal human hepatic L02 cells. Biol Pharm Bull. 2013;36:1080–1087. doi: 10.1248/bpb.b12-01069. [DOI] [PubMed] [Google Scholar]
- 87.Gaidi G., Miyamoto T., Laurens V., Lacaille-Dubois M.A. New acylated triterpene saponins from Silene fortunei that modulate lymphocyte proliferation. J Nat Prod. 2002;65:1568–1572. doi: 10.1021/np020105a. [DOI] [PubMed] [Google Scholar]
- 88.Bonjar S. Evaluation of antibacterial properties of some medicinal plants used in Iran. J Ethnopharmacol. 2004;94:301–305. doi: 10.1016/j.jep.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 89.Mukherjee P.K., Saha K., Bhattacharya S., Giri S.N., Pal M., Saha B.P. Studies on antitussive activity of Drymaria cordata Willd. (Caryophyllaceae) J ethnopharmacol. 1997;56:77–80. doi: 10.1016/s0378-8741(97)01512-2. [DOI] [PubMed] [Google Scholar]
- 90.Ameziane N., Boubaker H., Boudyach H., Msanda F., Jilal A., Benaoumar A.A. Antifungal activity of Moroccan plants against citrus fruit pathogens. Agron Sustain Dev. 2007;27:273–277. [Google Scholar]
- 91.Galeotti F., Barile E., Curir P., Dolci M., Lanzotti V. Flavonoids from carnation Dianthus caryophyllus and their antifungal activity. Phytochem Lett. 2008;1:44–48. [Google Scholar]
- 92.Ragetli H.W.J., Weintraub M. Purification and characteristics of a virus inhibitor from Dianthus caryophyllus L. II. Characterization and mode of action. Virology. 1962;18:241–248. doi: 10.1016/0042-6822(62)90010-7. [DOI] [PubMed] [Google Scholar]
- 93.Barakat A.B., Shoman S.A., Dina N., Alfarouk O.R. Antiviral activity and mode of action of Dianthus caryophyllus L. and Lupinus termes L. seed extracts against in vitro herpes simplex and hepatitis A viruses infection. J Microbiol Antimicrob. 2010;2:23–29. [Google Scholar]
- 94.Taniguchi T. Isolation and characteristics of a virus inhibitor in Dianthus barbatus seeds. Proc Kansai Pl Prot Soc. 1986;28:1–5. [Google Scholar]
- 95.Mamadalieva N.Z., Ul’chenko N.T., Yuldasheva N.K., Zhanibekov A.A., Egamberdieva D.R., Glushenkova A.I. Neutral lipids and biological activity of the CHCl3 extract of the aerial part of Silene guntensis. Chem Nat Compd. 2010;46:621–622. [Google Scholar]
- 96.Djeridane A., Yousfi M., Brunel J.M., Stocker P. Isolation and characterization of a new steroid derivative as a powerful antioxidant from Cleome arabica in screening the in vitro antioxidant capacity of 18 Algerian medicinal plants. Food Chem Toxicol. 2010;48:2599–2606. doi: 10.1016/j.fct.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 97.Karamian R., Ghasemlou F. Screening of total phenol and flavonoid content, antioxidant, and antibacterial activities of the methanolic extracts of three Silene species from Iran. Intl J Agri Crop Sci. 2013;5:305–312. [Google Scholar]
- 98.Cordell G.A., Lyon R.L., Fong H.H., Benoit P.S., Farnsworth N.R. Biological and phytochemical investigations of Dianthus barbatus cv. “China Doll” (Caryophyllaceae) Lloydia. 1977;40:361–363. [PubMed] [Google Scholar]
- 99.Zheng M.S., Hwang N.K., Kim D.H., Moon T.C., Son J.K., Chang H.W. Chemical constituents of Melandrium firmum Rohrbach and their anti-inflammatory activity. Arch Pharmacal Res. 2008;31:318–322. doi: 10.1007/s12272-001-1158-9. [DOI] [PubMed] [Google Scholar]
- 100.Lacaille-Dubois M.A., Hanquet B., Cui Z.H., Lou Z.C., Wagner H. Acylated triterpene saponins from Silene jenisseensis. Phytochemistry. 1995;40:509–514. doi: 10.1016/0031-9422(95)00222-s. [DOI] [PubMed] [Google Scholar]
- 101.Prestle J., Hornung E., Schonfelder M., Mundry K.W. Mechanism and site of action of a ribosome-inactivating protein type 1 from Dianthus barbatus which inactivates Escherichia coli ribosomes. FEBS Lett. 1992;297:250–252. doi: 10.1016/0014-5793(92)80549-v. [DOI] [PubMed] [Google Scholar]
- 102.Stripe F. Ribosome-inactivating proteins. In: Wiley R.G., Lappi D.A., editors. Molecular neurosurgery with targeted toxins. Humana Press; Totowa: 2005. pp. 9–29. [Google Scholar]
- 103.Lee M.Y., Shin I.S., Seo C.S., Lee N.H., Ha H.K., Son J.K. Effects of Melandrium firmum methanolic extract on testosterone-induced benign prostatic hyperplasia in Wistar rats. Asian J Androl. 2012;14:320. doi: 10.1038/aja.2011.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stocker P., Yousfi M., Djerridane O., Perrier J., Amziani R., El Boustani S. Effect of flavonoids from various Mediterranean plants on enzymatic activity of intestinal carboxylesterase. Biochimie. 2004;86:919–925. doi: 10.1016/j.biochi.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 105.Plotnikov M.B., Aliev O.I., Vasil’ev A.S., Maslov M.Y., Suslov N.I., Zibareva L.N. Hemorheological and cerebroprotective activity of Lychnis chalcedonica L. extract in rats with cerebral ischemia. Bull Exp Biol Med. 2005;139:60–63. doi: 10.1007/s10517-005-0212-7. [DOI] [PubMed] [Google Scholar]
- 106.Sun B., Morikawa T., Matsuda H., Tewtrakul S., Wu L.J., Harima S. Structures of new β-carboline-type alkaloids with antiallergic effects from Stellaria dichotoma. J Nat Prod. 2004;67:1464–1469. doi: 10.1021/np040080a. [DOI] [PubMed] [Google Scholar]
- 107.Chidrawar V.R., Patel K.N., Sheth N.R., Shiromwar S.S., Trivedi P. Antiobesity effect of Stellaria media against drug induced obesity in Swiss albino mice. Ayu. 2011;32:576. doi: 10.4103/0974-8520.96137. [DOI] [PMC free article] [PubMed] [Google Scholar]