Abstract
Background
The current study was designed to investigate the influence of monofloral Indian mustard bee pollen (MIMBP) and processed monofloral Indian mustard bee pollen (PMIMBP) supplementation on chronic swimming exercise-induced oxidative stress implications in the gastrocnemius muscle of Wistar rats.
Methods
MIMBP was processed with an edible lipid-surfactant mixture (Captex 355:Tween 80) to increase the extraction of polyphenols and flavonoid aglycones as analyzed by UV spectroscopy and high performance liquid chromatography-photo diode array. Wistar rats in different groups were fed with MIMBP or PMIMBP supplements at a dose of 100 mg/kg, 200 mg/kg and 300 mg/kg individually, while being subjected to chronic swimming exercise for 4 weeks (5 d/wk). Various biochemical [superoxide dismutase (SOD), glutathione (GSH), malonaldehyde (MDA), nitric oxide (NO), and total protein content], mitochondrial (Complex I, II, III, and IV enzyme activity), and molecular (myostatin mRNA expression) parameters were monitored in the gastrocnemius muscle of each group.
Results
Administration of both MIMBP (300 mg/kg) and PMIMBP (100 mg/kg, 200 mg/kg, and 300 mg/kg) wielded an antioxidant effect by significantly improving SOD, GSH, MDA, NO, and total protein levels. Further MIMBP (300 mg/kg) and PMIMBP (200 mg/kg and 300 mg/kg) significantly improved impaired mitochondrial Complex I, II, III, and IV enzyme activity. Significant down-regulation of myostatin mRNA expression by MIMBP (300 mg/kg) and PMIMBP (200 mg/kg and 300 mg/kg) indicates a muscle protectant role in oxidative stress conditions.
Conclusion
The study establishes the antioxidant, mitochondrial upregulatory, and myostatin inhibitory effects of both MIMBP and PMIMBP in exercise-induced oxidative stress conditions, suggesting their usefulness in effective management of exercise-induced muscular stress. Further, processing of MIMBP with an edible lipid-surfactant mixture was found to improve the therapeutic efficiency of pollen.
Keywords: bee pollen, lipid, oxidative stress, skeletal muscle, surfactant
1. Introduction
Skeletal muscles are highly sensitive to a variety of external stimuli, especially exercise. Heavy endurance training in the form of forced swimming exercise induces oxidative stress due to increased production of reactive oxygen or nitrogen species in skeletal muscle tissues.1 Increased oxidative stress triggers exercise-induced muscle injury on account of heavy eccentric contractions. The elevated intracellular reactive oxygen species (ROS) aggravates exercise-induced muscular weakness and fatigue.2 Furthermore, strenuous exercise may induce mitochondrial dysfunction which can cause oxidative damage and injury to tissues.3, 4, 5, 6 Myostatin, a transforming growth factor-β superfamily, plays a vital role in the regulation of skeletal muscle mass. Rigorous exercise modulates myostatin mRNA expression in skeletal muscles affecting muscle mass.7 Therefore, in order to combat the implications of oxidative stress during physical activity, many athletes and sportspeople need muscle protectant supplements.
Bee pollen is plant pollen which has been collected by honeybees. It is known to be a source of nutrients such as proteins, amino acids, carbohydrates, vitamins, polyphenols, flavonoids, etc. On account of its distinct composition, pollen are reported to exhibit an array of pharmacological properties such as antioxidant, immunomodulatory, anti-inflammatory, antimicrobial, antiatherosclerotic, antiallergic, antiosteoporosis, hepatoprotective, sedative, etc.8, 9, 10, 11 Owing to the rich nutrient content, bee pollen is claimed to be a useful food or dietary supplement for sportspeople and athletes,8 however very little scientific evidence is available to support this claim.12 In this context, the current study was undertaken to investigate at biochemical, mitochondrial, and molecular levels, the muscle protectant ability of bee pollen, which has not been explored to date.
Despite the high nutritional content documented for bee pollen, ambiguities regarding the digestibility of pollen by humans have been observed. In general, pollen structure is comprised of nutrient rich cytoplasm enclosed within a tough pollen coat consisting of a cellulosic inner layer, intine, and an external layer, exine, made of sporopollenin. The ridged sporopollenin matrix composition of the pollen coat makes it rigid, thereby resisting its decay and digestion.13 For this reason most of the nutritional qualities of pollen pass through the gut without being absorbed.14 This probed the hypothesis that the cracking of pollen or the pollen coat would improve the availability of the engulfed nutrients for digestion.8 Several biological, chemical, and mechanical methods have been reported for breaking the pollen wall.15, 16, 17, 18, 19 The success of such methods is limited by the associated drawbacks which include the degradation of nutrients by enzymatic or chemical treatments, the need for sophisticated instrumentation, and higher costs involved for mechanical techniques. Therefore the need for an effective and green processing technique to obtain ruptured pollen with better availability of nutrients remains unmet.
The current study was designed with two main objectives: (1) to develop a processing method for bee pollen, using the edible lipid (Captex 355) and surfactant (Tween 80), to produce processed pollen with improved availability of nutrients which would exert beneficial effects; and (2) to investigate the influence of neat and processed bee pollen supplementation on chronic swimming exercise-induced oxidative stress implications in the gastrocnemius muscle of Wistar rats. Several biochemical [malonaldehyde (MDA), superoxide dismutase (SOD), glutathione (GSH), total protein content, and nitrite level], mitochondrial (Complex I, II, III, and IV enzyme activity), and molecular (myostatin mRNA expression) parameters were monitored.
2. Methods
2.1. Bee pollen
In order to ensure uniform physicochemical, organoleptic and nutritional properties with minimal variations, bee pollen from a monofloral source, the Indian mustard crop Brassica juncea [monofloral Indian mustard bee pollen (MIMBP)], were used in this study. The nutritional and chemical composition for the MIMBP has been established in previous study.20 Pollen were collected from the 24 Parganas district of West Bengal, India (22° 35′ N, 88° 44′ E), they were identified and authenticated by the Central Bee Research and Training Institute, Pune, India [Voucher Specimen No (1/WB/2012)]. The collected pollen were dried at < 40 °C and stored in a freezer at −15 °C throughout the study. The pollen pellets were powdered and sieved through a 200 μm mesh before analysis.20
2.2. Chemicals
Analytical standards such as gallic acid, quercetin, kaempferol, Folin-Ciocalteu phenol reagent, diammonium sulfate, meta-phosphoric acid, ortho-phosphoric acid, analytical reagent (AR) grade solvents ethanol, ethyl acetate, and methanol were procured from Merck, New Jersey, USA. Captex 355 was a generous gift sample from Abitec Corp. Cleveland, OH, USA. Polyoxyethylene sorbitan monolaurate (Tween 80) AR grade was purchased from Merck. An RNA extraction and reverse transcriptase-polymerase chain reaction (RT-PCR) kit was purchased from Biotools B&M Labs, Madrid, Spain. The primers myostatin and β-actin were synthesized and purchased from Amnion Biosciences Pvt. Ltd., Bengaluru, India. All the reagents and solvents used in this study were AR grade.
2.3. Animals
Adult male Wistar rats (150–200 g) and Swiss albino mice (18–22 g) were obtained from the National Institute of Biosciences, Pune, India. The animals were housed at 24 ± 1 °C, with a relative humidity of 45–55% and a 12 hour/12 hour dark/light cycle. The animals had free access to standard pellet chow (Pranav Agro industries Ltd., Sangli, India) and filtered water throughout the experimental protocol. The experimental protocol (No. CPCSEA/40/12) was approved by the Institutional Animal Ethics Committee and performed according to the guidelines of the Committee for Control and Supervision of Experimentation on Animals, Government of India.
2.4. Processing of MIMBP with an edible lipid-surfactant mixture: Development of processed monofloral Indian mustard bee pollen
A fixed amount (1,000 mg) of MIMBP was mixed with different weight (mg) ratios of Captex 355:Tween 80 (500:500, 500:750, 750:500, and 750:750) separately in mortars. Individually the blends were vigorously triturated for a period of 10 minutes to ensure complete wetting and uniform mixing of MIMBP powder with the lipid-surfactant mixture to produce processed pollen. For analysis, individual processed pollen masses were dispersed into distilled water (20 mL) and then mixed using a vortex mixer for 10 minutes. The solutions were diluted with ethanol (99%, 10×), filtered through a 0.45 μm membrane filter, and subjected to determination of total polyphenol content. The final selection of lipid-surfactant composition was made on the basis of the observed highest total amount of polyphenol contained by a processed pollen sample.
2.5. Determination of total polyphenol content
Total polyphenol content of all processed pollen samples was determined by the Folin-Ciocalteu colorimetric method.21 Briefly, 1 mL of sample solution was mixed with Folin-Ciocalteu phenol reagent (1 mL). An aqueous solution of sodium carbonate (7%, 10 mL) was added to the mixture followed by dilution to 25 mL with distilled water. After 90 minutes of incubation at room temperature, absorbance was measured at 760 nm using a JASCO V-630 UV-visible spectrometer, (Tokyo, Japan). The blank solutions were made by using corresponding lipid-surfactant mixtures without pollen so as to nullify any possible interference of the lipid-surfactant mix. The total polyphenol content was expressed in terms of mg gallic acid equivalent (GAE)/kg of pollen.
2.6. Determination of flavonoid aglycone content by high performance liquid chromatography
The processed MIMBP (PMIMBP) sample exhibiting the highest total polyphenol content was subjected to determination of free flavonoid aglycone marker compounds using the process described in a previous study.20 Briefly, PMIMBP (2,500 mg) was mixed with ethyl acetate (25 mL). To this diammonium sulfate (40%, 12.5 mL) and meta-phosphoric acid (20%, 2.5 mL) were added and subjected to shaking for 20 minutes. The mixture was filtered through a 0.45 μm membrane filter and transferred into a separating funnel. The organic phase was collected by repeating the extraction process. The collected organic phase was concentrated to dryness under reduced pressure at < 40 °C. The residue was reconstituted with methanol (1.5 mL), filtered through a 0.45 μm nylon syringe filter and subjected to high performance liquid chromatography (HPLC) analysis as per a previously developed method in the laboratory using the Jasco HPLC system (Tokyo, Japan). A Thermo Hypersil BDS C18 guard column (30 mm × 4.6 mm, 5 μm) coupled to a Thermo-Hypersil GOLD C18 RP column (250 mm × 4.0 mm, 5 μm) was used with a Jasco PU2089 Plus quaternary gradient pump, Jasco multiwavelength detector (PDA, MD-2010Plus), Chrompass software (version 1.8.6.1), and a Rheodyne injector with a 20 μL loop. Flow rate was maintained at 1 mL/min at ambient temperature. The mobile phase was comprised of water adjusted to pH 3.0 with ortho-phosphoric acid (Solvent A) and methanol (Solvent B) mixed using a linear gradient system; initially 30% Solvent B, 30–50% Solvent B at 5 minutes, 50–70% Solvent B at 10 minutes, 70–75% Solvent B at 15 minutes followed by an isocratic 75% Solvent B at 17 minutes. Solvent B was decreased to 30% over the next 3 minutes and held constant until the end of the 25 minute of run. Detection was performed in the range of 200–400 nm and chromatograms were extracted at 370 nm. Quantification was done using the calibration curve of the standard solution.20
2.7. Scanning electron microscopy
MIMBP and PMIMBP were observed under the scanning electron microscope (SEM), Oxford Instruments (Inca X Sight Model No. 6650-M, Abingdon, Oxfordshire, UK). Around 30 pollen grains from both samples were studied. The samples were placed on a 12 mm carbon grid attached to a SEM specimen mount and were sputter coated with a layer of gold/palladium.
2.8. Acute oral toxicity test
An acute oral toxicity test for MIMBP and PMIMBP was performed according to Organisation for Economic Co-operation and Development (OECD) guidelines number AOT 425. Accordingly, a total of 110 Swiss albino mice of either sex with an average body weight in the range of 28–30 g were randomly distributed into the vehicle control group, MIMBP treated, and PMIMBP treated groups. Five animals were assigned to each group. Each of the MIMBP and PMIMBP treated groups received oral doses of 55 mg/kg, 175 mg/kg, 550 mg/kg, 1,750 mg/kg, and 2,000 mg/kg, respectively for 14 days. The vehicle control group received gum acacia (10 mg/kg) per oral dose. The animals were continuously monitored for 42 hours to detect any changes in behavioral, respiratory or autonomic responses, restlessness, convulsions, tremors, salivation, diarrhea, and mortality.
2.9. Experimental protocol
The Wistar rats were randomly divided into nine different groups with six rats in each group as follows:
(1) Nonexercised groups
-
Group I:
Normal: Rats were administered a single daily oral dose of vehicle gum acacia (10 mg/kg, for 4 weeks) and were not subjected to any chronic swimming exercise.
-
Group II:
Per se: Rats were administered a single daily oral dose of PMIMBP (300 mg/kg, for 4 weeks) and were not subjected to any chronic swimming exercise.
(2) Exercised groups
-
Group III:
Exercised control: Rats were administered a single daily oral dose of vehicle gum acacia (10 mg/kg, for 4 weeks) and subjected to chronic swimming exercise.
-
Group IV:
MIMBP (100 mg/kg) + exercise: Rats were administered a single daily oral dose of MIMBP (100 mg/kg in gum acacia, for 4 weeks) and subjected to chronic swimming exercise.
-
Group V:
MIMBP (200 mg/kg) + exercise: Rats were administered a single daily oral dose of MIMBP (200 mg/kg in gum acacia, for 4 weeks) and subjected to chronic swimming exercise.
-
Group VI:
MIMBP (300 mg/kg) + exercise: Rats were administered a single daily oral dose of MIMBP (300 mg/kg in gum acacia, for 4 weeks) and subjected to chronic swimming exercise.
-
Group VII:
PMIMBP (100 mg/kg) + exercise: Rats were administered a single daily oral dose of PMIMBP (100 mg/kg, for 4 weeks) and subjected to chronic swimming exercise.
-
Group VIII:
PMIMBP (200 mg/kg) + exercise: Rats were administered a single daily oral dose of PMIMBP (200 mg/kg, for 4 weeks) and subjected to chronic swimming exercise.
-
Group IX:
PMIMBP (300 mg/kg) + exercise: Rats were administered a single daily oral dose of PMIMBP (300 mg/kg, for 4 weeks) and subjected to chronic swimming exercise.
The animals were subjected to chronic swimming exercise as per the reported protocol with minor modifications.7 All groups (except Groups I and II) were subjected to intensive daily swimming exercise for 4 weeks, 5 days per week with gradually increasing intensity and duration of swimming stimulus. The rats swam together for 60–90 minutes per day during the 1st week in a transparent, cylindrical polypropylene tank (40 × 24 cm2) containing water to a level of 30 cm at 25 ± 2 °C. Group swimming induces vigorous muscle activity as the rats climb over each other unlike when swimming alone. An external weight corresponding to 1.5% of the animal's body weight was attached to the base of the tail during the 2nd week and the rats swam for 90 minutes per day. Subsequently during the 3rd week and 4th week, the rats swam for 90 minutes per day with a tail-weight that increased weekly to 3% and 6% of the animal's body weight, respectively. After the initial vigorous movements during the first few minutes of water exposure, the rats balance themselves on their tails by rotating their bellies thereby reducing the swimming activity. Therefore in order to provide continuous stimuli during chronic swimming, the attached external weight was gradually increased each week. The MIMBP and PMIMBP were administered daily each morning before the swimming exercise.
2.10. Tissue sampling
Upon completion of the 4th week of swimming exercise, the rats were immediately sacrificed by cervical dislocation. The gastrocnemius muscles were excised from hind limbs of the rats, frozen quickly in liquid nitrogen and stored at −80 °C. The isolated muscle tissue samples were weighed and divided into three parts. In total between 30 mg and 40 mg of tissue was subjected to reverse transcriptase-polymerase chain reaction (RT-PCR) studies, another portion of tissue was used for analysis of oxidative markers and mitochondrial enzyme determination. The gastrocnemius muscles were allocated for histopathological analysis.
2.11. Biochemical analysis
The isolated gastrocnemius muscle tissues were cut into small pieces, placed in a chilled sucrose solution (0.25 M) and homogenized in Tris buffer (pH 7.4, 10 mM) to a concentration of 10% w/v in a tissue homogenizer (Remi Motors, Mumbai, India). The homogenates were subjected to centrifugation at 13,000 g at 4 °C for 25 minutes using an Eppendorf 5810-R high speed cooling centrifuge. The clear supernatant was subjected to analysis of lipid peroxidation, SOD, GSH reduction, total protein content, and nitrite level according to previously described methods.22, 23
2.12. Mitochondrial enzymes estimation
Gastrocnemius muscle mitochondrial isolation was performed according to a previously described method.24 Briefly, the gastrocnemius muscle (1 g) was homogenized in buffer and the homogenates were centrifuged at 13,000 g at 4 °C for 5 minutes. Pellets were suspended in isolation buffer with ethylene glycol tetra acetic acid (EGTA) and spun at 13,000 g at 4 °C for 5 minutes. The resulting supernatants were transferred into new tubes and topped off with isolation buffer with EGTA and respun at 13,000 g at 4 °C for 10 minutes. Pellets containing pure mitochondria were resuspended in isolation buffer without EGTA and subjected to mitochondrial enzyme measurement. Mitochondrial enzymes Complex-I (NADH dehydrogenase) and Complex-II (succinate dehydrogenase) activities were measured spectrophotometrically according to a previously described method.25 Mitochondrial redox activity (Complex-III) i.e., MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-H-tetrazolium bromide] reduction rate was used to assess the activity of the mitochondrial respiratory chain in isolated mitochondria. It was determined according to a previously described method.26 Mitochondrial Complex-IV (cytochrome oxidase) activity was assayed in gastrocnemius muscle mitochondria according to a previously described method.27
2.13. RNA extraction and RT-PCR analysis
The RNA extraction and RT-PCR analysis was performed as per a previous study using a standard protocol provided by Biotools B&M Labs.28 Briefly, the isolated gastrocnemius muscle tissue (30–40 mg) was disrupted in liquid nitrogen using a mortar and pestle. To this, lysis buffer (350 μL) and β-mercaptoethanol (3.5 μL) were added followed by vigorous vortexing. The lysate was loaded into a filtering column in a collection tube and centrifuged for 1 minute at 11,000 g. The clarified lysate was mixed with 70% ethanol (350 μL) and loaded into the RNA binding column in a collection tube followed by centrifugation for 30 seconds at 11,000 g. Desalting buffer DBR (350 μL) and rDNase reaction mixture (95 μL) were added and incubated at room temperature for 15 minutes. Following successive washings with wash buffer, RNA was resuspended in ribonuclease-free water (60 μL) and centrifuged. The elute containing pure RNA was stored at −80 °C until analysis.
2.14. cDNA preparation
Single-stranded cDNA was synthesized from total cellular RNA using RT-PCR. Briefly, total RNA (2 μL) was treated with 100 mM magnesium sulfate solution (10 μL), primers (5 μL), and PCR astringent (12 μL). The volume was made up to 50 μL with nuclease free water. The primers were myostatin (5′-ATCTGAGAGCCGTCAAGACTCC-3′, 5′-CAGTCAAGCCCAAAGTCTCTCC-3′ base pair-340) and β-actin (5′-GGCATCGTGATGGACTCCG-3′, 5′GCTGGAAGGTGGACAGCGA-3′ base pair-760) synthesized by Amnion Biosciences Pvt. Ltd. The amplification of β-actin served as a control for sample loading and integrity. PCR products were detected by electrophoresis on a 1.5% agarose gel containing ethidium bromide. The size of amplicons was confirmed by using a 100 bp ladder (Amnion Biosciences Pvt. Ltd.) as a standard size marker. The amplicons were visualized and images were captured using a gel documentation system (Alpha Innotech Inc.,San Leandro, California, USA). Gene expression was assessed semiquantitatively by generating densitometry data for band intensities in different sets of experiments and analyzing the gel images using the software ImageJ program (version 1.33, Bethesda, Maryland, USA). The band intensities were compared with constitutively expressed β-actin. The intensity of myostatin mRNAs was standardized against that of the β-actin mRNA from each sample and results were expressed as the myostatin mRNA/β-actin mRNA ratio.
2.15. Histopathological analysis
The excised gastrocnemius muscle tissues were stored in 10% formalin for 24 hours. The specimens were dehydrated and placed in xylene for 1 hour (3 times) and later in ethyl alcohol (70%, 90%, and 100% respectively) for 2 hours. Tissue specimens were cut into sections of 3–5 μm thickness and were stained with hematoxylin and eosin (H&E). The specimens were mounted on a slide using a distrene phthalate xylene (DPX) medium. The sections were examined under a light microscope.
2.16. Statistical analysis
Data values were expressed as the mean ± standard error mean (SEM). Data analysis was performed using software (version 5.0, GraphPad, San Diego, CA, USA). Data of biochemical parameters were analyzed using one-way analysis of variance (ANOVA) followed by Dunnett's test for post hoc analysis. A p value < 0.05 was considered to be statistically significant. Gel images were analyzed using Image J 1.37, NIH, USA software.
3. Results
3.1. Characterization of PMIMBP
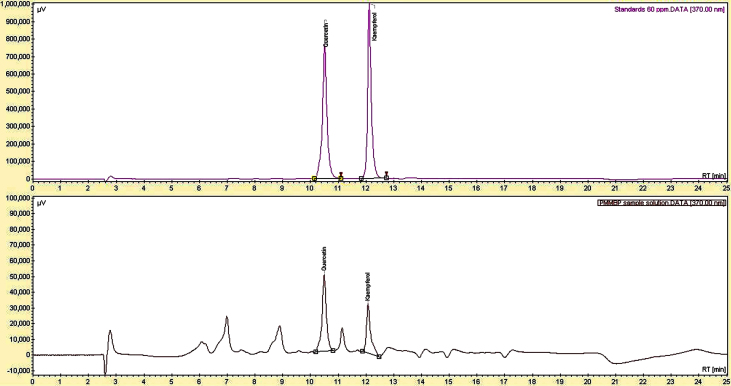
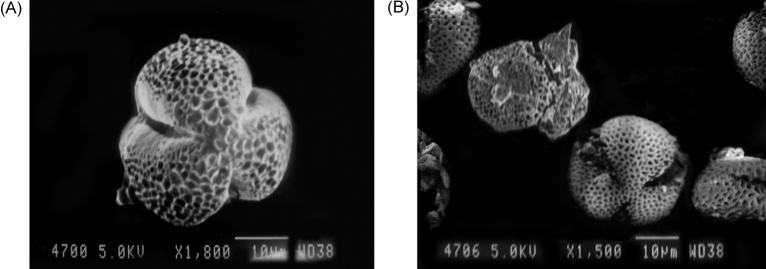
Table 1 shows the observed yields of total polyphenol content for processed pollen samples produced using different ratios of Captex 355 and Tween 80. The composition of lipid-surfactant mixture was found to be decisive in the extraction of polyphenols from the pollen sample. The pollen sample with the highest total polyphenol content (31,233.55 ± 1,634.45 mg GAE/kg) was processed with Captex 355:Tween 80 at a weight ratio of 500:750 and was selected for further investigation (hereafter referred to as PMIMBP). All the samples were yellowish brown in color and were readily dispersed in distilled water upon vortexing before analysis. HPLC analysis showed that the PMIMBP comprised 88.94 ± 3.15 mg/kg and 130.21 ± 2.55 mg/kg of kaempferol and quercetin, respectively. Fig. 1 illustrates the stacked view of representative chromatograms for the standards and PMIMBP sample solution. The SEM analysis showed subolate to subprolate shape and medium trizonocolpate along with ambtrilobed fossaperture characteristic to MIMBP. The exine part of PMIMBP was found to be partially ruptured which may be as a result of the process of vigorous trituration in the presence of a lipid-surfactant mixture (Fig. 2A, 2B).
Table 1.
Total polyphenol content for processed pollen samples
| Amount of Captex 355 (mg) | Amount of Tween 80 (mg) | Total polyphenol content expressed as (mg GAE/kg) |
|---|---|---|
| 500 | 500 | 21,347.59 ± 1,444.59 |
| 500 | 750 | 31,233.55 ± 1,634.45 |
| 750 | 500 | 25,345.39 ± 1,386.13 |
| 750 | 750 | 27,589.91 ± 1,456.23 |
Data are presented as mean ± SD; n = 3.
GAE, gallic acid equivalents.
Bold values show the highest value of Total Polyphenol content at specific ratio.
Fig. 1.
Stacked view of representative chromatograms obtained for the standards and the PMIMBP sample solution.
PMIMBP, processed monofloral Indian mustard bee pollen.
Fig. 2.
Scanning electron microscope image for (A) MIMBP sample; (B) PMIMBP sample. MIMBP, monofloral Indian mustard bee pollen;PMIMBP, processed monofloral Indian mustard bee pollen.
3.2. Acute oral toxicity test
Table 2 shows the survival data for the acute toxicity study of MIMBP and PMIMBP. No mortality was observed in any group at the administered doses. Furthermore, no signs or symptoms of toxicity were observed during the entire period. Both MIMBP and PMIMBP were found to be safe up to 2,000 mg/kg.
Table 2.
Acute oral toxicity (AOT) study for MIMBP and PMIMBP: Survival data
| Treatment (dose in mg/kg) | Sex | Dead/total | Dead (%) | Toxicity symptoms/adverse effects |
|---|---|---|---|---|
| Vehicle control | Male | 0/5 | 0 | None |
| Female | 0/5 | 0 | None | |
| MIMBP (55) | Male | 0/5 | 0 | None |
| Female | 0/5 | 0 | None | |
| MIMBP (175) | Male | 0/5 | 0 | None |
| Female | 0/5 | 0 | None | |
| MIMBP (550) | Male | 0/5 | 0 | None |
| Female | 0/5 | 0 | None | |
| MIMBP (1,750) | Male | 0/5 | 0 | None |
| Female | 0/5 | 0 | None | |
| MIMBP (2,000) | Male | 0/5 | 0 | None |
| Female | 0/5 | 0 | None | |
| PMIMBP (55) | Male | 0/5 | 0 | None |
| Female | 0/5 | 0 | None | |
| PMIMBP (175) | Male | 0/5 | 0 | None |
| Female | 0/5 | 0 | None | |
| PMIMBP (550) | Male | 0/5 | 0 | None |
| Female | 0/5 | 0 | None | |
| PMIMBP (1,750) | Male | 0/5 | 0 | None |
| Female | 0/5 | 0 | None | |
| PMIMBP (2,000) | Male | 0/5 | 0 | None |
| Female | 0/5 | 0 | None |
MIMBP, monofloral Indian mustard bee pollen; PMIMBP, processed monofloral Indian mustard bee pollen.
3.3. Effect of MIMBP and PMIMBP treatment on body weight and relative gastrocnemius muscle weight
A significant decrease (p < 0.001) in the body weight of exercised control rats as compared to normal rats was observed. A significant reduction (p < 0.01) in the relative gastrocnemius muscle weight of exercised control rats was observed in comparison to the normal groups. Rats administered with MIMBP (200 mg/kg and 300 mg/kg) showed significant (p < 0.05 and p < 0.001) and dose dependent inhibition of decrease in body weight when compared to exercised control rats. Relative weight of the gastrocnemius muscle increased significantly (p < 0.05) in MIMBP (300 mg/kg) treated rats when compared to that of exercised control rats. When compared with exercised control rats, PMIMBP (100 mg/kg, 200 mg/kg, and 300 mg/kg) treated rats showed a significant increase (p < 0.001) in body weight whereas the relative gastrocnemius muscle weight was significantly increased (p < 0.05) in the PMIMBP (300 mg/kg) treated rats. Per se treated animals did not show any significant change in body weight and relative gastrocnemius muscle weight when compared to that of normal rats (Table 3).
Table 3.
Effect of MIMBP and PMIMBP on chronic swimming induced alteration in body weight and gastrocnemius muscle weight in rats.*
| Treatment | Body weight (g) | Absolute gastrocnemius muscle weight (g) | Relative gastrocnemius muscle weight† |
|---|---|---|---|
| Normal | 252.60 ± 4.22 | 1.82 ± 0.07 | 0.0072 ± 0.00028 |
| Exercised control | 182.40 ± 2.63‡ | 1.19 ± 0.09‡ | 0.0064 ± 0.00048‡ |
| MIMBP (100) + Exercise | 199.00 ± 2.42 | 1.27 ± 0.15 | 0.0064 ± 0.00076 |
| MIMBP (200) + Exercise | 204.40 ± 5.62|| | 1.35 ± 0.08 | 0.0066 ± 0.00038 |
| MIMBP (300) + Exercise | 217.80 ± 2.87¶ | 1.57 ± 0.04|| | 0.0072 ± 0.00012|| |
| PMIMBP (100) + Exercise | 213.80 ± 1.93¶ | 1.31 ± 0.12 | 0.0061 ± 0.00058 |
| PMIMBP (200) + Exercise | 219.60 ± 3.20¶ | 1.54 ± 0.06|| | 0.0070 ± 0.00023 |
| PMIMBP (300) + Exercise | 226.40 ± 9.01¶ | 1.70 ± 0.07¶ | 0.0076 ± 0.00062** |
| Per se | 248.40 ± 7.38 | 1.84 ± 0.09 | 0.0076 ± 0.00056 |
Data are expressed as mean ± standard error mean.
Data were analyzed by one-way analysis of variance followed by Dunnett's test.
Relative gastrocnemius muscle weight represents the ratio of absolute gastrocnemius muscle weight to the body weight.
p < 0.01, §p < 0.001 as compared to normal rats.
p < 0.05 and ¶p < 0.001 as compared to exercise control rats.
MIMBP, monofloral Indian mustard bee pollen; PMIMBP, processed monofloral Indian mustard bee pollen.
3.4. Effect of MIMBP and PMIMBP treatment on SOD and GSH levels
Significant reduction (p < 0.001) in SOD and GSH levels of the gastrocnemius muscle was observed in the exercised control group as compared to that of normal rats. MIMBP (200 mg/kg and 300 mg/kg) treatment significantly inhibited SOD reduction (p < 0.05 and p < 0.001 respectively). Furthermore, it significantly prevented reduction in GSH levels as compared to that of exercised control rats (p < 0.05). PMIMBP (100 mg/kg, 200 mg/kg, and 300 mg/kg) treated rats showed a significant and dose dependent increase in SOD level as compared to that of exercised control rats (p < 0.05, p < 0.001, and p < 0.001, respectively). PMIMBP (200 mg/kg and 300 mg/kg) treatment showed a significant and dose dependent increase in GSH levels as compared to exercised control rats (p < 0.01 and p < 0.001). There was no significant change in SOD and GSH levels in per se treated animals when compared with normal rats (Table 4).
Table 4.
Effect of MIMBP and PMIMBP on chronic swimming induced alteration in SOD, GSH, MDA, NO and total protein in gastrocnemius muscle in rats.*
| Treatment | SOD (U/mg of protein) |
GSH (μg/mg of protein) |
MDA (nm/mg of protein) |
NO (μg/ml) |
Total protein (mg/gm) |
|---|---|---|---|---|---|
| Normal | 23.53 ± 0.45 | 1.86 ± 0.14 | 2.98 ± 0.32 | 97.66 ± 8.61 | 18.50 ± 2.69 |
| Exercised control | 4.17 ± 0.57|| | 0.22 ± 0.03|| | 20.10 ± 0.65|| | 512.2 ± 14.99|| | 74.50 ± 7.84|| |
| MIMBP (100) + Exercise | 6.31 ± 0.55 | 0.45 ± 0.06 | 19.54 ± 0.53 | 499.5 ± 11.01 | 63.50 ± 5.62 |
| MIMBP (200) + Exercise | 8.34 ± 0.91† | 0.57 ± 0.06† | 18.80 ± 0.43 | 464.7 ± 7.70 | 63.50 ± 6.96 |
| MIMBP (300) + Exercise | 10.15 ± 0.84‡ | 0.62 ± 0.07† | 17.04 ± 0.74‡ | 444.6 ± 8.83‡ | 47.50 ± 3.76‡ |
| PMIMBP (100) + Exercise | 8.58 ± 0.71† | 0.44 ± 0.03 | 16.84 ± 0.46‡ | 452.5 ± 14.43‡ | 67.5 ± 3.26 |
| PMIMBP (200) + Exercise | 14.89 ± 2.07§ | 0.73 ± 0.04‡ | 13.12 ± 0.71§ | 383.8 ± 13.46§ | 43.0 ± 6.68§ |
| PMIMBP (300) + Exercise | 17.24 ± 0.69§ | 1.27 ± 0.13§ | 9.58 ± 1.09§ | 241.0 ± 20.00§ | 31.0 ± 3.58§ |
| Per se | 21.73 ± 1.49 | 1.69 ± 0.11 | 3.82 ± 0.32 | 93.88 ± 6.64 | 27.00 ± 2.15 |
Data are presented as mean ± standard error mean.
Data were analyzed by one-way analysis of variance followed by Dunnett's test.
p < 0.05, ‡p < 0.01 and §p < 0.001 as compared to exercise control rats.
p < 0.001 as compared to normal rats.
GSH, glutathione; MDA, malonaldehyde; MIMBP, monofloral Indian mustard bee pollen; NO, nitric oxide; PMIMBP, processed monofloral Indian mustard bee pollen; SOD, superoxide dismutase.
3.5. Effect of MIMBP and PMIMBP treatment on MDA, nitric oxide, and total protein level
There was a significant increase (p < 0.001) in MDA, nitric oxide (NO), and total protein level in the gastrocnemius muscle of exercised control group rats as compared to normal rats. The MIMBP (300 mg/kg) treatment showed significant reduction in MDA, NO, and total protein level as compared to that of exercised control rats (p < 0.01). The PMIMBP (100 mg/kg, 200 mg/kg, and 300 mg/kg) treatment showed a significant and dose dependent decrease in MDA and NO level as compared to vehicle rats (p < 0.01, p < 0.001, and p < 0.001, respectively). When compared with exercised control rats, treatment with PMIMBP (200 mg/kg and 300 mg/kg) showed a significant reduction (p < 0.001) in the total protein level. Per se treated animals did not show any significant alteration in MDA, NO, and total protein level as compared to normal rats (Table 4).
3.6. Effect of MIMBP and PMIMBP treatment on mitochondrial complex enzymes
Exhaustive chronic swimming stress for 4 weeks significantly impaired mitochondrial complex enzyme activities in exercised control rats as compared to normal group rats (p < 0.001). MIMBP (300 mg/kg) treatment significantly restored mitochondrial Complex-I, -III, and -IV enzyme activities as compared to the exercised control group (p < 0.01). However MIMBP (200 mg/kg and 300 mg/kg) produced a significant increase in mitochondrial Complex-II enzyme activity as compared to that of the exercised control group (p < 0.01). Furthermore, PMIMBP (200 mg/kg and 300 mg/kg) significantly and dose dependently inhibited the exhaustive swimming stress induced alteration in mitochondrial Complex-I, -II, -III, and -IV enzyme activities as compared to that of exercised control rats (p < 0.05 and p < 0.001). However, the per se group did not produce any significant alteration in mitochondrial Complex-I, -II, -III, and -IV enzyme activities as compared to normal rats (Table 5).
Table 5.
Effect of MIMBP and PMIMBP on chronic swimming induced alteration in mitochondrial enzyme activity in gastrocnemius muscle in rats.*
| Treatment | Complex-I (nmol of NADH oxidized/min/mg protein) | Complex-II (mmol/mg protein) | Complex-III (MTT assay) (OD at 540 nm) | Complex-IV (nmol cyto-C oxidized/min/mg protein) |
|---|---|---|---|---|
| Normal | 22.34 ± 3.61 | 71.20 ± 4.25 | 0.39 ± 0.04 | 6,071 ± 173.3 |
| Exercised control | 4.10 ± 0.75† | 14.85 ± 3.49† | 0.09 ± 0.01† | 1,387 ± 431.3† |
| MIMBP (100) + Exercise | 4.91 ± 0.63 | 27.90 ± 2.66 | 0.10 ± 0.008 | 1,277 ± 390.5 |
| MIMBP (200) + Exercise | 4.70 ± 1.05 | 31.77 ± 2.84§ | 0.16 ± 0.01 | 2,598 ± 570.4 |
| MIMBP (300) + Exercise | 10.60 ± 0.82§ | 36.39 ± 5.05§ | 0.20 ± 0.008§ | 4,498 ± 487.5§ |
| PMIMBP (100) + Exercise | 6.90 ± 0.68 | 20.93 ± 2.48 | 0.14 ± 0.01 | 1,884 ± 727.6 |
| PMIMBP (200) + Exercise | 13.05 ± 1.85§ | 36.78 ± 3.57‡ | 0.018 ± 0.02‡ | 4,068 ± 382.0‡ |
| PMIMBP (300) + Exercise | 17.92 ± 1.36|| | 58.46 ± 3.76|| | 0.33 ± 0.03|| | 5,578 ± 1104|| |
| Per se | 19.99 ± 1.59 | 73.07 ± 4.10 | 0.40 ± 0.02 | 5,925 ± 806.1 |
Data are expressed as mean ± standard error mean.
Data was analyzed by one-way analysis of variance followed by Dunnett's test.
p < 0.001 as compared to normal rats.
p < 0.05, §p < 0.01, and ||p < 0.001 as compared to exercise control rats.
MIMBP, monofloral Indian mustard bee pollen; PMIMBP, processed monofloral Indian mustard bee pollen.
3.7. Effect of MIMBP and PMIMBP treatment on myostatin expression in gastrocnemius muscle
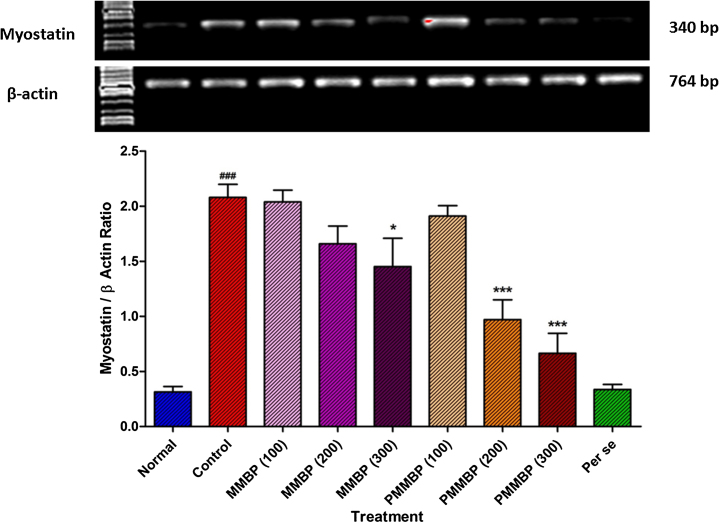
Significant upregulation of myostatin mRNA expression in the gastrocnemius muscle of exercised control rats upon 4 weeks of exhaustive swimming stress was observed as compared to that of normal group rats (p < 0.001). MIMBP (300 mg/kg) treatment showed significant downregulation in myostatin mRNA expression as compared to that of exercised control group (p < 0.05). The PMIMBP (200 mg/kg and 300 mg/kg) treatment showed significant inhibition in exhaustive swimming stress induced upregulation of myostatin mRNA expression as compared to that of the exercised control group (p < 0.001). No significant alteration in the myostatin mRNA expression of the per se group was observed when compared with that of normal group (Fig. 3).
Fig. 3.
Effect of MIMBP and PMIMBP on chronic swimming induced alteration in reverse transcriptase analysis of protein expression of myostatin.
Data are expressed as mean ± standard error mean.
Data was analyzed by one-way analysis of variance followed by Dunnett's test.
# p < 0.05 as compared to normal rats. *p < 0.05 as compared to exercise control rats.
MIMBP, monofloral Indian mustard bee pollen; PMIMBP, processed monofloral Indian mustard bee pollen.
3.8. Effect of MIMBP and PMIMBP treatment on histopathological alteration in rat gastrocnemius muscle
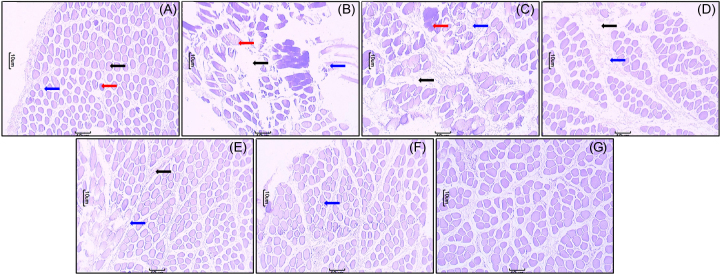
Histological findings of the gastrocnemius muscle of the normal group of rats stained with H&E revealed the presence of bundles of muscle fibers (black arrow) separated by connective tissue perimysium (blue arrow). The connective tissue endomysium containing blood vessels (red arrow) was detected among the muscle fibers (Fig. 4A). Gastrocnemius muscle from exercised control rats showed splitting of the myofibers (red arrow) in addition to focal areas with cellular infiltration (blue arrow) and containing myofibers characterized by fragmentation of the sarcoplasm and necrosis (black arrow). The exercised control group showed few blood vessels as compared to the normal group (Fig. 4B). Rats treated with MIMBP (200 mg/kg) showed the presence of cellular infiltration (blue arrow), splitting of the myofibers (red arrow), and necrosis (black arrow) (Fig. 4C). These features were halted by MIMBP (300 mg/kg) treatment (Fig. 4D). However, PMIMBP (200 mg/kg) treated rats showed the presence of cellular infiltration (blue arrow) and necrosis (black arrow) but the structure of myofibers remained intact (Fig. 4E). PMIMBP (300 mg/kg) treated rats showed minor infiltration of inflammatory cells (blue arrow) with a normal myofibers structure evident as compared to that of the exercised control group (Fig. 4F). Fig. 4G depicts the normal architecture of a gastrocnemius muscle from per se treated animals. Qualitatively the histological investigation suggests that supplementation of PMIMBP inhibits the increase in thickness of muscle fibers (Fig. 4E, 4F) as compared to that of the exercised control group.
Fig. 4.
Effect of MIMBP and PMIMBP on chronic swimming induced alteration in histopathological analysis of gastrocnemius muscles. Photomicrographs of sections of gastrocnemius muscles from rats stained with hematoxylin & eosin. Gastrocnemius muscles microscopic image of (A) normal rat, (B) exercise control rat, (C) MIMBP (200 mg/kg) + exercise rat, (D) MIMBP (300 mg/kg) + exercise rat, (E) PMIMBP (200 mg/kg) + exercise rat, (F) PMIMBP (300 mg/kg) + exercise rat, and (G) Per se treated rats (microscopic examination under 100 × light microscopy).
MIMBP, monofloral Indian mustard bee pollen; PMIMBP, processed monofloral Indian mustard bee pollen.
4. Discussion
In the current study, the processing of MIMBP with an edible lipid-surfactant mixture (Captex 355:Tween 80) was found to improve the availability of engulfed nutrients. Polyphenols, the most important group of antioxidant principles, have previously been reported to improve the swimming performance of rodents in forced conditions.29, 30, 31 Therefore, the PMIMPB samples were evaluated for total polyphenol content. The protective role against several kinds of oxidative stresses has been well documented for kaempferol, a popular flavonoid.32, 33, 34 Quercetin is another potent flavonoid effective in alleviating oxidative stress conditions.29, 35, 36, 37 The MIMBP was reported to be a rich source of these flavonoids as principal marker components.20 Therefore the content of these potent flavonoid aglycones from PMIMBP was determined. Lipid based systems in the form of a mixture of oil and surfactants have been reported to improve the solubility and bioavailability of poorly soluble actives.38 Several lipid excipients comprising medium chain triglycerides (MCTs) have been documented for improving the poor solubility of phytoconstituents and thereby increasing their extraction efficiency.39, 40, 41 Also, surfactants, on account of their amphiphilic nature comprising both hydrophobic and hydrophilic moieties, have been employed for extraction of several phytocompounds especially phenolics.42, 43, 44 Taking the ability of MCT oils and surfactants to improve solubility and extraction efficiency into account, the current study used a combination of the edible oil Captex 355 with the surfactant Tween 80, for extraction of polyphenols entrapped in the MIMBP shell. According to a previous study, the MIMBP, when subjected to a conventional extraction method in ethanol (on a rotating mechanical shaker at 33,987 g for 30 minutes at 70 °C), obtained polyphenols of about 18,286.1 ± 374.0 mg GAE/kg. Furthermore, the MIMBP was found to contain the flavonoids kaempferol (65.4 ± 0.5 mg/kg) and quercetin (51.4 ± 0.4 mg/kg).20 The extraction of polyphenols from herbal drug matrices is influenced by several factors such as temperature, extraction time, solvent-to-solid ratio, type of solvent, etc.45, 46 In the case of MIMBP the tough pollen shell hinders the process of extraction due to its rigid composition and insoluble nature. In the current study, observed higher yields of polyphenols and flavonoid aglycones for PMIMBP can be attributed to the properties of Captex 355 and Tween 80, respectively. Captex 355 is a MCT obtained by esterification of glycerin and fatty acids (mainly caprylic and capric acid).47 It exhibits high lipophilicity and thereby possesses an ability to engulf a wide range of nonpolar polyphenolic compounds. It also contributes to providing stability of polyphenols against oxidation. Tween 80, a nonionic, less toxic, and stable surfactant comprising of polyoxyethylene-(20)-sorbitan monooleate has proven to be an efficient extractant for polyphenols from apple pomace.45 Tween 80, on account of its solubilizing effect, has contributed to the enhanced extraction of polyphenols in two possible ways: (1) by reducing the surface tension thereby enhancing the release of entrapped polyphenols and (2) by facilitating increased contact between polyphenols and the oil component of the lipid-surfactant mixture which ultimately solubilizes in the aqueous phase.45 The initial stage in the process of extraction is penetration of the extractant into a herbal matrix by the wetting of substances present within the cells. Surfactants reduce surface tension and facilitate wetting and swelling of the plant material which intensifies the process of mass transfer resulting in improved extraction of active substances. Similar improvements in the extraction of the flavonoid rutin from Japanese pagoda tree buds by using surfactants has been reported.48 Increased extraction of total polyphenols and flavonoid aglycones for PMIMBP can be attributed to the enhanced solubilization of polyphenolic moieties in the oil-surfactant mixture. The hydrophilic polyoxyethylene chain of Tween 80 together with the lipophilic caprylic-capric triglyceride composition of Captex 355 provides good solvent properties for engulfing the polyphenolic moieties. Similar improvements in the solubility of the flavonoid quercetin in the mixture of MCT Capmul MCM and Tween 20 has been documented.49 The trituration was found to be an important step for uniform mixing and homogenization of the lipid-surfactant mixture with the MIMBP inducing partial rupture of the pollen coat (as observed in the SEM image Fig. 2B). This might have contributed to intensifying the mass transfer of polyphenols into the lipid matrix improving the extraction efficiency. The rigid sporopollenin matrix of the pollen shell (comprising of polyterpene) is reported to be solubilized in to a mixture of a hydrolysable ester such as Tween and a hydrophilic solvent.50 This suggests the possible solubilization of the sporopollenin components of the pollen shell into the lipid-surfactant mixture thereby contributing to enhanced mass transfer of polyphenolics into the lipid matrix.
The observed results of biochemical analysis in terms of SOD, GSH, MDA, NO, and total protein levels confirmed an antioxidant effect of both MIMBP and PMIMBP in oxidative stress induced situations in the gastrocnemius muscle of Wistar rats. Forced swimming stress has been documented to be a continuous stressor with both psychological and physiological components.29 The exhaustive swimming stress for 4 weeks decreased the body mass and reduced relative weight of the gastrocnemius muscle in exercised control rats, which can be attributed to a decreased fat component.7 During exercise, energy demand exceeds around 35 times that required at rest.51 Therefore the oxygen intake increases greatly during muscular activity. This leads to the generation of a ROS that is considered responsible for muscle fatigue during exercise. Different mechanisms which contribute to the generation of free radicals may include: (1) the elevation of intramuscular calcium during high intensity exercise which activates protease enzymes which convert xanthine dehydrogenase into xanthine oxidase. This consumes molecular oxygen instead of NAD+, and thereby produces superoxide radicals; (2) intermittent hypoxia and reoxygenation in exercised muscles during cyclic contractions and relaxations may convert molecular oxygen into a superoxide radical; (3) strenuous physical exercise may lead to temporary ischemia in the muscles exerting damage. This induces activation of leukocytes which may stimulate the production of free radicals; (4) ischemic or hypoxic conditions stimulate NO synthase activity, leading to the generation of NO radicals; and (5) the activation of phospholipase A2 releases arachidonic acid from phospholipids. Ciclooxygenase, when it reacts with arachidonic acid generates hydroxyl radicals.51, 52 Further excessive exercise results in muscle glycogen depletion due to the preferential use of intramuscular triacylglycerol and circulating lipids by the skeletal muscle.53 Moreover the increased breakdown of glycogen leads to intracellular accumulation of lactic acid which dissociates into lactate and H+. This proton accumulation due to acidosis contributes to exercise-induced oxidative stress.54 Enhanced levels of ROS in oxidative stressed conditions promote contractile dysfunction leading to skeletal muscle fatigue. Furthermore, the prolonged and intense exercise can induce oxidative damage to cellular constituents.55 Therefore the skeletal muscles need a better antioxidant shield against potential damage that occurs during and after exercise. An antioxidant enzyme SOD, existing in the mitochondria and cytosol, reduces the oxygen radical (O2.) generated during exhaustive stress to H2O and thus scavenges the free radical.28 The GSH shields tissues and cells against generated free radicals by converting H2O2 to H2O. Observed reduced levels of SOD and GSH in the exercise control group of rats indicate the progression of oxidative stress and disturbed balance between pro- and antioxidative enzymes in the cells. Both MIMBP and PMIMBP supplementation inhibited the reduction of SOD and GSH levels indicating restoration of the oxidative balance in the muscles. It was evident that the groups treated with PMIMBP exerted a greater increase in SOD and GSH levels when compared to groups treated with MIMBP. The elevated levels of MDA i.e., enhanced lipid peroxidation in the gastrocnemius muscle of exercised control group rats, indicate oxidative damage to the tissue.1, 56 Furthermore, the increased NO levels in the muscle tissues are suggestive of stressed conditions.22 MIMBP supplementation at a high dose of 300 mg/kg was found to be effective in reducing MDA and NO levels while the PMIMBP exerted progressive downregulation of the same.
Impaired mitochondrial function contributes to the development of oxidative stress by decreasing oxidative phosphorylation and adenosine triphosphate (ATP) generation along with a marked rise in free radicals.3 During exercise the oxygen requirement of muscles increases. The oxygen consumed by the mitochondria in particular, undergoes one electron reduction generating superoxide radicals. The process of energy (ATP) production in mitochondria is catalyzed by the membrane bound protein complexes NADH dehydrogenase, succinate dehydrogenase, and ubiquinol cytochrome C oxidoreductase. NADH dehydrogenase is responsible for the transfer of electrons into the electron transport chain (ETC). It catalyzes the dehydrogenation of NADH generated through oxidation of numerous NADP-linked dehydrogenase reactions. The succinate dehydrogenase (SDH) is another enzyme responsible for the transfer of electrons into the ETC. It plays a key role in neuronal energy metabolism. Significant reduction in Complex-I and Complex-II activities of the exercise control group of rats can be ascribed to an increased ROS which inhibits the catalytic function of the enzymes.3, 4, 5 MIMBP (300 mg/kg) and PMIMBP (200 mg/kg and 300 mg/kg) prevented the attenuation of active NADH dehydrogenase. Furthermore, both MIMBP and PMIMBP prevented the attenuation of mitochondrial SDH activity. The improved activity of Complex-I and -II suggests there was an increase in the rate of transfer of electrons into the ETC.5 Cytochrome C catalyzes electron transport from ubiquinone to cytochrome oxidase. Cytochrome oxidase in the presence of reduced cytochrome C and oxygen transfers a proton into the mitochondrial inner membrane. Significant reduced activity of Complex-IV enzyme of the exercise control group of rats can be attributed to the oxidative stress induced by an increased ROS.3, 4 MIMBP (300 mg/kg) and PMIMBP (200 mg/kg and 300 mg/kg) significantly reversed the reduced cytochrome oxidase activity. Results of a MTT assay indicate that there was a significant decline in Complex-III activity of the exercise control group of rats which reflects impaired mitochondrial respiration. Both MIMBP and PMIMBP were found effective in restoring the decline in mitochondrial respiration due to oxidative stress. These findings suggest that the MIMBP and PMIMBP render their antioxidant effect through up regulation of mitochondrial enzyme activity.
Myostatin exerts an inhibitory role on skeletal muscle development and growth.57, 58 It regulates the number of myofibers formed during the development and postnatal growth of muscles. Several studies have reported alterations in the myostatin mRNA expression of rodents when exposed to rigorous exercise in the form of acute and short term swimming training,7 treadmill running,59 and chronic wheel running.60 This evidence has shown decreased myostatin mRNA expression in the muscles of rodents when exposed to exercise or training. However in the current study a significant increase in myostatin expression at mRNA level in the exercised control group of rats when subjected to intense swimming exercise for 4 weeks was observed. This can be attributed to the development of oxidative stress in the gastrocnemius muscle due to the excessive generation of ROS during chronic swimming exercise. The elevated ROS generation activates intracellular cytokines such as tumor necrosis factor-α which in turn stimulate myostatin expression.61, 62 Similarly, increased myostatin mRNA and protein expression upon heavy resistance training for 12 weeks has been documented by Willoughby.63 Overexpression of myostatin leads to muscle wasting.2, 64 It was accompanied by an observed reduction in the relative muscle and body weight of exercised control rats. This finding is in agreement with the previously stated negative role of myostatin in regulating muscle mass. Evident increases in the muscle mass and body weight of rats from Groups VI, VII, VIII, and IX as compared to that of the exercised control group confirmed the myostatin inhibitory role of both MIMBP and PMIMBP supplements suggesting their protective influence against muscle wasting conditions. Exercise induces alterations in muscle fiber morphometry and capillarization in tissues.65 There is a need for detailed morphometrical assessment of the exercised gastrocnemius muscles to trace and quantitate the effects of MIMBP and PMIMBP supplementation. Moreover, apart from myostatin, the influence of other genes such as fibroblast growth factors (fgf-18 and fgf-20), and atrogenes involved in growth, development, and degradation of skeletal muscles65 need to be analyzed.
In summary, the findings of this study highlight the antioxidant influence of MIMBP (and PMIMBP) at a biochemical and mitochondrial level in the gastrocnemius muscle of Wistar rats. Additionally, the observed myostatin inhibitory effect suggests muscle protectant ability. Along with the polyphenols, MIMBP also comprises other nutrients such as proteins, carbohydrates, etc.20 These nutrients might also have contributed to the observed beneficial effects. Based on the evident improvement in therapeutic efficiency of PMIMBP over MIMBP, it is believed that MCT and the surfactant composition of a lipid matrix might have contributed to improving the bioavailability of pollen nutrients; however systematic studies to determine their levels circulating in the blood and available at the site of absorption need to be undertaken.
In conclusion, this study for the first time establishes the muscle protectant role of neat and processed bee pollen supplementation on exercise-induced oxidative stress in the gastrocnemius muscle of Wistar rats. Processing of MIMBP using an edible lipid-surfactant mixture proved useful for improving the availability of engulfed nutrients which exert beneficial effects. The mitochondrial upregulating effects of MIMBP and PMIMBP were apparent. Further myostatin inhibitory effects of MIMBP and PMIMBP were established suggesting their role in preventing muscle wasting conditions.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
The authors wish to thank the University Grants Commission, India for providing financial assistance for the work carried out. The authors are also thankful to Dr. Lakshmi Rao, the Central Bee Research and Training Institute, Pune, India for support in identification and authentication of pollen samples through discussions during the project.
Contributor Information
Anant Paradkar, Email: a.paradkar1@bradford.ac.uk.
Kakasaheb Mahadik, Email: krmahadik@rediffmail.com.
References
- 1.Thirumalai T., Therasa S.V., Elumalai E., David E. Intense and exhaustive exercise induce oxidative stress in skeletal muscle. Asian Pac J Trop Dis. 2011;1:63–66. [Google Scholar]
- 2.Smith R.C., Lin B.K. Myostatin inhibitors as therapies for muscle wasting associated with cancer and other disorders. Curr Opin Support Palliat Care. 2013;7:352. doi: 10.1097/SPC.0000000000000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar A., Prakash A., Dogra S. Centella asiatica attenuates D-galactose-induced cognitive impairment, oxidative and mitochondrial dysfunction in mice. Int J Alzheimer's Dis. 2011:2011. doi: 10.4061/2011/347569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hagl S., Kocher A., Schiborr C., Eckert S.H., Ciobanu I., Birringer M. Rice bran extract protects from mitochondrial dysfunction in guinea pig brains. Pharmacol Res. 2013;76:17–27. doi: 10.1016/j.phrs.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Surapaneni D.K., Adapa S.R.S.S., Preeti K., Teja G.R., Veeraragavan M., Krishnamurthy S. Shilajit attenuates behavioral symptoms of chronic fatigue syndrome by modulating the hypothalamic–pituitary–adrenal axis and mitochondrial bioenergetics in rats. J Ethnopharmacol. 2012;143:91–99. doi: 10.1016/j.jep.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Lima F.D., Stamm D.N., Della-Pace I.D., Dobrachinski F., de Carvalho N.R., Royes L.F.F. Swimming training induces liver mitochondrial adaptations to oxidative stress in rats submitted to repeated exhaustive swimming bouts. PloS One. 2013;8:e55668. doi: 10.1371/journal.pone.0055668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsakas A., Bozzo C., Cacciani N., Caliaro F., Reggiani C., Mascarello F. Effect of swimming on myostatin expression in white and red gastrocnemius muscle and in cardiac muscle of rats. Exp physiol. 2006;91:983–994. doi: 10.1113/expphysiol.2006.033571. [DOI] [PubMed] [Google Scholar]
- 8.Campos M., Frigerio C., Lopes J., Bogdanov S. What is the future of bee-pollen. J ApiProduct ApiMed Sci. 2010;2:131–144. [Google Scholar]
- 9.Yıldız O., Can Z., Saral O., Yulug E., Ozturk F., Aliyazıcıoglu R. Hepatoprotective potential of chestnut bee pollen on carbon tetrachloride-induced hepatic damages in rats. Evid Based Complement Alternat Med. 2013:1–9. doi: 10.1155/2013/461478. doi:10.1155/2013/461478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yildiz O., Karahalil F., Can Z., Sahin H., Kolayli S. Total monoamine oxidase (MAO) inhibition by chestnut honey, pollen and propolis. J Enzyme Inhib Med Chem. 2013:1–5. doi: 10.3109/14756366.2013.843171. [DOI] [PubMed] [Google Scholar]
- 11.Žilić S., Vančetović J., Janković M., Maksimović V. Chemical composition, bioactive compounds, antioxidant capacity and stability of floral maize (Zea mays L.) pollen. J Funct Foods. 2014;10:65–74. [Google Scholar]
- 12.Maughan R., Evans S. Effects of pollen extract upon adolescent swimmers. Br J Sports Med. 1982;16:142–145. doi: 10.1136/bjsm.16.3.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diego-Taboada A., Beckett S.T., Atkin S.L., Mackenzie G. Hollow pollen shells to enhance drug delivery. Pharmaceutics. 2014;6:80–96. doi: 10.3390/pharmaceutics6010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roulston T., Cane J.H. Pollen nutritional content and digestibility for animals. Plant Systematics Evol. 2000;222:187–209. [Google Scholar]
- 15.Xu X., Sun L., Dong J., Zhang H. Breaking the cells of rape bee pollen and consecutive extraction of functional oil with supercritical carbon dioxide. Innovative Food Science Emerging Technol. 2009;10:42–46. [Google Scholar]
- 16.Loewus F.A., Baldi B.G., Franceschi V.R., Meinert L.D., McCollum J.J. Pollen sporoplasts: Dissolution of pollen walls. Plant Physiol. 1985;78:652–654. doi: 10.1104/pp.78.3.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fíla J., Čapková V., Feciková J., Honys D. Impact of homogenization and protein extraction conditions on the obtained tobacco pollen proteomic patterns. Biologia Plantarum. 2011;55:499–506. [Google Scholar]
- 18.Wang X., Wang H., Liu Y., You J., Suo Y. Extraction of pollen lipids by SFE-CO2 and determination of free fatty acids by HPLC. Eur J Lipid Sci Technol. 2009;111:155–163. [Google Scholar]
- 19.Fang K., Wang Y., Yu T., Zhang L., Baluska F., Samaj J. Isolation of de-exined pollen and cytological studies of the pollen intines of Pinus bungeana Zucc. Ex Endl. and Picea wilsonii Mast. Flora-Morphol Distrib Funct Ecol Plants. 2008;203:332–340. [Google Scholar]
- 20.Ketkar S.S., Rathore A.S., Lohidasan S., Rao L., Paradkar A.R., Mahadik K.R. Investigation of the nutraceutical potential of monofloral Indian mustard bee pollen. J Integrative Med. 2014;12:379–389. doi: 10.1016/S2095-4964(14)60033-9. [DOI] [PubMed] [Google Scholar]
- 21.Singleton V.L., Orthofer R., Lamuela-Raventos R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods in Enzymology. 1999;299:152–178. [Google Scholar]
- 22.Kandhare A.D., Raygude K.S., Ghosh P., Ghule A.E., Bodhankar S.L. Neuroprotective effect of naringin by modulation of endogenous biomarkers in streptozotocin induced painful diabetic neuropathy. Fitoterapia. 2012;83:650–659. doi: 10.1016/j.fitote.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Kandhare A.D., Raygude K.S., Ghosh P., Ghule A.E., Bodhankar S.L. Therapeutic role of curcumin in prevention of biochemical and behavioral aberration induced by alcoholic neuropathy in laboratory animals. Neurosci Lett. 2012;511:18–22. doi: 10.1016/j.neulet.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 24.Berman S.B., Hastings T.G. Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria. J Neurochem. 1999;73:1127–1137. doi: 10.1046/j.1471-4159.1999.0731127.x. [DOI] [PubMed] [Google Scholar]
- 25.King T.E., Howard R.L. The preparation and some properties of a reduced diphosphopyridine nucleotide dehydrogenase from the snake venom digest of a heart muscle preparation. J Biol Chem. 1962;237:1686–1698. [PubMed] [Google Scholar]
- 26.Liu Y., Fiskum G., Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J Neurochem. 2002;80:780–787. doi: 10.1046/j.0022-3042.2002.00744.x. [DOI] [PubMed] [Google Scholar]
- 27.Sottocasa G.L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria a biochemical and morphological study. J Cell Biol. 1967;32:415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kandhare A.D., Shivakumar V., Rajmane A., Ghosh P., Bodhankar S.L. Evaluation of the neuroprotective effect of chrysin via modulation of endogenous biomarkers in a rat model of spinal cord injury. J Nat Med. 2014:1–18. doi: 10.1007/s11418-014-0840-1. [DOI] [PubMed] [Google Scholar]
- 29.Haleagrahara N., Radhakrishnan A., Lee N., Kumar P. Flavonoid quercetin protects against swimming stress-induced changes in oxidative biomarkers in the hypothalamus of rats. Eur J Pharmacol. 2009;621:46–52. doi: 10.1016/j.ejphar.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 30.Kan N.W., Huang W.C., Lin W.T., Huang C.Y., Wen K.C., Chiang H.M. Hepatoprotective effects of Ixora parviflora extract against exhaustive exercise-induced oxidative stress in mice. Molecules. 2013;18:10721–10732. doi: 10.3390/molecules180910721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swamy M., Naveen S., Farhath K. Antifatigue mechanism of green tea polyphenols in rat subjected to forced swimming test. Int J Adv Pharm Res. 2011;2:133–137. [Google Scholar]
- 32.Chen A.Y., Chen Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013;138:2099–2107. doi: 10.1016/j.foodchem.2012.11.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saw C.L.L., Guo Y., Yang A.Y., Paredes-Gonzalez X., Ramirez C., Pung D. The berry constituents quercetin, kaempferol, and pterostilbene synergistically attenuate reactive oxygen species: Involvement of the Nrf2-ARE signaling pathway. Food Chem Toxicol. 2014;72:303–311. doi: 10.1016/j.fct.2014.07.038. [DOI] [PubMed] [Google Scholar]
- 34.Shakya G., Manjini S., Hoda M., Rajagopalan R. Hepatoprotective role of kaempferol during alcohol-and ΔPUFA-induced oxidative stress. J Basic Clinical Physiol Pharmacol. 2014;25:73–79. doi: 10.1515/jbcpp-2013-0051. [DOI] [PubMed] [Google Scholar]
- 35.Abarikwu S.O. Protective effect of quercetin on atrazine-induced oxidative stress in the liver, kidney, brain, and heart of adult Wistar rats. Toxicol Int. 2014;21:148. doi: 10.4103/0971-6580.139794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaiswal N., Rizvi S.I. Onion extract (Allium cepa L.), quercetin and catechin up-regulate paraoxonase 1 activity with concomitant protection against low-density lipoprotein oxidation in male Wistar rats subjected to oxidative stress. J Sci Food Agri. 2014 doi: 10.1002/jsfa.6620. [DOI] [PubMed] [Google Scholar]
- 37.Chen Y., Islam A., Abraham P., Deuster P. Single-dose oral quercetin improves redox status but does not affect heat shock response in mice. Nutr Res. 2014;34:623–629. doi: 10.1016/j.nutres.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Pouton C.W. Lipid formulations for oral administration of drugs: non-emulsifying, self-emulsifying and ‘self-microemulsifying’ drug delivery systems. Eur J Pharma Sci. 2000;11:S93–S98. doi: 10.1016/s0928-0987(00)00167-6. [DOI] [PubMed] [Google Scholar]
- 39.Aher S., Biradar S., Gopu C., Paradkar A. Novel pepper extract for enhanced P-glycoprotein inhibition. J Pharm Pharmacol. 2009;61:1179–1186. doi: 10.1211/jpp/61.09.0007. [DOI] [PubMed] [Google Scholar]
- 40.Ketkar S., Gopu C., Badgujar L., Paradkar A., Mahadik K. Male sexual behavior improving effect of lipid based extract of Mucuna Pruriens in rats. Pharmacologyonline. 2011;1:1–14. [Google Scholar]
- 41.Sathiyanarayanan L., Paradkar A.R., Mahadik K.R. In vivo and in vitro antioxidant activity of lipid based extract of Bacopa monniera Linn. compared to conventional extract and traditional preparation. Eur J Integrat Med. 2010;2:93–101. [Google Scholar]
- 42.Gilda S., Kanitkar M., Bhonde R., Paradkar A. Activity of water-soluble turmeric extract using hydrophilic excipients. LWT-Food Sci Technol. 2010;43:59–66. [Google Scholar]
- 43.Hosseinzadeh R., Khorsandi K., Hemmaty S. Study of the effect of surfactants on extraction and determination of polyphenolic compounds and antioxidant capacity of fruits extracts. PloS one. 2013;8:e57353. doi: 10.1371/journal.pone.0057353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yazdi A.S. Surfactant-based extraction methods. TrAC Trends Anal Chem. 2011;30:918–929. [Google Scholar]
- 45.Ajila C., Brar S., Verma M., Tyagi R., Valéro J. Solid-state fermentation of apple pomace using Phanerocheate chrysosporium–Liberation and extraction of phenolic antioxidants. Food Chem. 2011;126:1071–1080. [Google Scholar]
- 46.Dent M., Dragović-Uzelac V., Penić M., Brnčić M., Bosiljkov T., Levaj B. The effect of extraction solvents, temperature and time on the composition and mass fraction of polyphenols in Dalmatian Wild Sage (Salvia officinalis L.) extracts. Food Technol Biotechnol. 2013:51. [Google Scholar]
- 47.Prajapati H.N., Dalrymple D.M., Serajuddin A.T. A comparative evaluation of mono-, di-and triglyceride of medium chain fatty acids by lipid/surfactant/water phase diagram, solubility determination and dispersion testing for application in pharmaceutical dosage form development. Pharma Res. 2012;29:285–305. doi: 10.1007/s11095-011-0541-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishikawa Y., Tokura T., Nakano N., Hara M., Niyonsaba F., Ushio H. Inhibitory effect of honeybee-collected pollen on mast cell degranulation in vivo and in vitro. J Med Food. 2008;11:14–20. doi: 10.1089/jmf.2006.163. [DOI] [PubMed] [Google Scholar]
- 49.Jain S., Jain A.K., Pohekar M., Thanki K. Novel self-emulsifying formulation of quercetin for improved in vivo antioxidant potential: Implications for drug-induced cardiotoxicity and nephrotoxicity. Free Radical Biol Med. 2013;65:117–130. doi: 10.1016/j.freeradbiomed.2013.05.041. [DOI] [PubMed] [Google Scholar]
- 50.Maack A. Soluble composition containing sporopollenin and the use thereof: U.S. Patent No. 7,182,965; 2007.
- 51.Åstrand P.-O., Rodahl K. McGraw-Hill; (New York): 1977. Textbook of work physiology: physiological bases of exercise. [Google Scholar]
- 52.Schneider C.D., Oliveira ARd. Oxygen free radicals and exercise: Mechanisms of synthesis and adaptation to the physical training. Revista Brasileira de Medicina do Esporte. 2004;10:308–313. [Google Scholar]
- 53.Kiens B., Richter E.A. Utilization of skeletal muscle triacylglycerol during postexercise recovery in humans. Am J Physiol-Endocrinol Metab. 1998;275:E332–E337. doi: 10.1152/ajpendo.1998.275.2.E332. [DOI] [PubMed] [Google Scholar]
- 54.Westerblad H., Allen D.G., Lännergren J. Muscle fatigue: lactic acid or inorganic phosphate the major cause? Physiology. 2002;17:17–21. doi: 10.1152/physiologyonline.2002.17.1.17. [DOI] [PubMed] [Google Scholar]
- 55.Powers S.K., Jackson M.J. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheesman K. Lipid peroxidation in biological systems. DNA and free radicals. Ellis Horwood, London. 1993;16:12–17. [Google Scholar]
- 57.Lee S.-J. Sprinting without myostatin: A genetic determinant of athletic prowess. Trends Genet. 2007;23:475–477. doi: 10.1016/j.tig.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 58.Lee S.-J. Regulation of muscle mass by myostatin. Annu Rev Cell Dev Biol. 2004;20:61–86. doi: 10.1146/annurev.cellbio.20.012103.135836. [DOI] [PubMed] [Google Scholar]
- 59.Wehling M., Cai B., Tidball J.G. Modulation of myostatin expression during modified muscle use. FASEB J. 2000;14:103–110. doi: 10.1096/fasebj.14.1.103. [DOI] [PubMed] [Google Scholar]
- 60.Matsakas A., Friedel A., Hertrampf T., Diel P. Short-term endurance training results in a muscle-specific decrease of myostatin mRNA content in the rat. Acta Physiol Scand. 2005;183:299–307. doi: 10.1111/j.1365-201X.2005.01406.x. [DOI] [PubMed] [Google Scholar]
- 61.Lenk K., Schur R., Linke A., Erbs S., Matsumoto Y., Adams V. Impact of exercise training on myostatin expression in the myocardium and skeletal muscle in a chronic heart failure model. Eur J Heart Failure. 2009;11:342–348. doi: 10.1093/eurjhf/hfp020. [DOI] [PubMed] [Google Scholar]
- 62.Sriram S., Subramanian S., Sathiakumar D., Venkatesh R., Salerno M.S., McFarlane C.D. Modulation of reactive oxygen species in skeletal muscle by myostatin is mediated through NF-κB. Aging cell. 2011;10:931–948. doi: 10.1111/j.1474-9726.2011.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Willoughby D.S. Effects of heavy resistance training on myostatin mRNA and protein expression. Med Sci Sports Exercise. 2004;36:574–582. doi: 10.1249/01.mss.0000121952.71533.ea. [DOI] [PubMed] [Google Scholar]
- 64.Whittemore L.A., Song K., Li X., Aghajanian J., Davies M., Girgenrath S. Inhibition of myostatin in adult mice increases skeletal muscle mass and strength. Biochem Biophys Res Commun. 2003;300:965–971. doi: 10.1016/s0006-291x(02)02953-4. [DOI] [PubMed] [Google Scholar]
- 65.Palstra A.P., Rovira M., Rizo D., Torrella J.R., Spaink H.P., Planas J.V. Swimming-induced exercise promotes hypertrophy and vascularization of fast skeletal muscle fibres and activation of myogenic and angiogenic transcriptional programs in adult zebrafish. BMC Genomics. 2014;15:1136. doi: 10.1186/1471-2164-15-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]