Abstract
Background
Enhanced intracellular Ca2+ signaling by stromal interaction molecule 1 (STIM1)-mediated store-operated Ca2+ entry (SOCE) is required for skeletal muscle differentiation. However, the contribution of STIM2, STIM1's analogue protein, on muscle cell differentiation has not been clearly elucidated. The present study aimed to explore the contribution of STIM2-mediated SOCE on C2C12 myoblast differentiation.
Methods
Changes in STIM2 expression level (reverse transcription-polymerase chain reaction and Western blotting) and SOCE activity ([Ca2+]i measurement) were measured during 3 days of in vitro differentiation of C2C12 skeletal myoblast. Transcriptional regulation of STIM2 by nuclear factor of activated T cells, cytoplasmic (NFATc) overexpression was observed, and the effect of STIM2 knockdown on NFAT transcriptional activity (luciferase assay) and myoblast differentiation was quantified.
Results
Increase of STIM2 protein level and enhanced SOCE activity were observed in differentiating myoblasts. Treatment with a SOCE blocker (2-APB) inhibited the differentiation. Overexpression of NFATc1 increased STIM2 expression and SOCE activity. Knockdown of STIM2 decreased NFAT transcriptional activity, SOCE activity, and differentiation of C2C12 myoblast.
Conclusion
It is suggested that STIM2-activated SOCE controls C2C12 myoblast differentiation.
Keywords: C2C12, myoblast differentiation, NFATc, STIM2, SOCE
1. Introduction
Differentiation of skeletal myoblasts into multinucleated myotubes is regulated by intracellular Ca2+ signaling.1, 2 The Ca2+ influx pathway that raises intracellular [Ca2+]i include voltage-gated Ca2+ channels and store-operated Ca2+ entry (SOCE).2, 3 SOCE involves Ca2+ entry channels that are activated by the depletion of sarcoplasmic reticulum (SR) Ca2+ stores.4, 5 Depletion of SR intraluminal Ca2+ is sensed by the SR transmembrane protein called stromal interaction molecule (STIM).4 The intraluminal facing Ca2+-binding domain of STIM senses the depletion of stored Ca2+, and is then activated to form homomultimeric complexes to make a clustered functa at the SR plasma membrane (PM) juction.5 The cytoplasmic domain of the clustered STIM proteins binds to a cytoplasmic domain of the PM Ca2+-permeable cation channel (Orai Ca2+ channel), and this causes the Orai channel to open and allow the entry of Ca2+ (SOCE-mediated Ca2+ influx).5 Many studies have demonstrated that SOCE-mediated Ca2+ signaling is required for an efficient muscle cell differentiation.3, 6, 7 In differentiated skeletal muscles, SOCE-mediated Ca2+ influx was known to contribute to the maintenance of repeated muscle contraction, especially under the higher frequency of muscle contraction.6, 7
Two isoforms of STIM proteins (STIM1 and STIM2) and three isoforms of Orai Ca2+ channels (Orai1, Orai2, and Orai3) have been discovered.5 STIM1 and Orai1 are the most abundant proteins that constitute SOCE in many different cells. Although STIM2 is an analogue protein of STIM1, its functional roles and contributions on the whole SOCE-mediated Ca2+ signaling are not clear. It has been proposed that STIM2 proteins possess a higher sensitivity to a decrease in intraluminal Ca2+ content; thus, it is easily activated by a partial depletion of SR Ca2+ stores.8, 9, 10, 11, 12, 13 Therefore, it has been hypothesized that STIM2 controls [Ca2+]i level in resting state or under a circumstance with a mild agonist stimulation.10, 11, 13 Meanwhile, STIM1 plays a significant role when SR Ca2+ stores are intensively depleted by strong agonist stimulation. It was suggested that crosstalk between STIM1 and STIM2 exists, and they have different potency for the activation of SOCE.11, 12 Recently, it has been suggested that STIM2 is activated under a mild depletion of Ca2+ stores at low agonist stimulation and promotes recruitment of STIM1 to the SR–PM junction.13 However, other previous studies raised questions about this specific role of STIM2. STIM2 has been suggested as an inhibitor of STIM1-mediated SOCE.14 The intraluminal Ca2+ binding affinity of the N terminus of STIM2 does not seem to be significantly different to that of STIM1.15
Our previous study on C2C12 myoblast differentiation demonstrated that STIM1-activated SOCE is required for an efficient C2C12 myoblast differentiation.16 We have shown that STIM1 knockdown suppresses C2C12 differentiation and STIM1 expression is positively regulated by the nuclear factor of activated T cells cytoplasmic 3 (NFATc3) transcription factor. The increased STIM1-mediated SOCE activity upon C2C12 differentiation stimulated Ca2+-dependent calcineurin/NFATc signaling and, in turn, it exerted positive feedback to the STIM1 protein expression level.16 As a result, the decreased activity of STIM1-mediated SOCE reduced NFAT-mediated transcriptional activity and finally inhibited differentiation. However, it is not clear whether STIM2 and STIM2-mediated SOCE activity controls C2C12 myoblast differentiation. Only a few studies have explored the roles of STIM2-regulated Ca2+ signaling in skeletal muscle differentiation and maturation.7 Therefore, the aim of the present study was to investigate the roles STIM2 on C2C12 myoblasts differentiation, mainly using a molecular knockdown study.
2. Methods
2.1. C2C12 myoblast differentiation and myotube quantification
The experimental protocols on C2C12 myoblast culture and differentiation have been described in previous studies.16, 17 In brief, C2C12 myoblasts were cultured in DMEM (Dulbecco's modified Eagle's medium; Welgen Inc., Worcester, MA, USA) growth medium supplemented with 15% fetal bovine serum, 1% penicillin, and streptomycin at 37 °C with 5% CO2. After reaching > 90% confluence, the growth medium was replaced with differentiation medium (DM; DMEM supplemented with 2% horse serum) to differentiate myoblasts into myotubes for the next 3 days. Differentiated myotubes were fixed with 4% cold paraformaldehyde (10 minutes) and permeabilized with cold methanol containing 0.3% H2O2 (10 minutes). Next, 5% horse serum was added to block the samples (30 minutes). The samples were incubated with anti-MyHC Ab at 4 °C (overnight) and then incubated with biotylated antimouse Ab (1:500, Jackson Immunoresearch, West Grove, PA, USA) at room temperature (2 hours). The samples were incubated with HRP-conjugated streptoavidin (1:1000, Jackson Immunoresearch) at room temperature (2 hours), and then developed with 3,3′-diaminobenzidine chromogen (Dako, Glostrup, Denmark). Myotube formation was quantified by counting the number of cells with mononucleus and multinucleated MyHC+ myotubes. The percentage of cells with nucleus number of 1–2, 3–7, or ≥ 8 MyHC+ myotubes is presented as a bar graph, where a higher percentage of multinucleated myotubes indicates an increase in differentiation.
2.2. Semiquantitative reverse transcription-polymerase chain reaction
Total RNA was extracted from C2C12 cells using the RNA STAT-60 (Tell-Test, Inc., Friendswood, TX, USA) reagent, following the instructions of the manufacturer; then, it was reverse-transcribed into cDNA single strand using a random hexamer primer SuperScript III RNase H-Reverse Transcriptase (Invitrogen, Waltham, MA, USA). The following DNA sequences of the primers were used for mouse STIM2 (363 bp). Forward: 5′-TGAGGATACCCTGCAGTGG -3′. Reverse: 5′-CAGTCTGCAGACTATCTAAG-3′. As a loading control, GAPDH (300 bp) with the following sequences were used. Forward: 5′-TGTCTTCACCATGGAG-3′. Reverse: 5′- CGGCCATCACGCCACAGCTT-3′.
2.3. Western blotting and antibodies
For cell protein extraction, the same protocols used in our previous study were followed.16 Total cell lysates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (6–10%) and transferred to a polymer of vinylidene fluoride (PVDF) membranes, and then incubated in a blocking solution (5% skim milk in Tris-buffered saline and 0.1% Tween 20) for 1 hour at room temperature. Immunoblotting was performed with the following antibodies (Ab): anti-STIM2 and anti-NFATc1 Ab, which were obtained from ProSci Company (Fort Collins, CO, USA); anti-β-actin Ab, which was purchased from Sigma (St. Louis, MO, USA); and anti-MyHCAb, which was obtained from Santa Cruz Biotechnology (Dallas, TX, USA). Unless otherwise stated, all chemicals used in this study were purchased from Sigma.
2.4. Stable knockdown of STIM2
To construct C2C12 cells with stable knockdown of STIM2, the shRNA target sequence for mouse STIM2 (accession number: NM_001081103.2) was selected using the Invitrogen shRNA design tool. The following DNA sequences of shSTIM2 were used. Forward: 5′-gatccc GGCCAGACACAGCTTCAGAA ttcaagaga TTCTGAAGCTGTGTCTGGC ttttttggaaa-3′. Reverse: 5′-agcttttccaaaaaa GCCAGACACAGCTTCAGAA tctcttgaa TTCTGAAGCTGTGTCTGGCC gg-3′. Oligonucleotides were cloned into p-Super.puro vector (Clontech, Mountain View, CA, USA). C2C12 myoblasts were transfected with shSTIM2 or p-Super.puro vector using lipofectamineTM2000 (Invitrogen) for 48 hours, and then puromycin (1 μg/mL)-resistant colonies were selected for a week. STIM2 knockdown (STIM2-KD) was confirmed with Western blot analysis.
2.5. Overexpression of NFATc
Each NFATc isoform (NFATc1, c2, c3, and c4) was transiently transfected into C2C12 cells by lipofectaminTM2000 (1.5 μg cDNA, 48 hours). The NFATc cDNA sources and validation method for NFATc overexpression were described in our previous study.9 After overexpressing the cells with NFATc isoforms, the changes in STIM2 expression level was confirmed with reverse transcription-polymerase chain reaction and Western blotting against STIM2.
2.6. Luciferase assay
Luciferase assay was performed to estimate the effect of STIM2 silencing on NFAT transcriptional activity. Luciferase reporter plasmids NFAT-Luc, a firefly luciferase reporter with a defined promoter, and renilla luciferase control plasmid were cotransfected into control or STIM2 knockdown C2C12 myoblasts (48 hours). Harvested myoblasts were lysed, and NFAT-Luc and renilla luciferase activities were assayed with the dual luciferase assay kit (Promega, Madison, WI, USA).
2.7. Measurement of [Ca2+]i and SOCE activity
SOCE-mediated Ca2+ influx was measured in C2C12 myoblasts or differentiated myotubes with Ca2+ fluorescent dye fura-2 (3 μM; Molecular Probes, Waltham, MA, USA), as we have previously described.16 Fura-2-loaded cells were bathed in normal Tyrode solution contained (in mM) 145 NaCl, 5 KCl, 2 CaCl2, 1.5 MgCl2, 10 glucose, and 20 HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; pH 7.4], and placed in a 1-mL temperature-controlled cuvette system (DeltaScan, PTI, Birmingham, NJ, USA). Fura-2 fluorescence ratios (F340/F380) were recorded and calculated into the [Ca2+]i by the treatment of Ca2+ ionomycin (Biomol, Hamburg, Germany) and ethylene glycol tetraacetic acid (EGTA) at the end of each measurement.18 To measure SOCE activity, cells were treated with an SR Ca2+-ATPase inhibitor thapsigargin (Tg, 1 μM) to deplete SR Ca2+ stores in Ca2+-free bathing solution (nominal Ca2+-free with 2.5 mM EGTA-containing Tyrode solution). To magnify the magnitudes of SOCE-mediated Ca2+ entry, 7.5 mM Ca2+ was reintroduced to the bath solution after depleting SR stores with Tg.
2.8. Statistical analysis
Data are presented as mean ± standard error of the mean with n, the sample numbers of experiments. Statistical significance was assessed using the unpaired Student t test, and p <0.05 was considered statistically significant.
3. Results
3.1. Increased expression of STIM2 during C2C12 differentiation
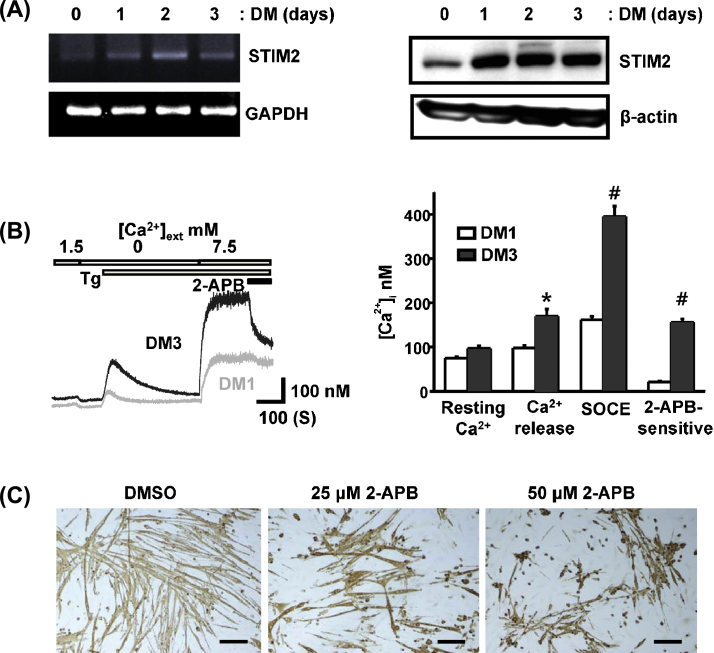
Both mRNA and protein expression level of STIM2 were gradually increased during the 3-day differentiation period (Fig. 1A). As the STIM2 expression level increased, the functional activity of SOCE was increased in the differentiated myotubes at 3 days of differentiation (DM3; Fig. 1B, left panel). The activated SOCE was efficiently inhibited by treatment with 2-APB (50 μM), a commonly used SOCE blocker. In addition, the differentiated myotubes at DM3 showed an increased resting [Ca2+]i level and a higher SR Ca2+ content (Fig. 1B, right panel). The contribution of SOCE-mediated Ca2+ signaling on C2C12 myotube formation was tested by treating the cells with 2-APB. Treatment with 2-APB (25–50 μM) during the 3-day differentiation period markedly inhibited the myotube formation (Fig. 1C), suggesting that SOCE activity is required for efficient differentiation.
Fig. 1.
Increased expression of STIM2 and SOCE activity during C2C12 myoblast differentiation. (A) Changes in mRNA (left panel) and protein expression (right panel) of STIM2 during 3 days of myoblast differentiation were quantified by RT-PCR and Western blotting, respectively. (B) Increasing SOCE activities were compared between the C2C12 cells at differentiation Day 1 (DM1) and Day 3 (DM3). Myotubes at DM3 showed much higher SOCE-mediated Ca2+ influx that is blocked by 50 μM 2-APB. Magnitude of SOCE recorded at DM1 was similar to the proliferating myoblast (data not shown). Higher resting [Ca2+]i and SR Ca2+ contents are shown in the myotubes at DM3. The SR Ca2+ stores were depleted by 1 μM thapsigargin in Ca2+-free bathing solution, and a higher extracellular Ca2+ (7.5 mM) was added back to the bath solution to magnify SOCE activities. *p < 0.05, #p < 0.01; n = 4. (C) C2C12 myoblasts were differentiated for 3 days within the culture medium that contained a SOCE blocker 2-APB (25 μM or 50 μM), and the degree of myotube formation was analyzed with MyHC+ immunohistochemistry at DM3. Scale bars = 100 μm.
RT-PCR, reverse transcription-polymerase chain reaction; SOCE, STIM1-mediated store-operated Ca2+ entry; SR, sarcoplasmic reticulum; STIM2, stromal interaction molecule 2.
3.2. NFATc regulation of STIM2 and SOCE
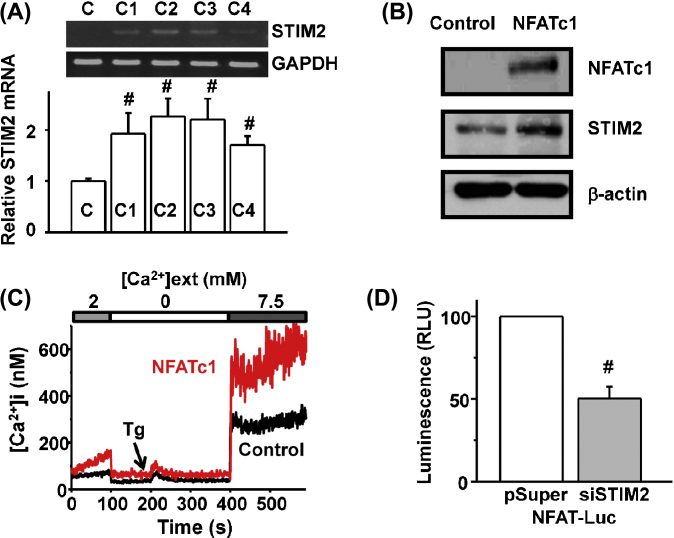
The STIM2 mRNA levels were increased by about 2-fold via overexpression of NFATc isoforms (NFATc1, c2, c3, and c4) in C2C12 myoblasts (p < 0.01; Fig. 2A). The degree of increase in STIM2 mRNA by NFATc was not different among isoforms. We overexpressed one of these NFTAc isoforms, NFATc1, into C2C12 myoblasts and measured the change in STIM2 protein level. NFATc1 overexpression resulted in an increase of the STIM2 protein level and a higher SOCE activity (Figs 2B, 2C). We tested whether NFATc and STIM2 have a reciprocal regulation mechanism, as the positive feedback relationship between NFATc3 and STIM1.16 As shown in Fig. 2D, basal NFAT transcriptional activity decreased to ∼50% in STIM2-KD cells. Altogether, the results suggest that NFATc positively regulates STIM2 protein expression and STIM2-mediated SOCE, and vice versa.
Fig. 2.
NFATc regulates the expression levels of STIM2 and SOCE activity of C2C12 myoblast. (A) The increased STIM2 mRNA levels were quantified by qRT-PCR in C2C12 myoblasts with overexpression of individual NFATc isoforms. Shown are means ± SD from two independent experiments (n = 4–6, #p < 0.01). (B) The increased STIM2 protein level by the transfection of NFATc1 was quantified by Western blotting in C2C12 myoblasts. (C) Representative [Ca2+]i measurement that shows an increase in SOCE activity in NFATc1-overexpressed myoblasts is plotted. (D) Decreased NFAT transcriptional activity in STIM2-KD myoblasts was measured by luciferase assay (3 independent experiments, n = 6, #p < 0.01).
KD, knockdown; NFATc, nuclear factor of activated T cells, cytoplasmic; qRT-PCR, quantitative reverse transcription-polymerase chain reaction; SD, standard deviation; SOCE, store-operated Ca2+ entry; STIM2, stromal interaction molecule 2.
3.3. STIM2-KD decreases SOCE activity and myoblast differentiation
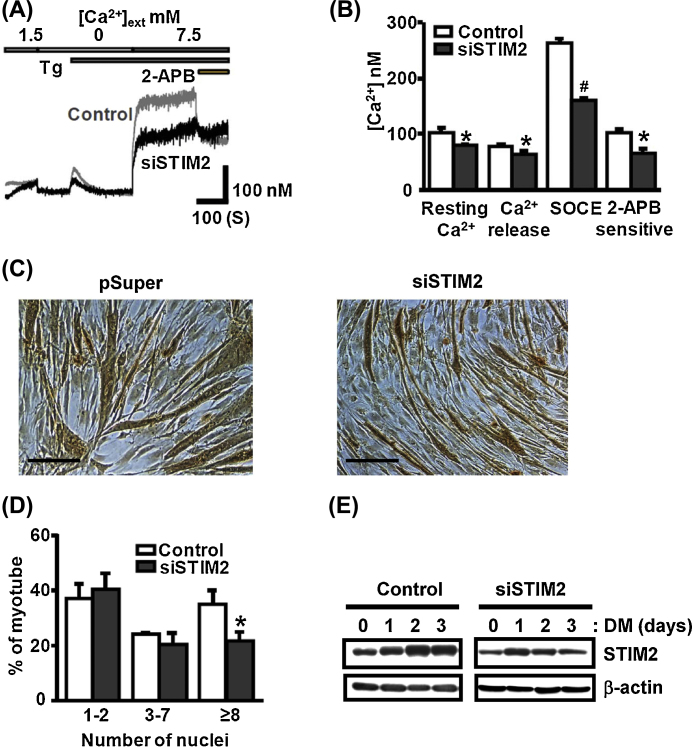
To evaluate the functional consequences of STIM2 silencing on SOCE activity and the muscle differentiation, we generated stable STIM2-KD C2C12 myoblasts, and they were induced to differentiate. In differentiated STIM2-KD myotubes, SOCE amplitudes were reduced to 61.5 ± 1.6% of control (p < 0.01; Figs. 3A, 3B). In addition, resting [Ca2+]i levels and the amounts of SR Ca2+ release by Tg were reduced to 78 ± 2.9% (p < 0.05) and 80.1 ± 9.5% of control (p < 0.05), respectively (Fig. 3B). Myoblast differentiation was reduced in STIM2-KD cells, which is characterized by the formation of relatively narrower myotubes (Fig. 3C). The percentage of MyHC+ myotubes with ≥ 8 nuclei was significantly reduced to 61.85 ± 16.8% of control (p < 0.05; Fig. 3D). When STIM2-KD myoblasts were differentiated into myotubes, lowered STIM2 protein levels were maintained during 3 days of differentiation (Fig. 3E). These results demonstrated that STIM2 silencing impairs SOCE activity and consequent C2C12 myoblast differentiation.
Fig. 3.
Knockdown (KD) of STIM2 reduces SOCE activity and impairs C2C12 myoblast differentiation. (A) Representative traces of [Ca2+]i measured at differentiation Day 3 (DM3) from control or STIM2-KD (siSTIM2) myotubes. (B) Summary bar graphs represent the changes in resting [Ca2+]i, SR Ca2+ release, SOCE activity, and 2-APB-sensitive SOCE component by knockdown of STIM2 (n = 3; *p < 0.05, #p < 0.01). (C) Representative images of MyHC+ cells taken from control and STIM2-KD myotubes at DM3. Shown are representative images from three independent experiments. Scale bars = 100 μm. (D) Quantification of nuclei number in MyHC+ cells at DM3. The percentage of MyHC+ myotubes with eight or more nuclei was 61.85 ± 16.8% of control (n = 3, *p < 0.05). (E) The decreased STIM2 protein expression level was maintained during 3 days of differentiation of STIM2-KD myoblasts. A representative immunoblotting is depicted from two independent experiments.
KD, knockdown; SOCE, store-operated Ca2+ entry; SR, sarcoplasmic reticulum; STIM2, stromal interaction molecule 2.
4. Discussion
The present study demonstrated that STIM2 can regulate the efficiency of C2C12 myoblast differentiation. As C2C12 myoblasts differentiate into multinucleated myotubes, an increased STIM2 expression (mRNA and protein) was accompanied with an increased SOCE activity (Fig. 1). This kind of expression pattern of STIM2 and SOCE looks almost identical to that of STIM1-mediated response in differentiating C2C12 cells.16 The inhibitory effect of 2-APB on SOCE and myoblast differentiation (Figs. 1B, 1C) indirectly indicated the important roles of SOCE-mediated Ca2+ signaling in myoblast differentiation. Because no pharmacological selectivity of 2-APB on STIM1- or STIM2-mediated SOCE was described, the data presented in Fig. 1 merely indicate the importance of SOCE-mediated Ca2+ signaling in C2C12 differentiation. To prove the relevance of STIM2 on muscle differentiation, we constructed STIM2-KD stable cells and then analyzed the functional consequences of STIM2 silencing on cellular [Ca2+]i, SOCE activity, NFAT transcriptional activity, and the myoblast differentiation (Fig. 3). Our knockdown data showed that STIM2-KD impaired C2C12 myoblast differentiation with a lower percentage of multinucleated myotubes. A reduced SOCE activity recorded in STIM2-KD myotubes (Fig. 3A) corresponded well to the lowered STIM2 protein expression (Figs. 3A, 3C). A lower level of resting [Ca2+]i was observed in STIM2-KD myotubes (Fig. 3B), which is in line with previous studies suggesting that STIM2 is an important regulator for maintaining the resting [Ca2+]i level. Our present results are in accordance with those of a previous study on human skeletal muscle differentiation, in which both STIM1 and STIM2 proteins are required for human myoblast differentiation and myotube excitation–contraction coupling.6, 7
Although we observed impaired C2C12 differentiation by knockdown of STIM2, the degree of impairment is relatively weaker than that of STIM1-KD C2C12 cells, as shown in our previous experiment.16 Therefore, it may be suggested that STIM1 is more important than STIM2 for differentiation of myotubes in vitro, at least in the murine C2C12 myoblast cell line. A major limitation of the present study with STIM2-KD is that we did not track the changes in the expression of STIM1 and its counterpart protein Orai1 from the differentiating STIM2-KD cells. If the amount of functional STIM1 protein is enough to compensate for the lack of STIM2, only a mild phenotypic change in SOCE-mediated Ca2+ signal and muscle differentiation could be expected. In other sets of experiments, we have observed that a reciprocal compensating interaction mechanism exists between STIM1 and STIM2 expression in C2C12 myoblasts (data not shown). In particular, a noticeable compensatory increase of STIM2 protein has been observed from STIM1 ablated cells. On the contrary, there was only a minor compensatory increase in the STIM1 protein level in STIM2 ablated cells (data not shown). Given that STIM1 is a major player of SOCE-controlled C2C12 differentiation,3, 16 STIM2 can be regarded as a supportive molecule to the STIM/SOCE-mediated signaling. However, this interpretation should be inspected carefully by in-depth studies, such as STIM-double knockout studies.
It has been well understood that intracellular Ca2+-calcineurin-NFAT signaling cascade is a key player for the control of myoblast differentiation.19, 20, 21 In the present study, STIM2 mRNA levels were upregulated ∼2-fold by transfected NFATc isoforms (NFATc1–c4) with no preference on a specific NFATc isoform (Fig. 2A). Transfected NFATc1 increased not only the amounts of STIM2 protein but also SOCE activity, suggesting that NFATc1 upregulates STIM2-activated SOCE (Figs. 2B, 2C). However, the present work did not test whether other NFATc isoforms, i.e., NFATc2–c4, can increase STIM2 protein level. Our previous study with C2C12 cells showed that the expression level of STIM1 (mRNA and protein) is exclusively upregulated by the NFATc3 isoform.16 In addition, the study demonstrated that a reciprocal positive feedback mechanism exists between NFATc3 and STIM1. In comparison with an exclusive control of STIM1 by NFTAc3,16, 21 our study may suggest that the transcriptional regulation of STIM2 by NFATc has no preference for a specific NFATc isoform, even if the signaling strength that comes from each isoform might be different. The fact that STIM2 silencing decreased NFATc transcriptional activity (Fig. 2D) suggests that STIM2 activation of SOCE is coupled to the Ca2+-dependent NFAT transcriptional activation of STIM2 expression. It means, therefore, that a positive feedback loop exists between NFATc and STIM2 in the course of myoblast differentiation processes, similar to the crosstalk between NFATc3 and STIM1.16 Taking into account that the activity of calcineurin-mediated transactivation of NFATc is dependent on the magnitude of intracellular [Ca2+]i,20, 21 the overall Ca2+-dependent control of the skeletal muscle differentiation can be significantly regulated by the expression regulation of STIM proteins or NFATc isoforms, in terms of protein levels.
Conflicts of interest
No conflicts of interest, financial or otherwise, are declared by the authors.
Acknowledgments
We thank Jin-Hyun Ahn (Sungkyunkwan University, Suwon, Korea), Anjana Rao (Harvard Medical School, Boston, MA, USA), and Sébastien Jauliac (INSERM, Paris, France) for NFATc constructs. This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (#2014R1A1A2056838).
References
- 1.Porter G.A., Makuck R.F., Rivkees S.A. Reduction in intracellular calcium levels inhibits myoblast differentiation. J Biol Chem. 2002;277:28942–28947. doi: 10.1074/jbc.M203961200. [DOI] [PubMed] [Google Scholar]
- 2.Arnaudeau S., Holzer N., Konig S., Bader C.R., Bernheim L. Calcium sources used by post-natal human myoblasts during initial differentiation. J Cell Physiol. 2006;208:435–445. doi: 10.1002/jcp.20679. [DOI] [PubMed] [Google Scholar]
- 3.Stiber J., Hawkins A., Zhang Z.S., Wang S., Burch J., Graham V. STIM1 signalling controls store-operated calcium entry required for development and contractile function in skeletal muscle. Nat Cell Biol. 2008;10:688–697. doi: 10.1038/ncb1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liou J., Kim M.L., Heo W.D., Jones J.T., Myers J.W., Ferrell J.E., Jr. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soboloff J., Rothberg B.S., Mades H.M., Gill D.L. STIM proteins: dynamic calcium signal transducers. Nat Rev Mol Cell Biol. 2012;13:549–565. doi: 10.1038/nrm3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darbellay B., Arnaudeau S., Konig S., Jousset H., Bader C., Demaurex N. STIM1- and Orai1-dependent store-operated calcium entry regulates human myoblast differentiation. J Biol Chem. 2009;284:5370–5380. doi: 10.1074/jbc.M806726200. [DOI] [PubMed] [Google Scholar]
- 7.Darbellay B., Arnaudeau S., Ceroni D., Bader C.R., Konig S., Bernheim L. Human muscle economy myoblast differentiation and excitation–contraction coupling use the same molecular partners. STIM1 and STIM2. J Biol Chem. 2010;285:22437–22447. doi: 10.1074/jbc.M110.118984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandman O., Liou J., Park W.S., Meyer T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell. 2007;131:1327–1339. doi: 10.1016/j.cell.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oh-Hora M., Yamashita M., Hogan P.G., Sharma S., Lamperti E., Chung W. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol. 2008;9:432–443. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thiel M., Lis A., Penner R. STIM2 drives Ca2+ oscillations through store-operated Ca2+ entry caused by mild store depletion. J Physiol. 2013;591:1433–1445. doi: 10.1113/jphysiol.2012.245399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gruszczynska-Biegala J., Kuznicki J. Native STIM2 and ORAI1 proteins form a calcium-sensitive and thapsigargin-insensitive complex in cortical neurons. J Neurochem. 2013;126:727–738. doi: 10.1111/jnc.12320. [DOI] [PubMed] [Google Scholar]
- 12.Shalygin A., Skopin A., Kalinina V., Zimina O., Glushankova L., Mozhayeva G.N. STIM1 and STIM2 proteins differently regulate endogenous store-operated channels in HEK293 cells. J Biol Chem. 2015;290:4717–4727. doi: 10.1074/jbc.M114.601856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ong H.L., de Souza L.B., Zheng C., Cheng K.T., Liu X., Goldsmith C.M. STIM2 enhances receptor-stimulated Ca2+ signaling by promoting recruitment of STIM1 to the endoplasmic reticulum–plasma membrane junctions. Sci Signal. 2015;8:ra3. doi: 10.1126/scisignal.2005748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soboloff J., Spassova M.A., Hewavitharana T., He L.P., Xu W., Johnstone L.S. STIM2 is an inhibitor of STIM1-mediated store-operated Ca2+ entry. Curr Biol. 2006;16:1465–1470. doi: 10.1016/j.cub.2006.05.051. [DOI] [PubMed] [Google Scholar]
- 15.Stathopulos P.B., Zheng L., Ikura M. Stromal interaction molecule (STIM) 1 and STIM2 calcium sensing regions exhibit distinct unfolding and oligomerization kinetics. J Biol Chem. 2009;284:728–732. doi: 10.1074/jbc.C800178200. [DOI] [PubMed] [Google Scholar]
- 16.Phuong T.T., Yun Y.H., Kim S.J., Kang T.M. Positive feedback control between STIM1 and NFATc3 is required for C2C12 myoblast differentiation. Biochem Biophys Res Commun. 2013;430:722–728. doi: 10.1016/j.bbrc.2012.11.082. [DOI] [PubMed] [Google Scholar]
- 17.Park S.Y., Kim M.H., Ahn J.H., Lee S.J., Lee J.H., Eum W.S. The stimulatory effect of essential fatty acids on glucose uptake involves both Akt and AMPK activation in C2C12 skeletal muscle cells. Korean J Physiol Pharmacol. 2014;18:255–261. doi: 10.4196/kjpp.2014.18.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen Y.H., Lee K.Y., Kim T.J., Kim S.J., Kang T.M. CD40 co-stimulation inhibits sustained BCR-induced Ca2+ signaling in response to long-term antigenic stimulation of immature B cells. Korean J Physiol Pharmacol. 2011;15:179–187. doi: 10.4196/kjpp.2011.15.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abbott K.L., Friday B.B., Thaloor D., Murphy T.J., Pavlath G.K. Activation and cellular localization of the cyclosporine A-sensitive transcription factor NF-AT in skeletal muscle cells. Mol Biol Cell. 1998;9:2905–2916. doi: 10.1091/mbc.9.10.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friday B.B., Mitchell P.O., Kegley K.M., Pavlath G.K. Calcineurin initiates skeletal muscle differentiation by activating MEF2 and MyoD. Differentiation. 2003;71:217–227. doi: 10.1046/j.1432-0436.2003.710303.x. [DOI] [PubMed] [Google Scholar]
- 21.Delling U., Tureckova J., Lim H.W., De Windt L.J., Rotwein P., Molkentin J.D. A calcineurin-NFATc3-dependent pathway regulates skeletal muscle differentiation and slow myosin heavy-chain expression. Mol Cell Biol. 2000;20:6600–6611. doi: 10.1128/mcb.20.17.6600-6611.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]