Abstract
Background
Propolis is a multicomponent active, complex resinous substance collected by honeybees (Apis mellifera) from a variety of plant sources. This study was designed to improve the antimicrobial efficacy of propolis by engineering a niosomal-based system for topical application.
Methods
Propolis was extracted in ethanol and screened for total polyphenol content. Propolis-loaded niosomes (PLNs) were prepared with varying concentrations of Span 60 and cholesterol. The PLNs were evaluated for physicochemical parameters, namely, vesicle size, entrapment efficiency, zeta potential, surface topography and shape, and stability, followed by screening for in vitro antimicrobial activity. The PLNs were formulated into propolis niosomal gel (PNG) using Carbopol P934 base and subjected to ex vivo skin deposition study.
Results
The ethanolic extract of propolis had high polyphenolic content (270 ± 9.2 mg GAE/g). The prepared PLNs showed vesicle size between 294 nm and 427 nm, and the percent entrapment in the range of 50.62–71.29% with a significant enhancement in antimicrobial activity against Staphylococcus aureus and Candida albicans. Enhanced antimicrobial activity of PLNs was attributed to the ability of niosomes to directly interact with the bacterial cell envelop thereby facilitating the diffusion of propolis constituents across the cell wall. The formulated PNG exhibited a twofold better skin deposition due to improved retention of niosomes in the skin.
Conclusion
The findings indicate that the engineering of a niosomal-based system for propolis enhanced its antimicrobial potential through topical application.
Keywords: antimicrobial, niosomes, nonionic surfactants, propolis
1. Introduction
Propolis is a complex resinous substance (sometimes referred to as bee glue) collected by honeybees, especially Apis mellifera, from a variety of plant sources including cracks in bark and leaf buds. Propolis is a strong adhesive material. As such, it is used by bees in the construction, maintenance, and protection of their hives.1, 2 It has a complex chemical composition and is known to be rich in polyphenols, flavonoids, waxes, resins, balsams, amino acids, oils, etc.3 Propolis is reported to have a wide array of pharmacological activities such as antioxidant, anticancer, antimicrobial, antiviral, immunomodulatory, wound healing, and antileishmanial properties.4, 5, 6, 7, 8, 9, 10, 11, 12 Despite its broad therapeutic potential, its complex resinous nature, low solubility, sticky consistency, and physical instability present a major hurdle with regard to its processing and formulation development.6, 10, 13
Several studies have reported on the broad-spectrum antimicrobial properties of propolis and its constituents.6, 7, 8, 11, 13, 14, 15, 16, 17 Propolis has shown strong bactericidal activity against several Gram-positive and Gram-negative bacteria. Propolis and some of its cinnamic and flavonoid components were found to uncouple the energy-transducing cytoplasmic membrane and to inhibit bacterial motility.14 Several studies have explored the antifungal properties of propolis.4, 15, 17 Although the mechanism of action for its antimicrobial effect is not yet clearly understood, some studies suggest that propolis constituents interfere with the division of bacterial cells through the formation of pseudomulticellular forms, cytoplasm disorganization, protein synthesis inhibition, and cell lysis.18 The antimicrobial effect of propolis is correlated with its complex composition comprising flavonoids; ferulic acid; caffeic acid derivatives such as caffeic acid phenyl ester (CAPE); hydroquinones; terpenic acids such as isopimaric, abietic and dehydroabietic acid; galangin; pinostrobin; and pinocembrin content.4, 6
The antimicrobial efficacy of propolis can be better used for treating several bacterial or fungal infections by fabricating a delivery system, which will prolong its diffusion and improve retention of its constituents in the skin through topical application.
Vesicular delivery systems such as niosomes are reported to improve the dermal or topical delivery of various poorly soluble actives by enhancing solubility and permeability along with retention into the skin.19, 20, 21 Niosomes enhance the residence time of drugs in the stratum corneum and epidermis while reducing the systemic absorption of the drug.21, 22, 23 These nonionic surfactant vesicles modify stratum corneum properties, improve the penetration of trapped substances across the skin, and act by reducing transepidermal water loss, thereby increasing smoothness by replenishing skin lipids, and thus, enhancing the potential of the entrapped drug administered topically.24, 25 Considering the evident characteristics of niosomes, it was hypothesized that developing a niosomal-based formulation for propolis would enhance its antimicrobial efficacy through topical application. Our group has previously traced a liposomal delivery system for propolis as a means of enhancing its hepatoprotective activity.26 This work was designed with the objective of developing a niosomal-based delivery system for propolis to improve its antimicrobial potential through topical application.
2. Methods
Propolis was generously supplied by Nature Laboratory Ltd. (Whitby, North Yorkshire, UK). Span 60, cholesterol, sodium hydroxide, and potassium dihydrogen phosphate were procured from Sigma-Aldrich Ltd. (Mumbai, India). Stearic acid was purchased from HiMedia Laboratories (Mumbai, India). Ethanol, acetonitrile, and methanol were purchased from Merck Ltd. (Mumbai, India). All the chemicals and solvent used in this study were of analytical grade.
2.1. Determination of total polyphenol content of propolis
The crude propolis (2 g) was extracted with absolute ethanol (40 mL) for 24 hours at room temperature by maceration using a mechanical shaker at 200 rpm followed by centrifugation. The supernatant obtained was filtered and concentrated under reduced pressure to obtain ethanolic extract of propolis (PEE).27 The total polyphenol content in the extract was determined by the Folin–Ciocalteu method.9, 28 In brief, the PEE (1 mL) was mixed with Folin–Ciocalteu's phenol reagent (1 mL). To this mixture, an aqueous solution of sodium carbonate (7%, 5 mL) was added and diluted to 25 mL with distilled water. Absorbance was measured at 760 nm using the JASCO V-630 UV–visible spectrometer, (Tokyo, Japan) after 90 minutes of incubation of the mixture at room temperature. The total polyphenol contents were expressed in terms of milligram gallic acid equivalents (GAE)/g.
2.2. Preparation of propolis-loaded niosomes
The ethanol injection method was optimized for preparing blank niosomes (empty vesicles) and propolis-loaded niosomes (PLNs, i.e., loaded vesicles). Different batches (J1, J2, J3, J4, J5, and J6) were prepared by varying molar ratios of Span 60 to cholesterol (1:0, 1:1, 1:1.5, 1:2, 1.5:1, and 2:1), respectively. In accordance with the molar ratios, measured amounts of Span 60, cholesterol, PEE (20 mg), and stearic acid (7 mg) were dissolved in 4 mL of ethanol. The mixture was injected using a syringe into 10 mL of distilled water maintained at 60–65 °C. The mixture was continuously stirred using a magnetic stirrer for 60 minutes to ensure complete evaporation of the solvent. The prepared niosomes were refrigerated for complete sealing of the surfactant bilayer. The niosomes were then characterized.
2.3. Characterization of niosomes
2.3.1. Vesicle-size analysis and size distribution
Mean vesicle size and size distribution of PLNs were determined using a particle-size analyzer (Malvern 2000SM; Malvern Instruments Ltd., Malvern, United Kingdom). The laser obscuration was maintained at 1–2.5% with the angle of detection at 90°. The experiment was performed in triplicate.
2.3.2. Entrapment efficiency
The entrapment efficiency (EE) of the prepared PLN was determined in terms of total polyphenol content of the PEE using the centrifugation method.21 The PLN dispersion in distilled water was centrifuged at 20,000 rpm at 4 °C for 1 hour. The supernatant was separated from the pellet and the amount of unentrapped drug in terms of total polyphenol content was determined by the Folin–Ciocalteu method.9, 28 The EE of the prepared PLN was calculated using the following equation:
where Ct is total polyphenol content of propolis and Cf is total polyphenol content of propolis in the supernatant.
2.3.3. Zeta potential determination
To assess the stability of the prepared niosomes, 1-mL aliquots of the prepared niosomal dispersions were diluted 100-fold with distilled water and assessed for zeta potential (ζ)using Zetasizer 300HsA (Malvern Instruments Ltd.).
2.3.4. Transmission electron microscopy
The surface topography and shape of the PLNs were observed by transmission electron microscopy. A drop of niosomal dispersion was placed on the copper mesh grid. Upon adsorption of sample after 15 minutes, the staining dye potassium phosphotungstate was dripped onto the film. The grid was dried under an infrared lamp for approximately 30 minutes and photographs were taken using a Zeiss EM 109 transmission electron microscope (Ostalbkreis, Germany).
2.3.5. Stability study
Optimized PLN dispersion (Batch J6) was subjected to a stability study at 25 ± 2 °C/60% relative humidity (RH) and 45 ± 2 °C/75% RH for 90 days. The physical stability was assessed in terms of vesicle size as described earlier.
2.4. Antimicrobial activity
The minimum inhibitory concentrations (MICs) for PLN and PEE were determined by serial tube dilution method7, 8 in the concentration range of 50–500 μg/mL. The antimicrobial activities of PLN and ethanolic solution of propolis were compared by determining the zone of inhibition using the agar gel diffusion method14 against Staphylococcus aureus (ATCC 6538P) and Candida albicans (ATCC 18804; both strains were supplied by the National Collection of Industrial Microorganisms, Pune, India). The bacterial culture was grown in a nutrient broth at 37 °C for 24 hours and the fungal culture was grown in Sabouraud dextrose broth, followed by incubation at 25 °C for 48 hours. Approximately 100 μL of cultures were seeded individually in 25-mL molten nutrient agar, mixed and poured into sterile Petri plates, and allowed to solidify. Different concentrations of PLN and PEE were added into the bore well (diameter: 8 mm) in the agar plates followed by incubation at 37 °C for 24 hours. The zone of inhibition was measured and recorded.
2.5. Preparation of propolis niosomal gel
The optimized PLN (Batch J6) was formulated into a gel for topical application consisting of 1% (w/w) Carbopol P934 gel base. The Carbopol was added gradually into the PLN (J6) dispersion with continuous stirring. The mixture was kept overnight to allow for swelling of the gel, which confirms complete hydration of polymer chains. The formed gel was analyzed for drug content and subjected to ex vivo skin deposition study. Similarly, a control gel comprising an equivalent amount of PEE in Carbopol was formulated. Drug content in terms of CAPE was determined for both by extracting 1 g of gel in ethanol, diluted with the mobile phase (acetonitrile:methanol) by high-performance liquid chromatography (HPLC) using a UV detector at 325 nm.29
2.6. Ex vivo skin deposition study
The ex vivo deposition study was carried out on Wistar rat skin according to the study protocol approved by the Institutional Animal Ethics Committee constituted under the Committee for the Purpose of Control and Supervision on Experimental Animals (India). A vertical Franz diffusion cell with a reservoir capacity of 22 mL was used for the study. The excised and defatted rat skin was mounted between the donor and receptor compartments of the vertical Franz diffusion cell with an effective permeation area of 1.5 cm2. The 2-mL receptor solution of phosphate buffer (pH 7.4) was continuously stirred using a magnetic bar and maintained at 37 °C for 24 hours. Individually, 1 g of propolis niosomal gel (PNG) and the control gel sample were applied over the skin into the donor compartment. After 24 hours, the skin was cleaned using a cotton cloth, finely divided, and subjected to homogenization with acetonitrile:methanol (50:50) and sonicated for 30 minutes. The mixture was centrifuged to sediment the cells and tissues of the skin. The supernatant was diluted using the mobile phase acetonitrile:methanol (50:50) and subjected to drug content determination in terms of CAPE using HPLC. The analyses were performed on a JASCO HPLC system (Tokyo, Japan) with a Thermo Scientific Hypersil GOLD C18 reversed-phase chromatography column (250 mm × 4.0 mm, 5 μm) using a UV-visible detector. Elution was carried out with a flow rate of 1 mL/min at ambient temperature. Detection was performed at 325 nm.
3. Results
3.1. Total polyphenol content of propolis
Propolis is commercialized in various regions of the world and is recognized as a vital source of constituents such as phenolics, which are responsible for several pharmacological effects.30 The PEE showed a high polyphenol content of 270 ± 9.2 mg GAE/g. Evaluating the polyphenol content is considered a means to determine the entrapment efficacy of developed PLN.
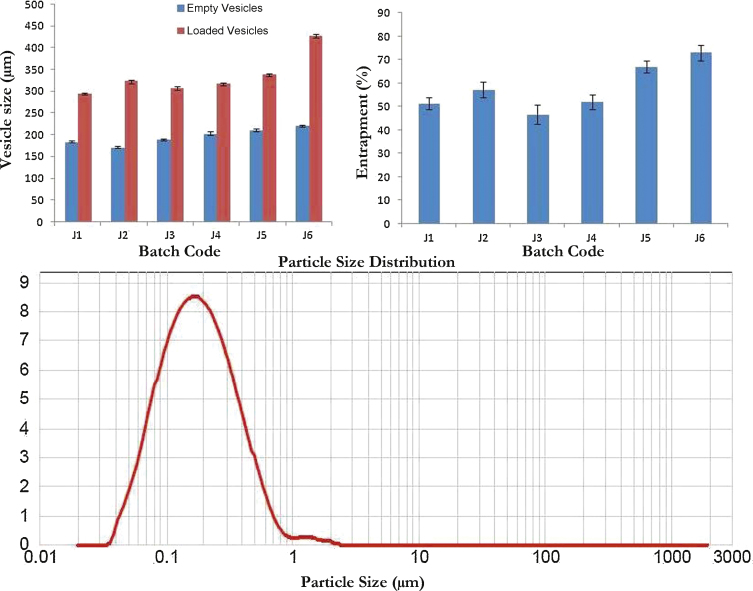
3.2. Vesicle size and EE
Table 1 presents the results of vesicle size and % EE for the prepared niosomes. A clear increase in vesicle size was observed for all batches of PLN compared with that of empty vesicles (Fig. 1). The results for vesicle-size analysis demonstrated a significant increase initially (p < 0.001) in the size of PLN Batch J2 compared with that of Batch J1. A significant decline (p < 0.001) in the size of PLN Batch J3 followed by a gradual increase in the size for Batches J4, J5, and J6 was observed. The vesicle size for PLN Batches J5 and J6 showed significant augmentation (p < 0.05 and p < 0.001, respectively) compared with that of Batch J2. The results of EE for PLN showed a significant decline (p < 0.05) for Batch J3 compared with that of Batch J2. A significant improvement (p < 0.001) in EE of PLN Batches J5 and J6 was observed compared with that of Batch J2.
Table 1.
Effect of variables on vesicle size and entrapment efficiency
| Batches | Span 60:cholesterol | Size of empty vesicles d(0.9) nm | Size of loaded vesicles d(0.9) nm | % Entrapment efficiency |
|---|---|---|---|---|
| J1 | 1:0 | 182 ± 3.08 | 294 ± 5.53 | 52.85 ± 1.95 |
| J2 | 1:1 | 173 ± 5.53 | 327 ± 3.53 * | 55.85 ± 2.49 |
| J3 | 1:1.5 | 189 ± 3.53 | 307 ± 2.52 | 50.62 ± 1.71 † |
| J4 | 1:2 | 195 ± 2.08 | 317 ± 3.15 | 50.96 ± 1.32 |
| J5 | 1.5:1 | 211 ± 4.53 | 334 ± 4.52 † | 67.31 ± 4.89 * |
| J6 | 2:1 | 217 ± 3.00 | 427 ± 5.52 * | 71.29 ± 5.32 * |
Values are expressed as mean ± standard deviation (n = 3) followed by one-way analysis of variance with Tukey's multiple comparison test.
p < 0.001.
p < 0.05.
Fig. 1.
Vesicle-size analysis and entrapment efficiency of propolis-loaded noisome batches.
3.3. Zeta potential determination
The zeta potential (ζ) for the prepared PLN was found to range from –33.2 mV to –38.8 mV.
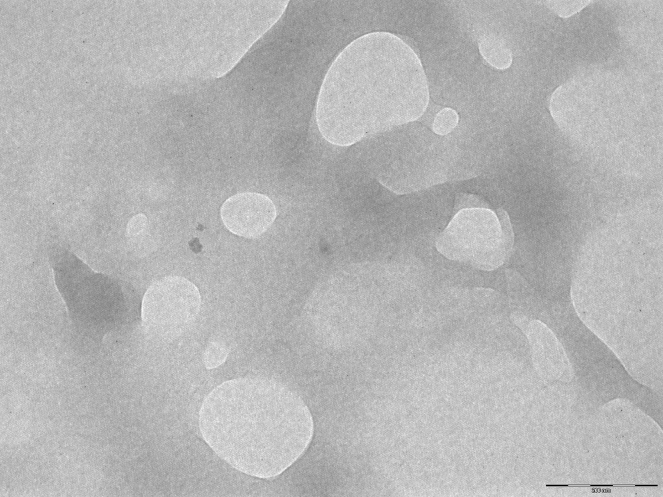
3.4. Transmission electron microscopy
The transmission electron microscopy image for PLN (Fig. 2) depicts a spherical shape and smooth surface. The size range of PLN was found to be between 294 nm and 427 nm.
Fig. 2.
Transmission electron microscopy image of propolis-loaded niosomes.
3.5. Stability study
The stability study for the optimized PLN (Batch J6) did not show a significant change in mean vesicle size over 90 days of study (data not shown). In addition, no signs of aggregation or sedimentation were observed, indicating the formation of stable niosomal dispersion.
3.6. Antimicrobial activity
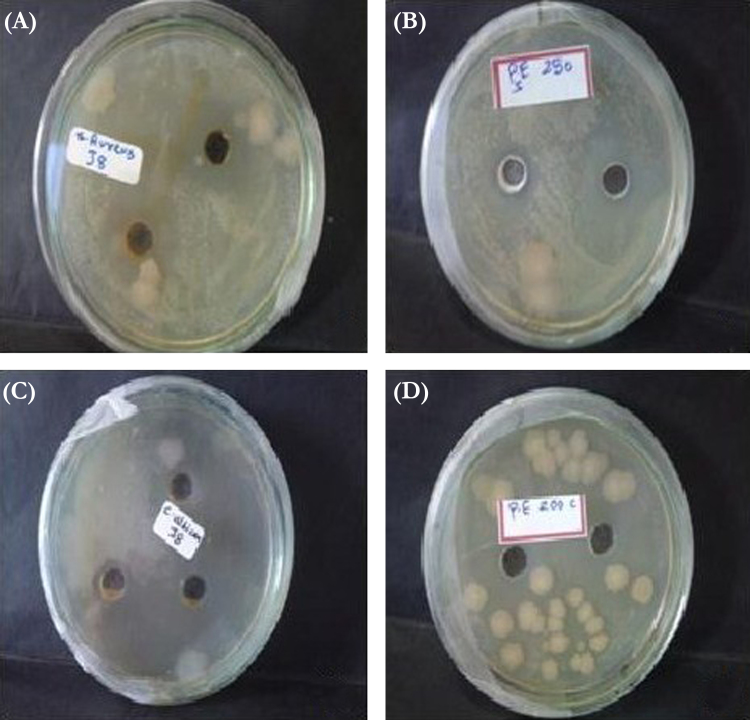
The antimicrobial activities of PLN (Batch J6) and the ethanolic solution of propolis were compared. The MIC of PLN against S. aureus and C. albicans was found to be lower in comparison with that of the ethanolic solution of propolis (Table 2). PLN significantly increased the zone of inhibition (p < 0.05; Table 2) against both S. aureus and C. albicans as displayed in Fig. 3.
Table 2.
Antimicrobial effect of PLN and ethanolic solution of propolis
| Formulation | Antibacterial activity against Staphylococcus aureus |
Antifungal activity against Candida albicans |
||
|---|---|---|---|---|
| MIC (μg/mL) | Zone of inhibition (mm) | MIC (μg/mL) | Zone of inhibition (mm) | |
| Ethanolic solution of propolis | 500 | 15.3 ± 1.53 | 400 | 10.7 ± 1.15 |
| PLNs | 300 | 25 ± 3.00 * | 300 | 20 ± 2.00 * |
Values are expressed as mean ± standard deviation (n = 3) followed by one-way analysis of variance with Tukey's multiple comparison test.
p < 0.05.
MIC, minimum inhibitory concentration; PLN, propolis-loaded noisome.
Fig. 3.
Antibacterial activity of: (A) propolis-loaded noisome (PLN); and (B) ethanolic solution of propolis. Antifungal activity of: (C) PLN and (D) ethanolic solution of propolis.
3.7. Ex vivo skin deposition study
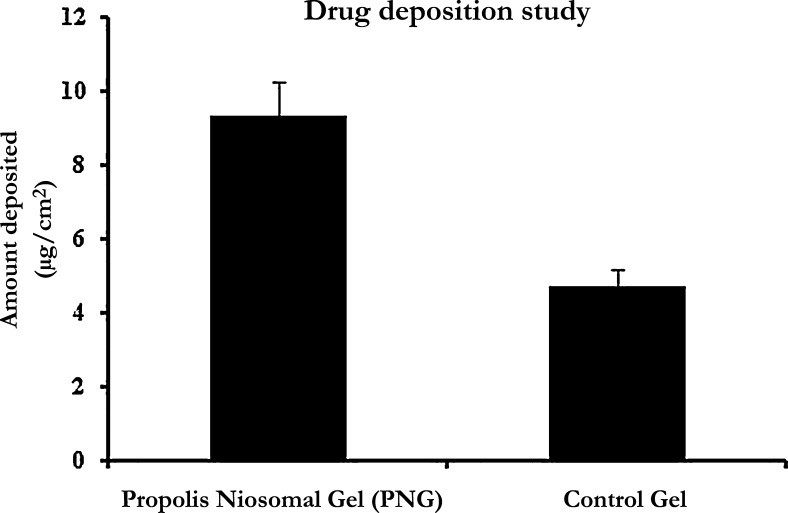
For ease of application and for improving their retention in the skin, the PLNs were formulated into gel using Carbopol P934 gel base. Because polyphenols are a group of compounds having variation in chemical structures with a wide range of polarity, they possess different coefficients of permeability. Therefore, CAPE, one of the major active phenolic constituents of propolis, was analyzed for ex vivo skin deposition study. The mean drug content in terms of CAPE from PNG and the control gel was found to be 55.92 ± 1.43 μg/g and 54.88 ± 3.09 μg/g, respectively. Estimation of mean drug deposition of PNG and the control gel in the excised rat skin was found to be 9.3 ± 2.0 μg/cm2 and 4.7 ± 1.6 μg/cm2, respectively.
4. Discussion
In this work, attempts have been made to develop niosomal-based delivery for propolis to improve its therapeutic efficacy through topical application. The ability of niosomes to improve dermal delivery of actives by enhancing their retention in the skin was thought to be useful for better achievement of therapeutic activity in terms of antimicrobial effect through topical application. Among various methods initially tried in this regard, the ethanol injection method produced PLN with desired physicochemical characteristics. At the outset, the optimal molar concentrations of Span 60 and cholesterol were determined to obtain stable niosomes free of aggregation, fusion, and sedimentation.
4.1. Influence of variables on vesicle size and EE
The amount of Span 60 and cholesterol showed profound influence over vesicle size and EE of niosomes. The observed significant decline (p < 0.001) in the size of PLN Batch J3 compared with that of Batch J2 can be attributed to the effect of cholesterol. Previous studies have shown that vesicle size reduces upon addition of cholesterol due to reduction in the curvature of vesicles because of interactive forces between Span 60 and cholesterol.31 However, further augmentation in the vesicle size of Batch J4 with an increased amount of cholesterol might be attributed to the increase in bilayer thickness on account of the probable association of the 3-OH group of cholesterol with propolis constituents. Similar effect was observed during fabrication of ciclopirox niosomes using cholesterol.32 Furthermore, the observed increase in PLN size for Batches J4, J5, and J6 can be attributed to the effect of Span 60. The diameter of niosomal vesicles is dependent on the length of alkyl chain of surfactants. Surfactants with longer alkyl chains produce larger vesicles.33 The long C18 stearyl chain of Span 60 contributes to larger vesicle size.31, 33 Further, the incorporation of propolis and its distribution within the bilayer might have contributed to the increase in the overall vesicle size of PLN compared with that of the empty vesicles. The EE of niosomes was found to be directly proportional to the concentration of Span 60, that is, as the concentration of surfactant increased linearly (J4, J5, J6), there was a significant increase (p < 0.001) in entrapment of propolis constituents as observed in Table 1. The increase in the EE of PLN was attributed to the higher amount of matrix available for propolis to be distributed within the bilayer with increasing concentrations of Span 60. In addition, increasing the molar concentration of cholesterol from 0 to 1 (J1, J2, J5, and J6; Table 1) improves the EE of niosomes owing to the membrane-stabilizing effect of cholesterol.31 The cholesterol effectively gets distributed between the bilayer and occupies void space, thereby decreasing the fluidity of the membrane and making it rigid.21, 31 Incorporation of cholesterol increases the bilayer hydrophobicity and stability of niosomes,34 reducing the permeability.35 This might lead to efficient trapping of propolis into the bilayers forming vesicles. It also decreased leakage from niosomes, thereby increasing the EE. Further increase in the cholesterol content (J3 and J4) declined the EE as it competes with propolis constituents for packing space within the bilayer (Fig. 1).31
Zeta potential reflects the charge present on the surface of the vesicle responsible for intervesicular repulsion, which prevents vesicle aggregation. The observed values for ζ for the prepared PLN indicate the formation of stable niosomes without intravesicular aggregation. The ethanol injection method is postulated to form small unilamellar vesicles.32 The transmission electron microscopy analysis confirmed the spherical shape and size of PLN. No significant change in the mean vesicle size and the absence of any signs of aggregation or sedimentation indicate the formation of stable niosomal dispersion.
The observed increase in the zone of inhibition with PLN (Table 2) against bacterial and fungal strains may be attributed to the ability of the vesicular delivery system to directly interact with the bacterial cell envelope, and thus, facilitating diffusion across the cell wall.29, 36 Vesicles such as liposomes have been reported to enhance the penetration of antibiotics by fusion with the bacterial cell.36 Furthermore, the niosomes protect an encapsulated drug from the action of bacterial enzymes as well as facilitate its diffusion across the bacterial cell wall.37 The MIC and zone of inhibition for PLN confirm the enhanced antimicrobial effect of propolis entrapped in niosomes compared with that of an ethanolic solution of propolis, thereby confirming its improved antimicrobial potential.
Drug deposition is a significant parameter that determines the performance of the topically delivered system. The efficacy of a topical delivery system is affected by its retention within the skin. The results of drug deposition indicate that PNG showed approximately twofold better skin deposition compared with that of the control gel (Fig. 4). The increase in skin deposition is directly attributed to the niosomal vesicles comprising nonionic surfactant. Niosomes are adsorbed on the outermost layer of the stratum corneum by forming stacks of bilayer on the top of the stratum corneum,22, 23, 34, 38 thereby enhancing the skin deposition shown by PNG as compared with the control gel.
Fig. 4.
Ex vivo skin deposition of propolis from propolis niosomal gel and the control gel.
5. Conclusion
This study demonstrates the successful formulation of a vesicular delivery system in the form of niosomes for multicomponent active propolis to improve its antimicrobial potential through topical application. The PLN possessed desired characteristics in terms of vesicle size, EE, and zeta potential. The amount of surfactant and cholesterol used exerts a profound effect on the vesicle size and EE. The formulated PNG demonstrates enhanced skin deposition in terms of CAPE, thereby proving the applicability of a niosomal delivery system to several multicomponent actives such as propolis to improve their therapeutic efficacy.
Conflicts of interest
The authors report no conflicts of interest.
Acknowledgments
The authors are thankful to Nature Laboratory Ltd. (Whitby, North Yorkshire, United Kingdom) for supplying the propolis sample.
Contributor Information
Kakasaheb R. Mahadik, Email: krmahadik@rediffmail.com.
Anant R. Paradkar, Email: a.paradkar1@bradford.ac.uk.
References
- 1.Alencar S.M., Oldoni T.L., Castro M.L., Cabral I.S., Costa-Neto C.M., Cury J.A. Chemical composition and biological activity of a new type of Brazilian propolis: red propolis. J Ethnopharmacol. 2007;113:278–283. doi: 10.1016/j.jep.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Fokt H., Pereira A., Ferreira A., Cunha A., Aguiar C. How do bees prevent hive infections? The antimicrobial properties of propolis. In: Méndez-Vilas A., editor. Vol. 1. Formatex; Badajoz, Spain: 2010. pp. 481–493. (Current research, technology and education. Topics in Applied Microbiology and Microbial Biotechnology). [Google Scholar]
- 3.Ghisalberti E. Propolis: a review [honey-bees] Bee World. 1979;60:59–84. [Google Scholar]
- 4.De Castro S.L. Propolis: biological and pharmacological activities. Therapeutic uses of this bee-product. Ann Rev Biol Sci. 2001;3:49–83. [Google Scholar]
- 5.Bankova V. Chemical diversity of propolis makes it a valuable source of new biologically active compounds. J ApiProd ApiMed Sci. 2009;1:23–28. [Google Scholar]
- 6.Burdock G. Review of the biological properties and toxicity of bee propolis (propolis) Food Chem Toxicol. 1998;36:347–363. doi: 10.1016/s0278-6915(97)00145-2. [DOI] [PubMed] [Google Scholar]
- 7.Hegazi A.G., Abd El Hady F.K. Egyptian propolis: 3. Antioxidant, antimicrobial activities and chemical composition of propolis from reclaimed lands. Z Naturforsch C. 2002;57:395–402. doi: 10.1515/znc-2002-3-432. [DOI] [PubMed] [Google Scholar]
- 8.Kujumgiev A., Tsvetkova I., Serkedjieva Y., Bankova V., Christov R., Popov S. Antibacterial, antifungal and antiviral activity of propolis of different geographic origin. J Ethnopharmacol. 1999;64:235–240. doi: 10.1016/s0378-8741(98)00131-7. [DOI] [PubMed] [Google Scholar]
- 9.Kumazawa S., Hamasaka T., Nakayama T. Antioxidant activity of propolis of various geographic origins. Food Chem. 2004;84:329–339. [Google Scholar]
- 10.Marcucci M.C. Propolis: chemical composition, biological properties and therapeutic activity. Apidologie (Celle) 1995;26:83–99. [Google Scholar]
- 11.Park Y.K., Koo M.H., Abreu J.A., Ikegaki M., Cury J.A., Rosalen P.L. Antimicrobial activity of propolis on oral microorganisms. Curr Microbiol. 1998;36:24–28. doi: 10.1007/s002849900274. [DOI] [PubMed] [Google Scholar]
- 12.Pontin K., Da Silva Filho A.A., Santos F.F., Silva M.L., Cunha W.R., Nanayakkara N.P. In vitro and in vivo antileishmanial activities of a Brazilian green propolis extract. Parasitol Res. 2008;103:487–492. doi: 10.1007/s00436-008-0970-z. [DOI] [PubMed] [Google Scholar]
- 13.Kalogeropoulos N., Konteles S.J., Troullidou E., Mourtzinos I., Karathanos V.T. Chemical composition, antioxidant activity and antimicrobial properties of propolis extracts from Greece and Cyprus. Food Chem. 2009;116:452–461. [Google Scholar]
- 14.Mirzoeva O., Grishanin R., Calder P. Antimicrobial action of propolis and some of its components: the effects on growth, membrane potential and motility of bacteria. Microbiol Res. 1997;152:239–246. doi: 10.1016/S0944-5013(97)80034-1. [DOI] [PubMed] [Google Scholar]
- 15.Oliveira A.C., Shinobu C.S., Longhini R., Franco S.L., Svidzinski T.I. Antifungal activity of propolis extract against yeasts isolated from onychomycosis lesions. Mem Inst Oswaldo Cruz. 2006;101:493–497. doi: 10.1590/s0074-02762006000500002. [DOI] [PubMed] [Google Scholar]
- 16.Motior Rahman M., Richardson A., Sofian-Azirun M. Antibacterial activity of propolis and honey against Staphylococcus aureus and Escherichia coli. Afr J Microbiol Res. 2010;4:1872–1878. [Google Scholar]
- 17.Ghasem Y.B., Ownagh A., Hasanloei M. Antibacterial and antifungal activity of Iranian propolis against Staphylococcus aureus and Candida albicans. Pak J Biol Sci. 2007;10:1343–1345. doi: 10.3923/pjbs.2007.1343.1345. [DOI] [PubMed] [Google Scholar]
- 18.Takaisi-Kikuni N.B., Schilcher H. Electron microscopic and microcalorimetric investigations of the possible mechanism of the antibacterial action of a defined propolis provenance. Planta Med. 1994;60:222–227. doi: 10.1055/s-2006-959463. [DOI] [PubMed] [Google Scholar]
- 19.Manconi M., Sinico C., Valenti D., Loy G., Fadda A.M. Niosomes as carriers for tretinoin. I. Preparation and properties. Int J Pharm. 2002;234:237–248. doi: 10.1016/s0378-5173(01)00971-1. [DOI] [PubMed] [Google Scholar]
- 20.Sahin N.O. Niosomes as nanocarrier systems. In: Reza Mozafari M., editor. Nanomaterials and nanosystems for biomedical applications. Springer; Dordrecht, The Netherlands: 2007. pp. 67–81. [Google Scholar]
- 21.Balakrishnan P., Shanmugam S., Lee W.S., Lee W.M., Kim J.O., Oh D.H. Formulation and in vitro assessment of minoxidil niosomes for enhanced skin delivery. Int J Pharm. 2009;377:1–8. doi: 10.1016/j.ijpharm.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 22.Hofland H.E.J., Bouwstra J.A., Ponec M., Boddé H.E., Spies F., Coos Verhoef J. Interactions of non-ionic surfactant vesicles with cultured keratinocytes and human skin in vitro: a survey of toxicological aspects and ultrastructural changes in stratum corneum. J Control Release. 1991;16:155–167. [Google Scholar]
- 23.Hofland H.E.J., Bouwstra J.A., Spies F., Boddé H.E., Nagelkerke J.F., Cullander C. Interactions between non-ionic surfactant vesicles and human stratum corneum in vitro. J Liposome Res. 1995;5:241–263. [Google Scholar]
- 24.Uchegbu I.F., Florence A.T. Non-ionic surfactant vesicles (niosomes): physical and pharmaceutical chemistry. Adv Colloid Interface Sci. 1995;58:1–55. [Google Scholar]
- 25.Uchegbu I.F., Vyas S.P. Non-ionic surfactant based vesicles (niosomes) in drug delivery. Int J Pharm. 1998;172:33–70. [Google Scholar]
- 26.Ambardekar R.V., Mahadik K.R., Paradkar A.R., Harsulkar A.M. Enhancement of hepatoprotective efficacy of propolis by fabrication of liposomes, as a platform nano-formulation for multi-component natural medicine. Curr Drug Deliv. 2012;9:477–486. doi: 10.2174/156720112802650653. [DOI] [PubMed] [Google Scholar]
- 27.Trusheva B., Trunkova D., Bankova V. Different extraction methods of biologically active components from propolis: a preliminary study. Chem Cent J. 2007;1:13. doi: 10.1186/1752-153X-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.González M., Guzmán B., Rudyk R., Romano E., Molina M.A. Spectrophotometric determination of phenolic compounds in propolis. Acta Farm Bonaer. 2003;22:243–248. [Google Scholar]
- 29.Ceschel G.C., Maffei P., Sforzini A., Lombardi Borgia S., Yasin A., Ronchi C. In vitro permeation through porcine buccal mucosa of caffeic acid phenetyl ester (CAPE) from a topical mucoadhesive gel containing propolis. Fitoterapia. 2002;73:S44–S52. doi: 10.1016/s0367-326x(02)00190-9. [DOI] [PubMed] [Google Scholar]
- 30.Marquele-Oliveira F., Fonseca Y.M., de Freitas O., Fonseca M.J. Development of topical functionalized formulations added with propolis extract: stability, cutaneous absorption and in vivo studies. Int J Pharm. 2007;342:40–48. doi: 10.1016/j.ijpharm.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 31.Nasseri B. Effect of cholesterol and temperature on the elastic properties of niosomal membranes. Int J Pharm. 2005;300:95–101. doi: 10.1016/j.ijpharm.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Shaikh K.S., Chellampillai B., Pawar A.P. Studies on nonionic surfactant bilayer vesicles of ciclopirox olamine. Drug Dev Ind Pharm. 2010;36:946–953. doi: 10.3109/03639040903585150. [DOI] [PubMed] [Google Scholar]
- 33.Manosroi A., Wongtrakul P., Manosroi J., Sakai H., Sugawara F., Yuasa M. Characterization of vesicles prepared with various non-ionic surfactants mixed with cholesterol. Colloids Surf B Biointerfaces. 2003;30:129–138. [Google Scholar]
- 34.Bernsdorff C., Wolf A., Winter R., Gratton E. Effect of hydrostatic pressure on water penetration and rotational dynamics in phospholipid-cholesterol bilayers. Biophys J. 1997;72:1264–1277. doi: 10.1016/S0006-3495(97)78773-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirby C., Clarke J., Gregoriadis G. Effect of the cholesterol content of small unilamellar liposomes on their stability in vivo and in vitro. Biochem J. 1980;186:591–598. doi: 10.1042/bj1860591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mugabe C., Azghani A.O., Omri A. Liposome-mediated gentamicin delivery: development and activity against resistant strains of Pseudomonas aeruginosa isolated from cystic fibrosis patients. J Antimicrob Chemother. 2005;55:269–271. doi: 10.1093/jac/dkh518. [DOI] [PubMed] [Google Scholar]
- 37.Moazeni E., Gilani K., Sotoudegan F., Pardakhty A., Najafabadi A.R., Ghalandari R. Formulation and in vitro evaluation of ciprofloxacin containing niosomes for pulmonary delivery. J Microencapsul. 2010;27:618–627. doi: 10.3109/02652048.2010.506579. [DOI] [PubMed] [Google Scholar]
- 38.Bonacucina G., Cespi M., Misici-Falzi M., Palmieri G.F. Rheological evaluation of silicon/Carbopol hydrophilic gel systems as a vehicle for delivery of water insoluble drugs. AAPS J. 2008;10:84–91. doi: 10.1208/s12248-008-9008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]