Abstract
Inducible defensive responses in plants are known to be activated locally and systemically by signaling molecules that are produced at sites of pathogen or insect attacks, but only one chemical signal, ethylene, is known to travel through the atmosphere to activate plant defensive genes. Methyl jasmonate, a common plant secondary compound, when applied to surfaces of tomato plants, induces the synthesis of defensive proteinase inhibitor proteins in the treated plants and in nearby plants as well. The presence of methyl jasmonate in the atmosphere of chambers containing plants from three species of two families, Solanaceae and Fabaceae, results in the accumulation of proteinase inhibitors in leaves of all three species. When sagebrush, Artemisia tridentata, a plant shown to possess methyl jasmonate in leaf surface structures, is incubated in chambers with tomato plants, proteinase inhibitor accumulation is induced in the tomato leaves, demonstrating that interplant communication can occur from leaves of one species of plant to leaves of another species to activate the expression of defensive genes.
Full text
PDF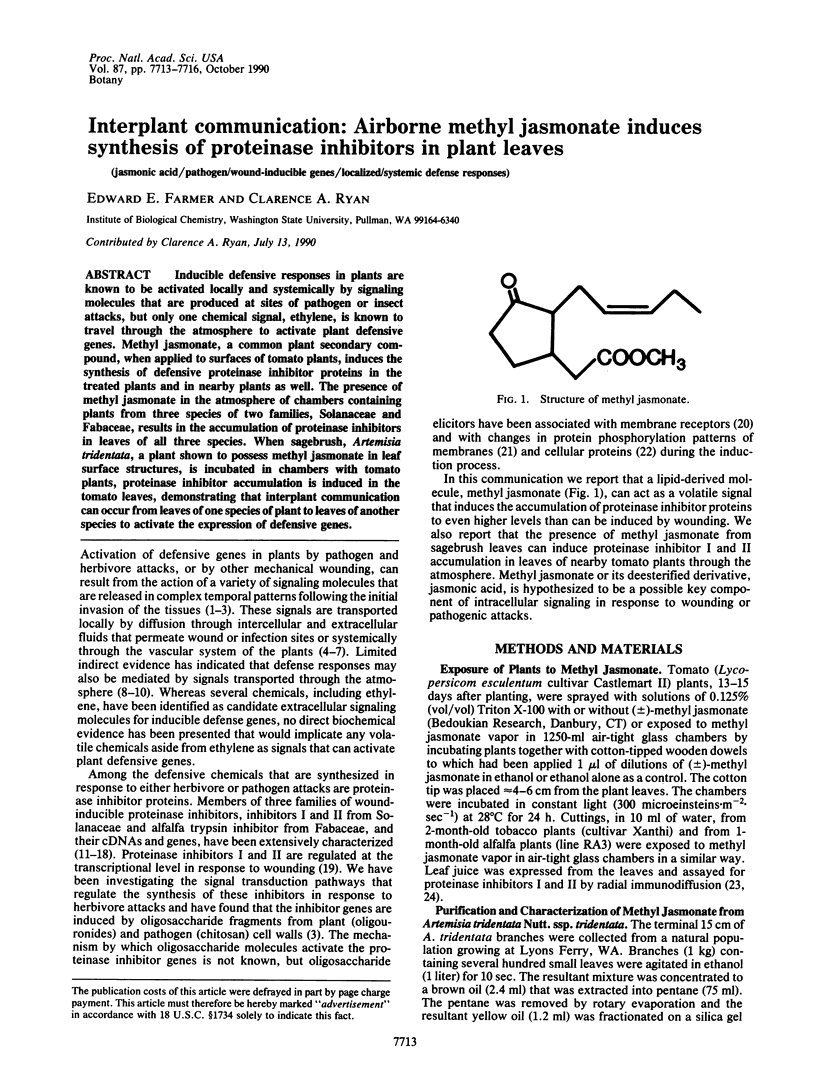

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin I. T., Schultz J. C. Rapid changes in tree leaf chemistry induced by damage: evidence for communication between plants. Science. 1983 Jul 15;221(4607):277–279. doi: 10.1126/science.221.4607.277. [DOI] [PubMed] [Google Scholar]
- Brown W. E., Ryan C. A. Isolation and characterization of a wound-induced trypsin inhibitor from alfalfa leaves. Biochemistry. 1984 Jul 17;23(15):3418–3422. doi: 10.1021/bi00310a006. [DOI] [PubMed] [Google Scholar]
- Bryant J., Green T. R., Gurusaddaiah T., Ryan C. A. Proteinase inhibitor II from potatoes: isolation and characterization of its protomer components. Biochemistry. 1976 Aug 10;15(16):3418–3424. doi: 10.1021/bi00661a004. [DOI] [PubMed] [Google Scholar]
- Dietrich A., Mayer J. E., Hahlbrock K. Fungal elicitor triggers rapid, transient, and specific protein phosphorylation in parsley cell suspension cultures. J Biol Chem. 1990 Apr 15;265(11):6360–6368. [PubMed] [Google Scholar]
- Farmer E. E., Pearce G., Ryan C. A. In vitro phosphorylation of plant plasma membrane proteins in response to the proteinase inhibitor inducing factor. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1539–1542. doi: 10.1073/pnas.86.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J. S., Pearce G., Merryweather J., Titani K., Ericsson L. H., Ryan C. A. Wound-induced proteinase inhibitors from tomato leaves. II. The cDNA-deduced primary structure of pre-inhibitor II. J Biol Chem. 1985 Jun 10;260(11):6561–6564. [PubMed] [Google Scholar]
- Graham J. S., Pearce G., Merryweather J., Titani K., Ericsson L., Ryan C. A. Wound-induced proteinase inhibitors from tomato leaves. I. The cDNA-deduced primary structure of pre-inhibitor I and its post-translational processing. J Biol Chem. 1985 Jun 10;260(11):6555–6560. [PubMed] [Google Scholar]
- Green T. R., Ryan C. A. Wound-Induced Proteinase Inhibitor in Plant Leaves: A Possible Defense Mechanism against Insects. Science. 1972 Feb 18;175(4023):776–777. doi: 10.1126/science.175.4023.776. [DOI] [PubMed] [Google Scholar]
- Kopp M., Rouster J., Fritig B., Darvill A., Albersheim P. Host-Pathogen Interactions : XXXII. A Fungal Glucan Preparation Protects Nicotianae against Infection by Viruses. Plant Physiol. 1989 May;90(1):208–216. doi: 10.1104/pp.90.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton M. A., Lamb C. J. Transcriptional activation of plant defense genes by fungal elicitor, wounding, and infection. Mol Cell Biol. 1987 Jan;7(1):335–341. doi: 10.1128/mcb.7.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Brown W. E., Graham J. S., Pearce G., Fox E. A., Dreher T. W., Ahern K. G., Pearson G. D., Ryan C. A. Molecular characterization and phylogenetic studies of a wound-inducible proteinase inhibitor I gene in Lycopersicon species. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7277–7281. doi: 10.1073/pnas.83.19.7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melville J. C., Ryan C. A. Chymotrypsin inhibitor I from potatoes. Large scale preparation and characterization of its subunit components. J Biol Chem. 1972 Jun 10;247(11):3445–3453. [PubMed] [Google Scholar]
- Ryan C. A. Oligosaccharide signalling in plants. Annu Rev Cell Biol. 1987;3:295–317. doi: 10.1146/annurev.cb.03.110187.001455. [DOI] [PubMed] [Google Scholar]
- Ryan C. A. Quantitative determination of soluble cellular proteins by radial diffusion in agar gels containing antibodies. Anal Biochem. 1967 Jun;19(3):434–440. doi: 10.1016/0003-2697(67)90233-3. [DOI] [PubMed] [Google Scholar]
- Sanchez-Serrano J. J., Keil M., O'Connor A., Schell J., Willmitzer L. Wound expression of a potato proteinase inhibitor II gene in transgenic tobacco plants. EMBO J. 1987 Feb;6(2):303–306. doi: 10.1002/j.1460-2075.1987.tb04754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W. E., Ebel J. Specific binding of a fungal glucan phytoalexin elicitor to membrane fractions from soybean Glycine max. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4117–4121. doi: 10.1073/pnas.84.12.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick P. E. Novel Regulation of Vegetative Storage Protein Genes. Plant Cell. 1990 Jan;2(1):1–6. doi: 10.1105/tpc.2.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornburg R. W., An G., Cleveland T. E., Johnson R., Ryan C. A. Wound-inducible expression of a potato inhibitor II-chloramphenicol acetyltransferase gene fusion in transgenic tobacco plants. Proc Natl Acad Sci U S A. 1987 Feb;84(3):744–748. doi: 10.1073/pnas.84.3.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautman R., Cowan K. M., Wagner G. G. Data processing for radial immunodiffusion. Immunochemistry. 1971 Oct;8(10):901–916. doi: 10.1016/0019-2791(71)90429-0. [DOI] [PubMed] [Google Scholar]
- Ueda J., Kato J. Isolation and Identification of a Senescence-promoting Substance from Wormwood (Artemisia absinthium L.). Plant Physiol. 1980 Aug;66(2):246–249. doi: 10.1104/pp.66.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick B. A., Zimmerman D. C. Biosynthesis of jasmonic Acid by several plant species. Plant Physiol. 1984 Jun;75(2):458–461. doi: 10.1104/pp.75.2.458. [DOI] [PMC free article] [PubMed] [Google Scholar]



