Abstract
Background
Magnolia officinalis cortex has been traditionally used to treat stomach and intestine diseases in traditional Korean medicine. In this study, we investigated the effect of water extract of M. officinalis cortex (WEMC) on osteoclast differentiation and function.
Methods
Phytochemical characterization of WEMC was performed by high-performance liquid chromatography analysis. Osteoclast differentiation of bone marrow-derived macrophages was determined by tartrate-resistant acid phosphatase activity assay. Receptor activator of nuclear factor-κB ligand (RANKL) signaling factors and transcription factors regulating osteoclast differentiation were analyzed by Western blot and real-time polymerase chain reaction. Bone resorption function of mature osteoclasts was examined by using culture plate coated with inorganic crystalline calcium phosphate. Furthermore, the in vivo effect of WEMC on osteoporosis was examined using RANKL-induced bone loss model, characterized by micro-computed tomography and bone metabolism marker analysis.
Results
WEMC inhibited RANKL-induced osteoclast differentiation and the bone resorbing activity of mature osteoclasts. WEMC contains gallic acid and honokiol as active constituents contributing to the inhibitory effect of WEMC on osteoclast differentiation. Further, WEMC suppressed RANKL-induced activation of p38 and nuclear factor-κB pathways and expression of osteoclastogenic transcription factors such as c-Fos for AP-1 and nuclear factor of activated T cells cytoplasmic 1. Ectopic overexpression of a constitutive active form of nuclear factor of activated T cells cytoplasmic 1 rescued the antiosteoclastogenic effect of WEMC. Consistent with the in vitro results, WEMC suppressed RANKL-induced trabecular bone loss in mice.
Conclusion
WEMC might have a therapeutic potential to treat pathological bone diseases due to increased osteoclast differentiation and function.
Keywords: Magnolia officinalis cortex, nuclear factor of activated T cells cytoplasmic 1, osteoclastogenesis, receptor activator for nuclear factor-κB ligand
1. Introduction
Regulation of bone remodeling between osteoclastic bone resorption and osteoblastic bone formation is critical to maintain bone mass, bone structural integrity, and mineral homeostasis in adults. Imbalance of the bone remodeling by excess osteoclastogenesis results in bone loss, as seen in various bone destructive diseases such as osteoporosis, rheumatoid arthritis, and osteolytic bone metastasis.1, 2 Osteoclasts are multinucleated bone resorbing cells derived from hematopoietic monocyte/macrophage precursor cells. Receptor activator for nuclear factor (KF)-κB ligand (RANKL), a tumor necrosis factor ligand superfamily member, is a key cytokine for osteoclast differentiation and function.3, 4 The binding of RANKL to RANK receptor on osteoclast precursors recruits tumor necrosis factor receptor-associated factor 6 to the cytoplasmic tail of RANK to trigger downstream signaling cascades including NF-κB and mitogen-activated protein kinase (MAPK) pathways. They subsequently lead to the activation of key transcription factors, AP-1 and NF of activated T cells cytoplasmic 1 (NFATc1), to regulate osteoclastic transcription.5, 6, 7, 8
There is a growing interest in medicinal herbs that have the potential to prevent or treat bone diseases with fewer side effects.9 Magnolia officinalis cortex named as Hubak in Traditional Korean Medicine has been used to strength the gastrointestinal tract, to treat ulcer, and to induce sedative effect.10 M. officinalis cortex is a key ingredient of several traditional medicines such as Pyengwisan and Banhahubaktang that are commercially available and widely prescribed at traditional medicine clinics in Korea.11, 12 Previous studies have shown that M. officinalis extracts have antibacterial,13 antiatherogenic,14 antineuroinflammatory,15 and anticancer effects.16 Moreover, M. officinalis cortex has several biological active components, including magnolol and honokiol, to have beneficial effects on ligature-induced periodontitis or arthritis by inhibiting osteoclastogenesis, suggesting an inhibitory potential of M. officinalis cortex on inflammatory-induced bone disease.17, 18 However, the effect of water extract of M. officinalis cortex (WEMC) on bone metabolism or RANK-related molecular mechanism has not been studied. In this study, we investigated the effects of WEMC on RANK signaling regulating osteoclast differentiation and function in osteoclast precursor cells to elucidate underlying action mechanism of WEMC. We also examined the in vivo effect of WEMC on osteoclast-mediated bone destruction using a murine model of RANKL-induced osteoporosis.
2. Methods
2.1. Reagents and antibodies
M. officinalis cortex was purchased from Yeongcheon herb (Yeongcheon, Korea). Alpha-modified minimal essential medium (α-MEM), fetal bovine serum, and penicillin/streptomycin were purchased from Gibco BRL Life Technologies (Grand Island, NY, USA). Macrophage colony-stimulating factor (M-CSF) and RANKL were obtained as described previously.19 Antibodies against phospho-JNK1/2 (Thr183/Tyr185), JNK, phospho-p38 (Thr180/Tyr182), p38, phospho-IκBα (Ser32), and IκBα were from Cell Singling Technology (Danvers, MA, USA). Antibodies against β-actin, c-Fos, and NFATc1 were from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
2.2. Preparation of WEMC
M. officinalis cortex was authenticated by Prof. K.H. Bae (Chungnam National University, Chungnam, Korea). A voucher specimen of M. officinalis cortex (No. W172) was deposited in the herbal bank of KM-Based Herbal Drug Research Group, Korea Institute of Oriental Medicine, Daejeon, Korea. M. officinalis cortex (50 g) was boiled for 3 hours in 1 L of distilled water. After filtration using testing sieves (150 μm; Retsch, Haan, Germany), the extract was lyophilized and stored at −20 °C prior to use. To prepare WEMC, the lyophilized powder (yield: 6.72%) was resuspended in distilled water, centrifuged at 10,000 × g for 15 minutes, and filtered through a 0.2 μm sterile filter.
2.3. High-performance liquid chromatography analysis
High-performance liquid chromatography (HPLC) analysis of WEMC was performed using the Waters HPLC 2695 system (Waters Co., Milford, MA, USA) consisting of a pump, autosampler, column oven, and photodiode array detector 996. Chromatographic separation was achieved in a RS-tech C18 column (4.6 mm × 250 mm, 5 μm, 35 °C). The mobile phase was 0.1% TFA (A) and 100% acetonitrile (B) with a step gradient elution (5 minutes, 5% B; 65 minutes, 100% B; 75 minutes, 100% B; 86 minutes, 5% B) at a flow rate of 1.0 mL/min. A mixture of marker components (gallic acid, syringin, honokiol, and magnolol; each 100 μg/mL) and WEMC (50 mg/mL) were dissolved in methanol and filtered through a 0.2 μm filter. For each sample, 10 μL was injected for the HPLC analysis.
2.4. Bone marrow macrophage culture and osteoclast differentiation
Bone marrow macrophages (BMMs) were obtained from mouse bone marrow cells and cultured in α-MEM complete medium containing 10% fetal bovine serum and 1% penicillin/streptomycin in the presence of M-CSF (60 ng/mL) as described previously.20 Cell viability of BMMs was determined using Cell Counting Kit-8 (Dojindo Molecular Technologies Inc., Rockville, MD, USA), after 2 days of BMM culture (1 × 104 cells/well in a 96-well plate) with WEMC and M-CSF (60 ng/mL). To induce osteoclast differentiation from BMMs, BMMs (1 × 104 cells/well) were cultured with M-CSF (60 ng/mL) and RANKL (100 ng/mL) for 4 days in 96-well plates. Cells were replenished with fresh medium and treatments on Day 3. To identify osteoclasts, cells were fixed in 10% neutral buffered formalin (Sigma-Aldrich, St. Louis, MO, USA) in phosphate-buffered saline (PBS), permeabilized with 0.1% Triton X-100 in PBS, and then stained with tartrate-resistant acid phosphatase (TRAP) buffer (50 mM sodium tartrate and 0.12 M sodium acetate, pH 5.2) containing naphthol AS-MX phosphate (0.1 mg/mL, Sigma-Aldrich) and fast red violet LB salt (0.5 mg/mL, Sigma-Aldrich). TRAP-stained cells were washed with distilled water and observed under a light microscope. TRAP-positive multinucleated cells containing more than three nuclei and > 100 μm in diameter were counted as osteoclasts.
2.5. Western blot analysis
BMMs were washed twice with ice-cold PBS and lysed in a protein extraction buffer (Millipore, Billerica, MA, USA) containing protease and phosphatase inhibitor cocktails (Roche Applied Science, Indianapolis, IN, USA) at 4 °C. Total cell lysates were obtained by centrifugation at 10,000 × g for 15 minutes at 4 °C. Protein concentration of lysates was determined with a BCA Protein Assay Kit (Thermo Fisher Scientific Inc., Rockford, IL, USA). Protein samples (30 μg) were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes (GE Healthcare, Little Chalfont, Buckinghamshire, UK). Membranes were blocked with blocking buffer, 5% nonfat dry milk in TBST (10 mM Tris-HCl pH 7.5, 150 mM NaCl, and 0.1% Tween 20), for 1 hour at room temperature, probed with the indicated primary antibodies (1/1000 dilution) overnight at 4 °C, and then washed with TBST three times for 10 minutes each. Membranes were then incubated with horseradish peroxidase-conjugated secondary antibodies (1/4000 dilution) for 1 hour at room temperature and washed with TBST three times. Chemiluminescent signals were detected on a LAS-4000 Luminescent Image Analyzer (Fuji Photo Film Co., Tokyo, Japan) with SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific Inc.).
2.6. Real-time quantitative polymerase chain reaction analysis
Total RNA was isolated with RNA-spin total RNA Extraction Kit (Bioneer, Daejeon, Korea) according to the manufacturer's protocol. cDNA was synthesized from 1 μg of total RNA with AccuPower RT-PreMix (Bioneer) according to the manufacturer's protocol. SYBR green-based quantitative polymerase chain reaction (PCR) amplification was performed on the Applied Biosystems 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) with cDNA diluted to 1:3, 10 pmol of primers, and AccuPower GreenStar qPCR Master Mix (Bioneer). The following mouse-specific primer sets were used: c-Fos, 5′-CGGGTTTCAACGCCGACTAC-3′ (forward) and 5′-AAAGTTGGCACTAGAGACGGACAGA-3′ (reverse); NFATc1, 5′-CCGTTGCTTCCA GAAAATAACA-3′ (forward) and 5′-TGTGGGATGTGAACTCGGAA-3′ (reverse); hypoxanthine phosphoribosyltransferase, 5′-CCTAAGATGAGCGCAAGTTGAA-3′ (forward) and 5′-CCACAGGACTAGAACACC TGCTAA-3′ (reverse). The PCR reaction consisted of 40 cycles of 94 °C for 20 seconds and 60 °C for 40 seconds. All reactions were run in triplicate, and data were analyzed using the 2−ΔΔCT method. Hypoxanthine phosphoribosyltransferase was used as an internal control to normalize RNA expression.
2.7. Retroviral gene transduction
To generate retroviral stocks, retroviral vectors, pMX-IRES-GFP (control vector) and pMX-CA-NFATc1-IRES-GFP encoding a constitutively active (CA) form of NFATc1, were transfected into Plat-E retroviral packaging cells (Cell Biolabs, San Diego, CA, USA). Viral supernatant was collected from culture media 48 hours after transfection. To infect BMMs with retrovirus, BMMs were incubated with retroviral supernatant together with polybrene (6 μg/mL, Sigma-Aldrich) and M-CSF (60 ng/mL) for 12 hours. BMMs infected were washed with α-MEM, cultured in α-MEM complete medium in the presence of M-CSF (60 ng/mL) for 1 day, and then treated as indicated.
2.8. Bone resorption assay
Primary calvarial osteoblasts were isolated from calvariae of newborn ICR mice (Samtako Bio Inc., Seoul, Korea) by using a sequential enzymatic digestion method described previously.21 To obtain mature osteoclasts, bone marrow cells (1.5 × 107 cells) and calvarial osteoblasts (1.5 × 106 cells) were cocultured with 1α,25-dihydroxyvitamin D3 (10 nM, Sigma-Aldrich) and prostaglandin E2 (100 nM; Sigma-Aldrich) for 6 days in a 10-cm culture dish coated with collagen gel (Cellmatrix type I-A; Nitta Gelatin Inc., Osaka, Japan). Mature osteoclasts were detached with 0.2% collagenase (Sigma-Aldrich), placed on an Osteo Assay Surface plate (Corning Inc., Corning, NY, USA), and allowed to settle for 2 hours, and then cultured with vehicle or WEMC for another 16 hours. Cells were stained for TRAP to identify osteoclasts. After removing cells with sodium hypochlorite, resorption pits were photographed and analyzed by using ImageJ software (National Institutes of Health, ML, USA).
2.9. Mouse model of osteoporosis
Animal experiments were handled in accordance with the Korea Food and Drug Administration Guide for the Care and Use of Laboratory Animals. The experiments were approved by the Institutional Animal Care and Use Committee at the Korea Institute of Oriental Medicine (Approval number; 12-121). Six-week-old male ICR mice (Samtako Bio Inc.) were housed at 22 ± 1 °C and 55 ± 10% humidity on a 12-hour light/dark cycle with unlimited access to food and water. After acclimatization for 1 week, mice were orally administered with vehicle (distilled water, n = 8 per group) or WEMC (0.25 g/kg and 0.75 g/kg of body weight, n = 7 per group) twice daily for 5 days. RANKL (1 mg/kg of body weight) or PBS was intraperitoneally injected on Day 3 and Day 4. After being fasted for 12 hours, blood samples and the right femora were harvested on Day 7. Serum C-terminal cross-linked telopeptide of type I collagen (CTX) and osteocalcin levels were measured using a RatLaps EIA kit (Immunodiagnostic Systems Inc., Fountain Hills, AZ, USA) and a mouse osteocalcin EIA kit (Biomedical Technologies Inc., Stoughton, MA, USA), respectively. Serum TRAP 5b activity was determined using the fluorogenic substrate naphthol AS-BI phosphate (Sigma-Aldrich) as described previously.19 Micro- computed tomography (CT) images of the distal femur of each mouse were acquired using the In-Vivo Micro-CT (SkyScan 1076; SkyScan N.V., Kontich, Belgium) at a resolution of 18 μm. The beam-hardening errors were corrected to improve the quality of the micro-CT images by flat-field correction prior to scanning and beam-hardening correction during reconstruction. Three-dimensional models of the trabecular bones were reconstructed using SkyScan CT Analyzer version 1.13 (Bruker Inc., WI, USA) to evaluate the alteration of bone. The structural parameters were measured at the distal femoral metaphysis between 0.54 mm and 1.46 mm distal to the growth plate.
2.10. Statistical analysis
Values are presented as mean ± standard deviation for the in vitro study and mean ± standard error of the mean for the in vivo study. Statistical significance of experimental results was analyzed by Student t test for comparison of two groups or analysis of variance followed by Dunnett test for comparison of multiple groups. A p value < 0.05 was considered significant.
3. Results
3.1. WEMC inhibits RANKL-induced osteoclast differentiation
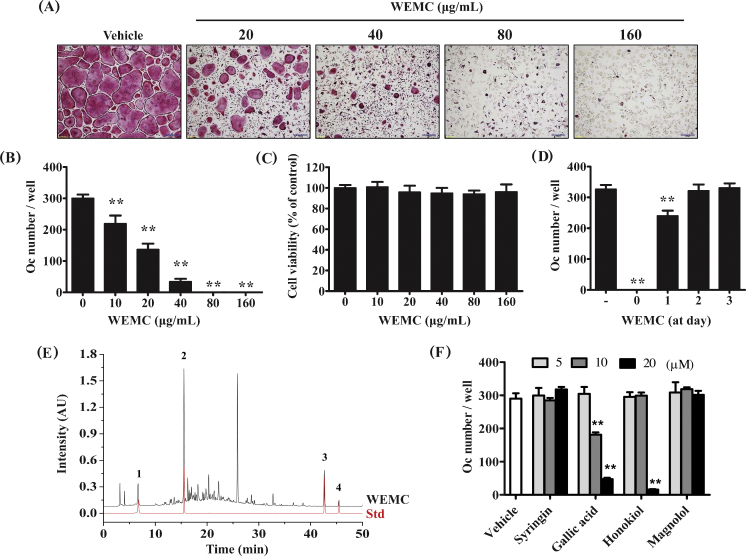
RANKL induces the differentiation of precursor cells such as mouse BMMs into osteoclasts in the presence of M-CSF. When BMMs were treated with RANKL for 4 days, TRAP-positive multinucleated osteoclasts were formed. WEMC dose-dependently inhibited RANKL-induced osteoclast formation in BMM cultures with nearly complete inhibition at 80 μg/mL (Fig. 1A–B). WEMC up to 160 μg/mL of concentration did not show any inhibitory effect on the growth of BMMs (Fig. 1C), suggesting that the inhibitory effect of WEMC was not due to cytotoxicity. When WEMC was added 1 day after RANKL stimulation, the inhibitory effect of WEMC on osteoclast differentiation was markedly decreased. WEMC did not inhibit RANKL-induced osteoclast formation when added on Day 2 and Day 3 (Fig. 1D). These results suggest that WEMC inhibits the early stage of RANKL-induced osteoclast differentiation.
Fig. 1.
Effect of WEMC on RANKL-induced osteoclast differentiation in BMMs. BMMs were cultured with vehicle (distilled water) or WEMC (20–160 μg/mL) in the presence of M-CSF (60 ng/mL) and RANKL (100 ng/mL) for 4 days. (A) Cultured cells were fixed and stained for TRAP activity. Scale bar, 200 μm. (B) TRAP-positive multinucleated cells containing more than three nuclei and > 100 μm in diameter were counted as osteoclasts. (C) BMMs were cultured with or without WEMC in the presence of M-CSF for 2 days, and cell viability was determined using Cell Counting Kit-8 assay. (D) BMMs were cultured in the presence of M-CSF and RANKL for 4 days. WEMC was added to the cultures at the indicated days, and the number of osteoclasts was counted on Day 4. (E) HPLC chromatograms of WEMC and a standard mixture of (1) gallic acid, (2) syringin, (3) honokiol, and (4) magnolol at 254 nm. (F) BMMs were cultured with the indicated compounds in the presence of M-CSF and RANKL for 4 days, and the number of osteoclasts was counted.
**p < 0.01 versus vehicle-treated control.
BMM, bone marrow macrophage; M-CSF, macrophage colony-stimulating factor; RANKL, receptor activator of nuclear factor-κB ligand; TRAP, tartrate-resistant acid phosphatase; WEMC, water extract of Magnolia officinalis cortex.
Previous phytochemical studies have shown that lignans and alkaloids are the major components of M. officinalis cortex.22, 23 We identified gallic acid, syringin, honokiol, and magnolol from WEMC by HPLC analysis, based on their HPLC retention times and UV absorption spectra (Fig. 1E). We examined whether these components mediate the antiosteoclastogenic action of WEMC. Among them, gallic acid showed the strongest inhibitory effect (Fig. 1F). Consistent with a previous report,24 honokiol inhibited RANKL-induced osteoclast formation in BMM cultures.
3.2. WEMC suppresses RANKL-induced NFATc1 expression in BMMs
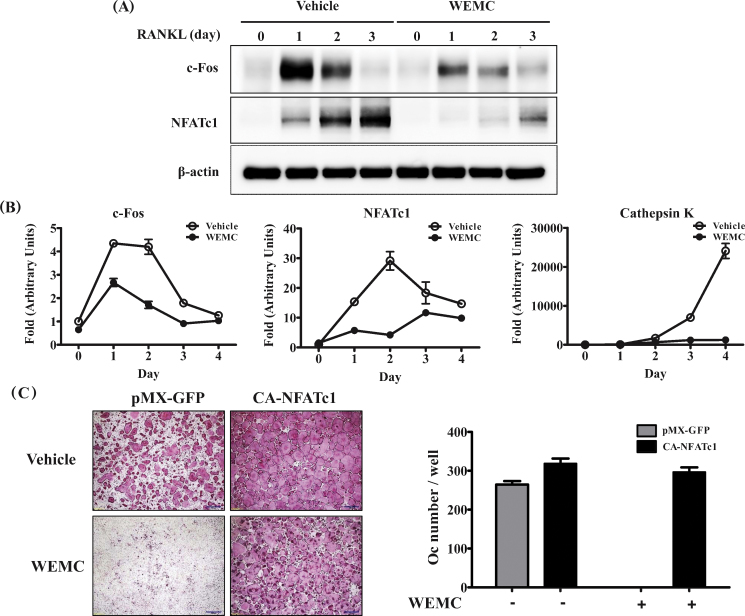
To investigate the mechanisms by which WEMC inhibits osteoclast differentiation, we assessed the impact of WEMC on the expression NFATc1, the master transcription factor for osteoclast differentiation.25 WEMC markedly inhibited RANKL-induced the mRNA and protein expression of NFATc1 induced by RANKL in BMMs (Fig. 2A, 2B). WEMC also suppressed the mRNA expression of cathepsin K, a downstream target of NFATc1 activation (Fig. 2B).26 To examine the antiosteoclastogenic action of WEMC caused by the downregulation of NFATc1, BMMs were retrovirally transduced with CA-NFATc1, which carries serine to alanine substitutions in the conserved serine-rich domain and all three serine–proline repeats.27 The ectopic expression of CA-NFATc1 blunted the inhibitory effect of WEMC (Fig. 2C). Since c-Fos is also upregulated during osteoclast differentiation and mainly functions to induce NFATc1 transcription,28 we next examined the effect of WEMC on c-Fos expression. WEMC suppressed RANKL-induced mRNA and protein expression of c-Fos (Fig. 2A, 2B).
Fig. 2.
Effect of WEMC on RANKL-induced c-Fos and NFATc1 expression in BMMs. BMMs were pretreated with vehicle or WEMC (80 μg/mL) for 3 hours, and then further cultured with RANKL (100 ng/mL). Total cell lysate or RNA was obtained at the indicated time points. (A) Total cell lysates (30 μg) were subjected to Western blot analysis with the indicated antibodies. β-actin was used as a loading control. (B) mRNA expression levels of c-Fos, NFATc1, and cathepsin K were analyzed by quantitative polymerase chain reaction. (C) BMMs infected with pMX-GFP (control retrovirus) or retrovirus encoding constitutively active NFATc1 were cultured with vehicle or WEMC (80 μg/mL) in the presence of M-CSF (60 ng/mL) and RANKL (100 ng/mL) for 4 days. The number of osteoclasts was counted.
BMM, bone marrow macrophage; M-CSF, macrophage colony-stimulating factor; NFATc-1, nuclear factor of activated T cells cytoplasmic 1; RANKL, receptor activator of nuclear factor-κB ligand; WEMC, water extract of Magnolia officinalis cortex.
3.3. WEMC affects RANKL-induced NF-κB and p38 activation in BMMs
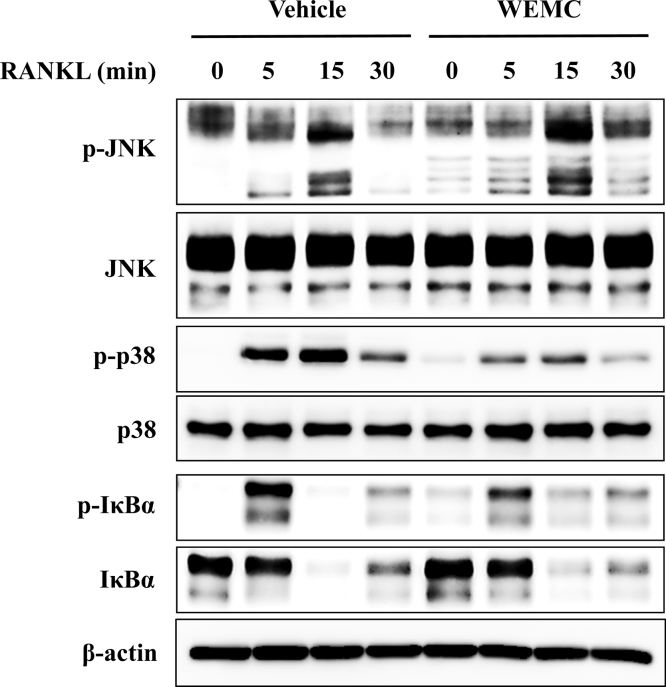
RANKL induces the activation of MAPK and NF-κB pathways, and these pathways are involved in c-Fos and NFATc1 expression.7, 8, 29 To elucidate the mechanisms underlying the inhibitory effect of WEMC on c-Fos and NFATc1 expression, we investigated the impact of WEMC on RANKL-induced activation of MAPK and NF-κB pathways. WEMC suppressed RANKL-induced phosphorylation of p38, but not JNK. WEMC slightly suppressed RANKL-induced activation of NF-κB pathway assessed by IκBα phosphorylation and degradation (Fig. 3). Thus, our results suggest that WEMC inhibits c-Fos and NFATc1 induction, at least in part, by suppressing RANKL-induced activation of p38 and NF-κB pathways.
Fig. 3.
Effect of WEMC on RANKL-induced activation of MAPK and NF-κB pathways in BMMs. BMMs were pretreated with WEMC (80 μg/mL) in the presence of M-CSF (60 ng/mL) for 3 hours and then incubated with RANKL (100 ng/mL) for indicated time points. Total cell lysate (30 μg) was subjected to Western blot analysis with the indicated antibodies.
BMM, bone marrow macrophage; M-CSF, macrophage colony-stimulating factor; RANKL, receptor activator of nuclear factor-κB ligand; WEMC, water extract of Magnolia officinalis cortex.
3.4. WEMC inhibits bone resorbing activity of osteoclasts
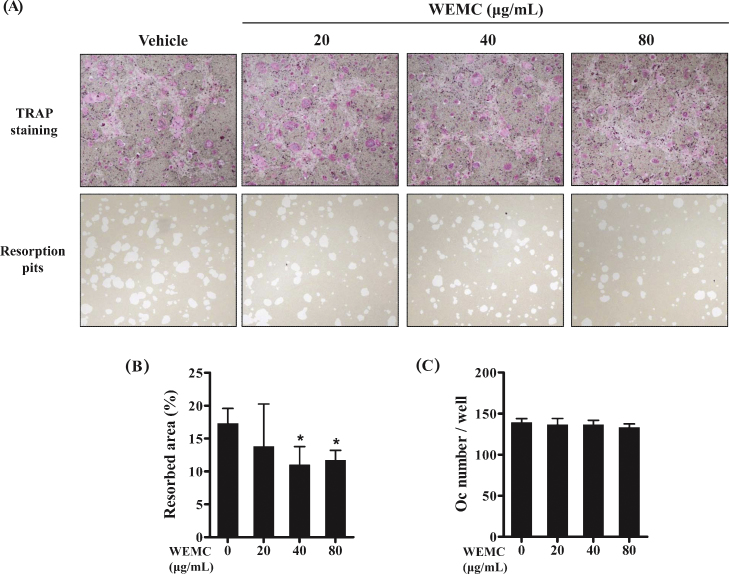
When attached to mineralized matrix, mature osteoclasts polarize their membrane and secrete hydrochloric acid and proteases into a sealed compartment, which degrades both the organic and the inorganic components of bone surface.2 We next asked whether WEMC affects bone resorption function of osteoclasts. Mature osteoclasts obtained from the coculture of osteoblasts and bone marrow cells were cultured on a plate coated with an inorganic crystalline calcium phosphate in the presence or absence of WEMC. After 16 hours of incubation, mature osteoclasts treated with vehicle generated numerous resorption pits. However, WEMC (40 μg/mL and 80 μg/mL) significantly decreased the resorbed area without affecting osteoclast number (Fig. 4A–C), suggesting that WEMC directly inhibits bone resorbing activity of mature osteoclasts.
Fig. 4.
Effect of WEMC on bone resorbing activity of mature osteoclasts. Mature osteoclasts obtained from the coculture of mouse calvarial osteoblasts and bone marrow cells were cultured on an Osteo Assay Surface plate coated with an inorganic crystalline calcium phosphate in the presence or absence of WEMC (20–80 μg/mL) for 16 hours. (A) Representative microscopic images of TRAP staining (upper panel) and resorption pits (lower panel). (B) Quantification of the resorbed areas. (C) The number of osteoclasts was counted.
*p < 0.05 versus vehicle-treated control.
TRAP, tartrate-resistant acid phosphatase; WEMC, water extract of Magnolia officinalis cortex.
3.5. WEMC attenuates RANKL-induced bone destruction
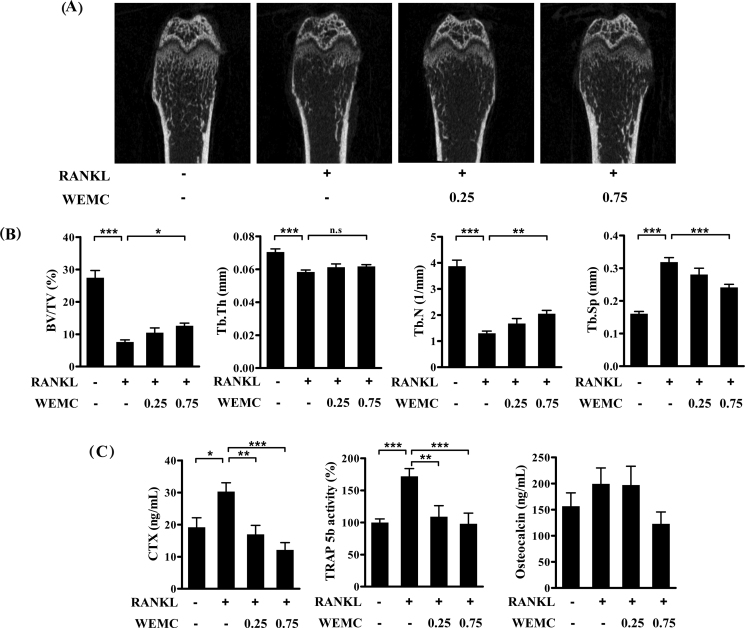
Having established that WEMC suppresses osteoclast differentiation and function, we next examined whether WEMC has a protective effect against bone destruction. We used a RANKL-induced osteoporosis mouse model. Intraperitoneal injections of RANKL rapidly induced trabecular bone loss with increases in serum CTX and TRAP 5b levels, which are markers of bone resorption and osteoclast number, respectively.30
Consistent with the previous results, intraperitoneal injections of RANKL caused a severe trabecular bone loss at the distal femoral metaphysis with decreases in trabecular bone volume, trabecular thickness, and trabecular number and an increase in trabecular separation. WEMC at 0.75 g/kg significantly attenuated RANKL-induced trabecular bone loss and architectural alterations, except trabecular thickness (Fig. 5A, 5B). In addition, WEMC at 0.25 g/kg and 0.75 g/kg prevented RANKL-induced increases in serum CTX levels and TRAP 5b activities. Serum osteocalcin levels, a maker of bone formation, were unchanged by either RANKL or WEMC (Fig. 5C).
Fig. 5.
Effect of WEMC on RANKL-induced bone destruction. Mice were orally administrated with WEMC (0.25 g/kg and 0.75 g/kg) twice per day for 5 days, and RANKL (1 mg/kg) was injected intraperitoneally on Day 3 and Day 4. Femora and sera were collected on Day 6. (A) Representative micro-CT images of the distal femur. (B) Bone volume/tissue volume (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), and trabecular separation (Tb.Sp) at the distal femoral metaphysis by micro-CT analysis. (C) CTX levels, TRAP 5b activity, and osteocalcin levels in serum.
*p < 0.05, **p < 0.01, ***p < 0.001.
CT, computed tomography; CTX, serum C-terminal cross-linked telopeptide of type 1 collagen; RANKL, receptor activator of nuclear factor-κB ligand; WEMC, water extract of Magnolia officinalis cortex.
4. Discussion
M. officinalis cortex has been used to treat liver disease, gastrointestinal disorders, anxiety, and allergic disease in traditional Korean medicine.31 In this study, we have demonstrated that WEMC inhibits osteoclast differentiation by inhibiting p38 and IκBα phosphorylation needed for NFATc1 expression. Our results also show that WEMC suppresses osteoclast resorption activity and trabecular bone loss.
NFATc1 expression is regulated by NF-κB, AP-1, and MAPK signaling, which is critical to determine the cell fate of osteoclasts at the early phase of RANKL-induced osteoclastogenesis. We found that WEMC significantly inhibits RANKL-induced NFATc1 and c-Fos expression, and partially inhibits IκB and p38 phosphorylation. Genetic study using NF-κB p50/p52 double knockout mice suggests that NF-κB is upstream of c-Fos, which binds to the NFATc1 promoter for its induction during the early phase of osteoclastogenesis.6, 28 In addition, IκB lacking its phosphorylation site blocks osteoclast differentiation and activation.32 It has also reported that an inhibition of IκB kinase (IKK) by IKK regulatory peptide inhibits RANKL-induced osteoclastogenesis and bone loss in arthritic model.33 Moreover, p38 activation participates to NF-κB transactivation by stimulating p65 phosphorylation for NFATc1 induction.34 Thus, it is reasonable to suggest that WEMC suppresses osteoclastogenesis by inhibiting NF-κB and p38 signaling pathway for NFATc1 expression.
When osteoclasts contact bone, they are polarized and dynamic cytoskeleton reorganization is initiated to form actin ring, accompanied with the secretion of hydroxycholide and several proteolytic enzymes to degrade collagenous bone matrix.35 Inhibitory peptide or genetic knockout of cytoskeletal signaling molecules mediated ανβ3 integrin signaling completely inhibits osteoclast maturation and bone resorption,36, 37 suggesting that cytoskeletal organization for actin ring formation is critical for bone resorption activity. We found that WEMC (40 μg/mL and 80 μg/mL) significantly inhibited RANKL-induced bone resorption by 30% when compared to vehicle-treated control. Thus, it seems that WEMC might partially inhibit bone resorbing activity of mature osteoclasts by affecting actin ring signaling.
Our micro-CT study demonstrated significantly increased trabecular bone volume and trabecular number, and decreased trabecular separation in WEMC administrated mice, resulting in the prevention of trabecular bone loss. It has been reported that intraperitoneal RANKL injection induces excess osteoclast number with increased resorption activity, which induces trabecular bone loss in mice.30 WEMC inhibited serum CTX and TRAP 5b levels, representative markers for osteoclast number and bone resorption activity, which is consistent with in vitro WEMC inhibitory activity. These results suggest that WEMC attenuates the in vivo bone destruction by suppressing osteoclast differentiation and bone resorption.
M. officinalis cortex contains several active compounds such as magnolol, honokiol, and other neolignan compounds, which have an inhibitory effect on osteoclastogenesis. Honokiol (3–30 μM) and magnolol (5–20 μM) inhibit RANKL-induced osteoclast formation in RAW264.7 cells.18, 24 Syringin (20 μM) prevents parathyroid hormone-induced osteoclast formation in the coculture of osteoblasts and bone marrow cells.38 In this study, we found that gallic acid and honokiol, but not magnolol and syringin, inhibited RANKL-induced osteoclast formation of BMMs, suggesting that these components might be active constituents contributing to the antiosteoclastogenic effect of WEMC. In addition, gallic acid inhibits proinflammatory cytokine production or gene expression by suppressing NF-κB activation.39, 40 Honokiol also inhibits collagen-induced arthritis by reducing the production of proinflammatory cytokines, MMP expressions, and oxidative stress.17 Given that the significance of inflammatory bone destruction and the inhibitory effect of active components in M. officinalis cortex on bone metabolism, further study needs an in-depth analysis for the therapeutic role of M. officinalis cortex in inflammatory-induced osteoporotic bone diseases.
In conclusion, we have demonstrated that WEMC inhibits osteoclast differentiation by suppressing RANKL-induced signaling pathways and decreasing bone resorbing activity of mature osteoclasts, thereby attenuating osteoclast-mediated bone loss. These results suggest that WEMC might be useful for the treatment of various bone destructive diseases associated with excessive bone loss.
Conflicts of interest
The authors declare there is no conflict of interests regarding the publication of this paper.
Acknowledgments
This work was supported by grants (K14050) from the Korea Institute of Oriental Medicine, Ministry of Science, ICT, and Future Planning, Korea.
References
- 1.Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007;7:292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- 2.Boyle W.J., Simonet W.S., Lacey D.L. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 3.Kong Y.Y., Yoshida H., Sarosi I., Tan H.L., Timms E., Capparelli C. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 4.Lacey D.L., Timms E., Tan H.L., Kelley M.J., Dunstan C.R., Burgess T. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 5.Gohda J., Akiyama T., Koga T., Takayanagi H., Tanaka S., Inoue J. RANK-mediated amplification of TRAF6 signaling leads to NFATc1 induction during osteoclastogenesis. EMBO J. 2005;24:790–799. doi: 10.1038/sj.emboj.7600564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamashita T., Yao Z., Li F., Zhang Q., Badell I.R., Schwarz E.M. NF-kappaB p50 and p52 regulate receptor activator of NF-kappaB ligand (RANKL) and tumor necrosis factor-induced osteoclast precursor differentiation by activating c-Fos and NFATc1. J Biol Chem. 2007;282:18245–18253. doi: 10.1074/jbc.M610701200. [DOI] [PubMed] [Google Scholar]
- 7.Huang H., Chang E.J., Ryu J., Lee Z.H., Lee Y., Kim H.H. Induction of c-Fos and NFATc1 during RANKL-stimulated osteoclast differentiation is mediated by the p38 signaling pathway. Biochem Biophys Res Commun. 2006;351:99–105. doi: 10.1016/j.bbrc.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Ikeda F., Nishimura R., Matsubara T., Tanaka S., Inoue J., Reddy S.V. Critical roles of c-Jun signaling in regulation of NFAT family and RANKL-regulated osteoclast differentiation. J Clin Invest. 2004;114:475–484. doi: 10.1172/JCI19657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banu J., Varela E., Fernandes G. Alternative therapies for the prevention and treatment of osteoporosis. Nutr Rev. 2012;70:22–40. doi: 10.1111/j.1753-4887.2011.00451.x. [DOI] [PubMed] [Google Scholar]
- 10.Jeong BS. Hyangyakdaesajeon (鄕藥大事典). Seoul: Younglimsa; 1990.
- 11.Kim J.W., Jeong B.J., Woo S.H., Shim H.J., Na E.J., Kim Y.H. A case report of ascites in liver cirrhosis with herbal prescription. Korean J Orient Int Med. 2006;27:962–968. [In Korean, English abstract] [Google Scholar]
- 12.Na B.J., Jung J.H., Choi C.M., Hong J.W., Kim T.H., Rhe J.W. Effects of Banhanhubak-tang (Banxiahoupotang) on patients with poststroke depression. Korean J Orient Int Med. 2005;26:563–574. [In Korean, English abstract] [Google Scholar]
- 13.Hu Y., Qiao J., Zhang X., Ge C. Antimicrobial effect of Magnolia officinalis extract against Staphylococcus aureus. J Sci Food Agric. 2011;91:1050–1056. doi: 10.1002/jsfa.4280. [DOI] [PubMed] [Google Scholar]
- 14.Chang W.C., Yu Y.M., Hsu Y.M., Wu C.H., Yin P.L., Chiang S.Y. Inhibitory effect of Magnolia officinalis and lovastatin on aortic oxidative stress and apoptosis in hyperlipidemic rabbits. J Cardiovasc Pharmacol. 2006;47:463–468. doi: 10.1097/01.fjc.0000211708.03111.6e. [DOI] [PubMed] [Google Scholar]
- 15.Lee Y.J., Choi D.Y., Yun Y.P., Han S.B., Kim H.M., Lee K. Ethanol extract of Magnolia officinalis prevents lipopolysaccharide-induced memory deficiency via its antineuroinflammatory and antiamyloidogenic effects. Phytother Res. 2013;27:438–447. doi: 10.1002/ptr.4740. [DOI] [PubMed] [Google Scholar]
- 16.Lee S.J., Kim H.M., Cho Y.H., Park K., Kim E.J., Jung K.H. Aqueous extract of Magnolia officinalis mediates proliferative capacity, p21WAF1 expression and TNF-alpha-induced NF-kappaB activity in human urinary bladder cancer 5637 cells; involvement of p38 MAP kinase. Oncol Rep. 2007;18:729–736. [PubMed] [Google Scholar]
- 17.Kim K.R., Park K.K., Chun K.S., Chung W.Y. Honokiol inhibits the progression of collagen-induced arthritis by reducing levels of pro-inflammatory cytokines and matrix metalloproteinases and blocking oxidative tissue damage. J Pharmacol Sci. 2010;114:69–78. doi: 10.1254/jphs.10070fp. [DOI] [PubMed] [Google Scholar]
- 18.Lu S.H., Huang R.Y., Chou T.C. Magnolol ameliorates ligature-induced periodontitis in rats and osteoclastogenesis: in vivo and in vitro study. Evid Based Complement Alternat Med. 2013:634095. doi: 10.1155/2013/634095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ha H., An H., Shim K.S., Kim T., Lee K.J., Hwang Y.H. Ethanol extract of Atractylodes macrocephala protects bone loss by inhibiting osteoclast differentiation. Molecules. 2013;18:7376–7388. doi: 10.3390/molecules18077376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J.H., Kim H.N., Yang D., Jung K., Kim H.M., Kim H.H. Trolox prevents osteoclastogenesis by suppressing RANKL expression and signaling. J Biol Chem. 2009;284:13725–13734. doi: 10.1074/jbc.M806941200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ichida F., Nishimura R., Hata K., Matsubara T., Ikeda F., Hisada K. Reciprocal roles of MSX2 in regulation of osteoblast and adipocyte differentiation. J Biol Chem. 2004;279:34015–34022. doi: 10.1074/jbc.M403621200. [DOI] [PubMed] [Google Scholar]
- 22.Shen C.C., Ni C.L., Shen Y.C., Huang Y.L., Kuo C.H., Wu T.S. Phenolic constituents from the stem bark of Magnolia officinalis. J Nat Prod. 2009;72:168–171. doi: 10.1021/np800494e. [DOI] [PubMed] [Google Scholar]
- 23.Guo Z.F., Wang X.B., Luo J.G., Luo J., Wang J.S., Kong L.Y. A novel aporphine alkaloid from Magnolia officinalis. Fitoterapia. 2011;82:637–641. doi: 10.1016/j.fitote.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 24.Hasegawa S., Yonezawa T., Ahn J.Y., Cha B.Y., Teruya T., Takami M. Honokiol inhibits osteoclast differentiation and function in vitro. Biol Pharm Bull. 2010;33:487–492. doi: 10.1248/bpb.33.487. [DOI] [PubMed] [Google Scholar]
- 25.Takayanagi H., Kim S., Koga T., Nishina H., Isshiki M., Yoshida H. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3:889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 26.Aliprantis A.O., Ueki Y., Sulyanto R., Park A., Sigrist K.S., Sharma S.M. NFATc1 in mice represses osteoprotegerin during osteoclastogenesis and dissociates systemic osteopenia from inflammation in cherubism. J Clin Invest. 2008;118:3775–3789. doi: 10.1172/JCI35711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neal J.W., Clipstone N.A. Glycogen synthase kinase-3 inhibits the DNA binding activity of NFATc. J Biol Chem. 2001;276:3666–3673. doi: 10.1074/jbc.M004888200. [DOI] [PubMed] [Google Scholar]
- 28.Matsuo K., Galson D.L., Zhao C., Peng L., Laplace C., Wang K.Z. Nuclear factor of activated T-cells (NFAT) rescues osteoclastogenesis in precursors lacking c-Fos. J Biol Chem. 2004;279:26475–26480. doi: 10.1074/jbc.M313973200. [DOI] [PubMed] [Google Scholar]
- 29.Ruocco M.G., Maeda S., Park J.M., Lawrence T., Hsu L.C., Cao Y. I{kappa}B kinase (IKK){beta}, but not IKK{alpha}, is a critical mediator of osteoclast survival and is required for inflammation-induced bone loss. J Exp Med. 2005;201:1677–1687. doi: 10.1084/jem.20042081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomimori Y., Mori K., Koide M., Nakamichi Y., Ninomiya T., Udagawa N. Evaluation of pharmaceuticals with a novel 50-hour animal model of bone loss. J Bone Miner Res. 2009;24:1194–1205. doi: 10.1359/jbmr.090217. [DOI] [PubMed] [Google Scholar]
- 31.Lee Y.J., Lee Y.M., Lee C.K., Jung J.K., Han S.B., Hong J.T. Therapeutic applications of compounds in the Magnolia family. Pharmacol Ther. 2011;130:157–176. doi: 10.1016/j.pharmthera.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 32.Abu-Amer Y., Dowdy S.F., Ross F.P., Clohisy J.C., Teitelbaum S.L. TAT fusion proteins containing tyrosine 42-deleted IkappaBalpha arrest osteoclastogenesis. J Biol Chem. 2001;276:30499–30503. doi: 10.1074/jbc.M104725200. [DOI] [PubMed] [Google Scholar]
- 33.Dai S., Hirayama T., Abbas S., Abu-Amer Y. The IkappaB kinase (IKK) inhibitor, NEMO-binding domain peptide, blocks osteoclastogenesis and bone erosion in inflammatory arthritis. J Biol Chem. 2004;279:37219–37222. doi: 10.1074/jbc.C400258200. [DOI] [PubMed] [Google Scholar]
- 34.Huang H., Ryu J., Ha J., Chang E.J., Kim H.J., Kim H.M. Osteoclast differentiation requires TAK1 and MKK6 for NFATc1 induction and NF-kappaB transactivation by RANKL. Cell Death Diff. 2006;13:1879–1891. doi: 10.1038/sj.cdd.4401882. [DOI] [PubMed] [Google Scholar]
- 35.Vaananen H.K., Zhao H., Mulari M., Halleen J.M. The cell biology of osteoclast function. J Cell Sci. 2000;113:377–381. doi: 10.1242/jcs.113.3.377. [DOI] [PubMed] [Google Scholar]
- 36.Kim H., Choi H.K., Shin J.H., Kim K.H., Huh J.Y., Lee S.A. Selective inhibition of RANK blocks osteoclast maturation and function and prevents bone loss in mice. J Clin Invest. 2009;119:813–825. doi: 10.1172/JCI36809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Faccio R., Novack D.V., Zallone A., Ross F.P., Teitelbaum S.L. Dynamic changes in the osteoclast cytoskeleton in response to growth factors and cell attachment are controlled by beta3 integrin. J Cell Biol. 2003;162:499–509. doi: 10.1083/jcb.200212082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li H., Miyahara T., Tezuka Y., Namba T., Nemoto N., Tonami S. The effect of Kampo formulae on bone resorption in vitro and in vivo. I. Active constituents of Tsu-kan-gan. Biol Pharm Bull. 1998;21:1322–1326. doi: 10.1248/bpb.21.1322. [DOI] [PubMed] [Google Scholar]
- 39.Kwon K.H., Murakami A., Ohigashi H. Suppressive effects of natural and synthetic agents on dextran sulfate sodium-induced interleukin-1beta release from murine peritoneal macrophages. Biosci Biotechnol Biochem. 2004;68:436–439. doi: 10.1271/bbb.68.436. [DOI] [PubMed] [Google Scholar]
- 40.Na H.J., Lee G., Oh H.Y., Jeon K.S., Kwon H.J., Ha K.S. 4-O-Methylgallic acid suppresses inflammation-associated gene expression by inhibition of redox-based NF-kappaB activation. Int Immunopharmacol. 2006;6:1597–1608. doi: 10.1016/j.intimp.2006.06.004. [DOI] [PubMed] [Google Scholar]