Abstract
Contrast sensitivity (CS) quantifies an observer’s ability to detect the smallest (threshold) luminance difference between a target and its surrounding. In clinical settings, printed letter contrast charts are commonly used, and the contrast of the letter stimuli is specified by the Weber contrast definition. Those paper-printed charts use negative polarity contrast (NP, dark letters on bright background) and are not available with positive polarity contrast (PP, bright letters on dark background), as needed in a number of applications. We implemented a mobile CS measuring app supporting both NP and PP contrast stimuli that mimic the paper charts for NP. A novel modified Weber definition was developed to specify the contrast of PP letters. The validity of the app is established in comparison with the paper chart. We found that our app generates more accurate and a wider range of contrast stimuli than the paper chart (especially at the critical high CS, low contrast range), and found a clear difference between NP and PP CS measures (CSNP>CSPP) despite the symmetry afforded by the modified Weber contrast definition. Our app provides a convenient way to measure CS in both lighted and dark environments.
Introduction
Contrast sensitivity (CS) is a measure of visual function that quantifies an observer’s ability to detect the smallest (threshold) luminance difference between a target and its surrounding. CS deficits are associated with visual disorders [1], a nd monitoring changes has been shown as an effective measure of treatment effectiveness [2, 3, 4]. Poor CS also results in significant decline in face recognition [5, 6], mobility [7, 8], and driving [9, 10].
Printed contrast letter charts, such as the Pelli-Robson (P-R) chart [11] or the Mars chart [12], are typically used in clinical settings. Both charts present dark letters over white backgrounds (i.e. negative polarity contrast, NP) of a fixed size (2.8°×2.5° for the P-R at 1 m distance) that gradually decrease in contrast from top to bottom. The Mars chart is used at shorter viewing distances (2° at 50 cm or 2.5° at 40 cm test distance). The number of letters correctly recognized by a patient is translated to a measured CS, which is often expressed in log units, LogCS. For both charts, the Weber definition [13] is used to specify the letters’ contrast.
The Weber definition used for specifying NP letter stimuli contrast is Cnegative = (Lletter Lbackground)/Lbackground. The CS is defined as the inverse of the measured contrast threshold: CS=1/Cthreshold.
Printed chart-based CS tests have several limitations: 1) only a few letters are available in a predefined sequence. Since a patient’s CS may need to be measured multiple times in studies, e.g. from different distances for different spatial frequencies, under different illumination conditions, and over time when monitoring disease progress or treatment with devices or medications, the chart-based measurements may be rendered invalid due to a subject involuntarily memorizing the letters. Surmounting this problem requires use of multiple versions of the charts. 2) Quality of the printed charts can deteriorate due to dirt, fingerprints stains, and print fading caused by exposure to ambient ultraviolet light. Quality decline is more critical for measuring low contrast (high CS range) because at that range the actual letter contrast is more sensitive to small changes in letter or background reflectance. 3) Achieving uniform chart illumination at the recommended ambient light level is often difficult. 4) Manual scoring and recording inevitably introduces errors.
Electronic display based letter CS charts may overcome these limitations, as random letters can be displayed at each trial, negating test memorization. The self-illumination characteristics of the display and relative ease of cleaning the display surface makes calibration and maintaining the letter contrast simpler and possible even without a light meter [14]. Operator error can be reduced by automatic scoring and recording. Letter size change is possible and simple within a range supported by the display’s resolution and size. Since modern mobile devices provide high quality electronic displays, CS measuring apps for these platforms opened a convenient way to conduct CS measure in clinics that can self-monitor the progress of the vision loss or treatment at home.
For android mobile devices (e.g. smart phone, tablet), only a few mobile CS measuring apps exist and the majority of them only report a vaguely defined measure of eye health (e.g. Contrast Sensitivity Test from healthcare4mobile, iCare Vision Test from iCare Eye Hospital, Eye Health from 4Ps Creators). Due to the difficulty inherent in calibrating each user’s screen, no absolute measure of LogCS is attempted. For apple devices, a few apps were developed using its Retina display. Dorr, et.al [15] developed an app for iPads to sample CS at various spatial frequencies to estimate a subject’s full CS function. It uses a Gabor target and computes the target stimuli contrast using the Michelson contrast definition. Kollbaum, et al [16] developed a letter CS chart for iPad, and reported good repeatability and agreement with Freiburg test on iPad, but found significantly higher LogCS scores than with the P-R paper chart. Furthermore, in both apps, the exact contrast calibration of the stimuli depended on the Retina display’s manufacturing quality control. Although luminance levels for each pixel value for the same Retina display are reasonably consistent within the same device (e.g. iPad vs. another iPad) [17], it may not be true among different generations of Retina displays (e.g. iPad 2 vs. iPad Air 2), as they have different intensity scales (ratio of the screen brightness relative to their maximum brightness) [18]. Therefore, even for Retina displays, individual display calibration [14] is essential to ensure the intended contrast of the stimuli to be properly represented.
The CS measures with NP stimuli provide baseline insight to the perceptibility of printed letters, shadows, and any other object with lesser light reflectance than its surround. On the other hand, CS measures with positive polarity (PP, light letters on dark background) stimuli provides a way to estimate the visibility of retro reflective objects at nighttime (e.g., stars), self-illuminating objects, and of the augmented information on optical see-through augmented devices such as head mounted displays (HMDs) or head up displays (HUDs). Importantly, we know of no app or chart that measures CS in PP condition.
We are studying the impact of cataracts on the visibility of pedestrians at night with and without glare from car headlights. Therefore, we wished to measure CS for PP objects under dark night driving conditions. Here, we described our implementation of a CS measurement app for a smart phone, which supports the CS measures for both PP and NP stimuli.
The app uses the conventional Weber contrast definition for specifying NP stimuli contrast, and a novel modified Weber contrast definition [19] for PP stimuli generation. The modified Weber definition used for specifying PP letter stimuli contrast is Cpositive = (Lletter − Lbackground)/Lletter. The app validity is verified against CS measurements made with the P-R chart.
Implementing CS Measuring App
Our implementation is intended to mimic the Pelli-Robson (P-R) chart [11] to preserve the operational familiarity and simplify selection of design parameters. Thus, we use three letters for each CS level (LogCS range of 0.15 to 2.25 with 0.15 LogCS steps), requiring calibration of only 15 contrast levels, and fixed size letter of 2.8°×2.5° at 80 cm distance.
Characterizing the Display
The CS measuring tool requires precise measurement of the display characteristics to enable accurate generation of stimulus contrast. Therefore, pixel value-luminance relations (Gamma function [20]) were measured, in a dark room, with a Minolta LS-0100 luminance meter (Osaka, Japan) for three common mobile display types (TFT, AMOLED, and IPS), while each display was displaying its 0–255 pixel value range.
The pixel value-luminance relations for the extreme pixel value ranges (0–5 and 250–255) are particularly important, as they are used for generating low contrast letter stimuli essentially needed to measure normal human CS in PP and NP, respectively. Therefore, for these ranges single pixel value steps were measured, while sparser steps were measured for intermediate pixel value range (two pixel value steps for ranges of 6–11 and 244–250, five pixel value steps for 15–25 and 230–240, ten pixel value steps for 30–50 and 200–230, and twenty pixel value steps for 50–150 and 180–200 pixel-value range).
Fig. 1 shows measured luminance as function of pixel value- with polynomial curves separately fitted for the three pixel value ranges (0–15, 15–240, 240–255) for an IPS (LG Optimus G Pro), AMOLED (Samsung Galaxy S3), and TFT (Motorola Atrix 4G) displays used in these smart phones.
Figure 1.
Measured pixel value- luminance correspondence with fitted polynomial curves for (a) low pixel range (0–15), (b) medium pixel range (15–240), and (c) high pixel range (240–255) for the three display types.
Usual exponential (Gamma function) fitting over the full pixel value range resulted in large errors at the extreme pixel value ranges, so separate curves were fitted to each corresponding pixel-value range. The order of the polynomial fitting was manually adjusted to produce good fits inside each pixel value range, while keeping good continuity at the pixel value range boundaries.
We chose the IPS display for our app implementation because the IPS display produced a smooth polynomial curve at both the high and low pixel value ranges, while the other displays exhibited bumps (sudden increase or decrease of luminance) at one of the extreme pixel value ranges.
The fitted pixel value-luminance correspondences are used to select proper pixel values to generate letter stimuli of a desired contrast. We used the resulting fitted functions to estimate the luminance of pixel values in the non-extreme range, as the impact of small estimation errors in that range on the letter contrast sensitivity measurement will be small. Also, it was shown in later testing of the CS measuring app that we only utilizes the pixel range of [254–155] and [1–18] to generate required letter stimuli for CS levels available in the paper charts.
Our initial implementation for the app is carried out for the two extreme background luminance values (e.g. pixel value=0 for PP and 255 for NP), then letter contrast is calculated for all other pixel values for both background luminance values.
Pixel values which result in the closest LogCS levels required for the CS measuring steps used in paper chart were selected. The pixel value-CS level lookup table were created this way was used when the letter stimuli are generated at runtime.
Initial calibration of the Electronic CS Chart
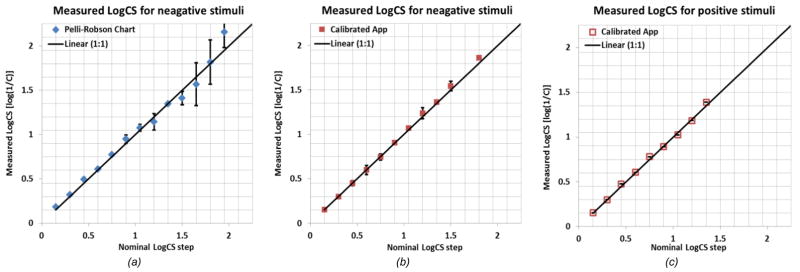
Fig. 2 shows the accuracy and range of the estimated CS with the P-R chart and our app using the initial calibration, as a function of the nominal CS steps. The diagonal line represents perfectly calibrated letter CS levels. The CS is computed based on the measured luminance for letters and background.
Figure 2.
LogCS (log (1/Cthreshold) of (a) the NP contrast letter stimuli measured from the Pelli-Robson paper chart, (b) our CS measuring app (on the IPS screen) with initial calibration, (b) PP contrast stimuli shown on our calibrated CS measuring app. The diagonal line represents perfectly calibrated chart. Error bars represent standard deviation of the measurement.
The calibrated app shows more consistent and accurate letter contrast values (Fig. 2b). The average CS errors relative to the nominal values of the printed P-R chart and our calibrated app are 0.070 and 0.017 in LogCS, respectively. The difference in CS error is significant t(11)=2.2, p<.001. Note that the error of displaying the correct contrasts in the P-R chart is much larger for the more important high LogCS range. The LogCS values measured with P-R chart for healthy (best corrected) 20/20, or better, eyes are within the range of 1.45 to 2.02 LogCS [21].
Our app with the initial calibration only supports measuring LogCS up to 1.86 for the NP (Fig. 2b), and even lower for the PP case (only up to 1.35 Log units) (Fig. 2c). However, since human vision CS is reduced in the dark [22, 23, 24, 25], our app’s reduced CS measuring range for PP condition may not be a limitation, as long as it covers the expected human CS performance in the PP. Indeed as shown in our preliminary data (see the performance validation section below) normal vision subjects cannot even reach 1.30 LogCS in PP CS measures.
For the NP CS measure, our app with the initial calibration could not generate stimuli at the required higher levels, above 1.65 LogCS, due to the limited dynamic range of the display at the bright end of the display, i.e. a single pixel value step above 1.5 LogCS reaches 1.85 LogCS (Fig. 2a). To generate sufficient contrast levels, we needed to expand the dynamic range to achieve luminance levels in steps that are smaller than the native one step difference.
Achieving Higher Dynamic Range
Most computer graphic systems use 8-bits or 265 levels per RGB color channels. In order to generate a certain grey level, the luminance from these R, G, and B channels are summed. The “bit-stealing” approach [26] is based on modifying one or two of the RGB component’s values independently to achieve intermediate luminance levels between consecutive grey levels, thus expanding the number of levels beyond what a conventional RGB system can display. The slight changes of the hues accompanied by such a small single channel luminance changes are not perceptible, especially under very low light levels. At such lighting conditions, signal from the rods dominate light perception, and they modulates color signal carried by cones [24].
This bit-stealing technique has been previously used to achieve the higher dynamic range needed to display the contrast levels needed for human threshold measurements [14].
Luminance of a color image is calculated by a weighted linear sum of R, G, and B luminance values, i.e. Y = 0.2126R + 0.7152G + 0.0722B, which accounts for the human luminance color perception with chromaticities of R. G. B channels, where the blue channel contributes the least, and green light contributes the most for perceived luminance [27]. Based on those weights, the order of the bit-stealing channel for the required amount of dynamic range can be assigned as following: bit-stealing is first done on the B-channel twice, then on the R-channel, and then again B-channel bit-stealing follows.
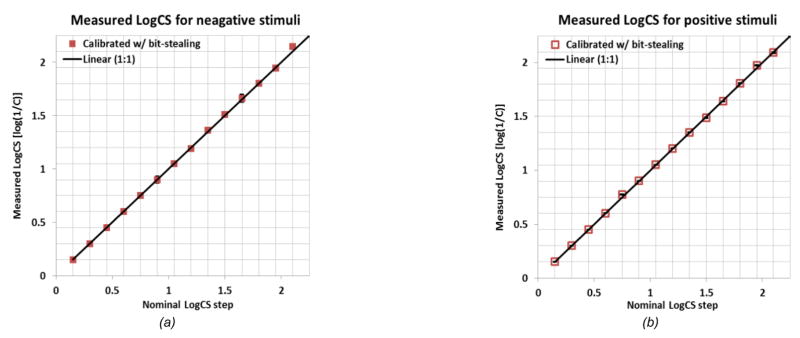
The increased dynamic range enables the generation of lower contrast stimuli necessary for measuring high LogCS levels. Fig. 3 shows the results of the calibration of our app with the bit-stealing applied. It shows better accuracy of stimulus contrast generation and increased range of measureable CS. Note that now the full range of contrast levels required to replicate the P-R chart (0.15 to 2.25 LogCS range with 0.15 Log unit steps) can be achieved for both polarities.
Figure 3.
Improved contrast accuracy of the CS measuring app using bit-stealing technique for (a) NP contrast letter stimuli, and (b) PP contrast stimuli. The diagonal line represents perfect calibration. Note that with bit-stealing technique, the full letter contrast range used by the P--R chart is now supported and overall accuracy of the contrast simulation is improved for both polarity CS measures.
Performance Validation of the CS App
The CS of two subjects with normal vision were measured repeatedly, with both our CS measuring app and the P-R chart, with and without a cataract simulation clip-on, over three days. The order of the measurement tools (PR-NP, APP-NP, and APP-PP) were counter balanced and the order of the viewing conditions (with or without clip-on, and binocular or monocular) were reversed each day.
Measurements were taken at the same time of day (at 1 PM). NP CS was measured with the P-R chart and our app under normal office ambient lighting condition, at 1m viewing distance. PP CS was measured with our app (on LG Optimus G Pro) with lighting turned off. The cataract simulation clip-on (SC) is used to illustrate the impact of light scatter on CS.
Fig. 4 compares the results obtained with the P-R chart and our app for NP contrast stimuli. There was close agreement between the LogCS values measured by the P-R chart and our app, with less than 0.02 Log units of non-significant difference, t(5)=0.78, p=2.24 for binocular, and 0.04 Log units difference, t(5)=2.43, p=.003 for monocular measurements. Note that a single letter stimulus difference on the P-R chart is 0.05 Log units.
Figure 4.
Measured CS for (a) subject 1 and (b) subject 2, with and without a simulated cataract (SC) clip-on, averaged over three measurements taken on three days. Binocular CS is higher than monocular CS. The P-R chart based CS measures are not different that of our app based measures in NP. Both subjects’ CS in PP condition is much lower (about 0.22 Log units) than the CS in NP condition.
The analysis of CS measurement repeatability (standard deviations of measured CS over the three days) showed that our app and the P-R chart perform are comparable (0.027 vs. 0.045 Log units, respectively), where the standard deviation was within a single letter score difference. Note that this also confirms that the measurement difference of 0.05 Log units is the expected variation for these types of CS measurements.
Comparing the results with and without the simulated cataract clip-on, it was observed that the cataract simulator clip-on caused overall 0.15 LogCS reduction in monocular measurements (t(11)=12.75, p<.001), where the significant CS reductions were shown for both NP (0.14 Log units reduction, t(5)=7.77, p<.001) and PP (0.17 Log units reduction, t(5)=10.76, p<.001) cases. However, LogCS reductions (effect of the light scatter) in NP and PP conditions are not significantly different (t(5)=1.07, p=.17).
Similar significant overall LogCS reductions (0.13 Log units, t(11)=8.64, p<.001) were observed in binocular CS measures, where significant LogCS reductions were shown for both NP (0.15 Log units, t(5)=8.72, p<.001) and PP (0.12 Log units, t(5)=5.60, p<.001) cases. Again, reduction in LogCS in both NP and PP cases with and without the clip-on were not significantly different (t(5)=1.07, p=.17). This result is somewhat surprising, as it was hypothesized that NP stimuli might induce more disability glare than that of the PP stimuli, so the effect of simulated cataract would be larger in NP.
In comparing the results of the CS measures in NP and PP conditions, it is clear that the measured LogCS for the PP condition is significantly lower in both monocular (0.19 Log units reduction, t(5)=9.09, p<.001) and binocular (0.25 Log units reduction, t(5)=14.00, p<.001) viewing. This finding highlights the need to measure a subject’s CS in both NP and PP contrast stimulus separately to estimate a subject’s visual performance in both environments.
For all CS measuring conditions, significant CS increases in binocular measures (0.10 Log units, t(23)=12.27, p<.001) compared to the monocular measures were observed, but the simulated cataract clip-on or polarity of stimulus contrast did not induce any significant effects (t(11)=1.12, p=0.29 and t(11)=2.04, p=0.07, respectively).
Note that about 42% of CS increase in binocular measure was expected due to the enhancement during binocular summation [28, 29,30], and our measurements also supports these previous founding.
Conclusion
We developed a CS measuring smartphone app that supports novel PP stimulus over a dark background, as well as NP stimuli over a light background. Using the bit-stealing technique, our app was able to generate the low contrast letter stimuli needed to measure human CS in both polarities.
The app produces more accurate contrast stimuli (especially in the high CS range) than the printed chart. The CS values measured by our app and the chart are also not significantly different from each other. We also demonstrated that the repeatability of our CS measurements was also on par with the chart based CS measure. This might indicate that the measurement steps on the paper P-R chart may be too large. A reduction of CS due to the simulated cataract clip-on was found but the expected stimulus polarity effect was not found. This suggests that both the effects of light scattering with respect to the luminance adaptation of human vision may be quite local.
Since our app is capable of generating wider range of contrast with finer CS resolution, finer steps of LogCS level may be implemented easily. To eliminate the need of physical measure of pixel value-luminance relations with a (rather expensive) light meter, the psychophysical method of contrast calibration [14] will be implemented, so that the contrast of stimuli can be correctly set for a given display.
Although the current target device is a smart phone, the implementation and calibration methods explained here can be applied to any electronic display.
Acknowledgments
Supported in part by the NIH grant, R01EY024075.
Biographies
Alex D. Hwang received BS in mechanical engineering from the University of Colorado Boulder (1999), and received MS (2003) and PhD in computer science from the University of Massachusetts Boston (2010). Since then, he has worked at Schepens Eye Research Institute in Boston MA. He became an Investigator and is appointed as Instructor of Ophthalmology at Harvard Medical School (2015). His work has focused on bioengineering and low vision rehabilitation.
Eli Peli received BSc and MSc in electrical engineering from Technician-Israel Institute of Technology (IIT), Haifa, Israel (1979), and OD from New England College of Optometry, Boston, MA (1983). Since then he has been at the Schepens Eye Research Institute where he is Senior Scientist and the Moakley Scholar in Aging Eye Research, and Professor of Ophthalmology at Harvard Medical School.
References
- 1.Woods RL, Tregear SJ, Mitchell RA. Screening for ophthalmic disease in older subjects using visual acuity and contrast sensitivity. Ophthalmology. 1998;105(12):2318–2326. doi: 10.1016/S0161-6420(98)91235-0. [DOI] [PubMed] [Google Scholar]
- 2.Trobe JD, Beck RW, Moke PS, Cleary PA. Contrast sensitivity and other vision tests in the optic neuritis treatment trial. American journal of ophthalmology. 1996;121(5):547–553. doi: 10.1016/s0002-9394(14)75429-7. [DOI] [PubMed] [Google Scholar]
- 3.Bulens C, Meerwaldt JD, Van der Wildt GJ, Van Deursen JBP. Effect of levodopa treatment on contrast sensitivity in Parkinson’s disease. Annals of neurology. 1987;22(3):365–369. doi: 10.1002/ana.410220313. [DOI] [PubMed] [Google Scholar]
- 4.Woo GC, Danziel CC. A pilot study of contrast sensitivity assessment of the CAM treatment of amblyopia. Acta ophthalmologica. 1981;59(1):35–37. doi: 10.1111/j.1755-3768.1981.tb06707.x. [DOI] [PubMed] [Google Scholar]
- 5.Owsley C, Sloane ME. Contrast sensitivity, acuity, and the perception of' real-world targets. British Journal of Ophthalmology. 1987;71(10):791–796. doi: 10.1136/bjo.71.10.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.West SK, Rubin GS, Broman AT, Munoz B, Bandeen-Roche K, Turano K. How does visual impairment affect performance on tasks of everyday life? The SEE Project Archives of Ophthalmology. 2002;120(6):774–780. doi: 10.1001/archopht.120.6.774. [DOI] [PubMed] [Google Scholar]
- 7.Marron JA, Bailey IL. Visual factors and orientation-mobility performance. American Journal of Optometry and Physiological Optics. 1982;59(5):413–426. doi: 10.1097/00006324-198205000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Long RG, Rieser JJ, Hill EW. Mobility in individuals with moderate visual impairments. Journal of Visual Impairment & Blindness. 1990;84:111–118. [Google Scholar]
- 9.Owsley C, McGwin G, Jr, Sloane M, Wells J, Stalvey BT, Gauthreaux S. Impact of cataract surgery on motor vehicle crash involvement by older adults. Jama. 2002;288(7):841–849. doi: 10.1001/jama.288.7.841. [DOI] [PubMed] [Google Scholar]
- 10.Bowers A, Peli E, Elgin J, McGWIN G, Jr, Owsley C. On-road driving with moderate visual field loss. Optometry & Vision Science. 2005;82(8):657–667. doi: 10.1097/01.opx.0000175558.33268.b5. [DOI] [PubMed] [Google Scholar]
- 11.Pelli DG, Robson JG. The design of a new letter chart for measuring contrast sensitivity. Clinical Vision Sciences. 1988;2:187–199. [Google Scholar]
- 12.Arditi A. Improving the design of the letter contrast sensitivity test. Investigative Ophthalmology & Visual Science. 2005;46(6):2225–2229. doi: 10.1167/iovs.04-1198. [DOI] [PubMed] [Google Scholar]
- 13.Peli E. Contrast in complex images. JOSA A. 1990;7(10):2032–2040. doi: 10.1364/josaa.7.002032. [DOI] [PubMed] [Google Scholar]
- 14.To L, Woods TL, Goldstein RB, Peli E. Psychophysical contrast calibration. Vision research. 2013;90:15–24. doi: 10.1016/j.visres.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dorr M, Lesmes LA, Lu ZL, Bex PJ. Rapid and Reliable Assessment of the Contrast Sensitivity Function on an iPad. Investigative ophthalmology & visual science. 2013;54(12):7266–7273. doi: 10.1167/iovs.13-11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kollbaum PS, Jansen ME, Kollbaum EJ, Bullimore MA. Validation of an iPad test of letter contrast sensitivity. Optometry & Vision Science. 2014;91(3):291–296. doi: 10.1097/OPX.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 17.Turpin A, Lawson DJ, McKendrick AM. PsyPad: A platform for visual psychophysics on the iPad. Journal of vision. 2014;14(3):16. doi: 10.1167/14.3.16. [DOI] [PubMed] [Google Scholar]
- 18.“iPad mini 3 and iPad Air 2 Image Contrast and Intensity Scales”, http://www.displaymate.com/Gamma_24.html & “iPad 2 and iPhone 4 Image Contrast and Intensity Scales”, http://www.displaymate.com/Gamma_2.html.
- 19.Hwang AD, Jung J, Peli E. Contrast and contrast sensitivity measures for positive and negative polarity targets. in preparation. [Google Scholar]
- 20.Peli E. Display nonlinearity in digital image processing for visual communications. Optical Engineering. 1992;31(11):2374–2382. [Google Scholar]
- 21.Mantyjarvi M, Laitinen T. Normal values for the Pelli-Robson contrast sensitivity test. Journal of Cataract & Refractive Surgery. 2001;27(2):261–266. doi: 10.1016/s0886-3350(00)00562-9. [DOI] [PubMed] [Google Scholar]
- 22.Barbur JL, Stockman A. Photopic, Mesopic, and Scotopic Vision and Changes in Visual Performance. In: Dartt DA, editor. Encyclopedia of the Eye. Vol. 3. Academic; 2010. pp. 323–331. [Google Scholar]
- 23.Richard H. Contrast thresholds of the human eye. JOSA A. 1946;36(11):624–643. doi: 10.1364/josa.36.000624. [DOI] [PubMed] [Google Scholar]
- 24.Zele AJ, Cao D. Vision under mesopic and scotopic illumination. Frontiers in psychology. 2014;5 doi: 10.3389/fpsyg.2014.01594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peli E, Yang J, Goldstein R, Reeves A. Effect of luminance on suprathreshold contrast perception. JOSA A. 1991;8(8):1352–1359. doi: 10.1364/josaa.8.001352. [DOI] [PubMed] [Google Scholar]
- 26.Tyler CW. Colour bit-stealing to enhance the luminance resolution of digital displays on a single pixel basis. Spatial vision. 1997;10(4):369–377. doi: 10.1163/156856897x00294. [DOI] [PubMed] [Google Scholar]
- 27.Anderson M, Chandrasekar S, Motta R, Stokes M. Technical report. International Color Consortium; 1996. A standard default color space for the internet: sRGB. [Google Scholar]
- 28.Gilchrist J, McIver C. Fechner’s paradox in binocular contrast sensitivity. Vision research. 1985;25(4):609–613. doi: 10.1016/0042-6989(85)90167-1. [DOI] [PubMed] [Google Scholar]
- 29.Pardhan S, Gilchrist J. Binocular contrast summation and inhibition in amblyopia. Documenta ophthalmologica. 1992;82(3):239–248. doi: 10.1007/BF00160771. [DOI] [PubMed] [Google Scholar]
- 30.Legge GE. Binocular contrast summation I. Detection and discrimination. Vision research. 1984;24(4):373–383. doi: 10.1016/0042-6989(84)90063-4. [DOI] [PubMed] [Google Scholar]