Abstract
Backgrounds
Corsica is a French island situated in the Mediterranean Sea. The island provides suitable natural conditions to study disease ecology, especially tick-borne diseases and emerging diseases in animals and ticks. The family Anaplasmataceae is a member of the order Rickettsiales; it includes the genera Anaplasma, Ehrlichia, Neorickettsia and Wolbachia. Anaplasmosis and ehrlichiosis traditionally refer to diseases caused by obligate intracellular bacteria of the genera Anaplasma and Ehrlichia. The aim of this study was to identify and estimate the prevalence of Anaplasmataceae species infecting domestic animals and ticks in Corsica.
Methods
In this study, 458 blood samples from sheep, cattle, horses, goats, dogs, and 123 ticks removed from cattle, were collected in Corsica. Quantitative real-time PCR screening and genetic characterisation of Anaplasmataceae bacteria were based on the 23S rRNA, rpoB and groEl genes.
Results
Two tick species were collected in the present study: Rhipicephalus bursa (118) and Hyalomma marginatum marginatum (5). Molecular investigation showed that 32.1% (147/458) of blood samples were positive for Anaplasmataceae infection. Anaplasma ovis was identified in 42.3% (93/220) of sheep. Anaplasma marginale was amplified from 100% (12/12) of cattle and two R. bursa (2/123). Several potentially new species were also identified: Anaplasma cf. ovis, “Candidatus Anaplasma corsicanum”, “Candidatus Anaplasma mediterraneum” were amplified from 17.3% (38/220) of sheep, and Anaplasma sp. marginale-like was amplified from 80% (4/5) of goats. Finally, one R. bursa tick was found to harbour the DNA of E. canis. All samples from horses and dogs were negative for Anaplasmataceae infection.
Conclusions
To our knowledge, this study is the first epidemiological survey on Anaplasmataceae species infecting animals and ticks in Corsica and contributes toward the identification of current Anaplasmataceae species circulating in Corsica.
Keywords: Corsica Island, Animals, Ticks, Anaplasma ovis, Anaplasma marginale, Anaplasma sp., Ehrlichia canis
Background
Bacteria from the genera Anaplasma and Ehrlichia are obligate intracellular bacteria transmitted by arthropods, mainly ticks, from one vertebrate host to another. Transmission usually occurs transstadially [1, 2], although transovarial transmission has been reported [3]. In the vertebrate host, the bacteria infect hematopoietic cells [4, 5]. Anaplasma and Ehrlichia can cause a persistent infection in vertebrate hosts, which allows these hosts to be reservoirs [1, 5]. Probable cases of Anaplasmataceae infection in domestic animals were known as early as the beginning of the twentieth century. However, wide interest in studying these bacteria arose when discovering species pathogenic for humans [1]. Anaplasma phagocytophilum was known to cause disease in domestic ruminants in Europe and the USA decades before its identification in humans [6]. The first European case of human anaplasmosis was reported in 1995 in Slovenia; after that, human cases have been reported in many countries of Europe [7–11]. Bovine anaplasmosis due to A. marginale results in the development of mild to severe anaemia and occurs in tropical and subtropical regions, including South and Central America, the United States, southern Europe, Africa, Asia and Australia [12]. In India, mortality due to bovine anaplasmosis is estimated at between 5 and 40% but may reach up to 70% during a severe outbreak [13]. The economic loss due to infections caused by Babesia and Anaplasma infections in India was estimated to be $57 million [14]. In Europe, A. marginale has spread up to the northern latitudes of Switzerland, Austria and Hungary [15]. Anaplasma centrale, a less pathogenic organism but closely related to A. marginale, was reported in cattle in Sicily, Italy [16], and from roe deer in Spain [17]. Anaplasma ovis is an intraerythrocytic pathogen of sheep, goats and wild ruminants [18]. It is thought to cause only mild clinical symptoms, thus being of minor economic importance [19]. Ovine anaplasmosis appears to be widespread and found in different regions of the world. The extent of the infection and the loss of livestock productivity remain poorly understood [19]. The historical record of this bacterium in Europe was established in Russia in 1929 and 1930 by Yakimoff et al. [19], and in France by Cuille et al. in 1935 and 1936 [19]. In 2007, A. ovis human infection was reported in a 27-year-old woman in Cyprus [20].
The management of vector-borne diseases requires increased communication between physicians and veterinarians, particularly when physicians are dealing with patients with unexplained febrile illnesses in an endemic area were pathogen like Anaplasmataceae largely interconnected in an epidemiological network involving animals, vectors and humans [21]. Corsica is a French island in the Mediterranean Sea close to the south-east French coast, Sardinia, and the west Italian coast. Highly endemic flora and fauna and endemic pathologies are characteristic in Corsica [22]. Recently, we reported the emergence of Toscana virus in dogs in this region [23] and West Nile virus in domestic animals [24]. Our main objective was to continue the epidemiologic investigation of neglected infectious diseases in animals. To date, the occurrence of Anaplasmataceae bacteria in Corsica in domestic animals has never been reported. The aim of this study was to screen for the presence and the prevalence of Anaplasmataceae species infecting and currently circulating in domestic animals and their ticks in this region.
Methods
Sampling
From 2014 to 2015, EDTA blood samples were obtained from domestic animals on different farms from 14 different areas situated on the east coast of Corsica, France (Fig. 1). Sheep and goats were sampled on the Aléria plain. Cattle and ticks were sampled from one farm in Centu Mezzini, Balagne (42°34′58.242″N, 8°58′38.015″E), whereas dogs and horses were sampled in different localities along the east coast of Corsica island, including Cap Corse (42°56′44″N, 9°26′28″E), Furiani (42°3932″N, 9°24′54″E), Biguglia (42°37′41″N, 9°25′14″E), Lucciana (42°32′48″N, 9°25′5″E), Vescovato (42°29′41″N, 9°26′26″E), Castellare (42°28′7″N, 9°28′27″E), Tallone (42°13′55″N, 9°24′53″E), Ghisonaccia (42°1′3″N, 9°24′20″E), Solenzara (41°55′36″N, 9°24′19″E), Lecci (41°40′48″N, 9°19′5″E), Borgo (42°33′17″N, 9°25′41″E), and Ventiseri-Solenzara (41°55′36″N, 9°24′19″E) (Table 1). Sheep blood samples (230) were collected from three farms. In two farms, the sheep appeared healthy; however, the farmers declared that their sheep experienced many health problems during the winter of 2014, including respiratory disorders and a drop in milk production. At the third sheep farm, the farmer declared that the sheep at his farm were currently unhealthy, with a variety of symptoms, including recurrent fever, abortion, and some sheep died. A cattle herd in Balagne consisted of 16 cows. The cows in this herd had pronounced anaemia with icterus, and some of them died in 2015. Goats (n = 5) were all sampled on one farm; they had anaemia and a drop in milk production. In addition, blood samples were collected from horses at a different ranch. Dogs sampled in the present study included hunting dogs, sheep dogs, military working dogs and some pet dogs. Animals were examined with the assistance of their owners. Blood samples were collected by a veterinarian. After transport to the laboratory in Marseille, all samples were stored at -80 °C.
Fig. 1.
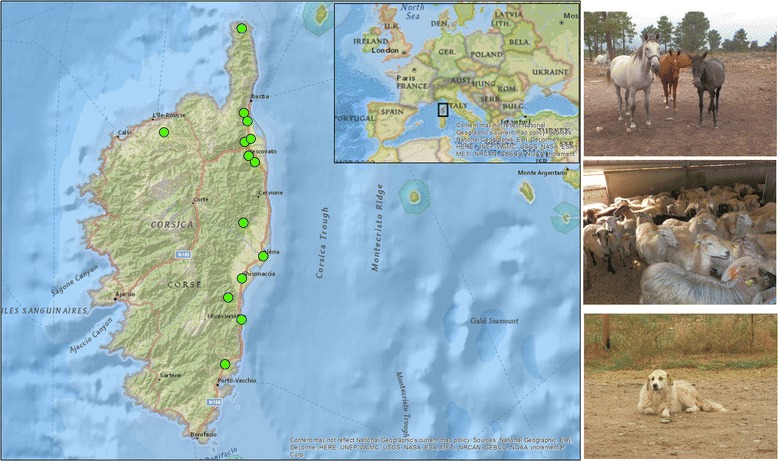
Map of Corsica, France, showing the study areas where the animals were sampled
Table 1.
Origin of animal and tick samples collected and investigated in this study
| Species | Number | Origin | Tick infestation | No. of ticks |
|---|---|---|---|---|
| 2014 | ||||
| Sheep | 201 | Aléria Plain | not found | – |
| Horse | 98 | East coast | not found | – |
| Dog | 73 | East coast | not found | – |
| 2015 | – | |||
| Sheep | 19 | Aléria Plain | not found | – |
| Cattle | 12 | Balagne | R. bursa | 118 |
| Hy. m. marginatum | 5 | |||
| Goat | 5 | Aléria Plain | not found | – |
| Dog | 50 | not found | – | |
| Totals | 458 | 123 | ||
Tick collection and identification
From the cattle farm, ticks were collected manually from adult cattle and stored in 70% ethanol until identification. Morphological identification was performed with a binocular microscope. Ticks were classified by family, genus and species using available taxonomic keys and morphometric tables [25, 26]. In addition, to confirm the morphological identification, three morphologically identified specimens of each species and all ticks that were not identified, or identified only to the genus level (engorged females and damaged ticks) were subjected to molecular identification using primers targeting the mitochondrial 12S rRNA gene, as previously described [27].
DNA extraction
DNA extraction was performed on the BioRobot EZ1 (Qiagen, Courtaboeuf, France) using a commercial EZ1 DNA Tissue Kit (Qiagen) according to the manufacturer’s instructions. DNA was extracted from 200 μl of blood from all animal samples. Ticks were recovered from ethanol, rinsed with distilled water and dried on sterile filter paper in a laminar-flow hood. Each tick was cut in half lengthways (the blades were discarded after each tick was cut). DNA was individually extracted from one half, and the remaining halves of the ticks were frozen at -80 °C for subsequent studies, as previously described [28].
PCR amplification
For the molecular identification of the species of selected ticks, the DNA samples were subjected to standard PCR to amplify a 360-base-pair (bp) fragment of the mitochondrial 12S rRNA gene (Table 2). To investigate the presence of Anaplasmataceae in Corsican ticks and domestic animals, DNA from ticks and blood were initially screened by a qPCR targeting the 23S rRNA gene. This qPCR has been reported to amplify most bacteria belonging to the family Anaplasmataceae [29]. Then, all positive samples were subjected to conventional PCR using the primers that amplify a 485 bp fragment of the 23S rRNA gene, as previously described [29]. In order to mine deeper into the identification of Anaplasmataceae species in domestic animals or ticks, positive samples were tested by PCR using Anaplasma genus-specific primers targeting the 525 bp fragment of the RNA polymerase subunit beta (rpoB) gene, and Ehrlichia genus-specific primers targeting the 590 bp fragment of the heat shock protein (groEl) gene [28] (Table 2).
Table 2.
Primers and probes used in this study
| Targeted microorganisms | Targeted gene | Primers and probea | Sequences 5′-3′ | Annealing temperature (°C) | References |
|---|---|---|---|---|---|
| qPCR | |||||
| Anaplasmataceae | 23S rRNA | TtAna-F TtAna-R TtAna-Sa |
TGACAGCGTACCTTTTGCAT GTAACAGGTTCGGTCCTCCA FAM-CTTGGTTTCGGGTCTAATCC-TAMRA |
60 | [28, 29] |
| Conventional PCR | |||||
| Anaplasmataceae | 23S rRNA | Ana23S-212f Ana23S-753r |
ATAAGCTGCGGGGAGTTGTC TGCAAAAGGTACGCTGTCAC |
55 | [28, 29] |
| Anaplasma spp. | rpoB | Ana-rpoBF Ana-rpoBR |
GCTGTTCCTAGGCTYTCTTACGCGA AATCRAGCCAVGAGCCCCTRTAWGG |
55 | [27] |
| Ehrlichia spp. | groEL | Ehr-groEL-F Ehr-groEL-R |
GTTGAAAARACTGATGGTATGCA ACACGRTCTTTACGYTCYTTAAC |
50 | [27] |
| Ticks | 12S rRNA | T1B T2A |
AAACTAGGATTAGATACCCT AATGAGAGCGACGGGCGATGT |
51 | [26] |
aProbe
PCR amplifications were performed as described previously [29, 30]. Briefly, quantitative real-time PCR assays were performed on the CFX96 Touch detection system (Bio-Rad, Marnes-la-Coquette, France) using the Takyon Master Mix according to the manufacturer’s instructions. The conventional PCRs were performed in automated DNA Thermal cyclers (GeneAmp PCR Systems Applied Biosystems, Courtaboeuf, France). The amplification reactions were performed under the following conditions: an initial denaturation step at 95 °C for 15 min, followed by 40 cycles consisting of 1 min denaturation at 95 °C, 1 min annealing at a corresponding temperature (Table 2) and a 1 min extension at 72 °C. A final extension cycle at 72 °C for 7 min was performed and the reactions were cooled at 15 °C. Distilled water was used as negative control. Positive control used was the DNA of A. phagocytophilum extracted from the supernatant of the continuous culture of this species in our laboratory, and E. canis DNA obtained from infected dogs sampled in Algeria [30]. After electrophoresis, the amplification products were visualised on 1.5% agarose gels stained with ethidium bromide and examined by UV transillumination. A DNA molecular weight marker (marker VI, Boehringer Mannheim, Mannheim, Germany) was used to estimate the size of the products.
Sequencing and phylogenetic analyses
Sequencing analyses were performed on the Applied Biosystems 3130xl Genetic Analyzer (Thermo Fisher Scientific, France) using the DNA sequencing BigDye Terminator Kit (Perkin-Elmer) according to the manufacturer’s instructions. The obtained sequences were assembled using ChromasPro 1.7 software (Technelysium Pty Ltd., Tewantin, Australia) and the sequences of primers were removed. Sequences obtained in this study were aligned with other ticks or Anaplasmataceae species sequences available on GenBank using CLUSTALW implemented on BioEdit v3 [31]. The sequence of 12S rDNA from ticks and the sequences of bacterial 23S rRNA, rpoB, and groEl genes were first aligned individually, gaps and missing data were eliminated, and then, for the sequences of Anaplasmataceae species, the alignment of the 23S rRNA with rpoB genes and 23S rRNA with groEl gene sequences were concatenated for phylogenetic tree construction for the Anaplasma and Ehrlichia species, respectively. Phylogenetic and molecular evolutionary analysis were inferred using the maximum likelihood method implemented on MEGA7 [32], with the complete deletion option, based on the Hasegawa-Kishino-Yano (HYK) model for nucleotide sequences. A discrete gamma distribution was used to model evolutionary rate differences among sites. Initial trees for the heuristic search were obtained automatically by applying the neighbor-joining and BIONJ algorithms to a matrix of pairwise distances estimated using the maximum composite likelihood (MCL) approach. Statistical support for internal branches of the trees was evaluated by bootstrapping with 1000 iterations.
Results
Tick identification and Anaplasmataceae screening
In total, 123 ticks were collected. Eighty-five removed ticks were identified as Rhipicephalus bursa, and 3 as Hyalomma marginatum. Thirty-five damaged ticks, including 32 engorged ticks, were only morphologically identified to the genus level as follows: 29 ticks Rhipicephalus sp., 2 Hyalomma sp., and 4 ticks were not identified. Two or three specimens from each tick identified at a species level were selected randomly, and all 35 damaged/engorged ticks were subjected to molecular identification. After 12S rDNA amplification and blast analysis, the six morphologically identified specimens were confirmed to be R. bursa and Hy. marginatum marginatum. From the 35 damaged ticks, 32 ticks were identified as R. bursa, and 3 were identified as Hy. m. marginatum. All 12S rDNA sequences of the R. bursa were identical to each other and showed 100% identity with R. bursa from Italy (KU51295, KC243833, AM410572), and 99% identity with R. bursa ticks reported from Spain (KC243834) (Fig. 1). All five sequences of Hy. m. marginatum were also identical to each other and showed 100% identity with Hy. m. marginatum from Italy (KC817304), Israel (KT391046), Morocco (AF150034) and Yemen (HE819515) (Fig. 2). Overall, the ticks collected in this study were as follows: 118 (95.9%) were identified as R. bursa; 75 were female, including 30 engorged females, and 43 were male. Five (4.1%) were identified as Hy. m. marginatum; two were engorged females, and three were male.
Fig. 2.
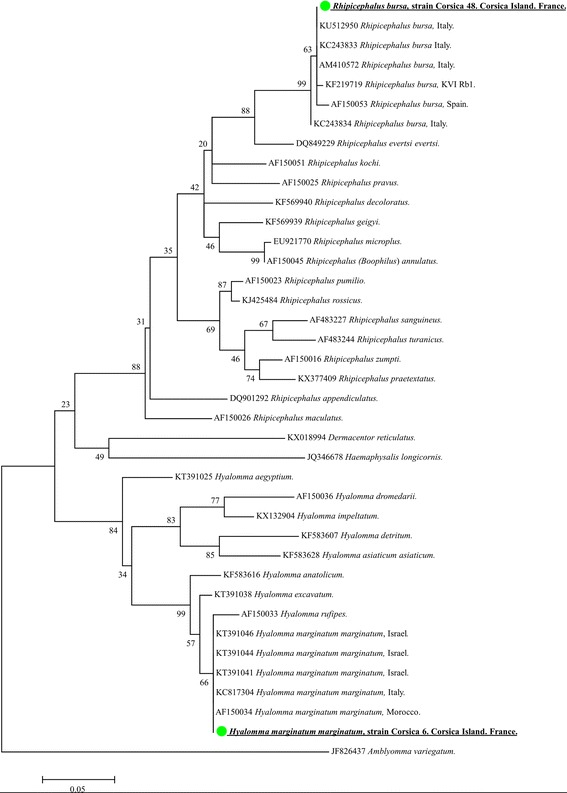
Phylogenetic tree showing the position of Rhipicephalus bursa and Hyalomma marginatum marginatum compared to other tick species. The evolutionary history was inferred by using the maximum likelihood method based on the Hasegawa-Kishino-Yano model. A discrete Gamma distribution was used to model evolutionary rate differences among sites [4 categories (+G, parameter = 0.2936)]. The analysis involved 39 nucleotide sequences. All positions containing gaps and missing data were eliminated. There was a total of 267 positions in the final dataset. The scale-bar represents a 5% nucleotide sequence divergence
Anaplasmataceae DNA was detected in three R. bursa of the 123 ticks examined (2.4%). After the 23S rRNA gene sequencing of the Anaplasmataceae DNA present in the three ticks, A. marginale was identified in two ticks. The two sequences of A. marginale were identical to each other and showed 100% homology with the A. marginale strain Dawn (CP006847) and Gypsy Plains (CP006846) reported from Australia, and 99% with the A. marginale strain Florida (CP001079) and St. Maries (CP000030) reported from the USA (Fig. 3). Finally, based on the 23S rRNA analysis, E. canis was identified from the third positive tick. These sequences presented 99% homology with the E. canis strain Jack (CP000107) reported from the USA (Fig. 4).
Fig. 3.
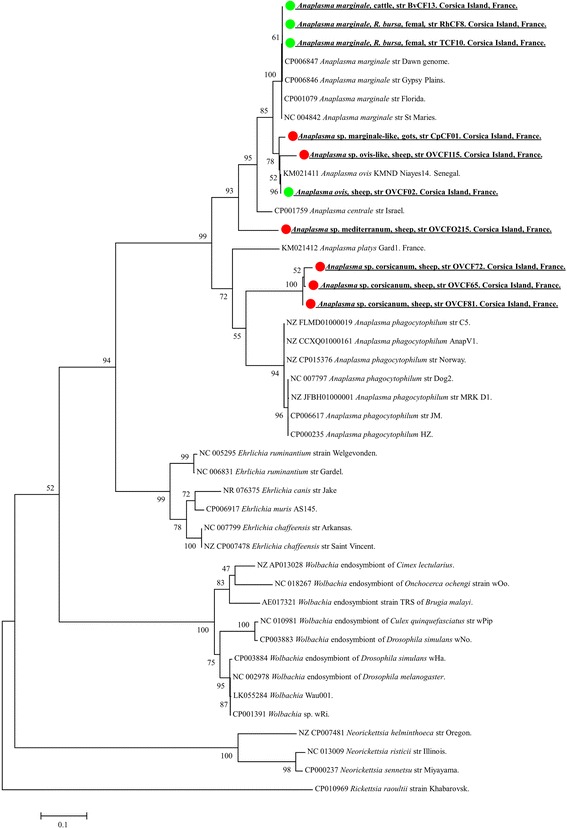
Phylogenetic tree showing the position of A. marginale amplified from R. bursa and cattle, A. ovis, “Ca. Anaplasma corsicanum”, Anaplasma sp. ovis-like, “Ca. Anaplasma mediterraneum” amplified from sheep and Anaplasma sp. marginale-like amplified from goats, compared to other species. The evolutionary history was inferred by using the maximum likelihood method based on the Hasegawa-Kishino-Yano model. A discrete Gamma distribution was used to model evolutionary rate differences among sites [2 categories (+G, parameter = 0.3880)]. The analysis involved 43 nucleotide sequences. All positions containing gaps and missing data were eliminated. There was a total of 861 positions in the final dataset. The scale-bar represents a 10% nucleotide sequence divergence
Fig. 4.
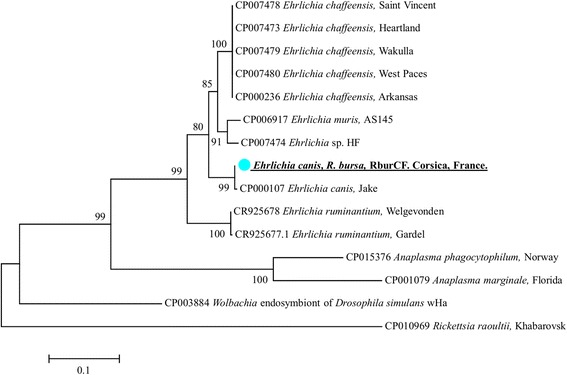
Phylogenetic tree showing the position of E. canis amplified from R. bursa compared to other Anaplasmataceae species. Evolutionary analyses were conducted using MEGA7 [32]. The concatenated 23S rRNA and the groEl genes of the Ehrlichia canis amplified in this study together with other sequences of Anaplasmataceae species available on GenBank. The evolutionary history was. A discrete Gamma distribution was used to model evolutionary rate differences among sites [4 categories (+G, parameter = 0.6567)]. The analysis involved 15 nucleotide sequences. All positions containing gaps and missing data were eliminated. There was a total of 1039 positions in the final dataset. The scale-bar represents a 5% nucleotide sequence divergence
Anaplasmataceae species screening from animal blood
The results are summarised in the (Table 3). Of the total of 458 blood samples analysed (Table 1), 32.1% (147) were positive for the initial 23S rRNA qPCR screening. The prevalence of Anaplasmataceae infections was as follows: sheep 59.5% (131/220), cattle 100% (12/12) and goats 80% (4/5), whereas all blood samples from horses and dogs were negative. Identification of bacterial species was achieved by amplification followed by sequencing of the portion of the 23S rRNA gene. Seventy-one percent (93/131) of Anaplasmataceae-positive sheep samples were infected by A. ovis. The 23S sequences obtained were identical to each other and showed 100% identity with A. ovis strain KMND Niayes-14 reported in sheep from Senegal [33]. All the other 38 qPCR-positive sheep (29%) were found to be infected by several as yet uncharacterised and potentially new species of Anaplasma. In 13/131 (9.9%) of infected sheep, the obtained sequences were identical to each other and showed only 96% identity with the A. ovis strain KMND Niayes-14. Due to the absence of additional data on this Anaplasma and genetic relatedness to A. ovis, we refer to this genotype here as Anaplasma cf. ovis. There were 3/131 (2.3%) sheep infected by another genotype of Anaplasma. These three sequences had 96–98% identity to each other and showed 91–94% identity with the A. phagocytophilum strain Norway Variant 2, reported from sheep in Norway (CP015376). We are provisionally calling this incompletely characterised bacterium “Candidatus Anaplasma corsicanum”. Finally, a third genotype was found infecting 22/131 (16.8%) sheep, sampled only in 2015. All sequences of this genotype were identical to each other and showed 95% identity with the A. centrale strain Israel (CP001759) reported from Israel (Fig. 3). We are provisionally calling this bacterium “Candidatus Anaplasma mediterraneum”.
Table 3.
Overall results and Anaplasmataceae species reported in the present study
| Species | Sheep | Cattle | Goats | Equine | Dogs | R. bursa | Hy. m. marginatum |
|---|---|---|---|---|---|---|---|
| A. ovis | 93/220 (71%) | 0 | 0 | 0 | 0 | 0 | 0 |
| A. marginale | 0 | 0 | 0 | 0 | 0 | 2/118 (1.7%) | 0 |
| Anaplasma cf. marginale | 0 | 12/12 (100%) | 4/5 (80%) | 0 | 0 | 0 | 0 |
| Anaplasma cf. ovis | 13/220 (9.9%) | 0 | 0 | 0 | 0 | 0 | 0 |
| “Canditatus Anaplasma corsicanum” | 3/220 (2.3%) | 0 | 0 | 0 | 0 | 0 | 0 |
| “Candidatus Anaplasma mediterraneum” | 22/220 (16.8%) | 0 | 0 | 0 | 0 | 0 | 0 |
| E. canis | 0 | 0 | 0 | 0 | 0 | 1/118 (0.8%) | 0 |
| Totals | 131/220 (59.5%) | 12/12 (100%) | 4/5 (80%) | 0 | 0 | 3/118 (2.5%) | 0 |
Data presented as No. of infected/No. of examined (Prevalence %)
All 12 cattle tested were positive in qPCR and conventional PCR (100%) for Anaplasmataceae bacteria. Sequencing analyses showed that all cattle were infected by A. marginale. The sequences were identical to each other, and also to the sequences of A. marginale identified in the R. bursa ticks removed from the same animals.
Finally, 4 of 5 goats were found to be infected by a potentially new species of Anaplasma similar to A. marginale. All sequences were identical to each other and showed 99% homology with the A. marginale strain Dawn (CP006847), A. centrale strain Israel (CP001759) and 99% with A. ovis strain KMND Niayas-14 (KM021411) (Fig. 3).
Additional characterisation of detected Anaplasmataceae bacteria was performed by amplification/sequencing of a portion of the rpoB gene (for Anaplasma-positive samples) or groEL gene (for Ehrlichia-positive samples). RpoB sequences from A. ovis-positive samples were also identical to each other and showed 100% identity with A. ovis strain KMND Nayes-14. rpoB sequences from two A. marginale-positive R. bursa ticks and the four other sequences obtained from cattle blood samples were identical to each other and showed 100% identity with A. marginale strain Dawn (CP006847) and Gypsy Plains (CP006846) and 99% with A. marginale strain Florida (CP001079) and St. Maries (CP000030). For the E. canis identified in one R. bursa tick, the DNA sample was amplified using groEl Ehrlichia genus-specific primers and sequenced. The sequence showed 99% homology with the E. canis strain Jack (CP000107) (Fig. 4).
Analysis of rpoB sequences of all three novel genotypes of Anaplasma produced results similar to the 23S gene analysis. RpoB sequences from A. cf. ovis showed 98% identity with A. ovis strain KMND Nayes-14. The three rpoB sequences from “Ca. Anaplasma corsicanum” had 99% identity to each other, and only 80% with A. phagocytophilum strains Norway Variant 2 (CP015376), Dog2 (CP006618), JM (CP006617) and HZ (CP000235). RpoB sequences of “Ca. Anaplasma mediterraneum” presented 84% identity with A. centrale strain Israel (CP001759). Finally, Anaplasma cf. marginale from four goats had rpoB sequences that shared 98% identity with A. ovis strain KMND Niayes-14 (KX155494) and strain RhburBas11 (KX155495), 93% with A. marginale strain Florida (CP001079), St. Maries (CP000030), 89% strain Dawn (CP006847) and Gypsy Plains (CP006846), and 87% with A. centrale strain Israel (CP001759) (Fig. 3).
Phylogenetic analyses of the potentially new species
The phylogenetic tree inferred from the Anaplasmataceae concatenated 23S rRNA, and the rpoB genes provide evidence that “Ca. Anaplasma corsicanum”, Anaplasma cf. ovis, “Ca. Anaplasma mediterraneum” from sheep and Anaplasma cf. marginale from goats could potentially be new species. “ Ca. Anaplasma corsicanum” clustered separately from the recognised species A. phagocytophilum, A. platys, A. ovis, A. marginale and A. centrale (Fig. 2). The sequence of Anaplasma cf. ovis from sheep and the sequence of Anaplasma cf. marginale from goats clustered together with the sequence of A. ovis strain KMND Niayes-14 from Senegal and A. ovis from sheep identified in this study with high bootstrap values and separately from the cluster of A. marginale species. Finally, the sequence of “Ca. Anaplasma mediterraneum” obtained from sheep form well-defined branches with high bootstrap values (93–95%) (Fig. 3).
All sequences obtained in the present study were submitted to GenBank under the following accession numbers: (i) for the 23S rRNA gene: Anaplasma ovis OVCF02 (KY498325), Anaplasma cf. ovis OVCF115 (KY498326), “Ca. Anaplasma corsicanum” OVCF72 (KY498327), “Ca. Anaplasma corsicanum” OVCF81 (KY498328), “Ca. Anaplasma corsicanum” OVCF65 (KY498329), “Ca. Anaplasma mediterraneum” OVCFO215 (KY498330), Anaplasma cf. marginale CpCF01 (KY498331), Anaplasma marginale BvCF13 (KY498332), A. marginale Rh.burCF08 (KY498334), A. marginale Rh.burCF10 (KY498335), Ehrlichia canis Rh.burCF07 (KY498333); (ii) for the rpoB gene: Anaplasma ovis OVCF02 (KY498325), Anaplasma cf. ovis OVCF115 (KY498336), “Ca. Anaplasma corsicanum” OVCF72 (KY498338), “Ca. Anaplasma corsicanum” OVCF81 (KY498339), “Ca. Anaplasma corsicanum” OVCF65 (KY498340), “Ca. Anaplasma mediterraneum” OVCFO215 (KY498341), Anaplasma cf. marginale CpCF01 (KY498342), Anaplasma marginale BvCF13 (KY498343), A. marginale Rh.burCF08 (KY498344), A. marginale Rh.burCF10 (KY498345); (iii) For the groEl gene: Ehrlichia canis Rh.burCF07 (KY498324). For tick species, the 12S rRNA sequences were submitted under the following accession number: Hy. m. marginatum (KY595783) and R. bursa (KY595784).
Discussion
Livestock farming in Corsica is an important economic activity involving approximately 150,000 sheep, 48,000 goats, 40,000 pigs and 70,000 cattle [22]. The significance of anaplasmosis in animals in Corsica is not yet known. Anaplasma infection may likely be neglected because of its unknown economic importance in small ruminants. To our knowledge, the present study is the first report of the incidence of Anaplasmataceae species in ticks and animals in Corsica. Furthermore, the presence and molecular traits of six species belonging to the genus Anaplasma from ruminants and ticks infecting cattle, and one Ehrlichia, are shown. The typical Mediterranean environment of Corsica with hot summers, along with the geographical location, favours the spread of seasonal tick infestations. Two tick species were collected and confirmed by the morphological and molecular investigation as R. bursa and Hy. m. marginatum (Fig. 1). Neither the tick fauna of Corsica nor the transmitted pathogens have been fully investigated. Here, ticks were only collected from cattle; infestation of other animals, including sheep, goats, horses and dogs, was not observed. A previous study demonstrated the presence of three species of the genus Hyalomma in Corsica: Hy. marginatum, Hy. aegyptium and Hy. rufipes [22]. While Hy. marginatum is found on many hosts, Hy. aegyptium was identified once in Corsica on a Testudo hermanni tortoise, while Hy. rufipes has been collected from migrating birds [22]. Recently, Hy. scupense was also identified and collected from Corsican cattle by Grech-Angelini et al. [22]. Rhipicephalus bursa was the most common tick infesting cattle in our study. This two-host species occurs in the entire Mediterranean, Adriatic and Aegean basins, including their islands, and North Africa [25, 34]. Rhipicephalus bursa prefers grassy slopes and low to medium altitude mountain slopes, as well as certain modified steppe and semi-desert environments [35]. However, this tick species is recorded in cold regions, including the Atlantic region of Europe, the French Basque country, Spanish Basque country, and north-west Portugal [28, 35]. Corsica is a typical Mediterranean ecosystem, which favours the spread of these ticks. Rhipicephalus bursa mature and adults infest many hosts, including cattle, sheep, goats and other domestic animals, whereas wild ungulates are the original host [36]. This species is a recognised vector of many pathogens, including Babesia ovis, Theileria spp., A. marginale and A. ovis [36]. DNA of Coxiella burnetii and A. phagocytophilum have also been amplified from these ticks [28, 35]. Here, the DNA of A. marginale was amplified from two engorged female ticks removed from cattle infected by A. marginale. Previous studies have reported A. marginale from R. bursa removed from cattle in Portugal [34], and from Iberian red deer and European wild boar in Spain [37]. It is likely that the presence of A. marginale DNA in these two ticks was due to the presence of this pathogen in the blood meal. However, the percentage of R. bursa-engorged females in our study was 42.9% (30/70 female); only two engorged ticks were found to harbour A. marginale.
Ehrlichia canis was amplified from one non-engorged R. bursa female. In Europe, E. canis is associated with the presence of the brown dog tick R. sanguineus [38]. However, in the Mediterranean area, E. canis has also been reported from R. bursa collected from goats in Sardinia, Italy [39] and Cediopsylla inaequalis collected from red foxes in Sicily, Italy [40]. In other European countries, there are reports of E. canis from D. marginatus collected from dogs, Ixodes canisuga collected from red foxes, and I. ricinus collected from vegetation in Hungary [41, 42]. Domestic animals are now recognised as the primary hosts of R. bursa [36]; however, the role of R. bursa and the other arthropod species in the transmission of E. canis remains unknown.
None of the five Hy. m. marginatum ticks were positive for Anaplasmataceae infection. However, in Spain, Hy. m. marginatum has been identified as a potential biological vector for A. marginale [43]. These ticks are also the vectors of Babesia caballi, causing babesiosis in horses and Theileria annulata infection under laboratory conditions [26]. Other studies are needed to clarify and list the pathogens associated with these ticks in Corsica.
The prevalence of Anaplasma spp. in our study was surprisingly high in ruminants. Based on the 23S rRNA gene molecular investigations, the individual prevalence observed was 59.5% in sheep, 100% in cattle, and 80% in goats. However, none of the canine or equine blood samples was positive. Genetic characterisation using 23S rRNA and the rpoB genes identified A. ovis, A. marginale, and several potentially new species, all belonging to the genus Anaplasma. These data confirm the relevance of ruminants as important hosts and reservoirs of different Anaplasma species in the Mediterranean ecosystem. The prevalence of Anaplasma spp. in ruminants examined by us was lower than the prevalence data reported from Sardinia [44]. The prevalence of A. marginale infection in cattle was higher than that observed in cattle in Sicily [45]; however, in that study, the number of samples analysed was greater than in our study. In sheep, the prevalence reported in our study was lower than that reported in Sicily [46].
Sheep, goats, and cattle sampled in this study manifested poor health. In sheep, most clinical manifestations observed were relapsing fever, drop in milk production and mortality. Molecular and phylogenetic analysis of sequences amplified from sheep blood samples were identified A. ovis, and three potentially new species, “Ca. Anaplasma corsicanum”, “Ca. Anaplasma mediterraneum”, and Anaplasma cf. ovis. In the Mediterranean area, A. ovis is reported to be endemic to Sicily [47, 48]. This pathogen has also been reported from Greece and Cyprus [21, 49]. In Europe, A. ovis has also been reported from Portugal, Hungary [19] and Slovakia [47]. Anaplasma ovis infection in the mouflon and the European roe deer has been reported from Cyprus and southern Spain, respectively [50, 51]. The main vector of A. ovis in Europe is R. bursa [28]. However, A. ovis DNA was amplified from I. ricinus removed from cattle in Hungary [15], Haemaphysalis sulcata removed from mouflons in Cyprus [50], and the sheep ked (Melophagus ovinus) and deer ked (Lipoptena cervi) in Hungary [48]. In addition, in provinces of Palermo and Ragusa (Italy), A. ovis was amplified from foxes, and a flea, Xenopsylla cheopis, removed from these foxes [40]. The role of these arthropods and insects in the transmission of A. ovis remains unclear. Anaplasmosis in sheep is usually subclinical. This bacterium can lead to severe infection with severe illness in sheep; severe illness can occur in some extreme conditions, such as the association with more than one parasitic disease or other stress factors [49, 52].
All cattle sampled in this study were infected with A. marginale. In this farm, the farmer reported mortality in his livestock. Anaemia and icterus were most observed in other cattle (Table 1). Bovine anaplasmosis due to A. marginale causes mild to severe anaemia, icterus, fever, weight loss, abortion and lethargy [53]. In Europe, A. marginale is mainly present in the Mediterranean region, alpine, and eastern areas [16]. In the Mediterranean region, the DNA of this bacterium has been amplified from D. reticulatus, D. marginatus, R. turanicus, Haemaphysalis punctata, Hy. m. marginatum and R. bursa [34, 37, 43]. Interestingly, A. marginale has been amplified from Xenopsylla cheopis removed from red foxes in Italy [40]. Outside of the Mediterranean region, A. marginale has been amplified from I. ricinus and Tabanus bovis in Hungary [15, 43]. The role of I. ricinus, Tabanus bovis and Xenopsylla cheopis in the transmission of A. marginale remains unclear.
The potentially new species “Ca. Anaplasma corsicanum” and “Ca. Anaplasma mediterraneum” have genetic features which are different from other species of the genus Anaplasma (Fig. 3). Phylogenetic analysis based on the concatenated 23S rRNA and rpoB genes showed that “Ca. Anaplasma corsicanum” is related to A. phagocytophilum, but clustered separately from recognised species. “Ca. Anaplasma mediterraneum” is related to A. centrale and forms a distinct subcluster. Two other identified genotypes, Anaplasma cf. ovis and Anaplasma cf. marginale, grouped with the sequences of A. ovis (Fig. 3). Interestingly, despite this grouping, Anaplasma cf. marginale is closer to A. marginale than to A. ovis, based on the 23S rRNA comparison. The rpoB encodes the RNA polymerase subunit beta and gives a better statistical score for differentiating between the closest species of Anaplasma spp., with more sequence variations [28]. The observed prevalence of the potentially new Anaplasma species in sheep was low (17.3%, 38/220) compared to the prevalence of A. ovis; however, 80% (4/5) of the goats sampled in this study were infected by Anaplasma cf. marginale. The importance of this amplified Anaplasma species remains to be understood.
Mortalities in animals were reported by the farmers in the sheep and cattle herds. Unfortunately, we did not have access to body tissue of fluid from the dead animals to perform a post-mortem diagnosis. The different reported symptoms and the results found in the present study with the high prevalence of A. marginale in cattle and A. ovis and the others amplified Anaplasma spp. in sheep and goats suggest that the mortalities can be linked to these Anaplasma species. However, other tick- or vector-borne diseases can also lead to mortalities like Piroplasmosis [54, 55]. In addition, co-infection by two or more pathogens can lead to increase the pathogenicity and clinical manifestations in animals and resultant varying outcomes on host health and survival [56]. The involvement or not involvement of the Anaplasmataceae species amplified in the present study should be considered with caution do to the possible implication of other pathogens.
In our study, we did not find A. phagocytophilum in animal or tick samples. Anaplasma platys and E. canis were also not found in dogs, although E. canis was found in Rh. bursa collected from a cow.
Conclusion
The present study demonstrates that ruminants in Corsica are a reservoir for multiple Anaplasma species, whereas R. bursa seems to be a vector of A. marginale in cattle. The prevalence of Anaplasma spp. infection was high. The use of quantitative real-time PCR complemented with sequencing and genetic characterisation using two genes, rpoB and groEl, revealed an interesting diversity of Anaplasma spp. infection in small ruminants and R. bursa, including potentially new species and E. canis in one R. bursa tick. Nevertheless, characterisation studies are needed to ascertain the pathogenesis and/or the zoonotic potential of the strains and their significance for animals and public health.
Acknowledgments
We are grateful to Emilie Fauconnier, Bernard Fabrizy, and Sandrine Ferrandi for their valuable help with the field work.
Funding
This study was supported by the AMIDEX project (No. ANR-11-IDEX-0001-02) funded by the “Investissements d’Avenir” French Government program, managed by the French National Research Agency (ANR) and Foundation Méditerranée Infection (www.mediterranee-infection.com). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The data supporting the conclusions of this article are included within the article.
Authors’ contributions
MD, DT, BD, FF, OM and DR designed the study. MD, BD, FF, OM designed and MD carried out the data analysis. MD and OM drafted the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
All animals sampled in this study were examined with the assistance of their owners. Blood samples were collected by a veterinarian.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- GroEl gene
Heat shock protein gene
- qPCR
Quantitative real-time polymerase chain reaction
- RpoB gene
RNA polymerase subunit beta gene
Contributor Information
Mustapha Dahmani, Email: mus.dahmani@gmail.com.
Bernard Davoust, Email: bernard.davoust@gmail.com.
Djamel Tahir, Email: djamel.tahir@yahoo.fr.
Didier Raoult, Email: didier.raoult@gmail.com.
Florence Fenollar, Email: florence.fenollar@univ-amu.fr.
Oleg Mediannikov, Email: olegusss1@gmail.com.
References
- 1.Rar V, Golovljova I. Anaplasma, Ehrlichia, and “ Candidatus Neoehrlichia” bacteria: Pathogenicity, biodiversity, and molecular genetic characteristics, a review. Infect Genet Evol. 2011;11:1842–1861. doi: 10.1016/j.meegid.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 2.de la Fuente J, Kocan KM, Blouin EF, Zivkovic Z, Naranjo V, Almazán C, et al. Functional genomics and evolution of tick-Anaplasma interactions and vaccine development. Vet Parasitol. 2010;167:175–186. doi: 10.1016/j.vetpar.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 3.Baldridge GD, Scoles GA, Burkhardt NY, Kurtti TJ, Munderloh UG, Baldridge GD, et al. Transovarial transmission of Francisella-like endosymbionts and Anaplasma phagocytophilum variants in Dermacentor albipictus (Acari: Ixodidae ) J Med Entomol. 2009;46:625–632. doi: 10.1603/033.046.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moniuszko A, Rückert C, Alberdi MP, Barry G, Stevenson B, Fazakerley JK, et al. Coinfection of tick cell lines has variable effects on replication of intracellular bacterial and viral pathogens. Ticks Tick Borne Dis. 2014;5:415–422. doi: 10.1016/j.ttbdis.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rikihisa Y. Anaplasma phagocytophilum and Ehrlichia chaffeensis: subversive manipulators of host cells. Nat Rev Microbiol. 2010;8:328–339. doi: 10.1038/nrmicro2318. [DOI] [PubMed] [Google Scholar]
- 6.Stuen S, Granquist EG, Silaghi C. Anaplasma phagocytophilum a widespread multi-host pathogen with highly adaptive strategies. Front Cell Infect Microbiol. 2013;3:31. doi: 10.3389/fcimb.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katargina O, Geller J, Alekseev A, Dubinina H, Efremova G, Mishaeva N, et al. Identification of Anaplasma phagocytophilum in tick populations in Estonia, the European part of Russia and Belarus. Clin Microbiol Infect. 2011;18:7–9. doi: 10.1111/j.1469-0691.2010.03457.x. [DOI] [PubMed] [Google Scholar]
- 8.Cochez C, Ducoffre G, Vandenvelde C, Luyasu V, Heyman P. Human anaplasmosis in Belgium: a 10-year seroepidemiological study. Ticks Tick Borne Dis. 2011;2:156–159. doi: 10.1016/j.ttbdis.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Väisänen E, Kuisma I, Phan TG, Delwart E, Lappalainen M, Tarkka E, et al. Granulocytic anaplasmosis acquired in Scotland. Emerg Infect Dis. 2014;20:7–9. doi: 10.3201/eid2006.131674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin H, Wei F, Liu Q, Qian J. Epidemiology and control of human granulocytic anaplasmosis: a systematic review. Vector Borne Zoonotic Dis. 2012;12:269–274. doi: 10.1089/vbz.2011.0753. [DOI] [PubMed] [Google Scholar]
- 11.Chochlakis D, Psaroulaki A, Kokkini S, Kostanatis S, Arkalati E, Karagrannaki E, et al. First evidence of Anaplasma infection in Crete, Greece. Report of six human cases. Clin Microbiol Infect. 2008;15:8–9. doi: 10.1111/j.1469-0691.2008.02695.x. [DOI] [PubMed] [Google Scholar]
- 12.Aubry P, Geale DW. A review of bovine anaplasmosis. Transbound Emerg Dis. 2011;58:1–30. doi: 10.1111/j.1865-1682.2010.01173.x. [DOI] [PubMed] [Google Scholar]
- 13.Ashraf QUA, Ullah A, Mehmood R, Ali M, Sadiq R, Ali M, et al. A report on the high prevalence of Anaplasma sp. in buffaloes from two provinces in Pakistan. Ticks Tick Borne Dis. 2013;4:395–398. doi: 10.1016/j.ttbdis.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Nair AS, Ravindran R, Lakshmanan B, Sreekumar C, Kumar SS, Raju R, et al. Bovine carriers of Anaplasma marginale and Anaplasma bovis in South India. Trop Biomed. 2013;30:105–112. [PubMed] [Google Scholar]
- 15.Hornok S, Micsutka A, Fernández de Mera IG, Meli ML, Gönczi E, Tánczos B, et al. Fatal bovine anaplasmosis in a herd with new genotypes of Anaplasma marginale, Anaplasma ovis and concurrent haemoplasmosis. Res Vet Sci. 2012;92:30–35. doi: 10.1016/j.rvsc.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Ceci L, Iarussi F, Greco B, Lacinio R, Fornelli S, Carelli G. Retrospective study of hemoparasites in cattle in Southern Italy by reverse line blot hybridization. J Vet Med Sci. 2014;76:869–875. doi: 10.1292/jvms.13-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.García-Pérez A, Oporto B, Espí A, del Cerro A, Barral M, Povedano I, et al. Anaplasmataceae in wild ungulates and carnivores in northern Spain. Ticks Tick Borne Dis. 2015;7:264–269. doi: 10.1016/j.ttbdis.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 18.de la Fuente J, Atkinson MW, Naranjo V, Fernández de Mera IG, Mangold AJ, Keating KA, et al. Sequence analysis of the Msp4 gene of Anaplasma ovis strains. Vet Microbiol. 2007;119:375–381. doi: 10.1016/j.vetmic.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Renneker S, Abdo J, Salih DE, Karagenç T, Bilgiç H, Torina, et al. Can Anaplasma ovis in small ruminants be neglected any longer? Transbound Emerg Dis. 2013;60(Suppl 2):105–112. doi: 10.1111/tbed.12149. [DOI] [PubMed] [Google Scholar]
- 20.Dimosthenis C, Ioannis I, Tselentis Y, Psaroulaki A. Human anaplasmosis and Anaplasma ovis Variant. Emerg Infect Dis. 2010;16:1031–1032. doi: 10.3201/eid1606.090175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dantas-Torres F, Chomel BB, Otranto D. Ticks and tick-borne diseases: a One Health perspective. Trends Parasitol Elsevier Ltd. 2012;28:437–446. doi: 10.1016/j.pt.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Grech-Angelini S, Stachurski F, Lancelot R, Boissier J, Allienne JF, Gharbi M, et al. First report of the tick Hyalomma scupense (natural vector of bovine tropical theileriosis) on the French Mediterranean island of Corsica. Vet Parasitol. 2016;216:33–37. doi: 10.1016/j.vetpar.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 23.Dahmani M, Alwassouf S, Grech-angelini S, Marié J, Davoust B, Charrel RN. Seroprevalence of Toscana virus in dogs from Corsica, France. Parasit Vectors. 2016;9:381. doi: 10.1186/s13071-016-1665-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maquart M, Dahmani M, Marié J-L, Gravier P, Leparc-Goffart I, Davoust B. First serological evidence of West Nile virus in horses and dogs from Corsica Island, France. Int J Infect Dis. 2016;53:58. doi: 10.1016/j.ijid.2016.11.148. [DOI] [PubMed] [Google Scholar]
- 25.Guglielmone AA, Robbins RG, Apanaskevich DA, Petney TN, Estrada-Peña A, Horak IG. The hard ticks of the World (Acari: Ixodidae: Ixodidae) Dordrecht: Springer; 2014. [Google Scholar]
- 26.Estrada-Peña A, Bouattour A, Camicas JL, Walker AR. Ticks of domestic animals in the Mediterranean region. Zaragoza: University of Zaragoza; 2004. [Google Scholar]
- 27.Beati L, Keirans JE. Analysis of the systematic relationships among ticks of the genera Rhipicephalus and Boophilus (Acari: Ixodidae) based on mitochondrial 12S ribosomal DNA gene sequences and morphological characters. J Parasitol. 2001;87:32–48. doi: 10.1645/0022-3395(2001)087[0032:AOTSRA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 28.Dahmani M, Davoust B, Rousseau F, Raoult D, Fenollar F, Mediannikov O. Natural Anaplasmataceae infection in Rhipicephalus bursa ticks collected from sheep in the French Basque Country. Ticks Tick Borne Dis. 2017;8:18–24. doi: 10.1016/j.ttbdis.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Dahmani M, Bernard D, Seghir BM, Fenollara F, Raoulta D, Mediannikov O. Development of a new PCR-based assay to detect Anaplasmataceae and the first report of Anaplasma phagocytophilum and Anaplasma platys in cattle from Algeria. Comp Immunol Microbiol Infect Dis. 2015;4:39–45. doi: 10.1016/j.cimid.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Dahmani M, Loudahi A, Mediannikov O, Fenollar F, Raoult D, Davoust B. Molecular detection of Anaplasma platys and Ehrlichia canis in dogs from Kabylie, Algeria. Ticks Tick Borne Dis. 2015;6:198–203. [DOI] [PubMed]
- 31.Hall TA. BioEdit: a user-frindly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser. 1999;41:95–8.
- 32.Kumar S, Stecher G, Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:msw054. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Djiba ML, Mediannikov O, Mbengue M, Thiongane Y, Molez J-F, Seck MT, et al. Survey of Anaplasmataceae bacteria in sheep from Senegal. Trop Anim Health Prod. 2013;45:1557–1561. doi: 10.1007/s11250-013-0399-y. [DOI] [PubMed] [Google Scholar]
- 34.Ferrolho J, Antunes S, Santos AS, Velez R, Padre L, Cabezas-Cruz A, et al. Detection and phylogenetic characterization of Theileria spp. and Anaplasma marginale in Rhipicephalus bursa in Portugal. Ticks Tick Borne Dis. 2016;7:443–448. doi: 10.1016/j.ttbdis.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Raele DA, Galante D, Pugliese N, De Simone E, Cafiero MA. Coxiella-like endosymbiont associated to the “Anatolian brown tick” Rhipicephalus bursa in Southern Italy. Microbes Infect. 2015;17:799–805. doi: 10.1016/j.micinf.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 36.Walker JB, Keirans JE, Horak IG. The genus Rhipicephalus (Acari, Ixodidae): a guide to the brown ticks of the world. Cambridge: Cambridge University Press; 2005.
- 37.De La Fuente J, Naranjo V, Ruiz-Fons F, Vicente J, Estrada-Peña A, Almazán C, et al. Prevalence of tick-borne pathogens in ixodid ticks (Acari: Ixodidae) collected from European wild boar (Sus scrofa) and Iberian red deer (Cervus elaphus hispanicus) in central Spain. Eur J Wildl Res. 2004;50:187–196. doi: 10.1007/s10344-004-0060-1. [DOI] [Google Scholar]
- 38.René-martellet M, Lebert I, Chêne J, Massot R, Leon M, Leal A, et al. Diagnosis and incidence risk of clinical canine monocytic ehrlichiosis under field conditions in Southern Europe. Parasit Vectors. 2015;8:3. doi: 10.1186/s13071-014-0613-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masala G, Chisu V, Foxi C, Socolovschi C, Raoult D, Parola P. First detection of Ehrlichia canis in Rhipicephalus bursa ticks in Sardinia, Italy. Ticks Tick Borne Dis. 2012;3:396–397. doi: 10.1016/j.ttbdis.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 40.Torina A, Blanda V, Antoci F, Scimeca S, Agostino RD, Scariano E, et al. A molecular survey of Anaplasma spp., Rickettsia spp., Ehrlichia canis and Babesia microti in foxes and fleas from Sicily. Transbound Emerg Dis. 2013;60:125–130. doi: 10.1111/tbed.12137. [DOI] [PubMed] [Google Scholar]
- 41.Real C, Sciences H, Animal S, Madrid D. Molecular evidence of Ehrlichia canis and Rickettsia massiliae in ixodid ticks. Acta Vet Hung. 2013;61:42–50. [DOI] [PubMed]
- 42.Hornok S, Meli ML, Perreten A, Farkas R, Willi B, Beugnet F, et al. Molecular investigation of hard ticks (Acari: Ixodidae) and fleas (Siphonaptera: Pulicidae) as potential vectors of rickettsial and mycoplasmal agents. Vet Microbiol. 2010;140:98–104. doi: 10.1016/j.vetmic.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 43.Bonnet S, de la Fuente J, Nicollet P, Liu X, Madani N, Blanchard B, et al. Prevalence of tick-borne pathogens in adult Dermacentor spp. ticks from nine collection sites in France. Vector Borne Zoonotic Dis. 2013;13:226–236. doi: 10.1089/vbz.2011.0933. [DOI] [PubMed] [Google Scholar]
- 44.Zobba R, Anfossi AG, Pinna Parpaglia ML, Dore GM, Chessa B, Spezzigu A, et al. Molecular investigation and phylogeny of Anaplasma spp. in Mediterranean ruminants reveal the presence of neutrophil-tropic strains closely related to A. platys. Appl Environ Microbiol. 2014;80:271–280. doi: 10.1128/AEM.03129-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de la Fuente J, Torina A, Caracappa S, Tumino G, Furlá R, Almazán C, et al. Serologic and molecular characterization of Anaplasma species infection in farm animals and ticks from Sicily. Vet Parasitol. 2005;133:357–362. doi: 10.1016/j.vetpar.2005.05.063. [DOI] [PubMed] [Google Scholar]
- 46.Torina A, Galindo RC, Vicente J, Di MV, Russo M, Aronica V, et al. Characterization of Anaplasma phagocytophilum and A. ovis infection in a naturally infected sheep flock with poor health condition. Trop Anim Health Prod. 2010;42:1327–1331. doi: 10.1007/s11250-010-9580-8. [DOI] [PubMed] [Google Scholar]
- 47.Víchová B, Majláthová V, Nováková M, Stanko M, Hvišcová I, Pangrácová L, et al. Anaplasma infections in ticks and reservoir host from Slovakia. Infect Genet Evol. 2014;22:265–272. doi: 10.1016/j.meegid.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Hornok S, de la Fuente J, Biró N, Fernández de Mera IG, Meli ML, Elek V, et al. First molecular evidence of Anaplasma ovis and Rickettsia spp. in keds (Diptera: Hippoboscidae) of sheep and wild ruminants. Vector Borne Zoonotic Dis. 2011;11:1319–1321. doi: 10.1089/vbz.2011.0649. [DOI] [PubMed] [Google Scholar]
- 49.Torina A, Caracappa S. Tick-borne diseases in sheep and goats: clinical and diagnostic aspects. Small Rumin Res. 2012;106:S6–S11. doi: 10.1016/j.smallrumres.2012.04.026. [DOI] [Google Scholar]
- 50.Ioannou I, Sandalakis V, Kassinis N, Chochlakis D, Papadopoulos B, Loukaides F, et al. Tick-borne bacteria in mouflons and their ectoparasites in Cyprus. J Wildl Dis. 2011;47:300–306. doi: 10.7589/0090-3558-47.2.300. [DOI] [PubMed] [Google Scholar]
- 51.de la Fuente J, Ruiz-Fons F, Naranjo V, Torina A, Rodríguez O, Gortázar C. Evidence of Anaplasma infections in European roe deer (Capreolus capreolus) from southern Spain. Res Vet Sci. 2008;84:382–386. doi: 10.1016/j.rvsc.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 52.Stuen S. Tick-borne infections in small ruminants in northern Europe. Small Rumin Res. 2013;110:142–144. doi: 10.1016/j.smallrumres.2012.11.022. [DOI] [Google Scholar]
- 53.Kocan KM, de la Fuente J, Blouin EF, Coetzee JF, Ewing S. The natural history of Anaplasma marginale. Vet Parasitol. 2010;167:95–107. doi: 10.1016/j.vetpar.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 54.Hornok S, Mester A, Takács N, Fernández de Mera IG, de la Fuente J, Farkas R. Re-emergence of bovine piroplasmosis in Hungary: has the etiological role of Babesia divergens been taken over by B. major and Theileria buffeli? Parasit Vectors. 2014;7:434. doi: 10.1186/1756-3305-7-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Decaro N, Larocca V, Parisi A, Losurdo M, Lia RP, Greco MF, et al. Clinical bovine piroplasmosis caused by Babesia occultans in Italy. J Clin Microbiol. 2013;51:2432–2434. doi: 10.1128/JCM.00713-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thumbi SM, Bronsvoort BMDC, Poole EJ, Kiara H, Toye PG, Mbole-Kariuki MN, et al. Parasite co-infections and their impact on survival of indigenous cattle. PLoS One. 2014;9:e76324. doi: 10.1371/journal.pone.0076324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the conclusions of this article are included within the article.


