Abstract
OBJECTIVE
We used targeted metabolomics in pregnant mothers to compare maternal metabolite associations with maternal BMI, glycemia, and insulin sensitivity.
RESEARCH DESIGN AND METHODS
Targeted metabolomic assays of clinical metabolites, amino acids, and acylcarnitines were performed on fasting and 1-h postglucose serum samples from European ancestry, Afro-Caribbean, Thai, and Mexican American mothers (400 from each ancestry group) who participated in the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study and underwent an oral glucose tolerance test at ∼28 weeks gestation.
RESULTS
K-means clustering, which identified patterns of metabolite levels across ancestry groups, demonstrated that, at both fasting and 1-h, levels of the majority of metabolites were similar across ancestry groups. Meta-analyses demonstrated association of a broad array of fasting and 1-h metabolites, including lipids and amino acids and their metabolites, with maternal BMI, glucose levels, and insulin sensitivity before and after adjustment for the different phenotypes. At fasting and 1 h, a mix of metabolites was identified that were common across phenotypes or associated with only one or two phenotypes. Partial correlation estimates, which allowed comparison of the strength of association of different metabolites with maternal phenotypes, demonstrated that metabolites most strongly associated with different phenotypes included some that were common across as well as unique to each phenotype.
CONCLUSIONS
Maternal BMI and glycemia have metabolic signatures that are both shared and unique to each phenotype. These signatures largely remain consistent across different ancestry groups and may contribute to the common and independent effects of these two phenotypes on adverse pregnancy outcomes.
Introduction
Pregnancy is characterized by profound changes in carbohydrate, fat, and protein metabolism to meet the physiological demands imposed by pregnancy and ensure fetal growth and development (1–3). Changes in insulin sensitivity are a hallmark of pregnancy and contribute to the metabolic changes, while nutrient transfer to the fetus impacts maternal metabolite levels (2,4). Maternal hyperglycemia and obesity further alter maternal metabolism (2,5).
Gestational diabetes mellitus (GDM) and maternal obesity are both associated with adverse pregnancy outcomes, including pregnancy-related hypertensive disorders, cesarean deliveries, and large-for-gestational-age infants (5,6). However, GDM confers a higher risk of preterm delivery compared with maternal obesity not complicated by diabetes (5,6), and maternal glycemia and BMI have an additive effect on birth weight and fetal adiposity (7–9). Together, these findings suggest that maternal glycemia and BMI may impact pregnancy outcomes through both common and independent mechanisms.
Pregnancy-related changes in metabolism, including those accompanying maternal hyperglycemia and obesity, are reflected in the maternal metabolome (10–14), which is determined by both intrinsic (e.g., genetic) and extrinsic (e.g., diet, exercise, etc.) factors (15,16). Whether the maternal metabolome associated with maternal BMI and glycemia varies, given their common and independent effects on pregnancy outcomes, is not known, nor is the similarity of these associations across ancestry groups with different genetic backgrounds and environments.
To address these questions, we used targeted metabolomics to compare associations of maternal metabolites with maternal BMI and glycemia in mothers from four ancestries and environments and determine whether common associations exist despite varied genetic and extrinsic factors.
Research Design and Methods
Data and Sample Collection
Maternal blood samples were obtained during a 75-g oral glucose tolerance test (OGTT) between 24 and 32 weeks gestation during the population-based Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study conducted during 2000–2006 at 15 international field centers (9,17). Fasting and 1-h glucose and C-peptide were measured. Insulin sensitivity was calculated using OGTT glucose and C-peptide measurements as previously described (18). Maternal samples were collected, processed, shipped, and stored centrally at −70°C prior to metabolomics assays (19).
Maternal anthropometrics, including height, weight, and mean arterial pressure, were measured by trained personnel using standardized procedures and calibrated equipment at OGTT. Maternal BMI was calculated using height and weight at OGTT. For 1,412 mothers in this study with self-reported pregestational weight, there was 92.4% correlation of OGTT BMI with pregestational BMI calculated using measured height and reported pregestational weight. Gestational age was calculated as previously described (9,17); self-identified ancestry, parity, and other demographic data were ascertained via questionnaire.
Metabolites were measured in maternal fasting and 1-h serum samples from 400 mothers of Afro-Caribbean, Mexican American, Northern European, and Thai ancestry. Mothers were sampled to span the range of maternal glucose and BMI (9). Results for Northern European ancestry mothers have been reported previously (20,21).
Conventional Metabolites and Targeted Metabolomics Assays
Conventional clinical metabolite and targeted metabolomics assays were conducted as previously described (18). In brief, conventional metabolites (lactate, triglycerides, β-hydroxybutyrate, glycerol, and nonesterified fatty acids [NEFA]) were measured on a Beckman-Coulter Unicell DxC 600 clinical analyzer. Targeted metabolomics assays for acylcarnitines and amino acids used tandem mass spectrometry with addition of known quantities of stable isotope-labeled internal standards on an Acquity TQD Triple Quadrupole system (Waters Corporation, Milford, MA). Sixty-two conventional and targeted metabolites were analyzed.
Statistical Analysis
Per-Metabolite Analysis
Acylcarnitine and β-hydroxybutyrate levels were log transformed for analysis. Outlying metabolite values, defined as five or more SDs from the mean, were excluded from analysis.
Metabolite levels were compared across ancestries using ANOVA, and K-means clustering was used to illustrate patterns in metabolite means across ancestries, with inputs consisting of differences between ancestry-specific and overall means, divided by ANOVA residual SE for each metabolite. K-means clustering is a method for partitioning data into groups that minimizes differences in values within clusters. Initially, data points are randomly placed into one of a prespecified number of groups. The mean value of the observations in each group is calculated, and points are reassigned to the group with the closest mean. This process proceeds iteratively until the mean value of each group no longer changes. To avoid arbitrarily prespecifying the number of groups (K), the “NbClust” R package was used (22). This package provides 30 data-driven indices for identifying the optimal number of clusters from a dataset. K was chosen to be the number most frequently identified among these 30 indices.
Associations between phenotypes and metabolites were identified using linear regression within ancestry group, treating maternal phenotypes as predictors and maternal metabolites as outcomes, adjusting for baseline covariates: field center, mean arterial pressure, maternal age, neonatal sex, sample storage time, and gestational age at OGTT. Maternal phenotypes included BMI (with and without glucose adjustment), fasting and 1-h glucose (with and without BMI adjustment), and insulin sensitivity (with and without BMI adjustment). False discovery rate (FDR) correction (23) was applied to ancestry-specific analyses; FDR-adjusted P values <0.05 were considered statistically significant.
Since comparison of regression model betas across maternal phenotypes and metabolites is complicated by different units of measurement and amounts of variability, partial correlation estimates (rp) were calculated for maternal phenotypes and metabolites, after adjusting for model covariates, to assist in interpreting strength of associations. Partial correlation estimates range in magnitude from 0 to 1 (no to perfect association) and represent association strength on a comparable scale for all maternal phenotypes and metabolites. Sample sizes of 400 for within-ancestry analyses afforded 90% power at two-sided α = 0.001 (roughly consistent with FDR adjustment for 62 metabolites to maintain overall 5% type I error) to detect partial correlations of 0.24, assuming up to 0.30 correlation of maternal phenotypes with other model covariates.
Meta-analysis
To combine effect estimates across analyses conducted for each ancestry group for a combined measure of association across all groups, random-effects meta-analysis was performed. Betas from per-metabolite analyses were combined across ancestry groups using random-effects meta-analysis with inverse variance weights and restricted maximum likelihood estimation for heterogeneity using the “metafor” R package (24). To test whether effects varied across ancestry groups, effect heterogeneity was described using I2 statistics, which measures the percentage of variability in a meta-analysis that is explained by heterogeneity across groups, and formally tested via Cochran Q tests, which test whether effects are heterogeneous (i.e., small P values indicate effect heterogeneity across groups) (25,26). FDR correction (23) was again applied to meta-analysis and Cochran Q test P values, and FDR-adjusted P values <0.05 were considered statistically significant. Meta-analyses of partial correlation coefficients were performed using Fisher z transformation (27). All statistical analyses were conducted using R (version 3.2.2).
Results
Maternal demographic data are presented in Supplementary Table 1. Mothers spanned the range of maternal glucose levels and BMI observed in HAPO. Analyses examined association of maternal fasting and 1-h metabolites with maternal 1) fasting or 1-h glucose, 2) BMI, and 3) insulin sensitivity.
Maternal Glucose
Association of maternal fasting and 1-h glucose with fasting and 1-h metabolites, respectively, was examined without and with adjustment for maternal BMI. Metabolites significantly associated with these phenotypes after BMI and FDR adjustment are shown in Fig. 1. Supplementary Tables 2–5 provide association data for all metabolites tested.
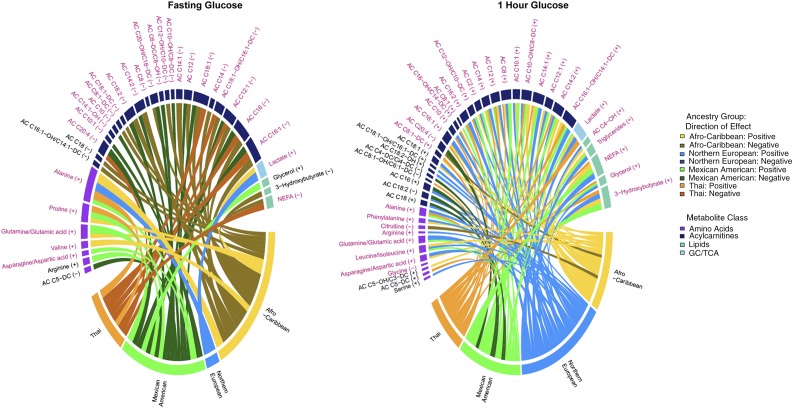
Figure 1.
Chord diagrams of fasting metabolite associations with fasting glucose (left panel) and 1-h metabolite associations with 1-h glucose (right panel) in the four ancestry groups. Fasting and 1-h metabolites associated with fasting and 1-h glucose, respectively, were adjusted for maternal BMI. Metabolites are arranged by metabolite class, and those in purple font were significantly associated with the respective phenotype in the meta-analysis. Metabolites with no connecting chords were significant in the meta-analysis but not in any one cohort. Metabolites in black font were significant in only one or two ancestry groups and not the meta-analysis. The width of the bar under each metabolite is proportional to the strength and number of associations across groups. Individual chords to each metabolite indicate associations that were significant in that ancestry group, with the width of the chord being proportional to the strength of the association. Positive and negative associations are indicated by lighter and darker colors as shown in the legend on the right. GC, glycolysis; TCA, tricarboxylic acid cycle.
In meta-analyses, metabolites associated with fasting and 1-h glucose were largely similar before and after maternal BMI adjustment, although the strength of some associations differed after adjustment. Associations of fasting triglycerides, leucine/isoleucine, arginine, and a long-chain acylcarnitine (C18) with fasting glucose were attenuated by adjusting for BMI (Supplementary Tables 2 and 3). One hour postglucose, a medium-chain acylcarnitine, C14-OH/C12-DC, was associated with 1-h glucose before but not after BMI adjustment (Supplementary Tables 4 and 5).
Metabolites positively associated with maternal fasting and 1-h glucose in meta-analyses before and after maternal BMI adjustment included the gluconeogenic substrates alanine, lactate, glutamine/glutamate, and asparagine/aspartate. Fasting valine, a branched-chain amino acid (BCAA), and proline, a gluconeogenic amino acid, were positively associated with FPG, whereas the BCAA leucine/isoleucine and aromatic amino acid phenylalanine were positively associated with 1-h maternal glucose. Glycine was negatively associated with 1-h glucose, consistent with its known association with insulin sensitivity (28).
In meta-analyses of lipid-related metabolites, NEFA were negatively associated with FPG, whereas both NEFA and triglycerides were positively associated with 1-h glucose. Similarly, several long- and medium-chain acylcarnitines associated with fasting and/or 1-h glucose were negatively associated with FPG and positively associated with 1-h glucose. 3-Hydroxybutyrate and its carnitine ester (C4-OH) were positively associated with 1-h but not fasting glucose. The carnitine ester of arachidonate (C20:4) was negatively associated with both fasting and 1-h glucose.
After BMI adjustment, Q tests did not indicate substantial heterogeneity across the four ancestry groups among metabolites significantly associated with FPG in meta-analysis (Supplementary Table 3). However, some ancestry-specific associations were evident (Fig. 1). At fasting, this included negative association of 3-hydroxybutyrate and a long-chain acylcarnitine in Afro-Caribbeans and positive association of glycerol and arginine and negative association of AC C5-DC, a product of serine/lysine/tryptophan metabolism and/or gut microbial action, in Mexican Americans. For 1-h metabolites, significant heterogeneity was evident for several metabolites after maternal BMI adjustment, notably, lipids, carnitine esters of long-chain fatty acids, and an amino acid (Supplementary Table 5), due largely to varied strengths of association. Ancestry-specific associations of serine and several long- and short-chain acylcarnitines were also present at 1 h.
Maternal BMI
Associations of maternal metabolites with maternal BMI were determined without and with glucose adjustment at the time point corresponding to metabolite measurement. Associations after glucose and FDR adjustment are shown in Fig. 2. Supplementary Tables 6–9 provide association data for all metabolites tested.
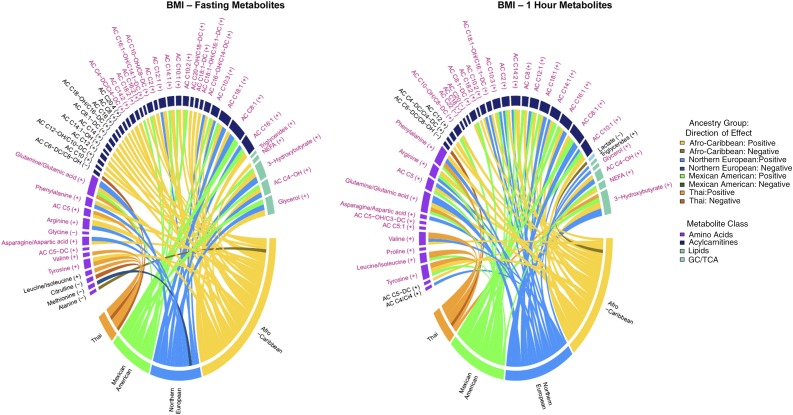
Figure 2.
Chord diagrams of fasting metabolite associations with maternal BMI (left panel) and 1-h metabolite associations with maternal BMI (right panel) in the four ancestry groups. Fasting and 1-h metabolites associated with maternal BMI were adjusted for fasting and 1-h glucose, respectively. Metabolites are arranged by metabolite class, and those in purple font were significantly associated with the respective phenotype in the meta-analysis. Metabolites with no connecting chords were significant in the meta-analysis but not in any one cohort. Metabolites in black font were significant in only one or two ancestry groups and not the meta-analysis. The width of the bar under each metabolite is proportional to the strength and number of associations across groups. Individual chords to each metabolite indicate associations that were significant in that ancestry group, with the width of the chord being proportional to the strength of the association. Positive and negative associations are indicated by lighter and darker colors as shown in the legend on the right. GC, glycolysis; TCA, tricarboxylic acid cycle.
Few differences were observed in metabolites associated with maternal BMI in meta-analyses before and after maternal glucose adjustment. The association of fasting lactate, leucine/isoleucine, and stearoyl carnitine (C18) with maternal BMI was attenuated by maternal FPG adjustment, whereas tyrosine and three carnitine esters, C14:2, C4-DC/Ci4-DC, and C10-OH/C8-DC, were associated with BMI only after FPG adjustment (Supplementary Tables 6 and 7). One hour postglucose, associations of three carnitine esters, C10, C16:2, and C16:1-OH/C14:1-DC, with maternal BMI were attenuated after maternal 1-h glucose adjustment (Supplementary Tables 8 and 9).
Multiple amino acids and their metabolites were associated with maternal BMI in meta-analyses after maternal glucose adjustment. Valine and a BCAA metabolite (C5) were associated at both fasting and 1 h and BCAAs and their carnitine esters (C5:1, C5-OH/C3-DC) at 1 h. Aromatic amino acids phenylalanine and tyrosine along with arginine, asparagine/aspartate, and glutamate/glutamine were positively associated with maternal BMI at fasting and 1 h. Finally, fasting glycine was negatively associated whereas 1-h proline was positively associated with maternal BMI.
Multiple lipid metabolites were associated with maternal BMI in meta-analyses. This included positive association of fasting triglycerides and fasting and 1-h 3-hydroxybutyrate, 3-hydroxybutyryl carnitine (C4-OH), NEFA, and glycerol. At both fasting and 1 h, carnitine esters of several mono- (C18:1 to C8:1) and polyunsaturated (C18:2, C14:2, and C10:2) long- and medium-chain fatty acids were positively associated with maternal BMI. The carnitine esters of several long-chain saturated fatty acids (C22, C20, and C18) were negatively associated and a medium-chain carnitine ester (C8) positively associated with maternal BMI at 1 h but not fasting. Finally, fasting and 1-h acetylcarnitine (C2), a by-product of glucose, amino acid, and fatty acid oxidation important for carnitine cycling in mitochondria, were positively associated with maternal BMI.
In ancestry-specific analyses, the most notable differences in maternal BMI associations after maternal glucose adjustment were in Thais across both time points and Afro-Caribbeans at fasting (Fig. 2 and Supplementary Tables 7 and 9). Overall, Thais showed fewer associations of metabolites with maternal BMI compared with other ancestry groups, with the exception of BCAA, which were positively associated in Thais but weakly or not associated in the other ancestries. The fasting state was notable for positive associations of multiple short- and long-chain acylcarnitines with maternal BMI in Afro-Caribbeans but not other ancestry groups.
Maternal Insulin Sensitivity
Associations of maternal metabolites with maternal insulin sensitivity were determined without and with maternal BMI adjustment at the time point corresponding to metabolite measurement. Associations after BMI and FDR adjustment are shown in Supplementary Fig. 1. Supplementary Tables 10 and 11 provide association data for all metabolites tested.
In meta-analyses, fasting and 1-h BCAA, their carnitine esters, and the aromatic amino acid phenylalanine were associated with maternal insulin resistance before and after BMI adjustment (except for 1-h leucine/isoleucine after BMI adjustment). Tyrosine was associated with insulin resistance only at fasting, and that association was attenuated after BMI adjustment. Glycine, which is positively associated with insulin sensitivity in nonpregnant populations (28,29), was positively associated with insulin sensitivity at fasting and 1 h, although associations were again attenuated at both time points after BMI adjustment. Otherwise, fasting and 1-h levels of gluconeogenic substrates, including alanine, arginine, proline, glutamate/glutamine, aspartate/asparagine, and lactate, were associated with insulin resistance.
Among lipid-related metabolites in meta-analyses, fasting and 1-h triglycerides were associated with insulin resistance, as was 1-h glycerol. 3-Hydroxybutyrate and NEFA were associated with insulin resistance 1 h postglucose, but these associations were attenuated after BMI adjustment. Otherwise, a number of fasting acylcarnitines of medium- and long-chain fatty acids were positively associated with insulin sensitivity; several of these associations were evident only after BMI adjustment.
Ancestry-specific associations were observed before and after adjustment for maternal BMI in all four ancestries. In general, there were more ancestry-specific associations at 1 h compared with fasting, and associations were largely with medium-/long-chain acylcarnitines and amino acid metabolites.
Metabolite Levels Across Ancestry Groups
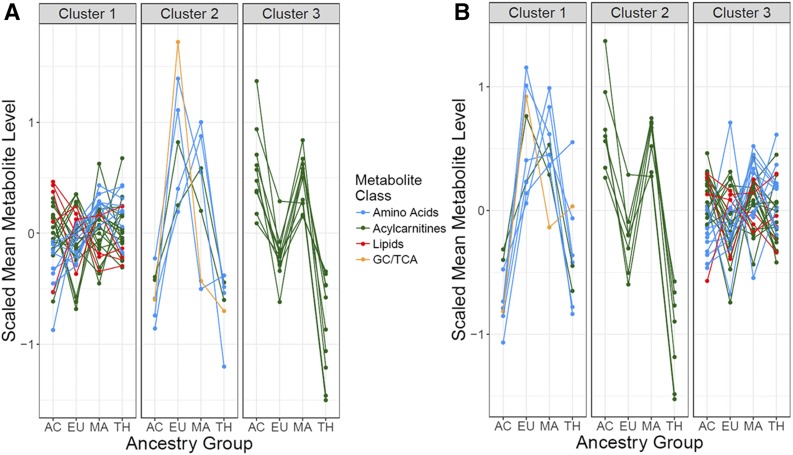
Given the different environments and genetic backgrounds of mothers from the four ancestry groups, subsequent analyses determined whether serum metabolite levels were similar or different across the ancestry groups. Summary statistics for serum levels of all metabolites in each ancestry group are provided in Supplementary Table 12. Patterns of serum levels of maternal metabolites across ancestry groups are shown for maternal metabolites significantly associated with one or more maternal phenotypes in Fig. 3. Three patterns were observed both fasting and 1 h postglucose. Some metabolites, including lactate, glycine, asparagine/aspartate, and phenylalanine together with additional amino acids and long-chain acylcarnitines, had highest levels in Europeans, lower levels in Afro-Caribbeans and Thais, and intermediate levels in Mexican Americans. A second group of medium- and long-chain acylcarnitines had highest levels in Afro-Caribbeans and Mexican Americans, with lower levels in Thais and Europeans. Levels of the third and largest group of metabolites, including amino acids, acylcarnitines, and lipids, were similar in all four ancestry groups.
Figure 3.
K-means cluster plot of metabolite levels in the four ancestry groups. Metabolites are clustered by patterns of variations in levels across ancestry groups, and lines representing different classes of metabolites are indicated in the figure. Fasting (A) and 1-h (B) metabolites present in the different clusters are as follows. A: Cluster 1—amino acids: alanine, arginine, leucine/isoleucine, proline, tyrosine, and valine; acylcarnitines: C2, C4-DC/Ci4-DC, C4-OH, C4/Ci4, C5, C5-DC, C8, C8-OH/C6-DC, C10, C10-OH/C8-DC, C12, C12-OH/C10-DC, C14, C14:1, C16, C16-OH/C14-DC, C16:1, C16:1-OH/C14-DC, C18:1-DC, C18:1-OH/C16:1-DC, and C20-OH/C18-DC; lipids: 3-hydroxybutyrate, glycerol, NEFA, and triglycerides. Cluster 2—amino acids: asparagine/aspartate, glutamine/glutamate, glycine, and phenylalanine; acylcarnitines: C18 and C18:1; glycolysis (GC)/tricarboxylic acid cycle (TCA): lactate. Cluster 3—acylcarnitines: C8:1, C8:1-DC, C10:1, C10:2, C10:3, C12:1, C14:2, C18:2, C20, and C20:4. B: Cluster 1—amino acids: alanine, asparagine/aspartate, glycine, ornithine, and phenylalanine; GC/TCA: lactate. Cluster 2—acylcarnitines: C8:1, C8:1-DC, C10:1, C10:2, C10:3, C12:1, and C18:2. Cluster 3—amino acids: arginine, citrulline, glutamine/glutamate, leucine/isoleucine, proline, tyrosine, and valine; acylcarnitines: C2, C4-OH, C4/Ci4, C5, C5-OH/C3-DC, C8, C10-OH/C8-DC, C12, C12-OH/C10-DC, C14, C14:1, C14:2, C16-OH/C14-DC, C16:1, C16:1-OH/C14-DC, C18:1-DC, C18:1-OH/C16:1-DC, C20-OH/C18-DC, C20, C20:4, and C22; lipids: 3-hydroxybutyrate, glycerol, NEFA, and triglycerides. AC, Afro-Caribbean; EU, European ancestry; MA, Mexican American; TH, Thai.
Unique and Common Metabolites Across Phenotypes
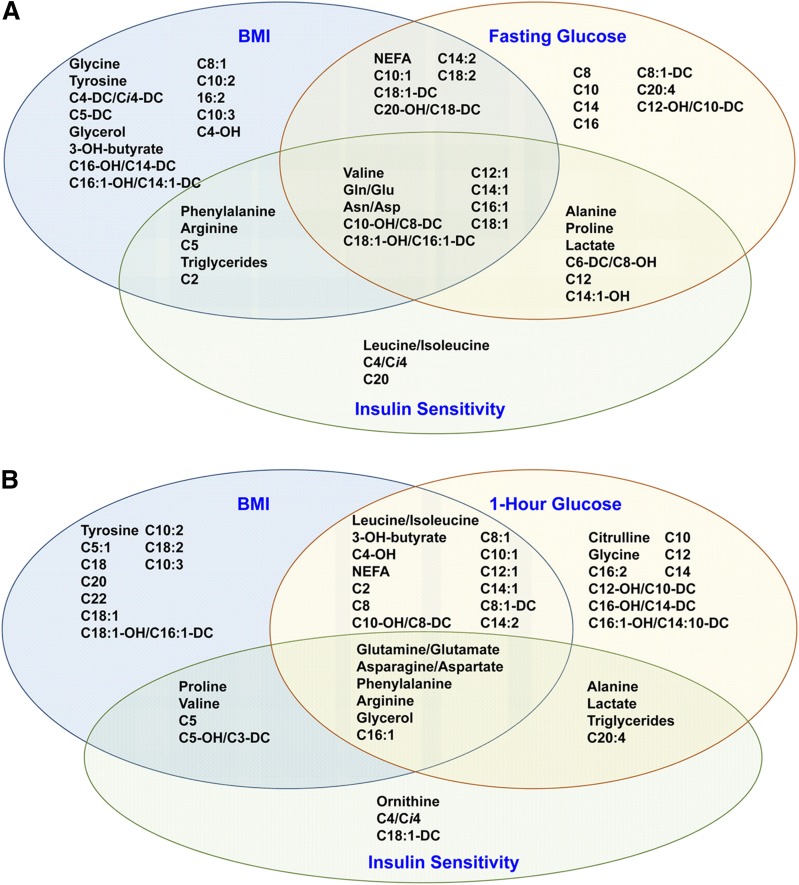
Maternal BMI, insulin sensitivity, and glucose are interrelated phenotypes. To identify common and unique associations, metabolites associated in meta-analyses with maternal glucose adjusted for BMI, BMI adjusted for glucose, and insulin sensitivity adjusted for BMI were considered, and overlap or lack thereof was assessed (Fig. 4).
Figure 4.
Venn diagram of fasting (A) and 1-h (B) metabolites significantly associated with either maternal BMI, fasting or 1-h glucose, and/or insulin sensitivity in a meta-analysis across the four ancestry groups.
At fasting, carnitine esters of several monounsaturated fatty acids (C12:1 to C18:1) were associated with all phenotypes, as were glutamine/glutamate, asparagine/aspartate, and valine. However, these carnitine esters were negatively associated with FPG but positively associated with maternal BMI and insulin resistance. Isoleucine/leucine, carnitine esters of their catabolites, and phenylalanine were associated with insulin resistance or both BMI and insulin resistance. Carnitine esters of arachidonate and several saturated fatty acids were associated with FPG, whereas tyrosine, glycine, and 3-hydroxybutyrate and its carnitine ester, as well as carnitine esters of polyunsaturated fatty acids, were associated with BMI. Overall, BMI demonstrated the most unique associations with fasting metabolites. FPG was associated with an intermediate number of unique metabolites, whereas insulin sensitivity had roughly equal overlap with BMI and glucose.
At 1 h, several metabolites were common across phenotypes, including glycerol, phenylalanine, arginine, glutamine/glutamate, asparagine/aspartate, and the carnitine ester of palmitoleate (Fig. 4B). Additional metabolites were associated with both BMI and 1-h glucose, including 3-hydroxybutyrate and its carnitine ester, BCAAs, and carnitine esters of several saturated and monounsaturated fatty acids. However, a number of metabolites were uniquely associated with either BMI or 1-h glucose. These were largely carnitine esters of fatty acids; however, tyrosine was associated with BMI whereas glycine and citrulline were associated with 1-h glucose. Triglycerides, arachidonoyl carnitine, and gluconeogenic substrates alanine and lactate were associated with 1-h glucose and insulin sensitivity, whereas proline, valine, and carnitine esters generated from BCAA metabolism were associated with maternal BMI and insulin sensitivity.
Partial correlation estimates (rp) were calculated to compare the relative strength of association of different metabolites with maternal phenotypes. In Supplementary Tables 13–18, fasting and 1-h metabolites are presented based upon their relative strength of association according to rp with the indicated phenotype. In the fasting state, gluconeogenic substrates, alanine, lactate, and proline, were among the metabolites most strongly associated with FPG, whereas 3-hydroxybutyrate and its carnitine ester, phenylalanine, and a medium-chain acylcarnitine (C8:1) were most strongly associated with maternal BMI (Supplementary Tables 13 and 14). Triglycerides, lactate, several amino acids (alanine, proline, glutamine/glutamate, arginine, asparagine/aspartate, and phenylalanine), and a BCAA metabolite (C5) were strongly associated with insulin resistance (Supplementary Table 15). At 1 h, 3-hydroxybutyrate was strongly associated with all three phenotypes (Supplementary Tables 16–18). NEFA and lactate exhibited strong association with 1-h glucose and insulin resistance, whereas phenylalanine, glutamine/glutamate, arginine, and the carnitine esters of monounsaturated fatty acids were strongly associated with maternal BMI. Medium- and long-chain carnitine esters were strongly associated with 1-h glucose, whereas triglycerides, glycerol, proline, alanine, glutamine/glutamate, and asparagine/aspartate exhibited strong association with insulin resistance.
Conclusions
Maternal hyperglycemia and obesity are associated with shared and unique adverse pregnancy outcomes due to metabolic disturbances that accompany these traits (5,30). Using targeted metabolomics in a multiancestry cohort of pregnant women, we have demonstrated that maternal BMI and glycemia have metabolic signatures that are, in part, shared but also unique to each phenotype and that these signatures largely remain consistent across ancestry groups. These analyses suggest that metabolites associated uniquely with maternal hyperglycemia or obesity may contribute to their independent effects on newborn outcomes and complement previous work demonstrating associations between the maternal metabolome and newborn outcomes in HAPO European ancestry mothers (20,21).
Fatty acids and their metabolites are important for fetal growth and development (31). Many of the metabolites associated with both maternal BMI and glycemia were lipid related, including NEFA and carnitine esters of several mono- (C10:1 to C18:1) and polyunsaturated fatty acids. Declining levels of free carnitine, acetylcarnitine, and short-chain carnitine esters and stable levels of medium- and long-chain carnitine esters during pregnancy have been reported (32), but their association with maternal phenotypes has not. We observed negative association of fasting NEFA and acylcarnitines with FPG but positive association with BMI. In contrast, 1 h postglucose, many of these same metabolites (e.g., AC C10:1 to C16:1 and NEFA) were positively associated with both maternal BMI and 1-h glucose.
Fatty acid oxidation is a primary source of carnitine esters, and lipolysis and fatty acid oxidation increase as plasma glucose decreases during fasting in pregnancy (2). Thus, the negative association of carnitine esters with FPG may reflect increased fatty acid mobilization and oxidation as glucose declines. Postglucose, greater insulin resistance, reflected by higher maternal BMI and 1-h glucose, may result in higher levels of fatty acids and their metabolic by-products secondary to inefficient suppression of lipolysis and incomplete fatty acid oxidation due to mitochondrial dysfunction (33,34). Mitochondrial dysfunction associated with higher maternal BMI and insulin resistance in the fasting state could also underlie the positive association of maternal BMI with by-products of fatty acid oxidation. One hour postglucose, additional lipid-related metabolites, e.g., glycerol and 3-hydroxybutyrate and its carnitine ester AC C4-OH, were also associated with both phenotypes. Similar to nonpregnant populations (29,35), BCAAs leucine/isoleucine were positively associated with both maternal BMI and 1-h glucose, presumably reflecting the impact of insulin resistance.
A novel aspect of this study is identification of metabolic signatures uniquely associated with maternal BMI and glycemia, including differences in metabolites most strongly associated with these phenotypes. Among metabolites positively associated with both fasting and 1-h glucose but not BMI were gluconeogenic substrates lactate and alanine, consistent with our earlier findings (18,20). Similar to findings with the monounsaturated long-chain acylcarnitines, carnitine esters of several medium- and long-chain saturated fatty acids were negatively associated with FPG and positively associated with 1-h glucose. This association of saturated fatty acid metabolites with maternal glucose is similar to findings in nonpregnant populations where higher levels of ACs C8, C10, and C12 trended with worse dysglycemia (normal vs. impaired FPG or glucose tolerance vs. type 2 diabetes), but no association with percent body fat was observed (36). Another fatty acid metabolite uniquely associated with maternal glycemia was the carnitine ester of arachidonate (AC C20:4). Arachidonate is important for fetal development and derived, in part, from transplacental transfer (31). We found a negative association of its carnitine ester with fasting and 1-h glucose, contrasting with higher levels of fasting arachidonate reported in women with GDM and impaired glucose tolerance (37). One potential explanation for this dichotomy would be impaired maternal arachidonate metabolism in the setting of maternal hyperglycemia resulting in higher arachidonate levels overall but lower levels of its carnitine ester.
Like maternal glucose, maternal BMI also has a unique metabolic signature that includes a number of carnitine esters, although with more mono- and polyunsaturated fatty acids than seen with glucose. Recently, the association of several plasma metabolites with maternal prepregnancy BMI across gestation in a cohort of 167 non-Hispanic and Hispanic ancestry women was reported (10). In the third trimester, associated metabolites included two n-6 long-chain fatty acids and 3-hydroxybutyrate. In contrast to findings in our larger cohort, no acylcarnitines or amino acids, except asparagine, were significantly associated in the third trimester (10). Similar to that report, we found an association of fasting 3-hydroxybutyrate and its carnitine ester with maternal BMI, but we found an association of additional lipid-related metabolites in the third trimester, e.g., glycerol and triglycerides, and amino acids, most notably the aromatic amino acids tyrosine and phenylalanine and by-products of BCAA metabolism (ACs C5:1, C5, and C5-OH/C3-DC). At 1 h, maternal BMI was associated with tyrosine, valine, and BCAA metabolic by-products (ACs C5:1, C5, and C5-OH/C3-DC). Aromatic amino acids together with BCAA are associated with type 2 diabetes risk (38). Metabolites uniquely associated with either maternal glycemia or BMI may account, in part, for the independent effect of these phenotypes on pregnancy outcomes.
We also examined insulin resistance. Maternal BMI, glycemia, and insulin resistance are interrelated; however, unlike maternal BMI and glycemia, insulin resistance is not associated with newborn outcomes after adjusting for maternal BMI (21). Multiple metabolites demonstrated joint association with insulin resistance and either maternal BMI or glycemia, but only fasting leucine/isoleucine and BCAA metabolite AC C4/Ci4 were uniquely associated with maternal insulin resistance. An earlier study demonstrated no association of metabolites with insulin resistance, but cohort size may explain this difference (10).
A second novel aspect of our study was determination of metabolite levels in women of different ancestries and environments who have varying intrinsic (e.g., genetic) and extrinsic (e.g., diet, exercise, and environment) exposures (16). Metabolomic studies across ancestry groups have been limited. In a previous study of urinary metabolites in cohorts from China, Japan, and Chicago, principal components analysis distinguished between metabolite profiles in the three cohorts, consistent with genetic, dietary, and gut microbiome differences across populations (39). A study comparing Hispanic and non-Hispanic pregnant women demonstrated similarities and differences in metabolite levels between the two populations across gestation (32). This is consistent with our findings of differences and similarities in metabolite levels between populations in the third trimester. Despite some differences in levels across ancestry groups, our meta-analyses identified multiple metabolites associated with maternal phenotypes. For example, whereas many metabolites associated with BMI were not associated within the Thai cohort, significance on meta-analysis suggests similar trends among all four cohorts, even when heterogeneity of effect size was detected. These data suggest that associations of maternal BMI, glucose levels, and insulin resistance with maternal metabolites are largely similar regardless of ancestral background and environment, consistent with intrinsic factors having a significant impact on the response of maternal metabolites to pregnancy. Determining whether these are genetic or other factors will require future studies.
Our study had multiple strengths. First, it included four ancestry groups, ensuring that results are applicable to diverse populations. Second, samples obtained both fasting and 1 h after glucose provided insight into two metabolic states. Third, a large sample size from each ancestry group was available. One limitation is that the metabolic signatures associated with the different phenotypes were based on data limited to a single time point early in the third trimester. Levels of some metabolites are dynamic during pregnancy (12,32,40,41), and trimester-specific associations of some metabolites with maternal BMI have been demonstrated (10). A second limitation was use of BMI measured at the OGTT, as this reflects fetal and placental elements together with maternal elements. However, as noted, OGTT BMI showed a 92.4% correlation with calculated pregestational BMI in our cohort.
In summary, consistent with clinical observations that associations of maternal BMI and glucose with adverse pregnancy outcomes are, in part, independent, these two phenotypes are associated with unique sets of metabolites. Although there is clear overlap in classes of metabolites associated with each phenotype, the metabolic signature of maternal glucose is enriched for gluconeogenic substrates whereas maternal BMI is associated with a number of lipid-related metabolites. Importantly, similar to nonpregnant populations, our findings are consistent with pregnancy-induced insulin resistance and its related phenotypes being associated with mitochondrial dysfunction. The relatively consistent associations across ancestry groups and environments suggest a common response of maternal metabolites to the metabolic changes of pregnancy.
Supplementary Material
Article Information
Funding. This study was funded by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK-095963) and from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01-HD-34242 and R01-HD-34243).
Duality of Interest. B.E.M. has received an honorarium from Johnson & Johnson. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. S.J. contributed to study concept and design and interpretation of data. M.N. and A.C.R. contributed to analysis and interpretation of data. J.R.B., M.J.M., R.D.S., O.R.I., L.P.L., B.E.M., and C.B.N. contributed to acquisition and interpretation of data. D.M.S. and W.L.L. made substantial contributions to all project components, including study concept and design, acquisition of data, and analysis and interpretation of data. All authors made critical intellectual contributions to drafting and/or revising the manuscript and all approved the final version. D.M.S. and W.L.L. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. These data have been presented, in part, in abstract form at the 76th Scientific Sessions of the American Diabetes Association, New Orleans, LA, 10–14 June 2016.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc16-2453/-/DC1.
See accompanying article, p. 902.
References
- 1.Butte NF. Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus. Am J Clin Nutr 2000;71(Suppl.):1256S–1261S [DOI] [PubMed] [Google Scholar]
- 2.Hadden DR, McLaughlin C. Normal and abnormal maternal metabolism during pregnancy. Semin Fetal Neonatal Med 2009;14:66–71 [DOI] [PubMed] [Google Scholar]
- 3.Lain KY, Catalano PM. Metabolic changes in pregnancy. Clin Obstet Gynecol 2007;50:938–948 [DOI] [PubMed] [Google Scholar]
- 4.Catalano PM. Trying to understand gestational diabetes. Diabet Med 2014;31:273–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson SM, Matthews P, Poston L. Maternal metabolism and obesity: modifiable determinants of pregnancy outcome. Hum Reprod Update 2010;16:255–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damm P, Houshmand-Oeregaard A, Kelstrup L, Lauenborg J, Mathiesen ER, Clausen TD. Gestational diabetes mellitus and long-term consequences for mother and offspring: a view from Denmark. Diabetologia 2016;59:1396–1399 [DOI] [PubMed] [Google Scholar]
- 7.Catalano PM, McIntyre HD, Cruickshank JK, et al.; HAPO Study Cooperative Research Group . The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes Care 2012;35:780–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.HAPO Study Cooperative Research Group Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) Study: associations with maternal body mass index. BJOG 2010;117:575–584 [DOI] [PubMed] [Google Scholar]
- 9.Metzger BE, Lowe LP, Dyer AR, et al.; HAPO Study Cooperative Research Group . Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991–2002 [DOI] [PubMed] [Google Scholar]
- 10.Hellmuth C, Lindsay KL, Uhl O, et al. Association of maternal prepregnancy BMI with metabolomic profile across gestation. Int J Obes 2017;41:159–169 [DOI] [PubMed] [Google Scholar]
- 11.Huynh J, Xiong G, Bentley-Lewis R. A systematic review of metabolite profiling in gestational diabetes mellitus. Diabetologia 2014;57:2453–2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Q, Würtz P, Auro K, et al. Metabolic profiling of pregnancy: cross-sectional and longitudinal evidence. BMC Med 2016;14:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diaz SO, Barros AS, Goodfellow BJ, et al. Following healthy pregnancy by nuclear magnetic resonance (NMR) metabolic profiling of human urine. J Proteome Res 2013;12:969–979 [DOI] [PubMed] [Google Scholar]
- 14.Pinto J, Barros AS, Domingues MR, et al. Following healthy pregnancy by NMR metabolomics of plasma and correlation to urine. J Proteome Res 2015;14:1263–1274 [DOI] [PubMed] [Google Scholar]
- 15.Suhre K, Gieger C. Genetic variation in metabolic phenotypes: study designs and applications. Nat Rev Genet 2012;13:759–769 [DOI] [PubMed] [Google Scholar]
- 16.Holmes E, Wilson ID, Nicholson JK. Metabolic phenotyping in health and disease. Cell 2008;134:714–717 [DOI] [PubMed] [Google Scholar]
- 17.HAPO Study Cooperative Research Group The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Int J Gynaecol Obstet 2002;78:69–77 [DOI] [PubMed] [Google Scholar]
- 18.Scholtens DM, Muehlbauer MJ, Daya NR, et al.; HAPO Study Cooperative Research Group . Metabolomics reveals broad-scale metabolic perturbations in hyperglycemic mothers during pregnancy. Diabetes Care 2014;37:158–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nesbitt GS, Smye M, Sheridan B, Lappin TR, Trimble ER; HAPO Study Cooperative Research Group . Integration of local and central laboratory functions in a worldwide multicentre study: experience from the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Clin Trials 2006;3:397–407 [DOI] [PubMed] [Google Scholar]
- 20.Scholtens DM, Bain JR, Reisetter AC, et al.; HAPO Study Cooperative Research Group . Metabolic networks and metabolites underlie associations between maternal glucose during pregnancy and newborn size at birth. Diabetes 2016;65:2039–2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandler V, Reisetter AC, Bain JR, et al. Associations of maternal BMI and insulin resistance with the maternal metabolome and newborn outcomes. Diabetologia 2017;60:518–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charrad M, Ghazzali N, Boiteau V, Niknafs A. NBClust: an R package for determining the relevant number of clusters in a data set. J Stat Softw 2014;61:1–36 [Google Scholar]
- 23.Benjamini Y, Hochberg Y. Controlling the false discovery rate - a practical and powerful approach to multiple testing. J R Stat Soc B 1995;57:289–300 [Google Scholar]
- 24.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010;36:1–48 [Google Scholar]
- 25.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–1558 [DOI] [PubMed] [Google Scholar]
- 26.Cochran WG. The combination of estimates from different experiments. Biometrics 1954;10:101–129 [Google Scholar]
- 27.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. Chichester, West Sussex, U.K., John Wiley & Sons, Ltd., 2009 [Google Scholar]
- 28.Gall WE, Beebe K, Lawton KA, et al.; RISC Study Group . Alpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS One 2010;5:e10883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009;9:311–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hod M, Kapur A, Sacks DA, et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on gestational diabetes mellitus: a pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet 2015;131(Suppl. 3):S173–S211 [DOI] [PubMed] [Google Scholar]
- 31.Haggarty P. Fatty acid supply to the human fetus. Annu Rev Nutr 2010;30:237–255 [DOI] [PubMed] [Google Scholar]
- 32.Lindsay KL, Hellmuth C, Uhl O, et al. Longitudinal metabolomic profiling of amino acids and lipids across healthy pregnancy. PLoS One 2015;10:e0145794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koves TR, Ussher JR, Noland RC, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 2008;7:45–56 [DOI] [PubMed] [Google Scholar]
- 34.Muoio DM. Metabolic inflexibility: when mitochondrial indecision leads to metabolic gridlock. Cell 2014;159:1253–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab 2012;15:606–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mai M, Tönjes A, Kovacs P, Stumvoll M, Fiedler GM, Leichtle AB. Serum levels of acylcarnitines are altered in prediabetic conditions. PLoS One 2013;8:e82459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen X, Scholl TO, Leskiw M, Savaille J, Stein TP. Differences in maternal circulating fatty acid composition and dietary fat intake in women with gestational diabetes mellitus or mild gestational hyperglycemia. Diabetes Care 2010;33:2049–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guasch-Ferré M, Hruby A, Toledo E, et al. Metabolomics in prediabetes and diabetes: a systematic review and meta-analysis. Diabetes Care 2016;39:833–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dumas ME, Maibaum EC, Teague C, et al. Assessment of analytical reproducibility of 1H NMR spectroscopy based metabonomics for large-scale epidemiological research: the INTERMAP Study. Anal Chem 2006;78:2199–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maitre L, Villanueva CM, Lewis MR, et al. Maternal urinary metabolic signatures of fetal growth and associated clinical and environmental factors in the INMA study. BMC Med 2016;14:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang M, Liang Q, Li H, et al. Normal pregnancy-induced amino acid metabolic stress in a longitudinal cohort of pregnant women: novel insights generated from UPLC-QTOFMS-based urine metabolomic study. Metabolomics 2016;12:131 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.