Abstract
OBJECTIVE
Our aim was to study the relation between the duration of full and any breastfeeding and risk of type 1 diabetes.
RESEARCH DESIGN AND METHODS
We included two population-based cohorts of children followed from birth (1996–2009) to 2014 (Denmark) or 2015 (Norway). We analyzed data from a total of 155,392 children participating in the Norwegian Mother and Child Cohort Study (MoBa) and the Danish National Birth Cohort (DNBC). Parents reported infant dietary practices when their child was 6 and 18 months old. The outcome was clinical type 1 diabetes, ascertained from nationwide childhood diabetes registries. Hazard ratios (HRs) were estimated using Cox regression.
RESULTS
Type 1 diabetes was identified in 504 children during follow-up, and the incidence of type 1 diabetes per 100,000 person-years was 30.5 in the Norwegian cohort and 23.5 in the Danish cohort. Children who were never breastfed had a twofold increased risk of type 1 diabetes compared with those who were breastfed (HR 2.29 [95% CI 1.14–4.61] for no breastfeeding vs. any breastfeeding for ≥12 months). Among those who were breastfed, however, the incidence of type 1 diabetes was independent of duration of both full breastfeeding (HR per month 0.99 [95% CI 0.97–1.01]) and any breastfeeding (0.97 [0.92–1.03]).
CONCLUSIONS
Suggestive evidence supports the contention that breastfeeding reduces the risk of type 1 diabetes. Among those who were breastfed, however, no evidence indicated that prolonging full or any breastfeeding was associated with a reduced risk of type 1 diabetes.
Introduction
Type 1 diabetes is a common disease in childhood, and the Nordic countries have some of the highest incidence rates in the world. Type 1 diabetes results from immune-mediated destruction of pancreatic β-cells, eventually leading to complete and lifelong dependence on exogenous insulin (1). Although genetic susceptibility variants play a role in the development of type 1 diabetes, increased incidence over the past 50 years strongly suggests an important role for nongenetic factors (2–4). The hypothesis that breastfeeding could protect against type 1 diabetes was proposed more than 30 years ago, supported by ecological and retrospective case-control data from Denmark and Norway (5). Human milk contains biologically active substances, including antibodies, cytokines, and hormones, that may influence the infant immune system (6). A number of biologically plausible mechanisms have been hypothesized for the potential protective effect of breast milk against type 1 diabetes, such as protection against potentially diabetogenic infections, postponed exposure to dietary antigens including cow’s milk, healthier infant gut microbiota, and optimal maturation of the infant gut (7).
Breastfeeding has been associated with a number of positive health outcomes, and a possible protective effect against type 1 diabetes in children has been cited as evidence for the importance of breastfeeding (8,9). Previous systematic reviews and meta-analyses of observational studies have suggested that breastfeeding more than 3 months (10,11), and exclusive breastfeeding for more than 2 weeks (12), are associated with an approximately 15–30% lower risk of type 1 diabetes. However, data were almost exclusively from case-control studies (8,10–12) and therefore susceptible to recall bias and selection bias. Recent prospective studies of genetically susceptible children have had limitedvsample sizes and inconsistent results (13–15). Therefore, the role of breastfeeding, and whether an optimal duration of breastfeeding exists with respect to risk of type 1 diabetes, is unclear. We set out to investigate whether an association exists between breastfeeding duration and risk of development of type 1 diabetes in two of the world’s largest birth cohorts from Norway and Denmark.
Research Design and Methods
Participants and Design
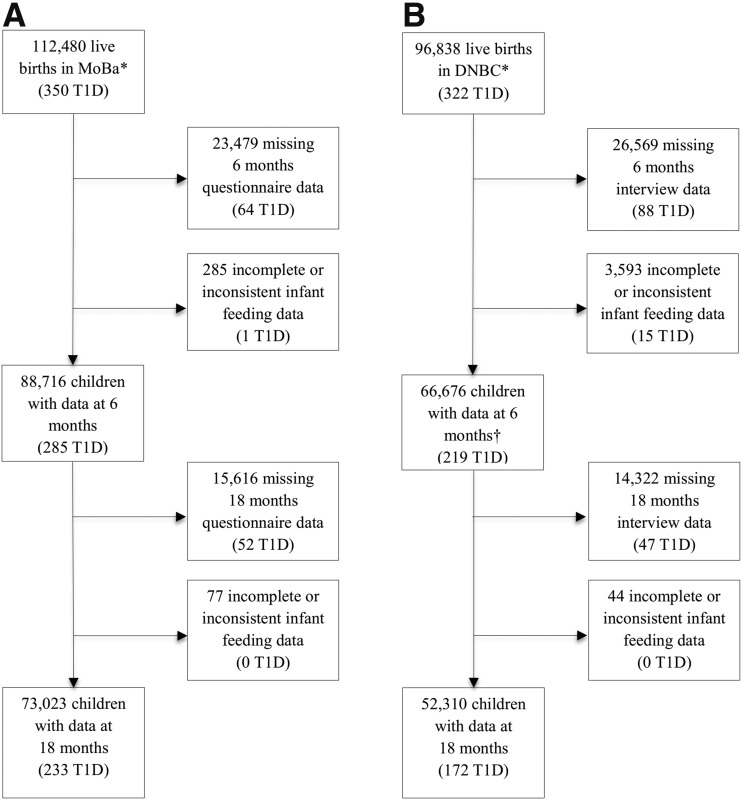
We used information from two population-based pregnancy cohorts, the Norwegian Mother and Child Cohort Study (MoBa) (16) and the Danish National Birth Cohort (DNBC) (17). MoBa participants were recruited across Norway from 1999 to 2008, and DNBC participants were recruited across Denmark from 1996 to 2003. A total of 155,392 children, of whom 504 developed type 1 diabetes, were eligible for the study (Fig. 1). Participants in both cohorts gave written informed consent. The establishment of and data collection in MoBa were approved by the Norwegian Data Inspectorate and the Regional Committee for Medical Research Ethics. DNBC was approved by the Danish Data Protection Agency and the National Committee on Biomedical Research Ethics.
Figure 1.
Flowchart showing participants from the MoBa (A) and the DNBC (B) included in the analysis. *In MoBa, 95,200 mothers participated during one or more pregnancies (40.6% of eligible pregnancies). In DNBC, 91,326 mothers participated during one or more pregnancies (about 30% of eligible mothers). †The DNBC interview when the infants were 6 months old did not include detailed questions about age at introduction of formula until April 2000, resulting in missing data for age at introduction of formula and thus also for duration of full breastfeeding for 12,402 of the 66,676 children whose mothers completed the 6-month interview (including 48 children with type 1 diabetes [T1D]).
Assessment of Breastfeeding and Infant Diet
Information about infant feeding was derived from mailed questionnaires administered to the mothers in MoBa and from structured telephone interviews of the mothers in DNBC; questionnaires and interviews were administered when the children were 6 and 18 months old. Information about breastfeeding in MoBa was derived from three main questions in the questionnaire, administered when the infants were aged 6 months. The first question asked what the child had to drink during the first week after birth; the options were breast milk, water, sugar water, formula, other, and don’t know. The second question, which was also included in the questionnaire administered at age 18 months, asked what kind of milk the child received during the first 6 and 18 months of life. The possible answers were breast milk, different types of formula, and other milk. The answers were reported at monthly intervals for the first 6 months and at 3-month intervals thereafter. For both the first and the second questions, more than one type of drink could be marked. The third question asked when the child was introduced to solid food, with a total of 17 different alternative responses.
Information about breastfeeding in DNBC was derived from four main questions in the interview administered at age 6 months. The first question was also included in the interview administered at age 18 months and asked whether the mother was currently breastfeeding. If not currently breastfeeding, she was asked whether the child was ever breastfed and, if yes, when the mother stopped breastfeeding her child. The second, third, and fourth questions asked when the child was introduced to different types of formula, other milk, and solid food, respectively. The answers were reported in months, weeks, and days.
Definitions of Breastfeeding
Full breastfeeding means that the infant received breast milk, allowing water-based drinks in addition to vitamin supplements, but not formula, solid foods, or semisolid foods. Any breastfeeding means that the infant received breast milk, regardless of complementary feeding.
Assessment of Study End Point: Type 1 Diabetes
We used time to clinical diagnosis of type 1 diabetes as the outcome. Data on type 1 diabetes in MoBa was obtained from the Norwegian Childhood Diabetes Registry (4) and the Norwegian Patient Registry. We linked DNBC to the Danish Childhood Diabetes Registry (18). In total, for the two cohorts, 504 children with type 1 diabetes with information on infant feeding at 6 months of age were identified during follow-up (Fig. 1).
Other Variables
We included as covariates in regression models parent and child characteristics that might influence both infant feeding practices and development of type 1 diabetes: parental type 1 diabetes (for DNBC, only maternal diabetes of any type), infant sex, mode of delivery, infant birth weight, gestational age at delivery, and the mother’s parity, age at delivery, education, smoking during pregnancy, and BMI before pregnancy. Parental characteristics were obtained from the baseline questionnaire at week 18 of pregnancy in MoBa, and from interviews at weeks 12 and 30 of pregnancy and at infant age 18 months in DNBC. Maternal type 1 diabetes was obtained from the Norwegian Patient Registry and the questionnaire at week 18 of pregnancy in MoBa, and maternal diabetes of any type was identified from the interview at week 12 of pregnancy in DNBC. In addition, paternal type 1 diabetes was available from the Norwegian Patient Registry for the MoBa cohort. Information on infant sex, mode of delivery, and infant birth weight was obtained from the national medical birth registries. Information on gestational age in MoBa was obtained from the Medical Birth Registry of Norway, and in DNBC from the interview at infant age 6 months.
Data Analysis
We used Cox regression analysis to estimate hazard ratios (HRs) with 95% CIs within each cohort. Follow-up time was counted from date of birth to the first date of type 1 diabetes diagnosis or the end of follow-up (9 December 2015 in MoBa and 6 November 2014 in DNBC). We used robust cluster variance estimation to correct for within-family correlation. We assessed and tested the proportional hazard assumption of the Cox model using Schoenfeld residuals. Results from the two cohorts were combined using a random effects model (the Metan procedure in Stata software version 14). We defined statistical significance as P < 0.05, or 95% CIs for the HR not including 1.00. Heterogeneity of associations between the two cohorts was examined using the Cochran Q test. In a sensitivity analysis, we assessed the impact of missing data using multiple imputation, as described in the Supplementary Data.
Results
Cohort characteristics are shown in Table 1. The mean age of the children at the end of follow-up was 10.2 years (range 0.7–15.9) in MoBa and 14.0 years (range 0.9–16.7) in DNBC. The mean age at clinical diagnosis of type 1 diabetes was 6.7 years (range 0.7–14.1) in MoBa, and 8.5 years (range 0.9–15.8) in DNBC. The overall incidence of type 1 diabetes per 100,000 person-years was 30.5 in MoBa and 23.5 in DNBC. The incidence of type 1 diabetes among participants excluded because of insufficient information on infant diet did not differ from the incidence among those included in this analysis (Supplementary Fig. 1). Furthermore, participants with and without complete information were generally similar with regard to background characteristics, except that mothers without complete information in MoBa had lower education and parity, and more frequently smoked during pregnancy than mothers with complete information in MoBa. In DNBC, children without complete information had a lower birth weight and were more likely to be born via cesarean delivery (Supplementary Table 1).
Table 1.
Characteristics of mothers and infants in the MoBa and the DNBC
| Characteristics | MoBa (n = 88,716) | DNBC (n = 66,676) |
|---|---|---|
| Mothers | ||
| Age (years), mean (SD) | 30.3 (4.5) | 30.5 (4.3) |
| Missing, n | 0 | 36 |
| History of diabetes* | ||
| No | 87,756 (98.9) | 63,844 (99.7) |
| Yes | 960 (1.1) | 210 (0.3) |
| Missing, n | 0 | 2,622 |
| Education† | ||
| >4 years of college (MoBa)/≥high school (DNBC) | 22,475 (25.6) | 34,120 (66.6) |
| ≤4 years of college (MoBa)/vocational training (DNBC) | 37,082 (42.3) | 10,868 (21.2) |
| <College (MoBa)/≤10th grade (DNBC) | 28,127 (32.1) | 6,272 (12.2) |
| Missing, n | 1,032 | 15,416 |
| Smoking during pregnancy | ||
| No | 80,445 (92.4) | 49,403 (74.1) |
| Yes | 6,620 (7.6) | 17,268 (25.9) |
| Missing, n | 1,651 | 5 |
| BMI before pregnancy (kg/m2) | ||
| <20.0 | 10,702 (12.5) | 10,319 (16.4) |
| 20.0–24.9 | 48,123 (56.2) | 34,986 (55.4) |
| 25.0–29.9 | 18,636 (21.8) | 12,458 (19.7) |
| ≥30.0 | 8,139 (9.5) | 5,357 (8.5) |
| Missing, n | 3,116 | 3,556 |
| Parity | ||
| 0 | 41,882 (47.2) | 29,581 (46.1) |
| 1 | 30,507 (34.4) | 23,814 (37.2) |
| ≥2 | 16,327 (18.4) | 10,712 (16.7) |
| Missing, n | 0 | 2,569 |
| Infants | ||
| Birth weight (g) | ||
| <3,000 | 11,743 (13.2) | 7,467 (11.3) |
| 3,000–3,499 | 25,424 (28.7) | 19,237 (29.0) |
| 3,500–3,999 | 32,216 (36.3) | 24,105 (36.3) |
| ≥4000 | 19,317 (21.8) | 15,573 (23.4) |
| Missing, n | 16 | 294 |
| Gestational age (weeks) | ||
| <37 | 5,311 (6.0) | 1,846 (3.8) |
| ≥37 | 83,362 (94.0) | 46,850 (96.2) |
| Missing, n | 43 | 17,980 |
| Mode of delivery | ||
| Vaginal delivery | 75,675 (85.3) | 56,139 (84.5) |
| Cesarean delivery | 13,041 (14.7) | 10,262 (15.5) |
| Missing, n | 0 | 275 |
| Sex | ||
| Female | 43,307 (48.8) | 32,676 (49.0) |
| Male | 45,409 (51.2) | 34,000 (51.0) |
| Missing, n | 0 | 0 |
Data are n (%) unless otherwise indicated. Percentage values are column percentages among those with nonmissing data for each variable.
*MoBa reported type 1 diabetes in both mothers and fathers. DNBC reported maternal diabetes only (diabetes of any type).
†DNBC did not have detailed data on maternal education after high school.
Breastfeeding was never initiated for 0.8% of MoBa participants and 2.4% of DNBC participants. Children who were never breastfed had lower birth weight and younger gestational age, and were more likely to be born via cesarean delivery than children who were breastfed. Moreover, their mothers had less education and were more likely to be obese (Supplementary Table 2). Full breastfeeding for 6 months or more was reported for 13.8% of infants in MoBa and 6.3% of infants in DNBC, whereas any breastfeeding for 12 months or more was reported for 38.5% of infants in MoBa and 20.2% of infants in DNBC (Supplementary Table 3).
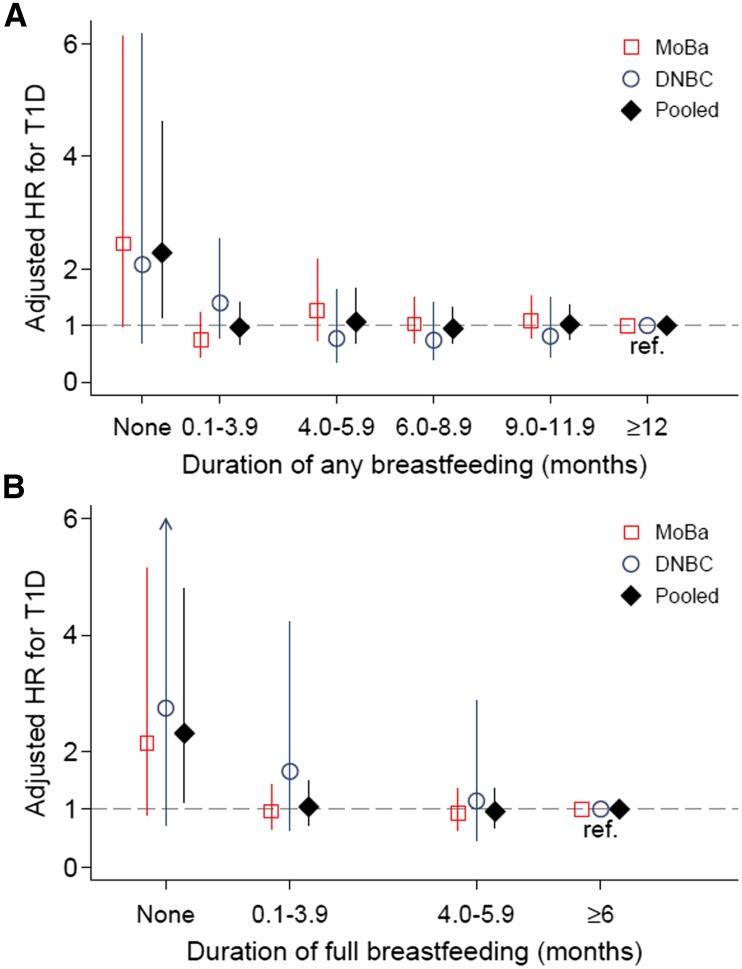
Never being breastfed was associated with a twofold higher risk for developing type 1 diabetes compared with those who were breastfed (HR 2.29 [95% CI 1.14–4.61] for no breastfeeding compared with any breastfeeding for ≥12 months; HR 2.31 [95% CI 1.11–4.80] for no breastfeeding compared with full breastfeeding for ≥6 months) (Fig. 2). However, we found no difference in type 1 diabetes risk by duration of either full or any breastfeeding for those who initiated at least some breastfeeding. The pooled HR (trend) per month was not significant for full breastfeeding (0.99 [95% CI 0.97–1.01]) or any breastfeeding (0.97 [95% CI 0.92–1.03]). Because of the nonlinearity (apparent threshold effect for no breastfeeding), we also estimated (post hoc) the trend in risk of type 1 diabetes per month increase in breastfeeding after excluding those not breastfed. This gave essentially unchanged trend estimates but higher P values (P > 0.70) (Supplementary Table 3).
Figure 2.
Association of duration of any breastfeeding (A) and full breastfeeding (B) with risk of type 1 diabetes (T1D) in the MoBa and DNBC. The vertical lines show the 95% CIs. The upper confidence limit was 10.4 (arrow in panel B). HRs were adjusted for the following covariates: parental type 1 diabetes, infant sex, mode of delivery, infant birth weight, and gestational age at delivery, as well as the mother’s parity, age at delivery, education, smoking during pregnancy, and BMI before pregnancy. Exact numbers and incidence rates are shown in Supplementary Table 3. A total of 14 children had type 1 diabetes among those with no breastfeeding, reflecting the relatively wide CIs for this group. The pooled trend-adjusted HR per month difference in any breastfeeding was 0.99 (95% CI 0.97–1.01) overall and 0.99 (95% CI 0.97–1.01; P = 0.89) after excluding those not breastfed at all. The pooled trend-adjusted HR per month difference in full breastfeeding was 0.97 (95% CI 0.92–1.03) overall and 0.99 (95% CI 0.94–1.05; P = 0.73) after excluding those not breastfed at all.
Furthermore, we found no associations of early or late introduction of formula or solid foods with type 1 diabetes (Table 2). Breastfeeding while introducing solid foods was also not associated with type 1 diabetes (Table 2).
Table 2.
Infant dietary exposure characteristics and risk of type 1 diabetes in the MoBa and the DNBC
| Characteristics | Total | Developed T1D | Incidence per 100,000 PY | Unadjusted HR (95% CI) | Adjusted HR (95% CI)* | P for trend |
|---|---|---|---|---|---|---|
| Age at introduction of formula (months) | ||||||
| MoBa, n | 88,716 | 285 | ||||
| <4 | 33,257 (37.5) | 119 (41.8) | 35.7 | 1.22 (0.95–1.56) | 1.10 (0.84–1.43) | |
| 4.0–5.9 | 9,827 (11.1) | 29 (10.2) | 28.8 | 0.97 (0.65–1.45) | 0.98 (0.65–1.48) | |
| ≥6 | 45,632 (51.4) | 137 (48.1) | 29.5 | 1 | 1 | |
| Missing, n | 0 | 0 | ||||
| Per month† | 1.01 (0.98–1.03) | 0.70 | ||||
| DNBC, n | 66,676 | 219 | ||||
| <4 | 15,528 (28.6) | 66 (38.6) | 31.8 | 1.41 (1.00–1.98) | 1.37 (0.87–2.17) | |
| 4.0–5.9 | 17,143 (31.6) | 36 (21.1) | 15.6 | 0.69 (0.46–1.03) | 0.67 (0.41–1.12) | |
| ≥6 | 21,603 (39.8) | 69 (40.4) | 23.0 | 1 | 1 | |
| Missing, n | 12,402 | 48 | ||||
| Per month† | 0.93 (0.85–1.01) | 0.10 | ||||
| Pooled‡ | ||||||
| <4 | 48,785 (34.1) | 185 (40.6) | 1.28 (1.05–1.57) | 1.16 (0.92–1.46) | ||
| 4.0–5.9 | 26,970 (18.9) | 65 (14.3) | 0.82 (0.62–1.09) | 0.84 (0.61–1.16) | ||
| ≥6 | 67,235 (47.0) | 206 (45.2) | 1 | 1 | ||
| Per month† | 1.00 (0.98–1.03) | |||||
| Age at introduction of any solid food (months)§ | ||||||
| MoBa, n | 88,716 | 285 | ||||
| <4 | 5,344 (6.0) | 15 (5.3) | 26.4 | 0.78 (0.44–1.37) | 0.73 (0.40–1.32) | |
| 4.0–5.9 | 66,703 (75.2) | 214 (75.1) | 31.8 | 0.96 (0.71–1.28) | 0.90 (0.66–1.23) | |
| ≥6 | 16,669 (18.8) | 56 (19.6) | 33.2 | 1 | 1 | |
| Missing, n | 0 | 0 | ||||
| Per month† | 1.04 (0.91–1.19) | 0.57 | ||||
| DNBC, n | 66,676 | 219 | ||||
| <4 | 4,985 (7.5) | 26 (11.9) | 36.9 | 2.37 (1.22–4.61) | 2.52 (0.85–7.51) | |
| 4.0–5.9 | 55,589 (83.4) | 180 (82.2) | 23.2 | 1.49 (0.85–2.62) | 1.90 (0.76–4.71) | |
| ≥6 | 6,091 (9.1) | 13 (5.9) | 15.5 | 1 | 1 | |
| Missing, n | 11 | 0 | ||||
| Per month† | 0.81 (0.62–1.05) | 0.11 | ||||
| Pooled‡ | ||||||
| <4 | 10,329 (6.6) | 41 (8.1) | 1.25 (0.81–1.92) | 0.97 (0.58–1.64) | ||
| 4.0–5.9 | 122,292 (78.7) | 394 (78.2) | 1.06 (0.81–1.37) | 0.97 (0.72–1.31) | ||
| ≥6 | 22,760 (14.6) | 69 (13.7) | 1 | 1 | ||
| Per month† | 0.99 (0.88–1.11) | |||||
| Breastfeeding while introducing any solid food | ||||||
| MoBa, n | 88,716 | 285 | ||||
| Yes | 63,850 (87.6) | 204 (87.5) | 31.4 | 0.97 (0.66–1.45) | 0.85 (0.55–1.30) | |
| No | 9,023 (12.4) | 29 (12.5) | 31.3 | 1 | 1 | |
| Missing, n | 15,843 | 52 | ||||
| DNBC, n | 66,676 | 219 | ||||
| Yes | 42,412 (69.1) | 131 (63.6) | 22.0 | 0.77 (0.58–1.02) | 0.73 (0.47–1.11) | |
| No | 18,932 (30.9) | 75 (36.4) | 28.5 | 1 | 1 | |
| Missing, n | 5,332 | 13 | ||||
| Pooled‡ | ||||||
| Yes | 106,262 (79.2) | 335 (76.3) | 0.83 (0.66–1.05) | 0.79 (0.58–1.07) | ||
| No | 27,955 (20.8) | 104 (23.7) | 1 | 1 |
Data are n (%) unless otherwise indicated.
*Adjusted for sex, birth weight, gestational age, cesarean delivery, parity, maternal smoking during pregnancy, maternal age, maternal BMI, and parental diabetes.
†Age at introduction of any solid foods after 6 months was set to 6.5 months in trend analysis using continuous months. Age at introduction of other milk after 18 months was set to 18.5 months.
‡No significant heterogeneity existed between the studies for any of the comparisons (all P values >0.05).
§In the MoBa cohort, no significant associations were found between type 1 diabetes (T1D) and age at introduction of or breastfeeding while introducing the following food groups: cereals, gluten-containing food, vegetables, meat/fish, and fruits/berries (data not shown). DNBC did not have information on specific food items. PY, person-years.
Sensitivity Analyses
The potential impact of missing data on our results was assessed using multiple imputation, as described in the Supplementary Data. Multiple imputation did not change any of the conclusions (Supplementary Fig. 2). Excluding children with a parent affected by diabetes also did not change the conclusion based on the entire sample (Supplementary Table 4). MoBa and DNBC consist mainly, but not exclusively, of ethnic Norwegians and Danes, respectively. For participants in MoBa, we repeated the main analyses after adjusting for ethnic group (ascertained by reported parental native language), and this did not change the conclusions (Supplementary Table 5). Information on ethnicity was not available in DNBC.
Conclusions
Our key findings are that children never breastfed had a twofold increased risk for developing type 1 diabetes, and that—perhaps most important—no clinically relevant difference in risk was found for those with different durations of either full or any breastfeeding among those who ever breastfed.
Comparison With Previous Studies
The majority of previous population-based studies of breastfeeding duration and risk of type 1 diabetes have been retrospective case-control studies (12), and the few population-based prospective studies (19–21) included few children with type 1 diabetes or had very limited information about breastfeeding (see Supplementary Table 6 for an overview of previous studies in the field with type 1 diabetes as the outcome). While a 2012 meta-analysis of mainly retrospective case-control studies suggested an approximately 15–30% reduced risk of type 1 diabetes in those breastfed for more than 3 months or exclusively breastfed for more than 2 weeks (12), we focused our comparison with previous publications on cohort studies, which are less prone to biases. Some evidence for associations of breastfeeding duration with type 1 diabetes have been found in genetically high-risk birth cohorts (13), but others did not find any such association (14,15). These prospective studies following children with genetic or familial risk included only a limited number of children who developed type 1 diabetes, compared with our study, and did not study the group who did not breastfeed. No significant associations have been reported between breastfeeding duration and islet autoimmunity, a surrogate end point for type 1 diabetes, in genetically high-risk birth cohorts (13,14,22,23). (See Supplementary Table 7 for a brief overview of published cohort studies of breastfeeding and islet autoimmunity.) The largest of the latter studies, Diabetes Prediction and Prevention (DIPP), reported suggestive but nonsignificant associations between duration of breastfeeding and islet autoimmunity, with HRs around 1.4 when comparing the lower with the upper third of the cohort distribution of breastfeeding duration (22). The trend estimates in our study can be used to compute HRs with CIs for different contrasts in breastfeeding duration. For a contrast of 3 months of any breastfeeding (e.g., comparing 9 and 12 months’ duration), the adjusted HR would be 0.97 (95% CI 0.82–1.15). For full breastfeeding, a 3-month difference (e.g., comparing 6 with 3 months’ duration) would give a model-based adjusted HR of 0.97 (95% CI 0.90–1.04). Because our 95% CIs for the trend analyses largely excluded relative risks of magnitudes suggested by previous retrospective or prospective studies, our results are not compatible with previously published results.
In line with previous population-based studies (13,15), we found no evidence for an association between age at introduction of formula and development of type 1 diabetes. This is also concurrent with results from the randomized Trial to Reduce IDDM in the Genetically at Risk (TRIGR) study, showing that weaning to a highly hydrolyzed formula, compared with regular cow’s milk formula, was not associated with a decreased risk of multiple islet autoantibodies (24). It should, however, be noted that the intervention in the TRIGR study did not directly address the effect of breastfeeding or intake of other complementary foods.
Only a few cohorts of genetically susceptible children have been analyzed with regard to age at introduction of solids in relation to type 1 diabetes (see the Supplementary Data for an overview of previous studies in the field). DNBC showed a tendency toward a higher risk for those who received solid foods before age 6 months, although this was not significant and not seen in MoBa or in the pooled analysis, supporting the suggestion that the DNBC results may have been due to chance. The lack of association between age at introduction of any solid food, or breastfeeding while introducing any solid food, and development of type 1 diabetes is in line with results from the Environmental Triggers of Type 1 Diabetes (MIDIA) study (13). However, introduction to any solid food before 4 months and after 6 months of age predicted development of type 1 diabetes in the Diabetes Autoimmunity Study in the Young (DAISY) (15). Breastfeeding while introducing wheat/barley was also associated with a lower risk of developing type 1 diabetes, but breastfeeding while introducing other solid foods was not (15). We found no association between the age at introduction of specific solid foods, including vegetables and fruits and berries, or breastfeeding while introducing specific solid foods, and type 1 diabetes. Virtanen et al. (22) reported from the DIPP study a significant association between early introduction of root vegetables and risk of islet autoimmunity, but they included no separate analysis with type 1 diabetes as the end point. Cultural differences in infant feeding practices could influence the ability to directly compare results between the different prospective studies. In line with Nordic nutrition recommendations (25), few of the infants in our study were introduced to any solid food before age 4 months (6.0% in MoBa and 7.5% in DNBC), in contrast to 43.0% in DAISY from Colorado (15). Our results are probably generalizable to many other industrialized countries, but may not be applicable to populations of non-European origin.
Strengths and Weaknesses
The main strengths of the study are the prospective design, large sample size, linkage to national registries with a high level of ascertainment, and inclusion of two independent cohorts with consistent results. Missing data on infant feeding was mainly the result of a loss to follow-up, as occurs in all cohorts based on voluntary participation and questionnaires or interviews. The similar risk of type 1 diabetes in those with or without complete infant feeding data, combined with multiple imputation analysis, suggest that bias resulting from missing data was not a serious problem. Relatively few children were not breastfed at all, and some uncertainty existed around the HR estimate for this specific group, which calls for a careful interpretation of this particular finding. While infant feeding practices were recorded typically many years before the children developed type 1 diabetes (no children developed type 1 diabetes before 6 months of age), the mothers inevitably had to recall their practices used a few months before the actual assessment. MoBa had information about type 1 diabetes in both the mother and the father, whereas DNBC had information about maternal diabetes of any type. While maternal type 1 diabetes is a potential confounder because it is associated with both the child’s risk of type 1 diabetes and possibly infant feeding, its actual impact as a confounder of our results is likely to be minor because maternal type 1 diabetes was very rare (<0.5% of the cohort). The study is observational, so we cannot exclude the possibility that unmeasured confounders influenced the results. On the other hand, no established environmental etiological factors are obvious confounders. While only a randomized trial design can eliminate confounding, it is difficult to imagine, for ethical and logistical reasons, a randomized controlled trial of breastfeeding duration with sufficient power to detect effects on type 1 diabetes.
Potential Mechanisms and Implications for Policy
Assuming the higher risk of type 1 diabetes in those who were not breastfed compared with those who were breastfed was not a result of bias or confounding, a possible interpretation of our findings is that substances in the colostrum mediate a protective effect. Colostrum is secreted the first 3–5 days after birth and contains high concentrations of immune factors, which decrease in mature milk (26). Breastfeeding could lead to a healthier infant gut microbiota profile at an early stage of life compared with infants who are never breastfed (27,28). Preliminary observations indicate that a difference in the gut microbiome exists between children with autoimmunity or type 1 diabetes and healthy children (29).
The finding with the most important implications for policy and public health is perhaps the lack of an association between the duration of either full or any breastfeeding and risk of type 1 diabetes. A possible preventive effect of a longer duration of breastfeeding is sometimes cited in reviews of the evidence for breastfeeding recommendations (8,9). This can lead to a sense of guilt among mothers of children who develop type 1 diabetes, if the mother did not fully comply with the recommendations for duration of breastfeeding. Our results suggest such mothers need not feel guilty. This is also an important message for clinicians diagnosing children with type 1 diabetes.
Conclusion
The results from two of the world’s largest birth cohorts provide suggestive evidence for the hypothesis that initiating breastfeeding may reduce the risk of type 1 diabetes. However, among those who were breastfed, our study provides strong evidence against a clinically important association with prolonging full or any breastfeeding and risk of type 1 diabetes.
Supplementary Material
Article Information
Acknowledgments. The authors are grateful to all families participating in the MoBa and the DNBC. The authors thank Dr. Inger Johanne Bakken, Norwegian Institute of Public Health, Oslo, Norway, for guidance in the use of data from the Norwegian Patient Register.
Funding. MoBa is supported by the Norwegian Ministry of Health and Care Services and the Ministry of Education and Research and by the National Institutes of Health National Institute of Environmental Health Sciences (contract no. N01-ES-75558) and National Institute of Neurological Disorders and Stroke (grant nos. UO1 NS 047537-01 and UO1 NS 047537-06A1). This substudy of type 1 diabetes, Prediction of Autoimmune Diabetes and Celiac Disease in Childhood by Genes and Perinatal Environment (PAGE), is funded by The Research Council of Norway (grant no. 221909/F20). N.A.L.-B. is supported by the Norwegian ExtraFoundation for Health and Rehabilitation (grant no. 2010/2/0012). K.S. is supported by the Oak Foundation, Geneva, Switzerland. P.R.N. is supported by the European Research Council, The Research Council of Norway, the University of Bergen, Helse Vest, and the Kristian Gerhard Jebsen Foundation. DNBC was supported by the Danish National Research Foundation, the Pharmacy Foundation, the Egmont Foundation, the March of Dimes Birth Defects Foundation, the Augustinus Foundation, the Health Foundation, and the Novo Nordisk Foundation.
The funders of the study had no role in collecting, analyzing, or interpreting data or in writing the manuscript.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. N.A.L.-B. wrote the manuscript. N.A.L.-B., S.D.S., K.S., A.-M.N.A., G.J., S.H., and L.C.S. designed the study. N.A.L.-B., S.D.S., K.S., A.-M.N.A., K.S.R., G.J., T.S., P.R.N., S.H., and L.C.S. interpreted the results. N.A.L.-B., S.D.S., and L.C.S. analyzed the data. N.A.L.-B. and L.C.S. reviewed the literature. K.S.R. and L.C.S. obtained funding. T.S. provided data from the Norwegian Childhood Diabetes Registry. All authors revised the manuscript. L.C.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc17-0016/-/DC1.
References
- 1.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet 2014;383:69–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patterson CC, Gyürüs E, Rosenbauer J, et al. Trends in childhood type 1 diabetes incidence in Europe during 1989–2008: evidence of non-uniformity over time in rates of increase. Diabetologia 2012;55:2142–2147 [DOI] [PubMed] [Google Scholar]
- 3.Harjutsalo V, Sund R, Knip M, Groop PH. Incidence of type 1 diabetes in Finland. JAMA 2013;310:427–428 [DOI] [PubMed] [Google Scholar]
- 4.Skrivarhaug T, Stene LC, Drivvoll AK, Strøm H, Joner G; Norwegian Childhood Diabetes Study Group . Incidence of type 1 diabetes in Norway among children aged 0–14 years between 1989 and 2012: has the incidence stopped rising? Results from the Norwegian Childhood Diabetes Registry. Diabetologia 2014;57:57–62 [DOI] [PubMed] [Google Scholar]
- 5.Borch-Johnsen K, Joner G, Mandrup-Poulsen T, et al. Relation between breast-feeding and incidence rates of insulin-dependent diabetes mellitus. A hypothesis. Lancet 1984;2:1083–1086 [DOI] [PubMed] [Google Scholar]
- 6.Hosea Blewett HJ, Cicalo MC, Holland CD, Field CJ. The immunological components of human milk. Adv Food Nutr Res 2008;54:45–80 [DOI] [PubMed] [Google Scholar]
- 7.Knip M, Virtanen SM, Åkerblom HK. Infant feeding and the risk of type 1 diabetes. Am J Clin Nutr 2010;91:1506S–1513S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Victora CG, Bahl R, Barros AJ, et al.; Lancet Breastfeeding Series Group . Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet 2016;387:475–490 [DOI] [PubMed] [Google Scholar]
- 9.Patnode CD, Henninger ML, Senger CA, Perdue LA, Whitlock EP. Primary care interventions to support breastfeeding: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016;316:1694–1705 [DOI] [PubMed] [Google Scholar]
- 10.Gerstein HC. Cow’s milk exposure and type I diabetes mellitus. A critical overview of the clinical literature. Diabetes Care 1994;17:13–19 [DOI] [PubMed] [Google Scholar]
- 11.Norris JM, Scott FW. A meta-analysis of infant diet and insulin-dependent diabetes mellitus: do biases play a role? Epidemiology 1996;7:87–92 [DOI] [PubMed] [Google Scholar]
- 12.Cardwell CR, Stene LC, Ludvigsson J, et al. Breast-feeding and childhood-onset type 1 diabetes: a pooled analysis of individual participant data from 43 observational studies. Diabetes Care 2012;35:2215–2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lund-Blix NA, Stene LC, Rasmussen T, Torjesen PA, Andersen LF, Rønningen KS. Infant feeding in relation to islet autoimmunity and type 1 diabetes in genetically susceptible children: the MIDIA study. Diabetes Care 2015;38:257–263 [DOI] [PubMed] [Google Scholar]
- 14.Chmiel R, Beyerlein A, Knopff A, Hummel S, Ziegler AG, Winkler C. Early infant feeding and risk of developing islet autoimmunity and type 1 diabetes. Acta Diabetol 2015;52:621–624 [DOI] [PubMed] [Google Scholar]
- 15.Frederiksen B, Kroehl M, Lamb MM, et al. Infant exposures and development of type 1 diabetes mellitus: the Diabetes Autoimmunity Study in the Young (DAISY). JAMA Pediatr 2013;167:808–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magnus P, Birke C, Vejrup K, et al. Cohort profile update: the Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol 2016;45:382–388 [DOI] [PubMed] [Google Scholar]
- 17.Olsen J, Melbye M, Olsen SF, et al. The Danish National Birth Cohort–its background, structure and aim. Scand J Public Health 2001;29:300–307 [DOI] [PubMed] [Google Scholar]
- 18.Thorsen SU, Eising S, Mortensen HB, et al.; Danish Childhood Diabetes Registry . Systemic levels of CCL2, CCL3, CCL4 and CXCL8 differ according to age, time period and season among children newly diagnosed with type 1 diabetes and their healthy siblings. Scand J Immunol 2014;80:452–461 [DOI] [PubMed] [Google Scholar]
- 19.Ievins R, Roberts SE, Goldacre MJ. Perinatal factors associated with subsequent diabetes mellitus in the child: record linkage study. Diabet Med 2007;24:664–670 [DOI] [PubMed] [Google Scholar]
- 20.Savilahti E, Saarinen KM. Early infant feeding and type 1 diabetes. Eur J Nutr 2009;48:243–249 [DOI] [PubMed] [Google Scholar]
- 21.Ponsonby AL, Pezic A, Cochrane J, et al. Infant anthropometry, early life infection, and subsequent risk of type 1 diabetes mellitus: a prospective birth cohort study. Pediatr Diabetes 2011;12:313–321 [DOI] [PubMed] [Google Scholar]
- 22.Virtanen SM, Takkinen HM, Nevalainen J, et al. Early introduction of root vegetables in infancy associated with advanced β-cell autoimmunity in young children with human leukocyte antigen–conferred susceptibility to type 1 diabetes. Diabet Med 2011;28:965–971 [DOI] [PubMed] [Google Scholar]
- 23.Norris JM, Barriga K, Klingensmith G, et al. Timing of initial cereal exposure in infancy and risk of islet autoimmunity. JAMA 2003;290:1713–1720 [DOI] [PubMed] [Google Scholar]
- 24.Knip M, Åkerblom HK, Becker D, et al.; TRIGR Study Group . Hydrolyzed infant formula and early β-cell autoimmunity: a randomized clinical trial. JAMA 2014;311:2279–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nordic Council of Ministers Nordic Nutrition Recommendations 2012: Integrating Nutrition and Physical Activity. 5th ed. Copenhagen, Nordic Council of Ministers, 2014 [Google Scholar]
- 26.Hassiotou F, Geddes D. Anatomy of the human mammary gland: current status of knowledge. Clin Anat 2013;26:29–48 [DOI] [PubMed] [Google Scholar]
- 27.Azad MB, Konya T, Maughan H, et al.; CHILD Study Investigators . Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ 2013;185:385–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bezirtzoglou E, Tsiotsias A, Welling GW. Microbiota profile in feces of breast- and formula-fed newborns by using fluorescence in situ hybridization (FISH). Anaerobe 2011;17:478–482 [DOI] [PubMed] [Google Scholar]
- 29.Knip M, Siljander H. The role of the intestinal microbiota in type 1 diabetes mellitus. Nat Rev Endocrinol 2016;12:154–167 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.