Abstract
OBJECTIVE
Hemoglobin A1c (HbA1c), a measure of average blood glucose level, is associated with the risk of dementia and cognitive impairment. However, the role of glycemic variability or glucose excursions in this association is unclear. We examined the association of glucose peaks in midlife, as determined by the measurement of 1,5-anhydroglucitol (1,5-AG) level, with the risk of dementia and 20-year cognitive decline.
RESEARCH DESIGN AND METHODS
Nearly 13,000 participants from the Atherosclerosis Risk in Communities (ARIC) study were examined. Dementia was ascertained from surveillance, neuropsychological testing, telephone calls with participants or their proxies, or death certificate dementia codes. Cognitive function was assessed using three neuropsychological tests at three visits over 20 years and was summarized as z scores. We used Cox and linear mixed-effects models. 1,5-AG level was dichotomized at 10 μg/mL and examined within clinical categories of HbA1c.
RESULTS
Over a median time of 21 years, dementia developed in 1,105 participants. Among persons with diabetes, each 5 μg/mL decrease in 1,5-AG increased the estimated risk of dementia by 16% (hazard ratio 1.16, P = 0.032). For cognitive decline among participants with diabetes and HbA1c <7% (53 mmol/mol), those with glucose peaks had a 0.19 greater z score decline over 20 years (P = 0.162) compared with those without peaks. Among participants with diabetes and HbA1c ≥7% (53 mmol/mol), those with glucose peaks had a 0.38 greater z score decline compared with persons without glucose peaks (P < 0.001). We found no significant associations in persons without diabetes.
CONCLUSIONS
Among participants with diabetes, glucose peaks are a risk factor for cognitive decline and dementia. Targeting glucose peaks, in addition to average glycemia, may be an important avenue for prevention.
Introduction
Diabetes is an established risk factor for cognitive impairment, with evidence showing that it affects performance in several cognitive domains and puts persons at increased risk of dementia (1,2). However, the pathophysiologic mechanisms underlying these associations are unclear.
Hemoglobin A1c (HbA1c) is a standard clinical measure used for the diagnosis and management of diabetes (3) and reflects the mean blood glucose level over the preceding 2–3 months. Several studies (4–6) have shown that the risk of dementia and cognitive decline increases at higher levels of HbA1c. However, HbA1c may not be the best measure for capturing some aspects of glycemia, such as glycemic peaks, which may be particularly relevant for cognitive function.
1,5-Anhydroglucitol (1,5-AG) is a monosaccharide similar to glucose in structure. It is obtained primarily from dietary sources, with soybeans containing a particularly high level of 1,5-AG, and rice, bread, beef, and other foods containing modest amounts (7). In the absence of hyperglycemia, serum and tissue levels of 1,5-AG remain stable, with intake balanced by urinary excretion (7). However, in the presence of hyperglycemia (levels above the renal filtration threshold of ∼180 mg/dL), 1,5-AG competes with glucose for renal reabsorption, which causes urine excretion to increase and serum levels to fall. As a result, 1,5-AG is inversely related to glucose and reflects hyperglycemic peaks over a short period of time (2–14 days) (8). In individuals with diabetes, 1,5-AG is highly correlated with postprandial hyperglycemia in those with HbA1c <7% (53 mmol/mol) (9), and studies have documented associations with microvascular and macrovascular disease and death that are independent of average blood glucose levels (10,11). It is likely that, in persons with diabetes, 1,5-AG reflects additional information on glycemic instability and hyperglycemic excursions not reflected in HbA1c or fasting glucose level. Fluctuating glucose levels have been shown to be more detrimental to neuronal cell functioning in vitro compared with consistently high or low levels (12) and have been linked cross-sectionally to cognitive function (13,14), but long-term prospective associations of glucose peaks with cognitive function and dementia have not been widely explored (15).
Our aim was to characterize the prospective association between glucose peaks, as measured by 1,5-AG, and 20-year cognitive decline and incident dementia in a community-based population. We hypothesized that low levels of 1,5-AG would be associated with a higher risk of dementia and faster long-term cognitive decline, independent of average glycemia as measured by HbA1c, and other risk factors for cognitive decline.
Research Design and Methods
Study Population
The Atherosclerosis Risk in Communities (ARIC) study is a prospective cohort of 15,792 community-dwelling adults sampled from the following four U.S. communities: suburbs of Minneapolis, MN; Washington County, MD; Forsyth County, NC; and Jackson, MS. The Forsyth County field center recruited both black and white participants, whereas the Jackson field center recruited only black participants. The other two field centers also selected participants by probability sampling; however, given the racial distribution in these regions at the time, a small percentage of nonwhite participants was selected. The first cohort examination took place in 1987–1989, with three additional visits roughly 3 years apart. A fifth visit was completed in 2011–2013. Institutional review boards at each study site reviewed and approved the study. Written informed consent was obtained from all participants.
The baseline for the current study is the second visit, which took place in 1990–1992. Of the 14,348 participants who attended visit 2, we excluded participants who were neither black nor white and nonwhites in Washington and Forsyth Counties (n = 91), participants missing 1,5-AG or HbA1c data (n = 1,250), or participants who had no follow-up time (n = 11), giving a sample of n = 12,996 for our analysis of incident dementia. For analysis of cognitive change, we did not exclude those participants missing follow-up time but further excluded persons missing cognitive testing at baseline (n = 172), for a final sample of 12,835.
Measurement of 1,5-AG and HbA1c
1,5-AG (GlycoMark, Winston-Salem, NC) was measured using a Roche Modular P800 system in 2012–2013 in stored serum samples originally collected at visit 2 (1990–1992). The interassay coefficient of variation was 5%, and the reliability coefficient using 610 masked duplicate samples was 0.99. Additionally, previous studies (16,17) have shown that this assay is reliable in long-term stored samples. HbA1c was measured in stored samples collected at visit 2 using high-performance liquid chromatography using the Tosoh 2.2 and the Tosoh G7 columns (18).
Definition of Diabetes and Prediabetes
Diabetes was defined on the basis of a self-reported physician diagnosis, the use of glucose-lowering medication, or a measured HbA1c level ≥6.5% (48 mmol/mol). Self-reported diabetes has been shown to be highly specific and reliable in the ARIC study population (19). Among participants classified as not having diabetes using this definition, we defined prediabetes as an HbA1c level in the range of 5.7–6.4% (39–46 mmol/mol).
Assessment of Incident Dementia and Cognitive Function
Methods of dementia classification have been described in detail previously (20,21). Briefly, an expert classification committee including physicians, neurologists, and neuropsychologists ascertained dementia status using visit-based data, including detailed neuropsychiatric tests at visit 5, previous visit cognitive testing, informant interviews, medical and family history, and information obtained by telephone contact with participants or their proxies. For persons who did not attend visit 5, participants were contacted to have their cognitive function assessed via the modified Telephone Interview for Cognitive Status (TICS-m) questionnaire (22). To ascertain dementia status for deceased participants or participants who could not be reached, the Clinical Dementia Rating (CDR) scale was administered to proxy informants familiar with the participant cognitive status. Last, cohort surveillance of hospitalizations and dementia codes on the death certificate were used to identify dementia cases. The TICS (23) and CDR (24) are validated measures of ascertaining cognitive performance and dementia status. Compared with in-person assessment, the TICS-m has a 50% sensitivity and 94% specificity for dementia, whereas hospitalization/death codes had 25% and 99% sensitivity and specificity, respectively (20).
For persons with dementia, the date was defined as the earliest of either the first occurrence of a hospitalization with an ICD-9 code for dementia, date of death (if dementia codes were present on death certificate), date of telephone contact with participant or their proxy indicating dementia, or date of visit 5 in 2011–2013 (if the participant had dementia at the visit but no earlier indication of dementia). Participants who attended visit 5 and were algorithmically classified as not having dementia (21) were censored at visit 5. Participants who did not attend visit 5 were censored at the last date where there was no known indication of dementia (telephone contact with the participant or their proxy or a hospitalization where no dementia was indicated).
Cognitive function was assessed at visits 2, 4, and 5 using the following three neuropsychological tests: Delayed Word Recall (23), Digit Symbol Substitution of the Wechsler Adult Intelligence Scale-Revised (25), and Word Fluency Test (26). Trained examiners administered the tests in a fixed order in a quiet room. Examiner performance was monitored by tape recording and was reviewed locally and centrally to ensure consistency with testing protocols.
In the Delayed Word Recall test, participants were presented with 10 common nouns and were asked to use each in a sentence. After the completion of all 10 words, a second exposure to each word was given. After a 5-min delay, participants were given 60 s to recall the words, with scores ranging from 0 to 10. For the Digit Symbol Substitution of the Wechsler Adult Intelligence Scale-Revised test, participants had 90 s to translate numbers (0–9) to symbols using a key. The score was calculated as the number of correctly translated symbols, with scores in this study ranging from 0 to 93. Finally, for the Word Fluency Test participants were given three 60-s trials to generate words beginning with the letters “F,” “A,” or “S,” excluding proper nouns. The total score for this test was the total number of words generated across the three trials.
We created a z score for each test at each visit, standardized to visit 2, by subtracting the test mean (at visit 2) and dividing by the SD (at visit 2). Next, we created a global composite z score by averaging the z score of the three tests and standardizing to visit 2.
Covariates
The following variables were evaluated as potential confounders: age; sex; race–field center (five categories: white persons from Minneapolis, Washington County, or Forsyth County; black persons from Forsyth County or from Jackson); education (less than high school, high school or vocational school, or college or above); cigarette smoking status (current, former, never); hypertension (yes/no, based on medication use, systolic blood pressure ≥140 mmHg, or diastolic blood pressure ≥90 mmHg); history of coronary heart disease (yes/no); history of stroke (yes/no); BMI; diabetes medication use (insulin, yes/no; sulfonylureas, yes/no); and apolipoprotein E4 (APOE4) genotype (coded as 0, 1, or 2 ε4 alleles). Education, race, and sex were evaluated at visit 1 (1987–1989). All other variables were assessed at visit 2.
Statistical Analysis
We dichotomized 1,5-AG at 10 μg/mL, a cut point recommended by the manufacturer and used in prior publications (10,11). Values <10 μg/mL are associated with glucose peaks above the average renal threshold (∼160–190 mg/dL) (7). We categorized participants into three groups according to diabetes status as no diabetes, diabetes and HbA1c <7% (53 mmol/mol), and diabetes and HbA1c ≥7% (53 mmol/mol). HbA1c <7% (53 mmol/mol) was chosen because it is the cutoff recommended by the American Diabetes Association as a “reasonable A1C goal” for persons with diabetes (3, p. S41). Together, the 1,5-AG and diabetes variables were used to create a six-level exposure variable (two levels of 1,5-AG by three levels of diabetes). We used an F test to examine the risk ratio modification by 1,5-AG of the association between the three-level diabetes variable with dementia and cognitive decline. We also modeled 1,5-AG continuously using linear splines with knots at the 5th, 35th, 65th, and 95th percentiles.
For analyses of incident dementia, we used Cox proportional hazards regression to estimate hazard ratios (HRs) and 95% CIs, using the Efron method to handle tied failure times. The follow-up time for incident dementia was through 31 December 2013. We verified the proportional hazards assumption by examining log-log plots and using a global test for zero slope of the scaled Schoenfeld residuals over time. We used two models that were specified as follows: model 1 was adjusted for age, race–field center, sex, and education; and model 2 was adjusted for the variables in model 1 plus hypertension, history of stroke, history of coronary heart disease, cigarette smoking status, drinking status, and APOE4. For analyses where the aim was to compare 1,5-AG groups (<10 vs. ≥10 µg/mL) within each diabetes group, we first stratified by diabetes group and within each group we additionally adjusted for HbA1c level.
For analyses of change in cognitive function, we used linear mixed-effects models, which account for the correlation of repeated measures over time. We modeled time since baseline (visit 2) using a linear spline with a knot at 6 years (the median time between visits 2 and 4). Models included a random intercept and two random slopes for time (one for each spline term), and the three random effects were assumed to be independent. We adjusted for age, age squared, race–field center, sex, education, hypertension, history of stroke, history of coronary heart disease, APOE4, cigarette smoking status, drinking status, and BMI, and included the interaction terms between each variable and time. From these linear mixed-effects models, we estimated cognitive decline at 20 years after baseline, which is consistent with prior ARIC publications (5,27).
Missing Data
A total of 469 participants at baseline (<4%) had missing values for one or more covariates used in incident dementia or cognitive change analyses. We used multiple imputation by chained equations to impute these missing baseline covariates. Additionally, for our analysis of cognitive decline, z scores of participants dropping out of the study over time were also imputed using multiple imputation by chained equations as previously described (28). Briefly, z scores were imputed using information collected during and outside of study visits, including annual follow-up telephone calls, community surveillance of hospitalizations, the TICS, or retrospective dementia ascertainment, where the CDR scale was given to the participant or a proxy, and covariates were included in our main analytic model. For participants alive at the time of visit 5, scores were imputed at the median visit date; for participants who were deceased by visit 5, scores were imputed 6 months prior to death. We calculated 10 imputations. Counts by participant vital status and visit attendance after baseline and characteristics by vital status at visit 5 are shown in Supplementary Tables 1 and 2, respectively.
Sensitivity Analyses
We conducted a number of sensitivity analyses to test the robustness of our findings. For analyses of incident dementia, we restricted our study population to only individuals who had at least one hospitalization (N = 10,646). This allowed us to examine whether associations were due to differential ascertainment of dementia between those with and without a hospitalization and addresses the fact that persons with diabetes may be more likely to be hospitalized and thus receive a diagnosis of dementia. We also performed stratified analyses by diabetes status and HbA1c groups by sex, race, and APOE4 alleles.
Results
At baseline among participants, the average age was 57 years, 56% were female, and 24% were black (Table 1). Among persons with diabetes and HbA1c <7%, participants with 1,5-AG concentration <10 μg/mL compared with those with 1,5-AG ≥10 μg/mL were less likely to be female (48% vs. 58%), black (33% vs. 43%), have less than a high school education (26% vs. 36%), have a history of stroke (2.4% vs. 5.1%), and be current smokers (18% vs. 23%). HbA1c level, BMI, hypertension, and baseline z scores were similar between these two groups, but fasting glucose level was higher in those with 1,5-AG concentration <10 μg/mL. Among persons with diabetes and HbA1c ≥7% (53 mmol/mol), participants with 1,5-AG concentration <10 μg/mL compared with those with 1,5-AG concentration ≥10 μg/mL were less likely to be female (57% vs. 67%), be black (48% vs. 58%), and have hypertension (61% vs. 66%). They also had higher baseline z scores (−0.55 vs. −0.71). Among persons with diabetes, those with 1,5-AG concentration <10 µg/mL appeared more likely to have 0 APOE4 alleles compared with persons with 1,5-AG concentration ≥10 µg/mL in both HbA1c groups; however, the χ2 test for differences across the groups was not statistically significant (Table 1) (P = 0.290).
Table 1.
Characteristics of study participants at baseline by diabetes status, HbA1c, and 1,5-AG categories
| Total | No diabetes* |
Diabetes* |
|||||
|---|---|---|---|---|---|---|---|
| HbA1c <7% |
HbA1c ≥7% |
||||||
| 1,5-AG ≥10 | 1,5-AG <10 | 1,5-AG ≥10 | 1,5-AG <10 | 1,5-AG ≥10 | 1,5-AG <10 | ||
| N (%) | 12,996 | 10,708 (82.4) | 576 (4.4) | 535 (4.1) | 125 (1.0) | 176 (1.4) | 876 (6.7) |
| 1,5-AG, μg/mL | 17.6 (6.7) | 19.5 (5.0) | 7.4 (2.0) | 17.9 (5.0) | 6.9 (2.4) | 15.4 (4.7) | 3.3 (2.5) |
| Fasting glucose, mg/dL | 114 (44.1) | 103 (11.2) | 104 (18.2) | 126 (24.0) | 143 (44.2) | 157 (36.3) | 243 (85.1) |
| HbA1c, % | 5.8 (1.2) | 5.4 (0.4) | 5.5 (0.4) | 6.2 (0.5) | 6.4 (0.5) | 7.6 (0.8) | 9.6 (1.9) |
| HbA1c, mmol/mol | 40 (13.4) | 36 (4.2) | 37 (4.4) | 44 (5.8) | 46 (5.4) | 59 (9.0) | 81 (20.1) |
| Prediabetes,† % | 17.0 | 19.1 | 26.9 | ||||
| Diabetes medication, % | |||||||
| Insulin‡ | 3.0 | 6.4 | 13.6 | 13.6 | 35.3 | ||
| Sulfonylureas | 3.8 | 14.0 | 28.8 | 26.1 | 38.7 | ||
| Diabetes duration, years§ | 5.0 (2.8–11.9) | 3.0 (2.7–8.9) | 6.0 (2.8–14.7) | 4.5 (2.8–9.2) | 5.9 (2.3–13) | ||
| Age, years | 56.9 (5.7) | 56.7 (5.7) | 57.3 (6.0) | 58.0 (5.8) | 58.6 (5.6) | 58.3 (5.7) | 58.1 (5.7) |
| Female, % | 56.3 | 55.6 | 65.6 | 58.1 | 48.0 | 66.5 | 57.2 |
| Black, % | 24.0 | 20.4 | 25.7 | 43.4 | 32.8 | 58.0 | 48.1 |
| Education, % | |||||||
| Less than high school | 21.3 | 19.2 | 17.4 | 35.6 | 26.4 | 39.2 | 36.2 |
| High school | 41.9 | 42.6 | 40.4 | 36.3 | 40.0 | 38.1 | 38.2 |
| College/vocational | 36.9 | 38.2 | 42.3 | 28.1 | 33.6 | 22.7 | 25.6 |
| BMI, kg/m2 | 28.0 (5.4) | 27.5 (5.1) | 27.0 (5.0) | 31.1 (6.1) | 31.0 (5.8) | 32.5 (6.5) | 31.6 (6.1) |
| eGFR, mL/min/1.73 m2 | 96.4 (15.7) | 96.4 (14.4) | 94.9 (18.4) | 96.8 (18.3) | 91.0 (25.2) | 97.8 (20.3) | 96.9 (21.9) |
| Hypertension, % | 35.6 | 32.0 | 32.4 | 57.1 | 54.0 | 65.9 | 60.7 |
| History of stroke, % | 1.9 | 1.4 | 2.1 | 5.1 | 2.4 | 4.6 | 5.2 |
| APOE4 alleles, % | |||||||
| 0 | 69.4 | 69.5 | 66.4 | 69.5 | 73.4 | 64.5 | 71.0 |
| 1 | 28.0 | 28.0 | 31.3 | 26.1 | 23.4 | 32.0 | 26.8 |
| 2 | 2.6 | 2.6 | 2.3 | 4.4 | 3.2 | 3.5 | 2.2 |
| Current smoker, % | 22.1 | 22.7 | 16.8 | 23.0 | 18.4 | 21.1 | 18.0 |
| Current drinker, % | 56.6 | 59.9 | 57.1 | 39.1 | 43.2 | 31.3 | 33.3 |
| Global z score | 0.00 (1.00) | 0.08 (0.97) | 0.05 (0.99) | −0.43 (1.01) | −0.48 (1.05) | −0.71 (0.98) | −0.55 (1.05) |
| Visit 5 status, N (%) | |||||||
| Attended | 5,869 (45.7) | 5,172 (48.8) | 275 (48.6) | 150 (28.5) | 33 (27.0) | 45 (26.0) | 194 (23.2) |
| Alive, did not attend | 3,411 (26.6) | 2,864 (27.0) | 143 (25.3) | 156 (29.7) | 35 (28.7) | 46 (26.6) | 167 (19.9) |
| Deceased | 3,550 (27.7) | 2,569 (24.2) | 148 (26.1) | 220 (41.8) | 54 (44.3) | 82 (47.4) | 477 (56.9) |
Values shown as mean (SD) or %, unless otherwise indicated. eGFR, estimated glomerular filtration rate.
*Diabetes was defined as a self-reported physician diagnosis of diabetes, use of glucose-lowering medication, or an HbA1c level ≥6.5% (by definition, persons in the “No diabetes” group have an HbA1c level <6.5% [48 mmol/mol]).
†Prediabetes was defined as HbA1c level 5.7–6.4% (39–46 mmol/mol).
‡Includes insulin plus another oral medication.
§Shown as median (25th to 75th percentiles).
Over a median follow-up period of 21 years, dementia developed in 1,105 participants. As expected, participants in whom dementia subsequently developed had lower cognitive z scores at each visit and greater change between visits compared with persons in whom dementia did not develop (Supplementary Table 3). In fully adjusted models, compared with persons with well-controlled diabetes and 1,5-AG concentration ≥10 μg/mL, persons with well-controlled diabetes and 1,5-AG concentration <10 μg/mL had a 33% higher risk of dementia, although this was not statistically significant (P = 0.285) (Table 2). Additionally, persons with poorly controlled diabetes and 1,5-AG concentration <10 μg/mL had an 86% higher risk of dementia (P = 0.011) (Table 2) compared with persons with 1,5-AG concentration ≥10 μg/mL. In persons without diabetes, the risk of dementia was not significantly higher in persons with 1,5-AG concentration <10 μg/mL compared with those with 1,5-AG concentration ≥10 μg/mL (HR 1.05, P = 0.754) (Table 2). The F test for risk-ratio modification by 1,5-AG was not statistically significant (P value-for-interaction = 0.140). We found similar, but attenuated, results when we performed analyses fully stratified by diabetes status with additional adjustment for HbA1c level (Supplementary Table 4) and when we restricted the population to only include participants with at least one hospitalization (Supplementary Table 5). Stratified analyses by sex (Supplementary Table 6), race (Supplementary Table 7), and APOE4 (Supplementary Table 8) showed similar trends, but subgroup sample sizes were small.
Table 2.
Adjusted HRs (95% CI) for the association of 1,5-AG categories with incident dementia by diabetes status (N = 12,996)
| Events/N (%) | Model 1 HR (95% CI) | P value† | Model 2 HR (95% CI) | P value† | ||
|---|---|---|---|---|---|---|
| No diabetes | ||||||
| 1,5-AG ≥10 μg/mL | 829/10,708 (7.7%) | 1 (reference) | 0.962 | 1 (reference) | 0.754 | |
| 1,5-AG <10 μg/mL | 48/576 (8.3%) | 1.01 (0.75, 1.35) | 1.05 (0.78, 1.40) | |||
| Diabetes | ||||||
| HbA1c <7%* | 1,5-AG ≥10 μg/mL | 60/535 (11.2%) | 1.34 (1.02, 1.75) | 0.359 | 1.27 (0.97, 1.67) | 0.285 |
| 1,5-AG <10 μg/mL | 19/125 (15.2%) | 1.71 (1.08, 2.70) | 1.69 (1.07, 2.67) | |||
| HbA1c ≥7%* | 1,5-AG ≥10 μg/mL | 19/176 (10.8%) | 1.41 (0.89, 2.23) | 0.020 | 1.31 (0.83, 2.07) | 0.011 |
| 1,5-AG <10 μg/mL | 130/876 (14.8%) | 2.49 (2.06, 3.02) | 2.44 (2.01, 2.97) |
HRs and CIs were estimated using Cox proportional hazards regression over a median follow-up time of 21 years. Diabetes was defined as a self-reported physician diagnosis of diabetes, use of glucose-lowering medication, or an HbA1c ≥6.5% (48 mmol/mol); model 1, adjusted for age, sex, education, and race-center; model 2, adjusted for the variables in model 1 plus hypertension, history of stroke, history of coronary heart disease, cigarette smoking status, drinking status, and APOE4.
†P value for test of the difference in HR between 1,5-AG concentrations of ≥10 and <10 μg/mL within diabetes status and HbA1c category.
*Equivalent to HbA1c level of 53 mmol/mol.
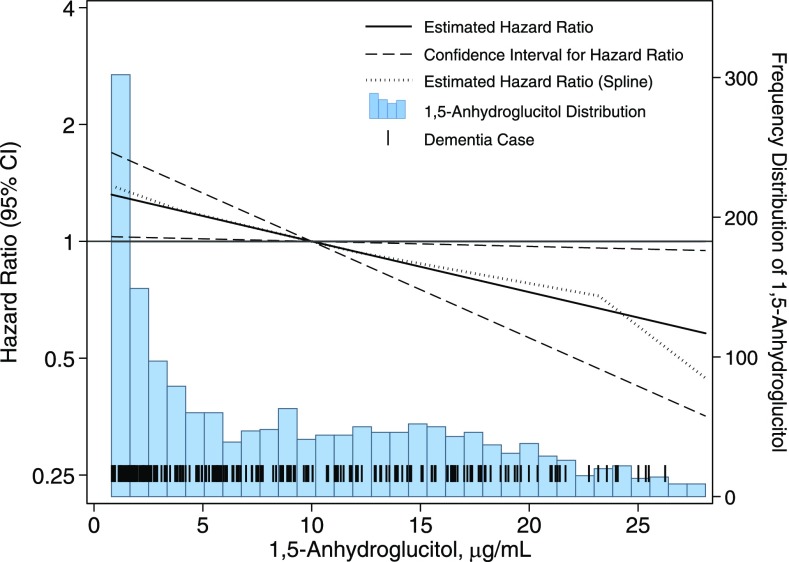
We observed similar trends for the association of 1,5-AG with dementia in persons with diabetes when 1,5-AG was modeled continuously (Fig. 1). We found an estimated 16% increased risk of dementia (HR 1.16, P value = 0.032) for each 5 μg/mL decrease in 1,5-AG. The association between 1,5-AG and dementia was similar when modeled using linear splines.
Figure 1.
Adjusted HRs (95% CI) for the association of 1,5-AG with incident dementia among persons with diabetes. HRs were estimated using Cox proportional hazards regression among persons with diabetes (N = 1,659) with adjustment for age, race (black/white), sex, education, hypertension (yes/no), history of stroke (yes/no), history of coronary heart disease (yes/no), APOE4 genotype (0, 1, or 2 alleles), and HbA1c. 1,5-AG was measured at baseline (1990–1992) and modeled continuously with the reference point of 1,5-AG set to the 60th percentile (∼10 μg/mL). We also modeled the association using linear splines with knots at the 5th, 35th, 65th, and 95th percentiles. Diabetes was defined as a self-reported physician diagnosis of diabetes, use of glucose-lowering medication, or an HbA1c level of ≥6.5% (48 mmol/mol). The median follow-up time was 18 years, and there were 217 cases of incident dementia.
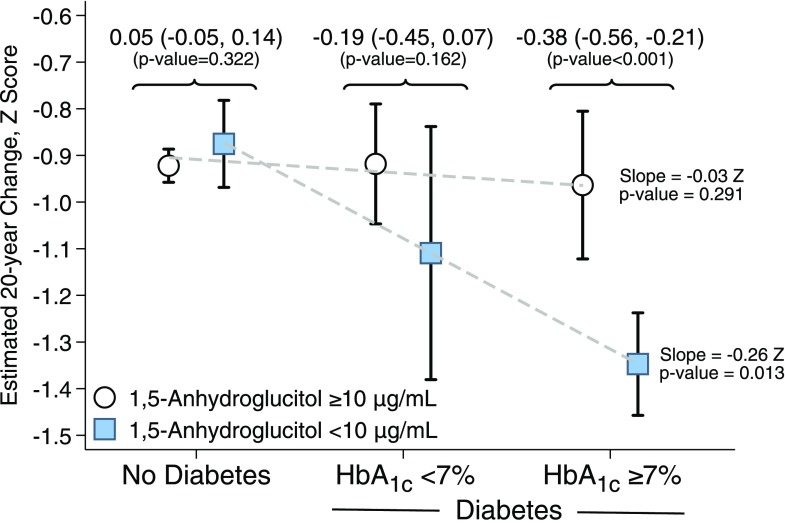
Figure 2 shows the estimated association between baseline categories of diabetes and 20-year cognitive decline. Among persons with diabetes and HbA1c level <7% (53 mmol/mol), persons with 1,5-AG concentration <10 μg/mL had a 0.19 greater z score decline compared with persons with 1,5-AG concentration ≥10 μg/mL (P = 0.162). Among persons with diabetes and HbA1c level ≥7% (53 mmol/mol), persons with 1,5-AG concentration <10 μg/mL had a 0.38 greater z score decline compared with persons with 1,5-AG concentration ≥10 μg/mL (P < 0.001). Participants with 1,5-AG concentration ≥10 μg/mL and diabetes, regardless of HbA1c status, had a similar decline in cognitive function compared with persons with 1,5-AG concentration ≥10 μg/mL without diabetes. However, there was a graded association among persons with 1,5-AG concentration <10 μg/mL, with a 0.26 z score decline per diabetes and HbA1c category (Fig. 2). The P value for interaction between the three diabetes categories and 1,5-AG was 0.035.
Figure 2.
Estimated association between baseline categories of diabetes and 20-year cognitive decline by diabetes, HbA1c, and 1,5-AG group. Estimates and 95% CIs are from linear mixed-effects models with adjustment for age, age squared, race–field center, sex, education, cigarette smoking status, drinking status, hypertension, history of stroke, history of coronary heart disease, APOE4 genotype, BMI, and interactions between these variables and time. Time since baseline (visit 2) was the time axis and was modeled with a linear spline with a knot at 6 years. A random intercept and two random slopes for time (one for each spline term) were included, and the three random effects were assumed to be independent. Slope: dashed lines indicate linear regression fit across the three diabetes groupings (no diabetes, diabetes HbA1c <7%, diabetes HbA1c ≥7%) and 1,5-AG category by creating an indicator that takes on the values 1, 2, or 3. Decline indicates the estimated 20-year cognitive decline per category. The P values comparing 1,5-AG concentrations ≥10 to <10 μg/mL are from analyses stratified by diabetes group (no diabetes, diabetes with HbA1c <7%, diabetes with HbA1c ≥7%) with additional adjustment for HbA1c. An HbA1c of 7% is equivalent to 53 mmol/mol.
Conclusions
In this community-based study, we found that low levels of 1,5-AG, indicative of glycemic peaks, were associated with an increased risk of dementia and greater cognitive decline over 20 years. In persons with diabetes, each 5 µg/mL increase in 1,5-AG was associated with a 16% increase in the risk of dementia. Among persons with diabetes and HbA1c <7% (53 mmol/mol), those with more glucose peaks had greater cognitive decline over 20 years compared with persons without peaks (z = 0.19 more decline). Although this value was not statistically significant (P = 0.162), the magnitude of this association was nearly as large as our previously reported association between diabetes and 20-year decline (5) (z = 0.23 more decline in participants with diabetes compared with those without). 1,5-AG appeared to modify the association between diabetes status and cognitive decline (P value-for-interaction = 0.035). Although persons without glucose peaks had little cognitive decline, even if they had diabetes or HbA1c level ≥7% (53 mmol/mol), more research is needed to investigate this finding. 1,5-AG did not seem to provide additional information about incident dementia or 20-year decline in persons without diabetes.
The mechanisms by which diabetes leads to cognitive impairment are not well understood. It is thought that hyperglycemia, hypoglycemia, and oxidative stress, among other factors, play important roles (29), but less attention has been given to the role of glycemic variability, and debate on its usefulness in clinical practice is ongoing (30,31). At the cellular level, fluctuations in glycemia have been shown to more adversely affect endothelial function and induce oxidative stress compared with sustained hyperglycemia (32,33), potentially leading to greater cerebrovascular damage and cognitive decline. A few studies using continuous glucose monitors have found associations between measures of glycemic variability and cognitive impairment and brain atrophy independent of both mean levels of glycemia and hypoglycemic episodes (13,14), but long-term prospective studies have not been conducted.
Studies using data from continuous glucose monitors have found moderate correlations between common measures of glycemic variability (e.g., mean amplitude of glycemic excursions and postprandial glucose excursions) and 1,5-AG (34,35). Glycemic variability is an aspect of glycemia that is not well captured by HbA1c, which is less sensitive to glycemic peaks compared with 1,5-AG. If glucose peaks in persons with diabetes contribute to long-term cognitive decline and dementia above and beyond average hyperglycemia, they may also offer additional targets for prevention. One randomized controlled trial (36) examining the effects of targeting postprandial glucose excursions showed the benefits for cognitive function, although sample sizes were small; additional studies in this area are needed. Last, glycemic peaks are common in older adults. A study of >3,200 participants with non–insulin-treated type 2 diabetes using in-home glucose readings over a 1-week period found that 84% of participants recorded at least one postprandial blood glucose concentration >160 mg/dL. Even among persons with an HbA1c level <7% (53 mmol/mol), 38% had a postprandial glucose concentration >160 mg/dL in >40% of the readings (37).
Our study has some limitations that should be considered when interpreting these results. First, ascertainment of dementia in participants not seen at the 2011–2013 examination was based in part on ICD-9 codes and the TICS-m. The low sensitivity of these methods implies that not all cases were captured, potentially biasing results toward the null and increasing SEs. Second, we had only a single measurement of 1,5-AG, although in the ARIC study it has been shown that total short-term variability of 1,5-AG (over a mean period of 6 weeks) is intermediate between fasting glucose and HbA1c (38). The single measurement of 1,5-AG also limited our ability to examine 1,5-AG as a time-varying exposure. Third, we had relatively few participants in some of our exposure groups, which limited our statistical power in some analyses. Fourth, cognitive decline may interfere with one’s ability to adequately manage one’s diabetes, resulting in worsening glycemic control (i.e., reverse causation), although our measurements of diabetes and 1,5-AG in midlife, when cognitive impairment is relatively rare, and over the subsequent 20 years of follow-up should mitigate this possibility. Fifth, we were unable to account for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis as either confounders or mediators. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis are strongly associated with diabetes (39), and emerging evidence suggests associations with Alzheimer’s disease and dementia (40). Last, over 20 years of follow-up a number of participants died or did not attend follow-up visits, potentially biasing associations of interest in a conservative direction. Although we used validated methods to account for this dropout, including the use of auxiliary information collected to characterize participants’ cognition during follow-up, these methods may not fully account for the effects of attrition.
Our study also has a number of strengths, including a large sample size of nearly 13,000 adults, comprehensive assessment of confounders at baseline, well-characterized and validated neuropsychological tests for cognitive function and dementia assessment during follow-up, and a median follow-up time of >20 years.
In summary, our study found that glucose peaks, as measured by 1,5-AG, were detrimental to long-term cognitive function in persons with diabetes. 1,5-AG was associated with an increased risk of dementia independent of mean glucose level and other risk factors for cognitive decline. Besides primary prevention of diabetes as a tool for preventing cognitive decline, these results suggest that glycemic peaks may provide an additional treatment target for persons with diabetes. More research is needed to confirm these findings and to determine the group of persons who are most likely to benefit from this targeted intervention.
Supplementary Material
Article Information
Acknowledgments. The authors thank the staff and participants of the ARIC study for their important contributions. Reagents for the 1,5-AG assays were donated by the GlycoMark Corporation.
Funding. The ARIC study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute (NHLBI) contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C, with the ARIC carotid MRI examination funded by U01HL075572-01). This research was supported by National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant R01-DK-089174 (to E.S.). E.S. was also supported by NIH/NIDDK grant K24-DK-106414. Neurocognitive data collection was supported by NHLBI and National Institute of Neurological Disorders and Stroke (NINDS) grants U01-HL-096812, HL-096814, HL-096899, HL-096902, and HL-096917, with previous brain MRI examinations funded by NINDS grant R01-HL-70825 from the NHLBI. A.M.R. was supported by NIH/NHLBI grant T32-HL-007024.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. A.M.R. contributed to the design of the study, was involved in the design and implementation of the statistical methods, conducted analyses, and drafted the manuscript. A.R.S., T.H.M., S.H.B., and J.A.D. were involved in the design and implementation of the statistical methods. E.S. contributed to the design of the study and the acquisition of data. All authors contributed to the interpretation of the results and provided critical revisions to the manuscript. A.M.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at EPI/Lifestyle 2016, Epidemiology and Prevention and Lifestyle and Cardiometabolic Health, 2016 Scientific Sessions of the American Heart Association, Phoenix, AZ, 1–4 March 2016.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc16-2203/-/DC1.
This article is featured in a podcast available at http://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1.Reijmer YD, van den Berg E, Ruis C, Kappelle LJ, Biessels GJ. Cognitive dysfunction in patients with type 2 diabetes. Diabetes Metab Res Rev 2010;26:507–519 [DOI] [PubMed] [Google Scholar]
- 2.Ryan JP, Fine DF, Rosano C. Type 2 diabetes and cognitive impairment: contributions from neuroimaging. J Geriatr Psychiatry Neurol 2014;27:47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes Association Standards of medical care in diabetes–2016. Diabetes Care 2016;39:S1–S10826696671 [Google Scholar]
- 4.Tuligenga RH, Dugravot A, Tabák AG, et al. Midlife type 2 diabetes and poor glycaemic control as risk factors for cognitive decline in early old age: a post-hoc analysis of the Whitehall II cohort study. Lancet Diabetes Endocrinol 2013;2:228–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rawlings AM, Sharrett AR, Schneider ALC, et al. Diabetes in midlife and cognitive change over 20 years: a cohort study. Ann Intern Med 2014;161:785–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crane PK, Walker R, Hubbard RA, et al. Glucose levels and risk of dementia. N Engl J Med 2013;369:540–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buse JB, Freeman JLR, Edelman SV, Jovanovic L, McGill JB. Serum 1,5-anhydroglucitol (GlycoMark): a short-term glycemic marker. Diabetes Technol Ther 2003;5:355–363 [DOI] [PubMed] [Google Scholar]
- 8.Yamanouchi T, Minoda S, Yabuuchi M, et al. Plasma 1,5-anhydro-D-glucitol as new clinical marker of glycemic control in NIDDM patients. Diabetes 1989;38:723–729 [DOI] [PubMed] [Google Scholar]
- 9.Won JC, Park CY, Park HS, et al. 1,5-Anhydroglucitol reflects postprandial hyperglycemia and a decreased insulinogenic index, even in subjects with prediabetes and well-controlled type 2 diabetes. Diabetes Res Clin Pract 2009;84:51–57 [DOI] [PubMed] [Google Scholar]
- 10.Selvin E, Rawlings A, Lutsey P, et al. Association of 1,5-anhydroglucitol with cardiovascular disease and mortality. Diabetes 2016;65:201–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selvin E, Rawlings AM, Grams M, Klein R, Steffes M, Coresh J. Association of 1,5-anhydroglucitol with diabetes and microvascular conditions. Clin Chem 2014;60:1409–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russo VC, Higgins S, Werther GA, Cameron FJ. Effects of fluctuating glucose levels on neuronal cells in vitro. Neurochem Res 2012;37:1768–1782 [DOI] [PubMed] [Google Scholar]
- 13.Rizzo MR, Marfella R, Barbieri M, et al. Relationships between daily acute glucose fluctuations and cognitive performance among aged type 2 diabetic patients. Diabetes Care 2010;33:2169–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui X, Abduljalil A, Manor BD, Peng C-K, Novak V. Multi-scale glycemic variability: a link to gray matter atrophy and cognitive decline in type 2 diabetes. PLoS One 2014;9:e86284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geijselaers SLC, Sep SJS, Stehouwer CDA, Biessels GJ. Glucose regulation, cognition, and brain MRI in type 2 diabetes: a systematic review. Lancet Diabetes Endocrinol 2015;3:75–89 [DOI] [PubMed] [Google Scholar]
- 16.Selvin E, Rynders GP, Steffes MW. Comparison of two assays for serum 1,5-anhydroglucitol. Clin Chim Acta 2011;412:793–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selvin E, Steffes MW, Ballantyne CM, Hoogeveen RC, Coresh J, Brancati FL. Racial differences in glycemic markers: a cross-sectional analysis of community-based data. Ann Intern Med 2011;154:303–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selvin E, Coresh J, Zhu H, Folsom A, Steffes MW. Measurement of HbA1c from stored whole blood samples in the Atherosclerosis Risk in Communities study. J Diabetes 2010;2:118–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider ALC, Pankow JS, Heiss G, Selvin E. Validity and reliability of self-reported diabetes in the Atherosclerosis Risk in Communities Study. Am J Epidemiol 2012;176:738–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knopman DS, Gottesman RF, Sharrett AR, et al. Mild cognitive impairment and dementia prevalence: the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). Alzheimers Dement (Amst) 2016;2:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atherosclerosis Risk in Communities. Manual 17. ARIC NCS neurocognitive exam (stages 2 and 3) [article online], 2012. Available from https://www2.cscc.unc.edu/aric/sites/default/files/public/manuals/17%20Neurocognitive%20Exam%20%28Stage%202%20and%203%29.pdf. Accessed 3 April 2014
- 22.Welsh KA, Breitner JCS, Magruder-Habib KM. Detection of dementia in the elderly using telephone screening of cognitive status. Cogn Behav Neurol 1993;6:103–110 [Google Scholar]
- 23.Knopman DS, Ryberg S. A verbal memory test with high predictive accuracy for dementia of the Alzheimer type. Arch Neurol 1989;46:141–145 [DOI] [PubMed] [Google Scholar]
- 24.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414 [DOI] [PubMed] [Google Scholar]
- 25.Wechsler D. WAIS-R, Wechsler Adult Intelligence Scale-Revised, Manual. San Antonio, TX, The Psychological Corporation, 1981 [Google Scholar]
- 26.Benton A, Hamsher K. Multilingual Aphasia Examination. 2nd ed. Iowa City, IA, AJA Associates, 1989 [Google Scholar]
- 27.Gottesman RF, Schneider ALC, Albert M, et al. Midlife hypertension and 20-year cognitive change. JAMA Neurol 2014;71:1218–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rawlings AM, Sang Y, Sharrett AR, et al. Multiple imputation of cognitive performance as a repeatedly measured outcome. Eur J Epidemiol 2017;32:55–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet 2012;379:2291–2299 [DOI] [PubMed] [Google Scholar]
- 30.Bergenstal RM. Glycemic variability and diabetes complications: does it matter? Simply put, there are better glycemic markers! Diabetes Care 2015;38:1615–1621 [DOI] [PubMed] [Google Scholar]
- 31.Hirsch IB. Glycemic variability and diabetes complications: does it matter? Of course it does! Diabetes Care 2015;38:1610–1614 [DOI] [PubMed] [Google Scholar]
- 32.Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006;295:1681–1687 [DOI] [PubMed] [Google Scholar]
- 33.Ceriello A, Esposito K, Piconi L, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 2008;57:1349–1354 [DOI] [PubMed] [Google Scholar]
- 34.Sun J, Dou J-T, Wang X-L, et al. Correlation between 1,5-anhydroglucitol and glycemic excursions in type 2 diabetic patients. Chin Med J (Engl) 2011;124:3641–3645 [PubMed] [Google Scholar]
- 35.Dungan KM, Buse JB, Largay J, et al. 1,5-Anhydroglucitol and postprandial hyperglycemia as measured by continuous glucose monitoring system in moderately controlled patients with diabetes. Diabetes Care 2006;29:1214–1219 [DOI] [PubMed] [Google Scholar]
- 36.Abbatecola AM, Rizzo MR, Barbieri M, et al. Postprandial plasma glucose excursions and cognitive functioning in aged type 2 diabetics. Neurology 2006;67:235–240 [DOI] [PubMed] [Google Scholar]
- 37.Bonora E, Corrao G, Bagnardi V, et al. Prevalence and correlates of post-prandial hyperglycaemia in a large sample of patients with type 2 diabetes mellitus. Diabetologia 2006;49:846–854 [DOI] [PubMed] [Google Scholar]
- 38.Parrinello CM, Lutsey PL, Couper D, et al. Total short-term variability in biomarkers of hyperglycemia in older adults. Clin Chem 2015;61:1540–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hazlehurst JM, Woods C, Marjot T, Cobbold JF, Tomlinson JW. Non-alcoholic fatty liver disease and diabetes. Metabolism 2016;65:1096–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de la Monte SM, Longato L, Tong M, Wands JR. Insulin resistance and neurodegeneration: roles of obesity, type 2 diabetes mellitus and non-alcoholic steatohepatitis. Curr Opin Investig Drugs 2009;10:1049–1060 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.