Type 1 diabetes (T1D) is associated with a 10-fold increase in cardiovascular disease (CVD) risk, due in part to dyslipidemia. Patients with poorly controlled T1D show an increase in total and LDL cholesterol as well as elevated apolipoprotein B (ApoB) levels. Moreover, even patients with well-controlled T1D can show increased levels of ApoB. Dyslipidemia is particularly important in females with T1D, as the atheroprotective lipoprotein profile and reduced risk of CVD usually observed in females without diabetes are lost in females with diabetes (1,2).
Proprotein convertase subtilisin/kexin type 9 (PCSK9) has recently emerged as a novel regulator of plasma cholesterol levels: gain-of-function PCSK9 mutations increase LDL cholesterol and CVD risk, whereas inhibitors of PCSK9 lower LDL cholesterol. Although PCSK9 is increased in individuals with obesity and type 2 diabetes (3,4), it is not known whether PCSK9 levels are also changed in individuals with T1D.
Here, we measured PCSK9 levels in a subset of a previously described cohort of T1D and control subjects (5). For the current study, subjects with serum available for PCSK9 measurement were matched for age and sex between each group using the frequency matching method. Subjects who reported taking statin medications at the time of the study were excluded from the analyses (n = 3, all from T1D group). Variance-stabilizing measures to transform nonnormal values were performed as appropriate for parametric analyses. χ2 analyses were performed to determine group differences for categorical variables. Independent t tests, one-way ANOVA, and ANCOVA were performed to test for mean differences between the groups. Correlations between PCSK9 and age, BMI, BMI z score, HbA1c, systolic blood pressure, diastolic blood pressure, triglycerides, total cholesterol, LDL cholesterol, HDL cholesterol, and ApoB were assessed in each group by measuring the Spearman correlation coefficient; in this case, to account for multiple testing, P < 0.002 was considered significant.
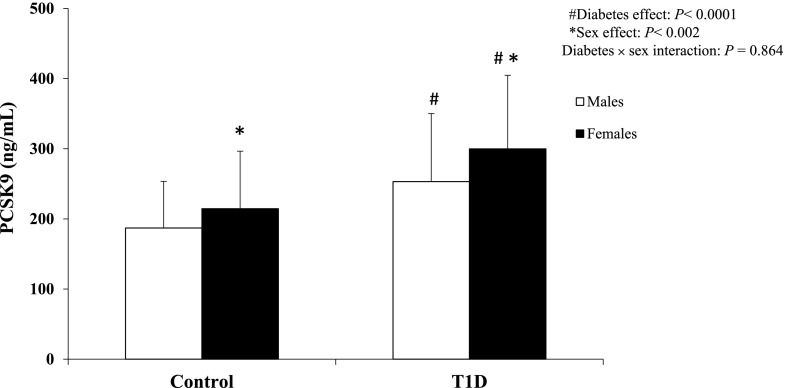
Patients with T1D showed higher systolic and diastolic blood pressure measurements and ApoB levels, but normal total cholesterol, triglycerides, LDL cholesterol, and HDL cholesterol (Table 1). Serum PCSK9 concentrations were significantly higher in females and individuals with T1D. Thus, PCSK9 levels increased from 187 ± 67 ng/mL in control group males to 215 ± 83 ng/mL in control group females, 253 ± 98 ng/mL in males with T1D, and 299 ± 106 ng/mL in females with T1D (Fig. 1) (P < 0.002 for the effects of sex; P < 0.0001 for the effects of T1D; and P = 0.864 for their interaction). In the control group, PCSK9 was significantly correlated with total cholesterol (rs = 0.51) and ApoB (rs = 0.40). In the T1D group, PCSK9 was significantly correlated with HbA1c (rs = 0.36), triglycerides (rs = 0.24), total cholesterol (rs = 0.39), LDL cholesterol (rs = 0.31), and ApoB (rs = 0.31).
Table 1.
Demographic and clinical characteristics of T1D case subjects and control subjects in the study population
| Patient characteristics | Control (n = 74) | T1D (n = 176) | P value* |
|---|---|---|---|
| Age (years) | 15.4 (2.2) | 15.2 (2.2) | 0.64 |
| Duration of diabetes (years) | — | 8.6 (2.9) | |
| Sex, female, n (%) | 42 (57) | 99 (56) | 0.92 |
| Race, % | 0.03 | ||
| Non-Hispanic white | 68 | 83 | |
| African American | 11 | 3 | |
| Hispanic | 11 | 6 | |
| Other | 10 | 9 | |
| Tanner stage, %¥ | 0.77 | ||
| 1 | 5 | 4 | |
| 2 | 13 | 10 | |
| 3 | 16 | 12 | |
| 4 | 24 | 25 | |
| 5 | 42 | 49 | |
| BMI (kg/m2) | 21.8 (4.2) | 22.4 (3.4) | 0.03 |
| BMI z score | 0.27 (1.0) | 0.55 (0.77) | 0.04 |
| HbA1c (%, mmol/mol) | 5.3 (0.3), 34 (2.9) | 9.0 (1.6), 75 (17.5) | <0.001 |
| Systolic BP (mmHg) | 109 (8) | 113 (9) | <0.001 |
| Diastolic BP (mmHg) | 64 (6) | 69 (6) | <0.001 |
| Total cholesterol (mg/dL) | 148 (28) | 159 (35) | 0.47 |
| LDL cholesterol (mg/dL) | 82 (23) | 90 (28) | 0.39 |
| HDL cholesterol (mg/dL) | 49 (9) | 52 (11) | 0.29 |
| Triglycerides (mg/dL) | 83 (42) | 87 (50) | 0.33 |
| ApoB (mg/dL) | 69 (19) | 77 (23) | 0.003 |
| PCSK9 (ng/mL) | 203 (77) | 279 (104) | 0.0001 |
Values are mean (SD), unless otherwise indicated. BP, blood pressure.
Comparisons between groups determined using t tests if variables were normally distributed or normality was achieved with log transformation. Boldface type indicates statistical significance where P < 0.05.
Tanner stage refers to pubic hair development by provider report.
Figure 1.
Comparison of PCSK9 levels by sex within each group. Data are mean and SD. Two-way ANOVA comparison results are also shown. #P < 0.0001, subjects with T1D vs. control subjects. *P < 0.002, females vs. males.
Three findings from this study should be noted. First, PCSK9 levels are higher in subjects with T1D than in control subjects without diabetes. The liver is the major source of serum PCSK9 (6), and insulin increases PCSK9 expression and secretion in hepatocytes (7). Consequently, plasma PCSK9 levels in adults with T1D are positively correlated with insulin dose (8). Conversely, mice with livers unable to respond to insulin because of a deletion of the hepatic insulin receptor show a marked decrease in plasma PCSK9 (9). Because individuals with T1D are relatively deficient in hepatic insulin signaling owing to the lack of endogenous insulin stimulation from the portal circulation, their increased levels of serum PCSK9 were somewhat surprising and suggest that factors other than insulin may also play an important role in the regulation of PCSK9. Second, among subjects with T1D, PCSK9 levels are correlated with HbA1c. Similar positive associations between PCSK9 and HbA1c have been observed in other T1D cohorts (8), and this result is consistent with the greater degree of dyslipidemia found in T1D patients with poor glycemic control. Finally, PCSK9 levels are higher among females, in both the control and T1D groups, consistent with prior studies of PCSK9 in other populations (3). It has been suggested that sex steroids may be responsible for the increase in PCSK9 observed in females (10). However, we compared PCSK9 levels in the female subjects who were Tanner stages 1–3 (prepubertal or early puberty, with presumed low sex steroid levels) versus Tanner stage 5 (postpuberty, or presumed high sex steroid levels) and found that neither the Tanner stage nor the interaction between Tanner stage and diabetes was significant.
In summary, our results in a youth cohort indicate that PCSK9 levels are higher in females compared with males, higher in subjects with T1D than in control subjects, and correlated with HbA1c. Future work will be necessary to understand the contribution of PCSK9 to the dyslipidemia and increased CVD risk associated with T1D, particularly in females.
Article Information
Funding. This study was supported by the National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases grant 5K12-DK-094721-04 (A.E.L.) and NIH National Heart, Lung, and Blood Institute grant R01-HL-109650 (S.B.B.). This research was also funded by JDRF (11-2007-694), NIH/National Center for Research Resources Colorado Clinical Translation and Science Institute (UL1-RR-025780), the Children’s Hospital Colorado Pediatric Clinical and Translational Research Center (M01-RR-00069), and the Diabetes Endocrinology Research Centers Core (P30-DK-57516). Its contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Duality of Interest. No conflicts of interest relevant to this study were reported.
Author Contributions. A.E.L., R.P.W., F.K.B., D.M.M., and S.B.B. designed the study. A.E.L. performed the PCSK9 assays and analyzed the data. F.K.B. facilitated sample and data transfer. P.R.K. conducted the statistical analysis. A.E.L. and S.B.B. wrote the manuscript. R.P.W., A.S.S., T.R.K., E.M.U., S.D.d.F., D.M.M., and L.M.D. reviewed and edited the manuscript. S.B.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
References
- 1.Walden CE, Knopp RH, Wahl PW, Beach KW, Strandness E Jr. Sex differences in the effect of diabetes mellitus on lipoprotein triglyceride and cholesterol concentrations. N Engl J Med 1984;311:953–959 [DOI] [PubMed] [Google Scholar]
- 2.Maahs DM, Hokanson JE, Wang H, et al. . Lipoprotein subfraction cholesterol distribution is proatherogenic in women with type 1 diabetes and insulin resistance. Diabetes 2010;59:1771–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lakoski SG, Lagace TA, Cohen JC, Horton JD, Hobbs HH. Genetic and metabolic determinants of plasma PCSK9 levels. J Clin Endocrinol Metab 2009;94:2537–2543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levenson AE, Shah AS, Khoury PR, et al. . Obesity and type 2 diabetes are associated with elevated PCSK9 levels in young women. Pediatr Diabetes. 17 January 2017 [Epub ahead of print]. DOI: 10.1111/pedi.12490 [DOI] [PMC free article] [PubMed]
- 5.Brown TL, Maahs DM, Bishop FK, Snell-Bergeon JK, Wadwa RP. Influences of gender on cardiovascular disease risk factors in adolescents with and without type 1 diabetes. Int J Pediatr Endocrinol 2016;2016:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaid A, Roubtsova A, Essalmani R, et al. . Proprotein convertase subtilisin/kexin type 9 (PCSK9): hepatocyte-specific low-density lipoprotein receptor degradation and critical role in mouse liver regeneration. Hepatology 2008;48:646–654 [DOI] [PubMed] [Google Scholar]
- 7.Costet P, Cariou B, Lambert G, et al. . Hepatic PCSK9 expression is regulated by nutritional status via insulin and sterol regulatory element-binding protein 1c. J Biol Chem 2006;281:6211–6218 [DOI] [PubMed] [Google Scholar]
- 8.Laugier-Robiolle S, Vergès B, Le Bras M, et al. . Glycaemic control influences the relationship between plasma PCSK9 and LDL cholesterol in type 1 diabetes. Diabetes Obes Metab 2017;19:448–451 [DOI] [PubMed] [Google Scholar]
- 9.Miao J, Manthena PV, Haas ME, et al. . Role of insulin in the regulation of proprotein convertase subtilisin/kexin type 9. Arterioscler Thromb Vasc Biol 2015;35:1589–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosh M, Gälman C, Rudling M, Angelin B. Influence of physiological changes in endogenous estrogen on circulating PCSK9 and LDL cholesterol. J Lipid Res 2015;56:463–469 [DOI] [PMC free article] [PubMed] [Google Scholar]