Abstract
OBJECTIVE
To determine the prevalence of and characteristics associated with metformin use among U.S. adults with prediabetes using the National Health and Nutrition Examination Survey (NHANES) 2005–2012.
RESEARCH DESIGN AND METHODS
The American Diabetes Association’s guidelines for metformin use in prediabetes have evolved, with 2017 recommendations suggesting metformin be considered in patients with prediabetes and additional risk factors (BMI ≥35 kg/m2, age <60 years, or prior gestational diabetes mellitus) or rising hemoglobin A1c (HbA1c). We estimated the age-adjusted prevalence of metformin use among individuals with prediabetes (defined by HbA1c 5.7–6.4%, fasting glucose 100–125 mg/dL, 2-h poststimulated glucose 140–199 mg/dL, or self-report) and used multivariate logistic regression to evaluate characteristics associated with metformin use.
RESULTS
Of 22,174 adults, 7,652 had prediabetes. The age-adjusted prevalence of metformin use among those with prediabetes was 0.7%. Metformin use was associated with higher mean BMI (35.1 kg/m2 vs. 29.6 kg/m2, P < 0.01) and higher glucose (fasting glucose 114 mg/dL vs. 105 mg/dL, P = 0.03; 2-h poststimulated glucose 155 mg/dL vs. 128 mg/dL, P = 0.003; and HbA1c 6.0% [42 mmol/mmol] vs. 5.6% [38 mmol/mmol], P < 0.01). Metformin use was low even among those with BMI ≥35 kg/m2, a group for whom metformin use is recommended. Metformin use did not vary by race, poverty-to-income ratio, or education.
CONCLUSIONS
Metformin use was <1% among U.S. adults with prediabetes and only slightly more common among those with additional risk factors for diabetes.
Introduction
Eighty-six million adults in the U.S. have prediabetes, and up to 70% of these individuals will eventually develop diabetes (1,2). In the Diabetes Prevention Program (DPP), the incidence of diabetes in the placebo group was 62% after a mean follow-up of 15 years (3). Considering the number of individuals who will progress from prediabetes to diabetes (2), there is limited evidence on whether patients with prediabetes are being managed with metformin. The American Diabetes Association (ADA) recommends an intensive diet and physical activity behavioral counseling program for all patients with prediabetes and suggests that metformin be considered in patients with additional risk factors (BMI ≥35 kg/m2, age <60 years, or history of gestational diabetes), largely based on subgroup analyses from the DPP (4,5). In 2017, the ADA also recommended that metformin be considered in those with prediabetes and a rising hemoglobin A1c (HbA1c) despite lifestyle intervention.
The prevalence of metformin use for prediabetes and the characteristics of patients currently being prescribed metformin for prediabetes are not well described. A prior study using National Health and Nutrition Examination Survey (NHANES) data demonstrated that >96% of identified individuals with impaired fasting glucose and impaired glucose tolerance fulfilled older, broader criteria for metformin consideration (6). Although the majority of people with prediabetes likely qualify for metformin use based on age and BMI (7), recent analyses conducted in selected populations in the U.S. have shown that metformin use in prediabetes is low (3.7% from claims data from UnitedHealthcare and <0.1% from electronic health data from Kaiser Permanente) (8,9).
Using data from NHANES 2005–2012, we evaluated the prevalence of metformin use and the characteristics associated with metformin use among U.S. adults with prediabetes. We hypothesized that the prevalence of metformin use for prediabetes would be low (<5%) and that higher BMI, younger age, comorbid conditions, and higher glucose and HbA1c levels would be associated with use of metformin among adults with prediabetes.
Research Design and Methods
Data Collection
We examined data from four consecutive 2-year cycles (2005–2006, 2007–2008, 2009–2010, and 2011–2012) of NHANES to estimate the prevalence of metformin use in prediabetes. NHANES is a biannual, stratified, multistage probability sample of the U.S. civilian, noninstitutionalized population (10). Participants undergo an in-home interview and a visit to a mobile examination center; nonresponse is accounted for in the sampling weights.
Age, race, sex, education level, health insurance coverage, smoking status, comorbid conditions (hypertension, coronary heart disease, and/or heart attack), and family history of diabetes were obtained through the household questionnaire. We used the poverty-to-income ratio, the ratio of a family’s income to the poverty threshold, to assess income level (11).
During the household interviews, interviewers asked the survey participants if they had taken prescription medications in the past month (12). If yes, the participant was asked to show the interviewer all medication containers. Medication names were entered and automatically matched to a prescription drug database. If a medication container was not available, the participant was asked to verbally list the medication name. The interviewer’s original entry and matched database drug name were saved under separate variables for quality control purposes (12). Participants were asked if they had ever been told by a doctor or health professional that they have “diabetes or sugar diabetes” with possible responses of “yes,” “no,” or “borderline.” If they answered “no,” then they were asked if they had ever been told any of the following: that they had “prediabetes, impaired fasting glucose, impaired glucose tolerance, borderline diabetes or that your blood sugar is higher than normal but not high enough to be called diabetes or sugar diabetes.”
For each mobile examination, participants were randomly selected to attend a morning, afternoon, or evening session at which height, weight, and systolic blood pressure were measured. HbA1c levels were measured at all sessions. Fasting triglycerides, LDL cholesterol, and fasting and 2-h plasma glucose from an oral glucose tolerance test were measured at the morning sessions only.
All study participants provided written informed consent. Study protocols were approved by the research ethics board of the National Center for Health Statistics.
Population
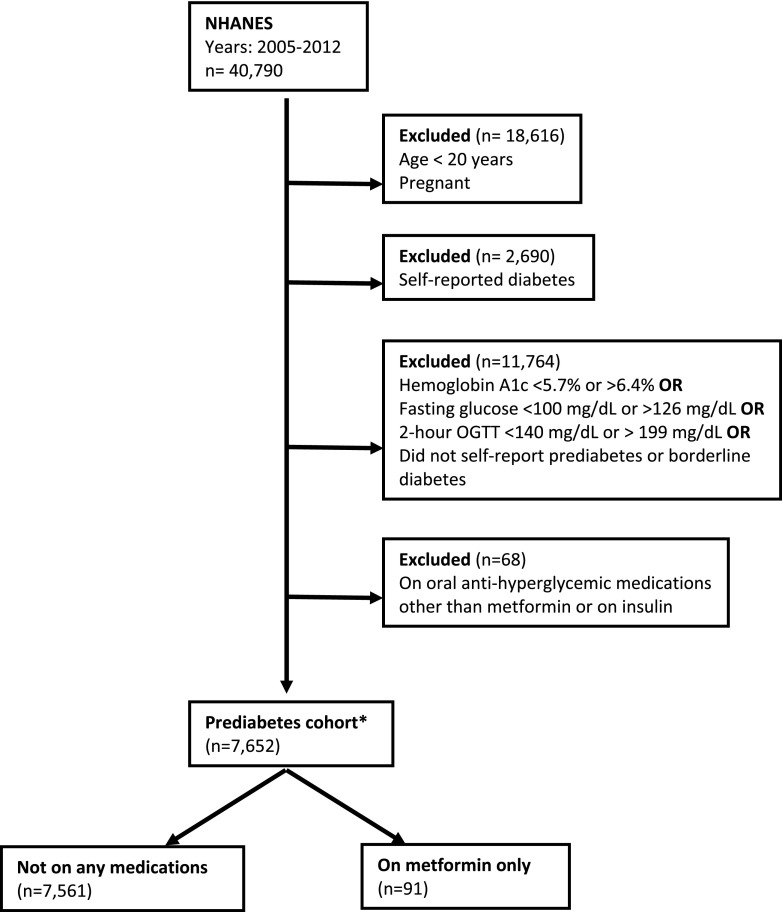
Participants were considered to have prediabetes based on 1) laboratory values (HbA1c 5.7–6.4% [39–46 mmol/mmol], fasting plasma glucose 100–125 mg/dL, or 2-h poststimulated plasma glucose 140–199 mg/dL) (13) without meeting any laboratory criteria for diabetes or 2) self-reported “borderline” diabetes or prediabetes as described above. We excluded persons younger than 20 years of age, those with self-reported diabetes, pregnant women, and persons who were on diabetes medications besides just metformin (Fig. 1).
Figure 1.
Study flow diagram. *Age-adjusted prevalence of prediabetes among adults was 40.8% (undiagnosed prediabetes, 36.4%; self-reported prediabetes, 4.6%). OGTT, oral glucose tolerance test.
Statistical Analysis
Our primary outcome of interest was the prevalence of metformin use among people with prediabetes. We estimated the age-adjusted prevalence of prediabetes in the overall population and the age-adjusted prevalence of metformin use among individuals with prediabetes using the 2000 Census population as the standard population structure (14). Sample weights from NHANES were used to represent the total civilian, noninstitutionalized U.S. population and to account for the complex survey design: we used the household interview weights for self-reported prediabetes, the mobile examination center weights for HbA1c, and the fasting plasma glucose and oral glucose tolerance test weights for fasting glucose and 2-h stimulated glucose, respectively (7). We used Taylor series linearization methods for variance estimation. We compared means and proportions using linear and logistic regression, respectively, in bivariate models. In the multivariate model, we adjusted for variables that were significantly associated with metformin use in bivariate analyses (insurance status, hypertension, overweight, HbA1c, and BMI).
Additionally, we examined the percentage of individuals who were on metformin based on different criteria for metformin use (Table 3). We also looked at metformin use by survey cycle and used survey-weighted logistic regression to calculate a P trend across survey cycles (15).
Table 3.
Percentage of individuals with prediabetes reporting metformin use by criteria for metformin use
| Risk factors for prediabetes | n* | N† | % (SE) on metformin‡ |
|---|---|---|---|
| Prediabetes by IFG, IGT, HbA1c, or self-report | 91 | 7,652 | 0.8 (0.008) |
| Prediabetes based on IGT alone | 1 | 285 | 0.8 (0.008) |
| Prediabetes based on IFG alone | 0 | 1,577 | 0 |
| Prediabetes based on HbA1c 5.7–6.4% alone | 18 | 3,065 | 0.6 (0.002) |
| Prediabetes based on HbA1c 6.0–6.4% alone | 13 | 1,411 | 0.9 (0.003) |
| Self-reported prediabetes alone | 0 | 0 | 0 |
| BMI ≥35 kg/m2 | 33 | 1,316 | 1.9 (0.005) |
| IFG and IGT + risk factor§ | 0 | 0 | 0 |
| IGT + risk factor‖ | 1 | 190 | 1.1 (0.1) |
| IFG + risk factor‖ | 0 | 1,178 | 0 |
| HbA1c 5.7–6.4% + risk factor‖ | 13 | 2,101 | 0.7 (0.002) |
| Self-reported prediabetes + risk factor‖ | 0 | 0 | 0 |
| IFG and IGT + risk factor‖ + HbA1c 6.0–6.4% | 0 | 179 | 0 |
IFG, impaired fasting glucose; IGT, impaired glucose tolerance.
*Represents unweighted n for those treated with metformin.
†Represents unweighted n for those treated or not treated with metformin.
‡Represents weighted percentage.
§2007 guidelines risk factors: age <60 years, BMI ≥35 kg/m2, family history of diabetes, elevated triglycerides, low HDL cholesterol, hypertension, or HbA1c ≥6%. We defined elevated triglycerides as ≥150 mg/dL and low HDL cholesterol as <40 mg/dL based on Adult Treatment Panel III guidelines (30).
‖2017 guidelines risk factors: age <60 years, BMI ≥35 kg/m2, or history of gestational diabetes mellitus (not available in this data set) and rising HbA1c despite lifestyle intervention (not available in this data set).
We performed a sensitivity analysis estimating the prevalence of metformin use among individuals with prediabetes defined by HbA1c criteria only. We also conducted two subgroup analyses of individuals with prediabetes who were on metformin stratified by BMI (≥35 and <35 kg/m2) and age (<60 and ≥60 years). Statistical analyses were performed using STATA software (version 13.1; StataCorp, College Station, TX) survey modules. All tests of significance were two tailed, with α-levels of 0.05.
Results
We identified 91 participants with prediabetes who were taking only metformin and 7,561 participants with prediabetes who were not taking any diabetes medications (age-adjusted prevalence of metformin use 0.7%) (Fig. 1). The age-adjusted prevalence of metformin use among individuals with prediabetes defined by HbA1c only was 1.0%. Metformin use for prediabetes was not associated with younger age but was associated with higher mean BMI (35.1 vs. 29.6 kg/m2, P < 0.01) and higher glucose (fasting glucose 114 vs. 105 mg/dL, P = 0.03; HbA1c 6.0% [42 mmol/mmol] vs. 5.6% [38 mmol/mmol], P < 0.01; 2-h poststimulated glucose 155 vs. 128 mg/dL, P = 0.003). More people with prediabetes who were on metformin had an HbA1c ≥6% compared with those not on metformin (51.0 vs. 14.5%; P < 0.001). In the multivariable model, adults with prediabetes on metformin had significantly higher odds of having an HbA1c ≥6 vs. <6% (adjusted odds ratio [OR] 4.56 [95% CI 2.48, 8.37]) and being informed of overweight status by a doctor (adjusted OR 3.27 [95% CI 1.68, 6.37]) (Table 2).
Table 2.
Adjusted ORs for characteristics associated with metformin use (versus nonuse of metformin) in prediabetes (n = 7,464)
| Characteristic | OR (95% CI)* | P |
|---|---|---|
| HbA1c ≥6% (ref. HbA1c <6%) | 4.56 (2.48, 8.37) | <0.001 |
| Have health insurance (ref. no health insurance) | 2.28 (0.85, 6.14) | 0.10 |
| History of hypertension (ref. no history of hypertension) | 1.22 (0.57, 2.59) | 0.61 |
| Identified as overweight by doctor (ref. not identified as overweight by doctor) | 3.27 (1.68, 6.37) | 0.001 |
| BMI ≥35 kg/m2 (ref. BMI <35 kg/m2) | 1.63 (0.78, 3.42) | 0.19 |
*Each OR adjusted for additional covariates: HbA1c ≥6%, health insurance status, history of hypertension, informed of overweight status by doctor, and BMI ≥35 kg/m2.
In our additional analyses, we found that the majority of people with prediabetes on metformin met more than one laboratory criterion for prediabetes (fasting glucose, HbA1c, or 2-h poststimulation plasma glucose) (Supplementary Table 1). While the additional risk factors delineated in the 2007 and 2017 ADA recommendations for metformin use were more common among those on metformin (Table 1), most people with those risk factors were not on metformin (Table 3).
Table 1.
Characteristics of U.S. adults with prediabetes on metformin compared with those not on diabetes preventive medication
| Characteristic | Prediabetes, on metformin (n = 91)* |
Prediabetes, not on any diabetes preventive medication (n = 7,561)† |
P‡ | ||
|---|---|---|---|---|---|
| Mean; SE or % (SE)§ | N‖ | Mean; SE or % (SE)§ | N‖ | ||
| Age (years) | 54.5; 1.9 | 50.8; 0.4 | 0.06 | ||
| Age <60 years | 63.3 (0.06) | 40 | 69.4 (0.009) | 4,372 | 0.30 |
| Female | 55.7 (0.08) | 47 | 45.9 (0.007) | 3,570 | 0.22 |
| Race | |||||
| White | 69.0 (0.05) | 38 | 70.2 (0.02) | 3,443 | |
| Black | 10.5 (0.03) | 20 | 10.4 (0.009) | 1,657 | |
| Other/multiracial | 7.8 (0.03) | 6 | 6.4 (0.006) | 562 | 0.66 |
| Mexican American | 7.5 (0.03) | 16 | 8.3 (0.009) | 1,198 | |
| Other Hispanic | 5.1 (0.02) | 11 | 4.7 (0.006) | 701 | |
| Have health insurance coverage | 92.0 (0.03) | 82 | 81.1 (0.009) | 5,837 | 0.008 |
| Education | |||||
| More than high school | 57.7 (0.07) | 36 | 55.7 (0.01) | 3,465 | |
| High school grad/GED or equivalent | 23.5 (0.06) | 23 | 24.6 (0.008) | 1,852 | 0.76 |
| Less than high school | 18.8 (0.04) | 32 | 19.6 (0.008) | 2,233 | |
| Income | |||||
| >200% of poverty level | 61.3 (0.07) | 41 | 62.1 (0.01) | 3,598 | |
| 100–200% of poverty level | 22.6 (0.06) | 26 | 19.6 (0.008) | 1,952 | 0.38 |
| Lower than poverty level | 13.4 (0.04) | 20 | 12.1 (0.006) | 1,371 | |
| BMI (kg/m2) | 35.1; 1.4 | 29.6; 0.1 | 0.005 | ||
| BMI (kg/m2) | |||||
| <35 | 53.6 (0.08) | 53 | 82.9 (0.007) | 6,115 | <0.001 |
| ≥35 | 46.4 (0.08) | 34 | 17.1 (0.007) | 1,330 | |
| Systolic blood pressure (mmHg) | 126.3; 2.4 | 124.1; 0.3 | 0.38 | ||
| Cholesterol (mg/dL) | |||||
| Total | 190.6; 6.7 | 201.0; 0.8 | 0.14 | ||
| HDL | 49.0; 2.2 | 52.0; 0.3 | 0.20 | ||
| LDL¶ | 116.5; 6.5 | 120.2; 0.8 | 0.56 | ||
| Triglycerides¶ | 171.1; 26.6 | 140.7; 2.3 | 0.33 | ||
| HbA1c, % (mean in mmol/mol) | 6.0; 0.07 (42) | 5.6; 0.009 (38) | <0.001 | ||
| HbA1c ≥6% | 51.0 (0.07) | 56 | 14.5 (0.006) | 1,879 | <0.001 |
| Fasting plasma glucose (mg/dL)¶ | 114.2; 4.0 | 105.1; 0.2 | 0.01 | ||
| 2-h poststimulation plasma glucose (mg/dL)¶ | 155.4; 8.7 | 127.7; 1.0 | 0.003 | ||
| Smoking | |||||
| Current smoker | 14.4 (0.06) | 12 | 21.1 (0.008) | 1,617 | |
| Ever smoker | 41.0 (0.07) | 36 | 27.4 (0.009) | 2,092 | 0.70 |
| Never smoker | 44.5 (0.07) | 43 | 51.5 (0.01) | 3,848 | |
| History of hypertension | 56.0 (0.08) | 61 | 36.2 (0.008) | 3,118 | 0.02 |
| History of heart disease and/or heart attack | 11.7 (0.05) | 9 | 6.0 (0.004) | 549 | 0.26 |
| Identified as overweight by doctor | 74.3 (0.05) | 61 | 36.3 (0.009) | 2,768 | <0.001 |
| Family history of diabetes | 41.3 (0.07) | 47 | 38.3 (0.007) | 3,006 | 0.67 |
GED, General Educational Development.
*N for subjects on metformin by variable: income, BMI, and systolic blood pressure, n = 87; HDL/total cholesterol, n = 84; history of heart attack, n = 89; family history of diabetes, n = 90.
†N for subjects not on metformin by variable: insurance, n = 7,554; education, n = 7,550; income, n = 6,921; BMI, n = 7,445; systolic blood pressure, n = 7,217; HDL/total cholesterol, n = 7,422; smoking, n = 7,557; hypertension, n = 7,548; history of heart attack, n = 7,523; overweight, n = 7,555; family history of diabetes, n = 7,392.
‡P value for comparison of means or proportions using linear or logistic regression, respectively, accounting for sampling weights.
§Represents weighted means or percentage.
‖Represents unweighted n.
¶Fasting laboratories available only for subjects who attended morning session: fasting glucose, n = 47 for metformin and n = 4,752 for no metformin; 2-h poststimulation plasma glucose, n = 5 for metformin and n = 4,192 for no metformin; for triglycerides, n = 46 for metformin and n = 4,725 for no metformin; for LDL cholesterol, n = 46 for metformin and n = 4,623 for no metformin.
We found that adults with prediabetes on metformin and BMI ≥35 kg/m2 tended to be white, have a smaller poverty-to-income ratio, and have lower mean HbA1c and fasting glucose compared with their counterparts with BMI <35 kg/m2 (Supplementary Table 2). In stratified analyses by age, individuals on metformin and age <60 years tended to have a higher mean BMI, higher HbA1c, and 2-h poststimulation plasma glucose; lower systolic blood pressure; and less insurance coverage and were less likely to have a history of heart disease or heart attack (Supplementary Table 3).
Compared with individuals not on any medications, adults with prediabetes on metformin were more likely to have health insurance coverage (92.0 vs. 81.1%; P < 0.01) and have comorbid conditions (self-reported hypertension 56.0 vs. 36.2%, P = 0.02) (Table 1), but these risk factors were not significantly associated with metformin use after multivariate adjustment (Table 2). Metformin use did not vary based on race, poverty-to-income ratio, or education level (Table 1). When examined by individual survey cycle year, metformin use was lowest during 2005–2006, with 0.4%, compared with subsequent cycles but did not vary significantly across cycles (P for trend = 0.17) (Supplementary Table 4).
Conclusions
Using nationally representative data from NHANES 2005–2012, we found that metformin use was uncommon, with <1% of U.S. adults with prediabetes reporting metformin use. Our findings are well below the expected usage of metformin in prediabetes based on risk factors (of those with prediabetes and not on metformin, 69% were younger than 60 years and 17% had a BMI ≥35 kg/m2). We did not find a significant secular trend in the prevalence of metformin use by NHANES cycle (Supplementary Table 4). The small number of people with prediabetes who were treated with metformin tended to have a higher HbA1c and have been informed of overweight status by a doctor. We did not identify disparities in metformin use based on race, poverty-to-income ratio, or education. Although people with prediabetes on metformin had a higher BMI compared with those not on metformin, having a BMI ≥35 kg/m2 was not independently associated with a higher prevalence of metformin use despite the ADA recommendations (4). Metformin use was not much more common among those with clear risk factors for progression to diabetes (as evident in Table 3), highlighting a missed opportunity for diabetes prevention.
Our findings add to the two prior studies published by Schmittdiel et al. (8) and Moin et al. (9), which used electronic health data (Kaiser) and health insurance claims data (UnitedHealthcare), respectively, to examine a similar question (8,9). Both studies showed that metformin use was low (<0.1–3.7%), but their findings were only generalizable to specific insured populations. Moin et al. (9) demonstrated that female sex, obesity, and a higher number of comorbid conditions were associated with metformin use. Additional factors were not evaluated given the limitations of claims data. In our study, we used a nationally representative database that includes prescription medication data that was confirmed during the in-person interview. Therefore, we avoided misclassification of metformin use, a limitation highlighted in one of the prior studies (9). Similar to prior analyses of NHANES, awareness of prediabetes status was low (16).
ADA recommendations for the consideration of pharmacotherapy for the prevention of diabetes have evolved over the past 10 years. In 2005, the ADA did not recommend metformin use for diabetes prevention because it was unclear that it was cost-effective (17). In 2007, the ADA convened an expert panel that concluded that metformin could be considered for people with impaired fasting glucose and impaired glucose tolerance with an additional risk factor, specifically, age <60 years, BMI ≥35 kg/m2, diabetes in first-degree relatives, elevated triglycerides, low HDL cholesterol, hypertension, or HbA1c ≥6.0% (18). Since 2012, the ADA has recommended that metformin be considered in those with impaired fasting glucose, impaired glucose tolerance, or a HbA1c of 5.7–6.4%, especially in individuals age <60 years, with BMI ≥35 kg/m2, or with a history of gestational diabetes mellitus (4). Clinicians may also be considering metformin based on a patient’s response to lifestyle intervention and may start metformin if a patient is not successful in lowering weight or fasting glucose with lifestyle change. Some evidence supports this strategy: a prior analysis of the DPP showed that it may be reasonable to consider metformin based on the amount of weight loss after 6 months of lifestyle change (19). As of 2017, the ADA added that metformin should be considered in those with rising HbA1c who are not responding to initial lifestyle change (4). It is important to note that the evidence on diabetes prevention underlying the ADA’s recommendations is based mainly on the DPP, which required participants to have elevated fasting glucose (95–125 mg/dL) and impaired glucose tolerance; therefore, the benefits of the lifestyle intervention and metformin may both be different for the population meeting criteria for prediabetes based on one abnormal test that is usually not a glucose tolerance test. In particular, while HbA1c was not used as an inclusion criterion for the DPP, it is likely that HbA1c is now being used to screen for prediabetes given the availability of diagnostic categories for prediabetes and diabetes based on HbA1c since 2010 (20). HbA1c does have some limitations in defining hyperglycemia in certain situations (e.g., anemia, specific medication use, different racial groups) and has high specificity but low sensitivity for diagnosing prediabetes and diabetes; this should also be considered in the interpretation of our results and in future work (21,22).
The argument that often has been raised against metformin therapy for prediabetes is that we are putting patients who are at risk for diabetes on a medication that treats diabetes and exposing them to a drug (and its potential side effects) for more time than is necessary. Furthermore, we know there is clinical inertia in treating and advancing therapy in diabetes (23,24), and we expect for this clinical inertia to be even greater with preventive therapy for prediabetes. However, first, prediabetes is associated with premature mortality (25) and autonomic neuropathy and idiopathic polyneuropathy (2). Second, the recommended initial management of both patients with diabetes and prediabetes is lifestyle change. Lifestyle change, irrespective of weight loss, has been shown to decrease the risk of developing diabetes (26). However, in the DPP, 35% of participants in the intensive lifestyle intervention arm were unable to achieve at least 5% weight loss in the first 6 months of the trial (5). If we apply that failure rate to the population of 86 million adults in the U.S. who have prediabetes, then there are roughly 30 million adults who might fail to achieve the prevention target for weight loss and could potentially benefit from metformin. Third, metformin has been widely studied and has a limited side effect profile, most commonly diarrhea, flatulence, nausea, and vomiting (average 28 vs. 16% in placebo arm, P = 0.01, during the DPP) (27). No cases of lactic acidosis were reported in the DPP Outcomes Study (27). Fourth, the 15-year follow-up of the DPP demonstrated that metformin reduced the incidence of diabetes significantly compared with placebo (hazard ratio 0.82 [95% CI 0.72, 0.93]; P = 0.001) (3). Fifth, the DPP Outcomes Study demonstrated that achieving a normal glucose level at least once, even if not sustained, can decrease the risk of developing diabetes compared with individuals who consistently had prediabetes (28). It is important to note that in the DPP Outcomes Study, while persons who did not develop diabetes had a lower risk of microvascular complications than those who did develop diabetes, to date, data are still lacking on whether diabetes prevention lowers the risk of macrovascular complications (3).
There are several limitations to this study. Using a single laboratory test to define prediabetes may overestimate the prevalence of prediabetes (and therefore underestimate the prevalence of metformin use in prediabetes); this would be of most concern with fasting and stimulated glucoses given their known variability (29). However, we also incorporated HbA1c data into our definition, which does not have significant variability. In our sensitivity analysis, we found that the prevalence of metformin use remained low (1.0%) even when we defined prediabetes by HbA1c only. History of gestational diabetes mellitus was not available for the entire cohort so was not included in this analysis. We were also unable to examine behavioral factors such as participation in a weight loss program, physical activity, or weight loss attempts. We included self-reported prediabetes in our definition of prediabetes, so it is possible that some of these people may have progressed to diabetes based on their laboratory results. However, all of the people with self-reported prediabetes had at least one laboratory result consistent with prediabetes (Supplementary Table 1). Finally, NHANES data may not be completely reflective of actual clinical practice, and the risk of diabetes in clinical populations may differ. However, using NHANES data allows for nationally representative estimates.
Overall, we found that the vast majority of U.S. adults with prediabetes, including those at high risk of developing diabetes, do not report use of metformin as preventive pharmacotherapy. Metformin is an effective, safe, and low-cost preventive pharmacotherapy option for patients with prediabetes. Ongoing efforts to reduce the risk of type 2 diabetes through intensive lifestyle change are important but must be augmented by other approaches. Further study on how to implement metformin as a preventive intervention for type 2 diabetes is needed to truly stem the tide of the diabetes epidemic.
Supplementary Material
Article Information
Funding. E.T. is supported by National Institutes of Health Clinical Center training grant T32 90047355. This study received analytic support from the Baltimore Diabetes Research Center (National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Disease, grant no. P30-DK-079637).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. E.T. analyzed data and wrote the manuscript. H.-C.Y. reviewed and edited the manuscript. N.M.M. contributed to the discussion and reviewed and edited the manuscript. E.T. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 76th Scientific Sessions of the American Diabetes Association, New Orleans, LA, 10–14 June 2016.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc16-1509/-/DC1.
This article is featured in a podcast available at http://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1.Centers for Disease Control and Prevention (CDC) Awareness of prediabetes--United States, 2005-2010. MMWR Morb Mortal Wkly Rep 2013;62:209–212 [PMC free article] [PubMed] [Google Scholar]
- 2.Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet 2012;379:2279–2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diabetes Prevention Program Research Group Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol 2015;3:866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Diabetes Association Prevention or delay of type 2 diabetes. Sec. 5. In Standards of Medical Care in Diabetes—2017. Diabetes Care 2017;40(Suppl. 1):S44–S47 [DOI] [PubMed] [Google Scholar]
- 5.Knowler WC, Barrett-Connor E, Fowler SE, et al.; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rhee MK, Herrick K, Ziemer DC, et al. Many Americans have pre-diabetes and should be considered for metformin therapy. Diabetes Care 2010;33:49–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA 2015;314:1021–1029 [DOI] [PubMed] [Google Scholar]
- 8.Schmittdiel JA, Adams SR, Segal J, et al. Novel use and utility of integrated electronic health records to assess rates of prediabetes recognition and treatment: brief report from an integrated electronic health records pilot study. Diabetes Care 2014;37:565–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moin T, Li J, Duru OK, et al. Metformin prescription for insured adults with prediabetes from 2010 to 2012: a retrospective cohort study. Ann Intern Med 2015;162:542–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National Health and Nutrition Examination Survey: plan and operations, 1999-2010. Vital Health Stat 1 2013;1:1–37 [PubMed]
- 11.National Health and Nutrition Examination Survey. 2005 - 2006 data documentation, codebook, and frequencies: demographic variables and sample weights (DEMO_D) [Internet], 2007. Available from https://wwwn.cdc.gov/Nchs/Nhanes/2005-2006/DEMO_D.htm#INDFMPIR. Accessed 15 March 2016
- 12.National Health and Nutrition Examination Survey. 2011 - 2012 data documentation, codebook, and frequencies: prescription medications (RXQ_RX_G) [Internet], 2014. Available from http://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/RXQ_RX_G.htm. Accessed 4 October 2016
- 13.American Diabetes Association Classification and diagnosis of diabetes. Sec. 2. In Standards of Medical Care in Diabetes—2015 Diabetes Care 2015;38(Suppl.):S8–S16 [DOI] [PubMed] [Google Scholar]
- 14.Task 1c: how to generate age-adjusted proportions or prevalence rates and means using Stata [Internet]. Available from http://www.cdc.gov/nchs/tutorials/NHANES/NHANESAnalyses/AgeStandardization/Task1c.htm. Accessed 20 October 2015
- 15.Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA 2015;314:1818–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karve A, Hayward RA. Prevalence, diagnosis, and treatment of impaired fasting glucose and impaired glucose tolerance in nondiabetic U.S. adults. Diabetes Care 2010;33:2355–2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Diabetes Association Standards of medical care in diabetes. In Clinical Practice Recommendations—2005. Diabetes Care 2005;28(Suppl. 1):S4–S36 [PubMed] [Google Scholar]
- 18.Nathan DM, Davidson MB, DeFronzo RA, et al.; American Diabetes Association . Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care 2007;30:753–759 [DOI] [PubMed] [Google Scholar]
- 19.Maruthur NM, Ma Y, Delahanty LM, et al.; Diabetes Prevention Program Research Group . Early response to preventive strategies in the Diabetes Prevention Program. J Gen Intern Med 2013;28:1629–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Diabetes Association Standards of medical care in diabetes--2010. In Clinical Practice Recommendations—2010. Diabetes Care 2010;33(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hare MJL, Shaw JE, Zimmet PZ. Current controversies in the use of haemoglobin A1c. J Intern Med 2012;271:227–236 [DOI] [PubMed] [Google Scholar]
- 22.Olson DE, Rhee MK, Herrick K, Ziemer DC, Twombly JG, Phillips LS. Screening for diabetes and pre-diabetes with proposed A1C-based diagnostic criteria. Diabetes Care 2010;33:2184–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ziemer DC, Miller CD, Rhee MK, et al. Clinical inertia contributes to poor diabetes control in a primary care setting. Diabetes Educ 2005;31:564–571 [DOI] [PubMed] [Google Scholar]
- 24.Bolen SD, Bricker E, Samuels TA, et al. Factors associated with intensification of oral diabetes medications in primary care provider-patient dyads: a cohort study. Diabetes Care 2009;32:25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seshasai SR, Kaptoge S, Thompson A, et al.; Emerging Risk Factors Collaboration . Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011;364:829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamman RF, Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 2006;29:2102–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diabetes Prevention Program Research Group Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care 2012;35:731–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phillips LS, Ratner RE, Buse JB, Kahn SE. We can change the natural history of type 2 diabetes. Diabetes Care 2014;37:2668–2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selvin E, Crainiceanu CM, Brancati FL, Coresh J. Short-term variability in measures of glycemia and implications for the classification of diabetes. Arch Intern Med 2007;167:1545–1551 [DOI] [PubMed] [Google Scholar]
- 30.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143–3421 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.