Abstract
Aging is associated with changes in numerous homeostatic functions, such as food intake, that are thought to be mediated by the hypothalamus. Orexin/hypocretin neurons of the hypothalamus regulate several physiological functions, including feeding, sleep and wakefulness. Evidence from both clinical and animal studies supports the notion that aging is associated with loss or dysregulation of the orexin system. Here, we used virus-mediated gene transfer to manipulate expression of orexin peptides in young and aged rats and examined behavioral and neurochemical correlates of food intake in these animals. Aged rats showed slower feeding latencies when presented with palatable food compared to young control rats, and these deficits were ameliorated by upregulation of orexin expression. Similarly, young animals treated with a virus designed to decrease preproorexin expression showed longer feeding latencies reminiscent of aged control rats. Feeding was also associated with increased acetylcholine, glutamate and GABA efflux in insular cortex of young control animals. Orexin upregulation did not restore deficits in feeding-elicited release of these neurotransmitters in aged rats, but did enhance basal neurotransmitter levels which may have contributed to the behavioral correlates of these genetic manipulations. These studies demonstrate that age-related deficits in behavioral and neurochemical measures of feeding are likely to be mediated, in part, by the orexin system. Because these same neurotransmitter systems have been shown to underlie orexin effects on cognition, treatments which increase orexin function may have potential for improving both physiological and cognitive manifestations of certain age-related disorders.
Keywords: orexin, hypocretin, insular cortex, microdialysis, feeding, aging
Introduction
Aging is associated with changes in physiological regulatory functions such as food intake, energy balance and sleep patterns (Bonnet and Arand, 1989, Morley, 1997, Espiritu, 2008, Manini, 2010). Although all of these functions are affected by both peripheral and central mechanisms, they are presumed to result, at least in part, from alterations in brain regions that underlie appropriate endocrine, behavioral and cognitive responses to whole-organism homeostatic challenges. Additionally, translational and clinical studies have demonstrated a relationship between homeostatic dysfunction in aging and subsequent cognitive decline, suggesting the possibility of common neurobiological mediators of these outcomes (Buchman et al., 2005, Johnson et al., 2006, Altena et al., 2010, Fadel et al., 2013).
The hypothalamus is the primary CNS node for homeostatic regulation and animal studies support a role for hypothalamic dysfunction in age-related alterations in metabolism and food intake (Wolden-Hanson, 2006). The hypothalamus is also highly heterogeneous, containing dozens of named nuclei and even more distinct neuronal populations as characterized by neurotransmitter or neuropeptide expression. The orexin (hypocretin) neuropeptide system, found within the lateral hypothalamus and perifornical area, has received much interest since its discovery in the late 1990’s for its role in regulating sleep-wake cycles, food intake, and stress and reward functions (de Lecea et al., 1998, Sakurai et al., 1998). Orexin neurons are lost in human narcolepsy with cataplexy (Siegel, 1999, Nishino et al., 2000) and are responsive to fluctuating levels of peripheral cues related to energy balance such as glucose (Burdakov et al., 2006) and leptin (Leinninger et al., 2011). An extensive literature has also implicated the orexin system in reward properties of abused drugs and natural reinforcers (Harris and Aston-Jones, 2006, Martin-Fardon et al., 2016) which may, in part, reflect orexin interactions with other neural signaling pathways classically associated with reward, such as dopamine or opioids (Espana, 2012, Muschamp et al., 2014). This diverse array of orexin-mediated functions has led to their description as “physiological regulators” that engage arousal and motivated behavior in response to whole organism homeostatic status (de Lecea et al., 2002, Li et al., 2014, Mahler et al., 2014). The potential significance of this neuropeptide system for age-related alterations in physiological regulation is further bolstered by accumulating literature demonstrating reductions in orexin neuron number, peptide expression, and receptor expression in aged animal models (Terao et al., 2002, Porkka-Heiskanen et al., 2004, Kessler et al., 2011). Clinical data suggests that human aging is also associated with alterations in orexin function that might be particularly exacerbated in disease states that comprise dysfunctions in both physiological regulation and cognition (Thannickal et al., 2007, Fronczek et al., 2012).
Orexin neurons project to a diverse array of forebrain targets that mediate the roles of these peptides in motivated behavior and arousal (Peyron et al., 1998). Several cortical areas are included in these projections, including the insular cortex (Hollander et al., 2008). The insular cortex is a site of integration of visceral and emotional processing, which has led to its description in primates as “interoceptive cortex”—a brain region that plays a key role in the conscious awareness of physiological status(Craig, 2002). This description is also consistent with demonstrated roles of the insular cortex in olfaction and gustation, which are also important for food intake (Augustine, 1996, Rolls, 2005, 2015).
In addition to receiving orexin afferents from the hypothalamus, the insular cortex receives input from several neuromodulatory transmitters, including the Ch4 subdivision of the basal forebrain cholinergic system (BFCS). Although Ch4 projections to the cortex are diffuse, reciprocal inputs to the BFCS from cortex are not, deriving disproportionately from a few areas which include the agranular insular cortex (Mesulam and Mufson, 1984, Zaborszky et al., 1997). Given the role of the BFCS in attentional processing, this circuitry may be important for biasing attentional resources toward external stimuli related to underlying physiological status. In addition to acetylcholine, however, effects of feeding-related stimuli on the insular cortex are likely to involve other neurotransmitter systems, including the primary fast-acting amino acid neurotransmitters, glutamate and GABA. Orexins modulate release of all three of these neurotransmitters in a variety brain regions and experimental contexts (John et al., 2003, Fadel et al., 2005, Thorpe et al., 2006, Stanley and Fadel, 2011).
Here, we altered expression of prepro-orexin in young and old rats to test the hypothesis that age-related alterations in behavioral and neurochemical correlates of basic feeding behavior may have an orexinergic basis. We further examined how acetylcholine, glutamate and GABA release in the insular cortex are modulated as a function of aging or alterations in orexin expression.
Experimental Procedures
Animals
Young (3 months old upon arrival) and aged (27 months old upon arrival) male Fisher 344/Brown Norway F1 hybrid rats obtained from the National Institute on Aging colony (Baltimore, MD) were single-housed on a 12–12 hr. light-dark cycle (lights on at 07:00 hr) with standard rat chow and water available ad libitum for at least one week prior to surgery or other experimental procedures. All in vivo experiments were initiated at least one hour after lights on and were concluded at least two hours prior to lights off. All animal care and use procedures were carried out in accordance with protocols written under the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at the University of South Carolina.
All animals were handled daily during the first week. During the second week, daily handling continued and food and water intake were recorded. Throughout the third week, the animals were mildly food restricted to achieve 95% of their free-fed body weight and habituated to microdialysis testing bowls for 2–3 hours/day (parabolic clear plastic bowls; Bioanalytical Systems, Inc., West Lafayette, IN). During habituation, all animals were trained to receive a single palatable treat (Bacon Softies; BioServe, Fleming, NJ) concurrent with 20 minutes of sudden darkness. This was done at the same time every day with half of the animals training at 13:30 hours and the other half at 14:30 hours. The time to start consuming the treat was recorded as the latency to feed. This or similar manipulations have been shown previously to produce robust increases in prefrontal cortical acetylcholine release concurrent with rapid approach and consumption of the palatable food (Fadel et al., 1996, Frederick-Duus et al., 2007). During the fourth week, animals continued with training and underwent stereotaxic surgery for hypothalamic virus injection and insular cortex guide cannula placement. Training did not occur on surgery day or one day post-op to allow for recovery. Following surgery, the animals were food restricted to achieve 85–90% of their original free-fed body weight. During weeks five and six, all rats received two microdialysis sessions, separated by an “off” day, concurrent with the feeding/darkness paradigm. After all experiments were completed, animals were deeply anesthetized with isoflurane and sacrificed via transcardial perfusion. Brains were removed, post-fixed in 4% paraformaldehyde for 48 hours, and then cryoprotected in 0.1 M phosphate buffer with 30% sucrose.
Surgery
Under sodium pentobarbital (60–65 mg/kg) or ketamine (80 mg/kg)/xylazine (8 mg/kg anesthesia, animals received bilateral intrahypothalamic injections of 0.2 μL (5 × 10E6 tu/μL) of preproorexin (PPOX) sense or antisense lentivirus or 0.2μL of control virus (GFP only) with the following stereotaxic coordinates relative to bregma (Paxinos and Watson, 2007): AP −2.5 mm, L+1.2 mm, DV −9.0 mm (young); AP −2.9 mm, L +1.6 mm, DV −9.4 mm (aged). Lentiviruses containing the rat PPOX cDNA inserted in either sense or antisense orientation and control transgene expression cassettes under a phosphoglycerate kinase-1(pgk-1) promoter were constructed at the University of South Carolina School of Medicine Viral Vector Core Facility. Fourteen aged animals received sense PPOX sense (AS group) and twelve received control GFP virus (AC group). Ten young animals received PPOX antisense (YAS group) designed to knockdown PPOX expression and ten received control GFP virus (YC group). At the same time a guide cannula (Bioanalytical Systems, Inc., West Lafayette, IN) was placed in the left agranular insular cortex (AIC) at the following stereotaxic coordinates relative to bregma (Paxinos and Watson, 2007): AP +2.0 mm, L +4.6 mm, DV −5.0 mm (young); AP +2.0 mm, L +5.0 mm, DV −5.4 mm (aged). Guide cannulas were affixed to the skull by stainless steel screws and dental cement. At the conclusion of surgery all animals received a subcutaneous injection of the analgesic buprenorphine (0.02 mg/kg) to ease post-operative pain.
In vivo microdialysis
Following two days of recovery, animals were habituated to microdialysis bowls for five days prior to the first microdialysis experiment. During microdialysis, stylets were removed from the guide cannulae and replaced with semipermeable probes (BAS, 30kDa cutoff) that extended 2.0 mm past the end of the cannula. Probes were perfused with artificial cerebral spinal fluid (aCSF; 150mM NaCl, 3.0 mM KCl, 1.7 mM CaCl2, 0.9 mM MgCl2, 4.9 mM D-glucose, plus 50 nM neostigmine bromide to promote reliable recovery of ACh) at a rate of 2.0 μL/minute. Collection of microdialysates in 15 min (30 μL) intervals began 2.5 to 3.0 hours after probe insertion. The microdialysis session consisted of four baseline collections, one stimulus collection (Bacon Softie with darkness), and four post-stimulus collections. Microdialysates were stored at −80 degrees C until analysis by HPLC with electrochemical detection as previously described with 10 μL of each dialysate used for as previously described for glutamate and GABA (Reznikov et al., 2007, Stanley and Fadel, 2011) and 20 μL used for ACh analysis (Fadel et al., 2005).
Histology
Cryoprotected brains were coronally sectioned (44 μm) using a freezing microtome. Representative sections through the rostrocaudal extent of the AIC were mounted and stained for acetylcholinesterase to verify probe placement (Figure 3D). All tissue for immunohistochemical analysis processed was processed according to previously described protocols with minor modifications (Frederick-Duus et al., 2007, Kessler et al., 2011). Hypothalamic sections were processed for GFP to verify virus expression. Briefly, free-floating sections were incubated in mouse anti-GFP antibody (1:1,000; Millipore, Inc., Temecula, CA; cat# MAB3580) for 48 hr. Tissue was then incubated with biotinylated donkey anti-mouse secondary antibody (1:1,000; Jackson ImmunoResearch Laboratories, West Grove, PA; cat# 715-065-151) for 1.5 hr followed by horseradish peroxidase-conjugated streptavidin (SHRP; 1:1,600; Jackson; cat# 016-030-084) for 1 hour. GFP labeling was visualized by adding hydrogen peroxide to the tissue sections in the presence of diaminobenzidine, generating a light brown stain in GFP-immunoreactive areas (Fig. 1A).
Figure 3.
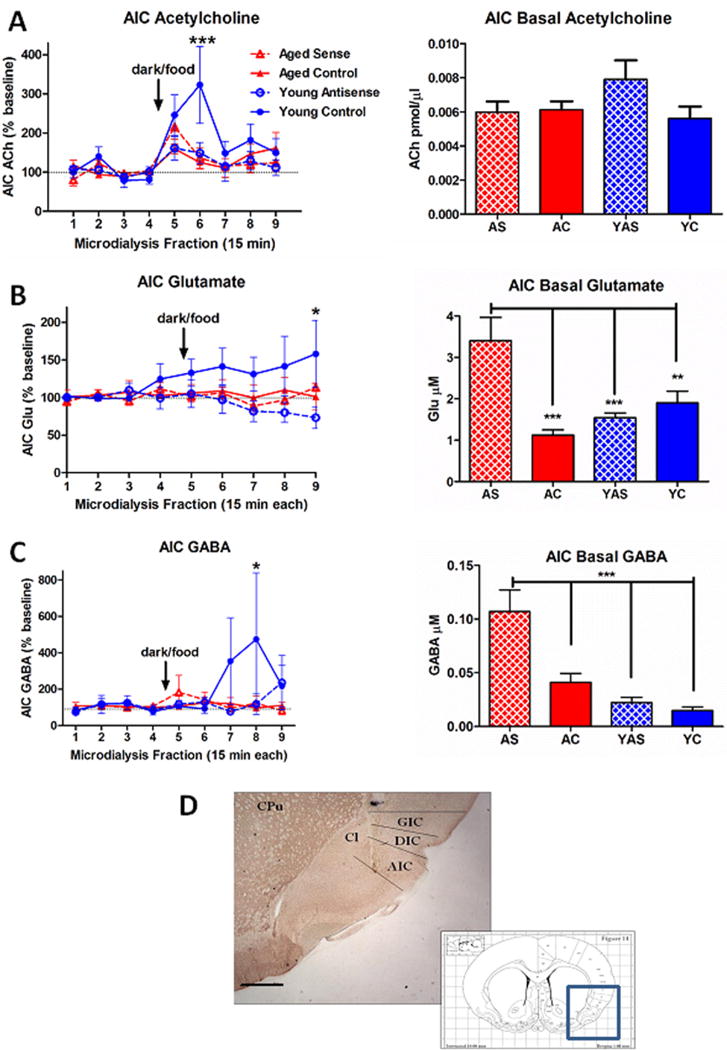
Modulation of neurotransmitter efflux in insular cortex following virus-mediated gene transfer and presentation of an entrained palatable food stimulus (darkness/food). A. (left) Young control (YC) rats showed a robust cholinergic response to presentation of the food stimulus that peaked during the second post-stimulus collection. This effect was abolished in aged control (AC) rats and young animals treated with preproorexin antisense (YAS). While there was a trend for preproorexin sense treatment to restore a response to the food stimulus in aged rats (AS), this did not reach significance. (right) Neither age nor virus treatment altered basal ACh levels insular cortex. ***p < 0.001 vs. baseline. B. (left) Following presentation of the food stimulus, YC rats showed a gradual increase in insular cortex glutamate levels that peaked and reached statistical significance during the final post-stimulus collection. (right) Treatment with preproorexin sense virus increased basal glutamate levels in insular cortex of AS rats relative to all other ages and treatment conditions. *p < 0.05 vs. baseline. C. (left) Presentation of the food stimulus was associated with a delayed increase in insular cortex GABA efflux in YC rats that reached significance only during the eighth collection (fourth post-stimulus collection). (right) Similar to glutamate, AS rats showed significant elevations in basal GABA levels in insular cortex relative to the other treatment conditions. *p < 0.05 vs. baseline. D. Representative histochemical verification of microdialysis probe placement. The probe tract typically extended through deeper layers of granular (GIC) and dysgranular (DIC) subdivisions of insular cortex at a level corresponding to roughly Bregma + 1.0 mm (inset; (Paxinos and Watson, 1998)) and had its greatest length in agranular insular cortex (AIC). Other abbreviations: CPu, caudate-putamen; Cl, claustrum. Scale bar, approximately 1.0 mm. Group sizes: N = 7–10 (AC), N = 6 (AS), N = 7–9 (YC), N = 7–8 (YAS).
Figure 1.
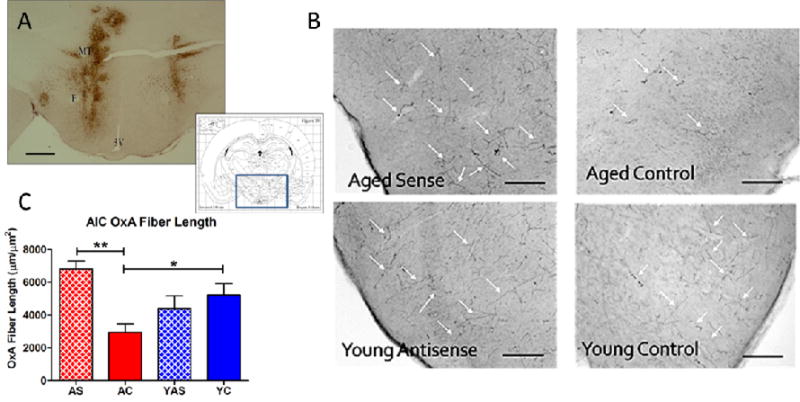
Modulation of orexin expression by lentivirus-mediated gene transfer. Bilateral infusions of lentivirus encoding preproorexin sense or antisense were made into the lateral hypothalamus and perifornical area. A. Immunohistochemical detection of GFP expression (brown staining) in hypothalamus following lentivirus infusions. The area encompassed in the photomicrograph, corresponding to roughly Bregma – 3.1 mm, is indicated by the blue box (inset; (Paxinos and Watson, 1998)). B. Immunohistochemical detection of orexin fiber labeling in insular cortex. Age/treatment are indicated in the lower left portion of each photomicrograph. White arrows indicate examples of orexin-immunoreactive fibers. C. Quantitation of orexin fiber expression following lentivirus-mediated modulation of orexin expression. Aged control rats had significantly lower levels of orexin expression (as measure by sum total fiber length within a defined region of insular cortex) than their young control counterparts. Treatment with the preproorexin sense virus significantly increased total orexin-positive fiber length to levels similar to young control animals. Abbreviations: F, fornix; MT, mammillothalamic tract; 3V, third ventricle; AS, aged preproorexin sense; AC, aged control; YAS, young preproorexin antisense; YC, young control. Scale bars, approximately 1 mm (A) and 500 μm (B). *p < 0.05. **p < 0.01. Group sizes: N = 12 (AC), N = 7 (AS), N = 10 (YC), N = 8 (YAS).
The efficacy of the lentivirus to modulate orexin expression was further verified by incubating rostral cortical sections (containing the AIC) in goat anti-OXA antibody (1:1,000; Santa Cruz Biotech, Santa Cruz, CA; cat# SC-8070) or rabbit anti-OXA (1:1,000; EMD Millipore; cat# PC362) for 48 hr followed by biotinylated donkey anti-goat (1:1,000 Jackson; cat# 705-005-003) or biotinylated donkey anti-rabbit (1:1,000 Jackson; cat# 711-065-152) for 1.5 hr followed by an incubation in SHRP as described above. Tissue was developed using nickel/cobalt-enhanced diaminobenzidine, generating a blue-black immunoprecipitate in orexin-positive fibers.
After dehydration and coverslipping, tissue sections were examined by light microscopy using Neurolucida (version 11.01.1) software (MicroBrightField; Wiliston, VT). Two serial sections per animal at approximately 1.6 mm rostral to bregma were analyzed in a 400,000 μm2 area within the AIC. All fibers were traced in a three dimensional plane and the total length per box was calculated by the software. An average was then calculated for each animal based on those numbers.
Statistical analysis
Baseline neurotransmitter levels in dialysates were calculated as the mean concentration of the four baseline collections for each animal. Data were then expressed as percent-of-baseline for each animal at each time point, then averaged within each treatment group. Group differences for each neurotransmitter analyte (Glutamate, GABA, and ACh) were revealed by repeated measures two-way analysis of variance (ANOVA) followed by Bonferroni planned comparisons. Differences in baseline neurotransmitter levels, total orexin-immunoreactive fiber length, and latency to feed were tested by one-way ANOVA with Tukey’s Multiple Comparison Test post-hoc analysis. In addition, a Pearson’s correlation was performed to on total orexin-immunoreactive fiber length and latency to feed. All values are expressed as the mean ± SEM. All statistical tests were done using GraphPad Prism version 5.02 (GraphPad Software Inc.; San Diego, CA).
Results
Virus expression
All virus treatment conditions (Control; PPOX Sense; PPOX Antisense) produced robust GFP expression in the hypothalamus surrounding the zone of infusion (Fig. 1A). Typically, bilateral GFP expression was observed in the medial hypothalamus, extending into the adjacent perifornical and lateral hypothalamus. In some cases, GFP expression extended dorsally along the infusion needle track to include the overlying zona incerta (Fig. 1A, left side). Modulation of orexin expression was further analyzed by quantifying OXA-immunopositive fiber length in the AIC (Fig. 1B,C), where one-way ANOVA yielded a significant effect of GROUP, F(3,32) = 5.789, p < 0.01. Post-hoc analysis revealed the AS group had significantly more OXA immunoreactivity in the AIC than AC animals, consistent with a virus-mediated enhancement of orexin expression in this target cortical area (p < 0.01). Furthermore, YC rats had significantly greater OXA immunoreactivity than AC animals (p < 0.05), consistent with previously-reported age-related reductions in orexin expression (Kessler et al., 2011). The YAS animals showed a trend for decreased OXA immunoreactivity relative to YC rats, but no significant differences were observed. Neither PPOX sense nor antisense expression altered food intake or weight relative to age-matched control animals.
Feeding latency
As previously reported (Frederick-Duus et al., 2007), young food-restricted control rats rapidly approached and consumed the palatable food stimulus (Fig. 2A). One way ANOVA revealed a main effect of GROUP (F(3,33) = 5.708, p = 0.0029) on feeding latency. Post hoc analyses indicated that the latency was significantly greater for AC and YAS rats compared to YC animals (p < 0.05 for both comparisons). Similarly, upregulation of PPOX expression in the aged rats significantly decreased latency to feed relative to age-matched controls (p < 0.05) and eliminated age-related differences with the young control animals (p > 0.05). In addition, Pearson correlation analysis revealed a highly significant negative correlation (r = −0.46; p = 0.005) between OXA fiber length within the insular cortex and latency to approach and consume the palatable food stimulus (Fig. 2B).
Figure 2.
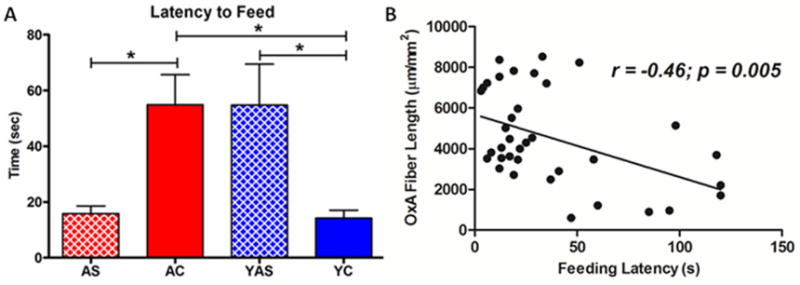
Latency (seconds ± SEM) to approach and consume a palatable food reward. A. Aged control (AC) rats showed significantly greater latency to approach and consume a palatable food reward (Bacon Softie) than their young control (YC) counterparts. Similarly, young rats treated with preproorexin antisense virus (YAS) showed significantly longer latency to approach and consume the food reward than YC rats. Treatment with preproorexin sense virus significantly reduced feeding latency in old rats to levels comparable to YC animals. *p < 0.05. B. Correlational analysis of all subjects revealed a highly significant negative correlation between insular cortex OxA fiber length and feeding latency. Group sizes: N = 12 (AC), N = 7 (AS), N = 10 (YC), N = 8 (YAS).
Microdialysis
For insular cortical ACh efflux, two-way ANOVA revealed a significant interaction between TIME and GROUP (F(3,23) = 1.775, p = 0.019) as well as a main effect of TIME (F(3,23) = 8.447, p < 0.0001). As we have previously reported in the prefrontal cortex of control animals (Frederick-Duus et al., 2007), presentation of the darkness/food stimulus in young, food-restricted rats produced a robust increase in insular cortex ACh levels that peaked during the second post-stimulus collection (Fig. 3A), confirmed by post hoc Bonferroni analysis (p < 0.001). Although aged rats that received the PPOX sense virus showed a trend toward increased ACh efflux during the first post-stimulus collection relative to age-matched controls, this did not reach significance (p = 0.102). Basal AIC ACh efflux did not differ as a function of age or virus condition (p > 0.05; Fig. 3A, right).
Presentation of the palatable food stimulus also increased insular glutamate levels in YC rats relative to YAS animals, although this increase was delayed and reached significance only at the final time point following the stimulus (p < 0.01; Fig. 3B). There were significant differences in basal glutamate levels in the insular cortex as well (F(3,29) = 11.15, p < 0.001), with the AS group showing elevated glutamate relative to each of the other groups (all p < 0.05; Fig. 3B, right).
Similar to glutamate, only the YC rats showed stimulus-elicited increases in insular cortex GABA efflux, with a delayed peak effect that was significantly greater than both YAS (p < 0.05) and AC (p < 0.01) groups at the eighth collection. Also similar to glutamate, there were significant group differences in basal GABA efflux (F(3,29) = 14.95, p < 0.001) with the AS group of rats showing significantly elevated basal levels of GABA in the insular cortex relative to each of the other groups (all p < 0.05; Fig. 3C, right).
Post-mortem acetylcholinesterase staining revealed that successfully targeted microdialysis probes were predominantly located in the deeper layers of the agranular insular cortex (AIC), with some dorsal extension into dysgranular and granular subregions of the insula (Fig. 3D).
Discussion
These experiments demonstrate that aged, food restricted rats have altered behavioral and neurochemical responses to a compound palatable food stimulus that may, in part, be mediated by deficits in orexin neurotransmission.
Virus-mediated modulation of orexin expression and feeding behavior
We used lentivirus-mediated gene transfer to increase preproorexin expression in aged rats and decrease preoproorexin expression in young rats. The primary measures of viral efficacy we utilized were expression of the reporter protein, GFP as well as orexin fiber length—a measure of orexin fiber density—in the primary orexin projection region of interest for our study, the insular cortex. By both measures, the preproorexin sense virus restored orexin expression in the insular cortex of aged rats to levels comparable to young control animals (Fig. 1). It is important to note that preproorexin expression in our animals was under the control of a non-specific (pgk) promoter. Thus, while our viral deposits were limited to the hypothalamic areas where orexin neurons are of greatest density, it is likely that there was some degree of ectopic expression. One candidate neuronal population for such expression might be MCH neurons, which are also distributed in the LH and dorsally-contiguous zona incerta and play a role in feeding (Qu et al., 1996, Hervieu, 2006). Orexin and MCH neurons share projections to areas that modulate reward and motivated behavior although their effects on these circuits may differ. In the nucleus accumbens, for example, both orexin and MCH promote food intake, but whereas intra-accumbens orexin increases activity and energy expenditure (Thorpe and Kotz, 2005, Teske et al., 2010), intra-accumbens MCH has no or negative effects on these measures (Georgescu et al., 2005, Guesdon et al., 2009). There are additional considerations with ectopic expression, including the potential decoupling of orexin co-release with co-expressed neurotransmitters such as glutamate (Torrealba et al., 2003, Henny et al., 2010) or dynorphin, which has been shown to be important in the context of orexin effects on reward (Muschamp et al., 2014). However, while ectopic orexin expression is an important caveat, an advantage to this approach is that it may have allowed us to overcome the limitation of a decreased number of orexin neurons in our aged rats, which we have previously shown have a naturally-occurring loss of 40–50% of this cell population (Kessler et al., 2011). In addition, we did not observe increased orexin fiber labeling in normally orexin-sparse brain regions, such as the caudate-putamen or cerebellum. Furthermore, it is likely that any functional effects of ectopic orexin expression would be limited to areas that are orexin-receptive under normal conditions, as there is little evidence for orexin acting on noncognate receptors.
To test whether reduction of preproorexin expression in young rats replicated the feeding behavior phenotype of aged rats, we employed a lentivirus containing the rat preproorexin cDNA inserted in antisense orientation relative to the pgk promoter. Transcription of long antisense RNAs effectively reduce gene expression in vivo, presumably by transcriptional repression or RNA interference (Davidkova et al., 1998, Weiss et al., 1999, Yeomans et al., 2005). However, in young rats treated with preproorexin antisense virus, the reduction in orexin fiber density in insular cortex did not reach statistical significance. This may reflect the limitations of immunohistochemistry for quantifying protein expression, as lightly immunoreactive fibers and darkly immunoreactive fibers of the same length would be assigned an equal measurement in our analysis. Additional factors in the failure to observe reduced orexin fiber density include 1) the fact that not all relevant, orexin-positive neurons in the tissue sections likely are transduced by the virus, limiting our ability to demonstrate knockdown across all fibers and 2) the possibility that the strength of preproorexin expression driven by the pgk promoter may be low relative to native expression. However, the dramatic effects of antisense expression on feeding latency and insular neurochemistry strongly argue for the effectiveness of our lentiviral approach to decrease functional correlates of orexin activity.
All animals in our feeding experiments were mildly food-restricted and received multiple exposures to the darkness/food stimulus. Latency to feed in this paradigm can be considered an index of motivated behavior (Wise and Raptis, 1985, Barbano and Cador, 2006), driven by a combination of homeostatic (energy balance deficit resulting from mild food restriction) and hedonic (highly palatable “Bacon Softie”) factors. Because all animals received multiple sessions with presentation of the food, there may also be elements of feeding as an entrained, learned behavior reflected in the latency measure. Several studies have demonstrated a convincing role for the orexin system in meditating cue-conditioned feeding (Petrovich et al., 2012, Cason and Aston-Jones, 2013, Cole et al., 2015, Keefer et al., 2016). Dissecting out the contributions of each of these—including how they are altered in aging—is beyond the scope of the current experiments but may be a fruitful avenue for future studies (Kmiec, 2006). Of greatest interest, however, is the age-related increase in latency and the ability of increased orexin expression to reduce latency in aged animals or orexin antisense to increase latency in young rats. The results of these bidirectional manipulations argue strongly that the age-related behavioral deficits we observed have an orexinergic basis, rather than resulting from some other factor such as motor impairment. Consistent with this hypothesis are previous demonstrations that orexins play important roles in food entrainment anticipatory behavior (Akiyama et al., 2004, Mieda et al., 2004).
Aging, orexin and neurochemical correlates of feeding in the insular cortex
Orexin neurons send robust projections to the basal forebrain, including its cholinergic components (Eggermann et al., 2001, Espana et al., 2001, Fadel et al., 2005). We have previously shown that orexin lesions or orexin antagonists attenuate behavioral and prefrontal cortical neurochemical responses to food-related cues in young rats (Frederick-Duus et al., 2007). Consistent with these observations, young animals treated with the preproorexin antisense virus in these experiments failed to show increased ACh release in the insular cortex upon food presentation. Prior work has shown that ACh in the insular cortex plays an important role in conditioned taste aversion and experiences of novel tastes (Miranda et al., 2003, Bermudez-Rattoni et al., 2004). However, we saw robust increases in insular ACh efflux in our young animals even though the palatable food stimulus was neither novel nor aversive. This may have been driven, in part, by the mild food restriction paradigm to which our animals were subjected. Thus, ACh release in the insular cortex is likely to reflect not just novelty, but motivational salience of a tastant or food-paired stimulus.
We observed no effect of orexin knockdown on basal ACh release in the insular cortex. This may reflect that orexin inputs to the BFCS are only acutely activated in response to food-paired stimuli. However, the physiological factors (e.g., decreased glucose levels) that mediate hunger-elicited activation of orexin neurons are likely to be present prior to food presentation (Yamanaka et al., 2003, Burdakov and Gonzalez, 2009). Thus, it seems more likely that orexin inputs to the BFCS do not drive basal cortical ACh release but only potentiate cholinergic activation in combination with other excitatory basal forebrain inputs associated specifically with food cues. The source of these other inputs is unclear but may include glutamatergic inputs from areas such as the prefrontal cortex or the insular cortex itself (Carnes et al., 1990, Gaykema et al., 1991, Zaborszky, 2002).
The food stimulus in young rats was associated with increased insular glutamate release relative to control animals, although, this effect did not reach statistical significance until the final collection period (Fig. 3B). The slow-developing nature of this response suggests that, under normal conditions, glutamate release in the insular cortex may reflect post-ingestive aspects of food-related stimuli (Oliveira-Maia et al., 2012). Interestingly, aged rats did not show acute increases in glutamate release in response to food presentation, nor was this response restored by increased orexin expression. Aged rats treated with the preproorexin virus did, however, show marked elevations in basal insular cortex glutamate levels relative to the other age and virus treatment conditions. It is tempting to speculate that basal elevations in insular glutamate animals may have facilitated processing of interoceptive cues related to hunger, thus promoting the lower feeding latency response in these animals. If so, this suggests that different presynaptic neurochemical mechanisms in the insula may converge on postsynaptic targets that facilitate appropriate behavioral responses to homeostatically-relevant stimuli. Consistent with this possibility, insular cortex ACh signaling has been shown to facilitate unconditioned taste-related signaling mediated in part by glutamatergic transmission (Bermudez-Rattoni et al., 2004). Whether such an interaction mediates the feeding latency responses in our animal model remains to be clearly demonstrated.
Similar to glutamate, insular cortex GABA levels rose slowly following food presentation in young control rats and only achieved significance well after the food stimulus (Fig. 3C). Although orexin knockdown in young rats abolished this effect, orexin upregulation in aged rats did not restore an acute GABAergic response. Again however, as with glutamate, aged rats treated with the preproorexin virus did show elevations in basal GABA levels in the insular cortex.
Future studies will be required to more definitively characterize the role of insular cortical orexin signaling, including the receptor mechanisms involved, in age-related alterations in feeding and cognition. Prior work has demonstrated that insular orexin transmission regulates nicotine reward (Hollander et al., 2008) and our work is consistent with a similar role in neurochemical and behavioral responses to food stimuli in food-restricted animals. In addition, it is likely that age-related reductions in orexin signaling in other brain regions underlying motivated behavior and cognition may also play a functionally significant role in these processes. Such candidates include the prefrontal cortex and ventral tegmental area (Fadel and Deutch, 2002, Lambe et al., 2005, Vittoz and Berridge, 2006, Cole et al., 2015).
In humans, aging is associated with alterations in several physiological functions, including experiencing of food reward (Morley, 2001) and subjective awareness of hunger state and other interoceptive phenomena thought to be mediated by the insula (De Castro, 1993). Multiple factors are likely to contribute to these deficits, including dysfunction of brain areas and circuits that underlie these important functions. However, the significance of age-related alterations in food intake and other aspects of physiological regulation is illustrated by clinical reports of an association between these deficits and subsequent likelihood of cognitive decline, including Alzheimer’s disease (Buchman et al., 2005, Johnson et al., 2006, Buchman et al., 2014). This link could be attributed to the fact that in addition to its role in regulation of physiological functions such as feeding and sleep-wake stability, the orexin system also promotes cognitive functions such as attention (Lambe et al., 2005, Deadwyler et al., 2007, Boschen et al., 2009, Fadel and Burk, 2010). The current studies further demonstrate that age-related alterations in feeding behavior and neurotransmission have an orexinergic basis, and suggest that orexin-based therapies may have utility in treating conditions which have both homeostatic and cognitive components. While virus-mediated upregulation of orexin expression is unlikely to find clinical application in the near future, additional means of enhancing central orexin transmission in aging may be feasible, including intranasal orexin delivery (Deadwyler et al., 2007, Baier et al., 2011, Dhuria et al., 2016) or development of systemically-deliverable small molecule orexin agonists (Scammell and Winrow, 2011).
Highlights.
Aged rats show deficits in orexin/hypocretin expression, feeding latency and insular cortex neurotransmitter release
Upregulated orexin expression in old rats partly restored behavioral and neurochemical correlates of food intake
Orexin antisense in young rats produced an “old” phenotype in measures of feeding latency and insular cortex neurochemistry
Orexins may integrate behavioral, neurochemical and cognitive responses to homeostatic deficits that manifest in aging
Acknowledgments
This work was supported by the National Institutes of Health (R01AG050518; R01AG030646).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- Akiyama M, Yuasa T, Hayasaka N, Horikawa K, Sakurai T, Shibata S. Reduced food anticipatory activity in genetically orexin (hypocretin) neuron-ablated mice. Eur J Neurosci. 2004;20:3054–3062. doi: 10.1111/j.1460-9568.2004.03749.x. [DOI] [PubMed] [Google Scholar]
- Altena E, Ramautar JR, Van Der Werf YD, Van Someren EJ. Do sleep complaints contribute to age-related cognitive decline? Prog Brain Res. 2010;185:181–205. doi: 10.1016/B978-0-444-53702-7.00011-7. [DOI] [PubMed] [Google Scholar]
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev. 1996;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Baier PC, Hallschmid M, Seeck-Hirschner M, Weinhold SL, Burkert S, Diessner N, Goder R, Aldenhoff JB, Hinze-Selch D. Effects of intranasal hypocretin-1 (orexin A) on sleep in narcolepsy with cataplexy. Sleep Med. 2011;12:941–946. doi: 10.1016/j.sleep.2011.06.015. [DOI] [PubMed] [Google Scholar]
- Barbano MF, Cador M. Differential regulation of the consummatory, motivational and anticipatory aspects of feeding behavior by dopaminergic and opioidergic drugs. Neuropsychopharmacology. 2006;31:1371–1381. doi: 10.1038/sj.npp.1300908. [DOI] [PubMed] [Google Scholar]
- Bermudez-Rattoni F, Ramirez-Lugo L, Gutierrez R, Miranda MI. Molecular signals into the insular cortex and amygdala during aversive gustatory memory formation. Cell Mol Neurobiol. 2004;24:25–36. doi: 10.1023/B:CEMN.0000012722.45805.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet MH, Arand DL. Sleep loss in aging. Clin Geriatr Med. 1989;5:405–420. [PubMed] [Google Scholar]
- Boschen KE, Fadel JR, Burk JA. Systemic and intrabasalis administration of the orexin-1 receptor antagonist, SB-334867, disrupts attentional performance in rats. Psychopharmacology (Berl) 2009;206:205–213. doi: 10.1007/s00213-009-1596-2. [DOI] [PubMed] [Google Scholar]
- Buchman AS, Wilson RS, Bienias JL, Shah RC, Evans DA, Bennett DA. Change in body mass index and risk of incident Alzheimer disease. Neurology. 2005;65:892–897. doi: 10.1212/01.wnl.0000176061.33817.90. [DOI] [PubMed] [Google Scholar]
- Buchman AS, Yu L, Wilson RS, Boyle PA, Schneider JA, Bennett DA. Brain Pathology Contributes to Simultaneous Change in Physical Frailty and Cognition in Old Age. J Gerontol A Biol Sci Med Sci. 2014 doi: 10.1093/gerona/glu117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdakov D, Gonzalez JA. Physiological functions of glucose-inhibited neurones. Acta Physiol (Oxf) 2009;195:71–78. doi: 10.1111/j.1748-1716.2008.01922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdakov D, Jensen LT, Alexopoulos H, Williams RH, Fearon IM, O’Kelly I, Gerasimenko O, Fugger L, Verkhratsky A. Tandem-pore K+ channels mediate inhibition of orexin neurons by glucose. Neuron. 2006;50:711–722. doi: 10.1016/j.neuron.2006.04.032. [DOI] [PubMed] [Google Scholar]
- Carnes KM, Fuller TA, Price JL. Sources of presumptive glutamatergic/aspartatergic afferents to the magnocellular basal forebrain in the rat. J Comp Neurol. 1990;302:824–852. doi: 10.1002/cne.903020413. [DOI] [PubMed] [Google Scholar]
- Cason AM, Aston-Jones G. Role of orexin/hypocretin in conditioned sucrose-seeking in rats. Psychopharmacology (Berl) 2013;226:155–165. doi: 10.1007/s00213-012-2902-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S, Mayer HS, Petrovich GD. Orexin/Hypocretin-1 Receptor Antagonism Selectively Reduces Cue-Induced Feeding in Sated Rats and Recruits Medial Prefrontal Cortex and Thalamus. Scientific reports. 2015;5:16143. doi: 10.1038/srep16143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Davidkova G, Zhou LW, Morabito M, Zhang SP, Weiss B. D2 dopamine antisense RNA expression vector, unlike haloperidol, produces long-term inhibition of D2 dopamine-mediated behaviors without causing Up-regulation of D2 dopamine receptors. J Pharmacol Exp Ther. 1998;285:1187–1196. [PubMed] [Google Scholar]
- De Castro JM. Age-related changes in spontaneous food intake and hunger in humans. Appetite. 1993;21:255–272. doi: 10.1006/appe.1993.1044. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Sutcliffe JG, Fabre V. Hypocretins/orexins as integrators of physiological information: lessons from mutant animals. Neuropeptides. 2002;36:85–95. doi: 10.1054/npep.2002.0892. [DOI] [PubMed] [Google Scholar]
- Deadwyler SA, Porrino L, Siegel JM, Hampson RE. Systemic and nasal delivery of orexin-A (Hypocretin-1) reduces the effects of sleep deprivation on cognitive performance in nonhuman primates. J Neurosci. 2007;27:14239–14247. doi: 10.1523/JNEUROSCI.3878-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhuria SV, Fine JM, Bingham D, Svitak AL, Burns RB, Baillargeon AM, Panter SS, Kazi AN, Frey WH, 2nd, Hanson LR. Food consumption and activity levels increase in rats following intranasal Hypocretin-1. Neurosci Lett. 2016;627:155–159. doi: 10.1016/j.neulet.2016.05.053. [DOI] [PubMed] [Google Scholar]
- Eggermann E, Serafin M, Bayer L, Machard D, Saint-Mleux B, Jones BE, Muhlethaler M. Orexins/hypocretins excite basal forebrain cholinergic neurones. Neuroscience. 2001;108:177–181. doi: 10.1016/s0306-4522(01)00512-7. [DOI] [PubMed] [Google Scholar]
- Espana RA. Hypocretin/orexin involvement in reward and reinforcement. Vitam Horm. 2012;89:185–208. doi: 10.1016/B978-0-12-394623-2.00010-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana RA, Baldo BA, Kelley AE, Berridge CW. Wake-promoting and sleep-suppressing actions of hypocretin (orexin): basal forebrain sites of action. Neuroscience. 2001;106:699–715. doi: 10.1016/s0306-4522(01)00319-0. [DOI] [PubMed] [Google Scholar]
- Espiritu JR. Aging-related sleep changes. Clin Geriatr Med. 2008;24:1–14. v. doi: 10.1016/j.cger.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Fadel J, Burk JA. Orexin/hypocretin modulation of the basal forebrain cholinergic system: Role in attention. Brain Res. 2010;1314:112–123. doi: 10.1016/j.brainres.2009.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111:379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Fadel J, Moore H, Sarter M, Bruno JP. Trans-synaptic stimulation of cortical acetylcholine release after partial 192 IgG-saporin-induced loss of cortical cholinergic afferents. J Neurosci. 1996;16:6592–6600. doi: 10.1523/JNEUROSCI.16-20-06592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J, Pasumarthi R, Reznikov LR. Stimulation of cortical acetylcholine release by orexin A. Neuroscience. 2005;130:541–547. doi: 10.1016/j.neuroscience.2004.09.050. [DOI] [PubMed] [Google Scholar]
- Fadel JR, Jolivalt CG, Reagan LP. Food for thought: the role of appetitive peptides in age-related cognitive decline. Ageing Res Rev. 2013;12:764–776. doi: 10.1016/j.arr.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick-Duus D, Guyton MF, Fadel J. Food-elicited increases in cortical acetylcholine release require orexin transmission. Neuroscience. 2007;149:499–507. doi: 10.1016/j.neuroscience.2007.07.061. [DOI] [PubMed] [Google Scholar]
- Fronczek R, van Geest S, Frolich M, Overeem S, Roelandse FW, Lammers GJ, Swaab DF. Hypocretin (orexin) loss in Alzheimer’s disease. Neurobiol Aging. 2012;33:1642–1650. doi: 10.1016/j.neurobiolaging.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Gaykema RP, van Weeghel R, Hersh LB, Luiten PG. Prefrontal cortical projections to the cholinergic neurons in the basal forebrain. J Comp Neurol. 1991;303:563–583. doi: 10.1002/cne.903030405. [DOI] [PubMed] [Google Scholar]
- Georgescu D, Sears RM, Hommel JD, Barrot M, Bolanos CA, Marsh DJ, Bednarek MA, Bibb JA, Maratos-Flier E, Nestler EJ, DiLeone RJ. The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus accumbens to modulate feeding behavior and forced-swim performance. J Neurosci. 2005;25:2933–2940. doi: 10.1523/JNEUROSCI.1714-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guesdon B, Paradis E, Samson P, Richard D. Effects of intracerebroventricular and intra-accumbens melanin-concentrating hormone agonism on food intake and energy expenditure. Am J Physiol Regul Integr Comp Physiol. 2009;296:R469–475. doi: 10.1152/ajpregu.90556.2008. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Henny P, Brischoux F, Mainville L, Stroh T, Jones BE. Immunohistochemical evidence for synaptic release of glutamate from orexin terminals in the locus coeruleus. Neuroscience. 2010;169:1150–1157. doi: 10.1016/j.neuroscience.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervieu GJ. Further insights into the neurobiology of melanin-concentrating hormone in energy and mood balances. Expert Opin Ther Targets. 2006;10:211–229. doi: 10.1517/14728222.10.2.211. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ. Insular hypocretin transmission regulates nicotine reward. Proc Natl Acad Sci U S A. 2008;105:19480–19485. doi: 10.1073/pnas.0808023105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John J, Wu MF, Kodama T, Siegel JM. Intravenously administered hypocretin-1 alters brain amino acid release: an in vivo microdialysis study in rats. J Physiol. 2003;548:557–562. doi: 10.1113/jphysiol.2002.038729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DK, Wilkins CH, Morris JC. Accelerated weight loss may precede diagnosis in Alzheimer disease. Arch Neurol. 2006;63:1312–1317. doi: 10.1001/archneur.63.9.1312. [DOI] [PubMed] [Google Scholar]
- Keefer SE, Cole S, Petrovich GD. Orexin/hypocretin receptor 1 signaling mediates Pavlovian cue-food conditioning and extinction. Physiol Behav. 2016;162:27–36. doi: 10.1016/j.physbeh.2016.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler BA, Stanley EM, Frederick-Duus D, Fadel J. Age-related loss of orexin/hypocretin neurons. Neuroscience. 2011;178:82–88. doi: 10.1016/j.neuroscience.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmiec Z. Central regulation of food intake in ageing. J Physiol Pharmacol. 2006;57(Suppl 6):7–16. [PubMed] [Google Scholar]
- Lambe EK, Olausson P, Horst NK, Taylor JR, Aghajanian GK. Hypocretin and nicotine excite the same thalamocortical synapses in prefrontal cortex: correlation with improved attention in rat. J Neurosci. 2005;25:5225–5229. doi: 10.1523/JNEUROSCI.0719-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinninger GM, Opland DM, Jo YH, Faouzi M, Christensen L, Cappellucci LA, Rhodes CJ, Gnegy ME, Becker JB, Pothos EN, Seasholtz AF, Thompson RC, Myers MG., Jr Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metab. 2011;14:313–323. doi: 10.1016/j.cmet.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Hu Z, de Lecea L. The hypocretins/orexins: integrators of multiple physiological functions. Br J Pharmacol. 2014;171:332–350. doi: 10.1111/bph.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Moorman DE, Smith RJ, James MH, Aston-Jones G. Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nat Neurosci. 2014;17:1298–1303. doi: 10.1038/nn.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manini TM. Energy expenditure and aging. Ageing Res Rev. 2010;9:1–11. doi: 10.1016/j.arr.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Cauvi G, Kerr TM, Weiss F. Differential role of hypothalamic orexin/hypocretin neurons in reward seeking motivated by cocaine versus palatable food. Addict Biol. 2016 doi: 10.1111/adb.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Neural inputs into the nucleus basalis of the substantia innominata (Ch4) in the rhesus monkey. Brain. 1984;107(Pt 1):253–274. doi: 10.1093/brain/107.1.253. [DOI] [PubMed] [Google Scholar]
- Mieda M, Williams SC, Sinton CM, Richardson JA, Sakurai T, Yanagisawa M. Orexin neurons function in an efferent pathway of a food-entrainable circadian oscillator in eliciting food-anticipatory activity and wakefulness. J Neurosci. 2004;24:10493–10501. doi: 10.1523/JNEUROSCI.3171-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda MI, Ferreira G, Ramirez-Lugo L, Bermudez-Rattoni F. Role of cholinergic system on the construction of memories: taste memory encoding. Neurobiol Learn Mem. 2003;80:211–222. doi: 10.1016/s1074-7427(03)00061-3. [DOI] [PubMed] [Google Scholar]
- Morley JE. Anorexia of aging: physiologic and pathologic. Am J Clin Nutr. 1997;66:760–773. doi: 10.1093/ajcn/66.4.760. [DOI] [PubMed] [Google Scholar]
- Morley JE. Decreased food intake with aging. J Gerontol A Biol Sci Med Sci. 2001;56(Spec No 2):81–88. doi: 10.1093/gerona/56.suppl_2.81. [DOI] [PubMed] [Google Scholar]
- Muschamp JW, Hollander JA, Thompson JL, Voren G, Hassinger LC, Onvani S, Kamenecka TM, Borgland SL, Kenny PJ, Carlezon WA., Jr Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proc Natl Acad Sci U S A. 2014;111:E1648–1655. doi: 10.1073/pnas.1315542111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- Oliveira-Maia AJ, de Araujo IE, Monteiro C, Workman V, Galhardo V, Nicolelis MA. The insular cortex controls food preferences independently of taste receptor signaling. Frontiers in systems neuroscience. 2012;6:5. doi: 10.3389/fnsys.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Petrovich GD, Hobin MP, Reppucci CJ. Selective Fos induction in hypothalamic orexin/hypocretin, but not melanin-concentrating hormone neurons, by a learned food-cue that stimulates feeding in sated rats. Neuroscience. 2012;224:70–80. doi: 10.1016/j.neuroscience.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Alanko L, Kalinchuk A, Heiskanen S, Stenberg D. The effect of age on prepro-orexin gene expression and contents of orexin A and B in the rat brain. Neurobiol Aging. 2004;25:231–238. doi: 10.1016/s0197-4580(03)00043-5. [DOI] [PubMed] [Google Scholar]
- Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, Mathes WF, Przypek R, Kanarek R, Maratos-Flier E. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380:243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- Reznikov LR, Grillo CA, Piroli GG, Pasumarthi RK, Reagan LP, Fadel J. Acute stress-mediated increases in extracellular glutamate levels in the rat amygdala: differential effects of antidepressant treatment. Eur J Neurosci. 2007;25:3109–3114. doi: 10.1111/j.1460-9568.2007.05560.x. [DOI] [PubMed] [Google Scholar]
- Rolls ET. Taste, olfactory, and food texture processing in the brain, and the control of food intake. Physiol Behav. 2005;85:45–56. doi: 10.1016/j.physbeh.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Rolls ET. Functions of the anterior insula in taste, autonomic, and related functions. Brain and cognition. 2015 doi: 10.1016/j.bandc.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Scammell TE, Winrow CJ. Orexin receptors: pharmacology and therapeutic opportunities. Annu Rev Pharmacol Toxicol. 2011;51:243–266. doi: 10.1146/annurev-pharmtox-010510-100528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM. Narcolepsy: a key role for hypocretins (orexins) Cell. 1999;98:409–412. doi: 10.1016/s0092-8674(00)81969-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley EM, Fadel JR. Aging-related alterations in orexin/hypocretin modulation of septo-hippocampal amino acid neurotransmission. Neuroscience. 2011 doi: 10.1016/j.neuroscience.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terao A, Apte-Deshpande A, Morairty S, Freund YR, Kilduff TS. Age-related decline in hypocretin (orexin) receptor 2 messenger RNA levels in the mouse brain. Neurosci Lett. 2002;332:190–194. doi: 10.1016/s0304-3940(02)00953-9. [DOI] [PubMed] [Google Scholar]
- Teske JA, Billington CJ, Kotz CM. Hypocretin/orexin and energy expenditure. Acta Physiol (Oxf) 2010;198:303–312. doi: 10.1111/j.1748-1716.2010.02075.x. [DOI] [PubMed] [Google Scholar]
- Thannickal TC, Lai YY, Siegel JM. Hypocretin (orexin) cell loss in Parkinson’s disease. Brain. 2007;130:1586–1595. doi: 10.1093/brain/awm097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe AJ, Doane DF, Sweet DC, Beverly JL, Kotz CM. Orexin A in the rostrolateral hypothalamic area induces feeding by modulating GABAergic transmission. Brain Res. 2006;1125:60–66. doi: 10.1016/j.brainres.2006.09.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe AJ, Kotz CM. Orexin A in the nucleus accumbens stimulates feeding and locomotor activity. Brain Res. 2005;1050:156–162. doi: 10.1016/j.brainres.2005.05.045. [DOI] [PubMed] [Google Scholar]
- Torrealba F, Yanagisawa M, Saper CB. Colocalization of orexin a and glutamate immunoreactivity in axon terminals in the tuberomammillary nucleus in rats. Neuroscience. 2003;119:1033–1044. doi: 10.1016/s0306-4522(03)00238-0. [DOI] [PubMed] [Google Scholar]
- Vittoz NM, Berridge CW. Hypocretin/Orexin Selectively Increases Dopamine Efflux within the Prefrontal Cortex: Involvement of the Ventral Tegmental Area. Neuropsychopharmacology. 2006;31:384–395. doi: 10.1038/sj.npp.1300807. [DOI] [PubMed] [Google Scholar]
- Weiss B, Davidkova G, Zhou LW. Antisense RNA gene therapy for studying and modulating biological processes. Cell Mol Life Sci. 1999;55:334–358. doi: 10.1007/s000180050296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Raptis L. Effects of pre-feeding on food-approach latency and food consumption speed in food deprived rats. Physiol Behav. 1985;35:961–963. doi: 10.1016/0031-9384(85)90266-5. [DOI] [PubMed] [Google Scholar]
- Wolden-Hanson T. Mechanisms of the anorexia of aging in the Brown Norway rat. Physiol Behav. 2006;88:267–276. doi: 10.1016/j.physbeh.2006.05.032. [DOI] [PubMed] [Google Scholar]
- Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, Tominaga M, Yagami K, Sugiyama F, Goto K, Yanagisawa M, Sakurai T. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- Yeomans DC, Levinson SR, Peters MC, Koszowski AG, Tzabazis AZ, Gilly WF, Wilson SP. Decrease in inflammatory hyperalgesia by herpes vector-mediated knockdown of Nav1.7 sodium channels in primary afferents. Hum Gene Ther. 2005;16:271–277. doi: 10.1089/hum.2005.16.271. [DOI] [PubMed] [Google Scholar]
- Zaborszky L. The modular organization of brain systems. Basal forebrain: the last frontier. Prog Brain Res. 2002;136:359–372. doi: 10.1016/s0079-6123(02)36030-8. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Gaykema RP, Swanson DJ, Cullinan WE. Cortical input to the basal forebrain. Neuroscience. 1997;79:1051–1078. doi: 10.1016/s0306-4522(97)00049-3. [DOI] [PubMed] [Google Scholar]


