Abstract
The aim of this research was to develop effective alternative therapies to reduce antibiotic use in animal agriculture. In this study, the efficacy of copper-modified palygorskite (CM-Pal) in preventing diarrhea caused by Salmonella was specifically examined both in vitro and in vivo. The CM-Pal was prepared with palygorskite (Pal) and copper nitrate. The antibacterial activity of the CM-Pal was detected by comparing the differences in cell numbers on plate count agar before and after adding the CM-Pal to Salmonella typhimurium cultures. Seventy ICR mice were then allocated into seven groups. Five groups (the treatment groups) were infected with S. typhimurium by intraperitoneal (i.p.) injection and were given Pal, CM-Pal, montmorillonite powder, gentamicin, and physiological saline, respectively. One group (the prevention group) was given CM-Pal before infection with S. typhimurium. Another group (the uninfected group) was not infected with S. typhimurium. The effects of Pal, CM-Pal, montmorillonite powder, and gentamicin on the treatment or prevention of diarrhea in the mice were examined by stool studies, fecal scoring, and assessment of growth performance and villus height. The CM-Pal had satisfactory anti-bacterial properties in vitro: the antibacterial rate was 100% after 2 h incubation with S. typhimurium NJS1 cultures (1×106 colony-forming units (CFU)/ml). In the in vivo experiment, the CM-Pal exerted superior effects in the treatment and prevention of diarrhea in mice compared with Pal, montmorillonite powder, and gentamicin. In the CM-Pal group, no mice showed signs of diarrhea at 24 h post infection (p.i.), and all mice fully recovered from infection. However, the Pal group, montmorillonite group, and gentamicin group only recovered after 48, 48, and 96 h, respectively. The villus height level in the CM-Pal treatment group recovered at 3 d p.i. However, the recovery time of the other groups was longer (at least 5 d). The CM-Pal prevention group had a better effect on weight gain than the other groups. This study suggested that CM-Pal may be an effective alternative to conventional antibiotics for the treatment and prevention of animal diarrhea caused by Salmonella.
Keywords: Copper, Palygorskite, Diarrhea, Salmonella
1. Introduction
Animal bacterial diarrhea is one of the more serious problems in the livestock and poultry industries. The main measure of treatment and prevention involves using antibiotics, which are an effective agent to control animal disease. However, the continuous use of an antibiotic increases the drug resistance exhibited by pathogenic bacteria. This resistance has become a more serious problem in recent years (Stahl et al., 2004). Because of the abuse of antibiotics, it is essential to find effective replacements that do not confer resistance (Hu and Xia, 2006).
Palygorskite (Pal) is a very important and useful type of industrial clay mineral belonging to the phyllosilicates. This mineral has a three-layer inverted structure, and iron commonly substitutes for aluminum and magnesium in the octahedral layer (Liu et al., 2015). The high surface area, the charge on the lattice, and the inverted structure confer properties of absorption, cation exchange capacity, and adhesive ability (Murray, 2000). These properties make Pal widely useful in the animal industry as an aflatoxin adsorbent, a tannin adsorbent, and an excipient in pharmaceutical preparations, and as an antacid, gastrointestinal protector, and anti-diarrheic (Isabel Carretero and Pozo, 2009; 2010; Zhang et al., 2013).
Copper ions (Cu2+) have good antibacterial properties. In particular, these ions can induce the inhibition of bacterial growth and have toxic effects on most microorganisms. Copper has seen widespread use in the preparation of antibacterial materials (Faundez et al., 2004; Kanhed et al., 2014). Copper-exchanged montmorillonite has antibacterial activity against Escherichia coli K88, and leads to improved growth performance and a decreased incidence of diarrhea in pigs (Xia et al., 2004; Zhou et al., 2004; Hu and Xia, 2006).
Considering these antecedents, this study aimed to evaluate the antibacterial and anti-diarrheic activity of copper-modified palygorskite (CM-Pal), which is characterized by the adsorptive ability of Pal and the antibacterial properties of copper.
2. Materials and methods
2.1. Preparation of CM-Pal
CM-Pal was prepared with Pal and copper nitrate as described previously, with some modifications (Hu and Xia, 2006). The Pal (Jiangsu Shenlite Biotechnology Co., Ltd., Jiangsu, China) was pre-treated in a muffle furnace at 350 °C for 2 h. Then, 10.0 g sample of Pal was dispersed in 200 ml of 0.15 mol/L copper nitrate solution. The dispersion was placed on a magnetic stirring apparatus at 90 °C and 200 r/min for 6 h. The CM-Pal was washed repeatedly with distilled water to remove the dissociative copper nitrate and then dried at 105 °C to a constant weight. The CM-Pal was subsequently pulverized to a powder using a mortar and filtered using a 200-mesh strainer.
Then, 0.1 g of the powdered CM-Pal sample was placed in a microwave digestion vessel. The sample was treated with a mixture of 7 ml HNO3, 3 ml HClO4, and 1 ml H2O2, and the microwave digestion vessel was kept in a microwave oven (ETHOS-TC, Milestone, Italy). The digestion and quantification of the metal content were performed in triplicate by inductively coupled plasma-optical emission spectrometry (ICP-OES) (Optimal 2100DV, Pekin Elmer, USA).
2.2. Preparation of the inoculum
Salmonella typhimurium NJS1 was used as the challenge strain. The medium contained the following components: 10 g/L peptone (Oxoid, UK), 5 g/L yeast extract (Oxoid, UK), and 5 g/L NaCl. The cultivation was conducted in 50 ml of medium in 250-ml conical flasks maintained at 37 °C. Incubation was carried out with agitation at 200 r/min for 24 h. The cultures were centrifuged at 12 000 r/min for 3 min and suspended in physiological saline. The final cell numbers were determined on plate count agar.
2.3. Detection of antibacterial activity of CM-Pal in vitro
Different amounts of CM-Pal and Pal (5, 10, and 20 mg) were added into 50-ml cultures (S. typhimurium NJS1, 1×106 colony-forming units (CFU)/ml) in 250-ml conical flasks, and the final concentration was 0.1, 0.2, and 0.4 mg/ml. The mixture was agitated at 200 r/min at 37 °C. Then, 1 ml of the mixture was used for detection of the total bacterial count at 0, 0.5, 1, 2, 4, and 6 h. The antibacterial rate (AR) of the CM-Pal was calculated from the difference in the cell numbers at 0 h and the others at the indicated time:
AR=(N 0−Nt)/N 0×100%,
where N 0 is the cell number at 0 h, and Nt is the cell number at time t (0.5, 1, 2, 4, and 6 h).
2.4. Infection experiments
The experiments were conducted using ICR mice (weighing 18–22 g). The animals were obtained from the Yangzhou Comparative Medicine Center (Yangzhou, China) and were kept at the animal laboratory for 5 d before the experiments. All animal experiments were approved by the Laboratory Animal Management Committee of Jiangsu Province, China.
Seventy ICR mice were divided into 7 groups (A–G). The experiment lasted for 12 d. Infection was induced on the 8th day by intraperitoneal (i.p.) injection of 1×107 CFU in 0.5 ml of a logarithmic-phase culture of S. typhimurium NJS1. Pal, CM-Pal, and montmorillonite powder (IPSEN, Tianjin, China) were suspended in distilled water to a final concentration of 8 mg/ml.
Group A (CM-Pal prevention group): a total of 0.5 ml of CM-Pal (8 mg/ml) was given orally by gavage once per day for 7 d before infection. CM-Pal was not used after infection until the end of the experiment. Group B (uninfected group): physiological saline was injected on the 8th day as a substitute for infection. Group C (Pal group), Group D (montmorillonite powder group), and Group E (CM-Pal treatment group): a total of 0.5 ml of Pal (8 mg/ml), 0.5 ml of montmorillonite powder (8 mg/ml), or 0.5 ml CM-Pal (8 mg/ml), respectively, was given twice per day after infection. Group F (gentamicin group): gentamicin (Huazhong Pharmaceutical Co., Ltd., Hubei, China) (0.4 mg/ml) was administered at a dose of 0.5 ml per mouse by gavage twice per day after infection. Group G (no treatment group): a total of 0.5 ml physiological saline was given twice per day after infection.
2.5. Effects of CM-Pal on the treatment or prevention of diarrhea in mice
Stools from each group were examined for the characteristics of diarrhea during the experiment. The incidence of diarrhea (%) was calculated as follows: (number of mice with diarrhea/number of all mice)×100%. Fecal scores were determined according to the diameter of the feces on filter paper, as described previously: 1, <10 mm; 2, 10–19 mm; 3, 20–30 mm; and 4, >30 mm (Zhou et al., 1994). The mice were allowed free access to food and water. The growth performance (total average weight) of each group was also measured.
2.6. Histopathological examination
The mice were euthanized via an injection of napental (100 mg/kg) through the intra-abdominal route. The duodenums were removed and fixed in 10% (v/v) formalin, dehydrated with an ethanol and toluene series, and embedded in paraffin wax via a routine process. All sections were stained with hematoxylin and eosin (H & E) and were examined histopathologically using an optical microscope. To better evaluate the duodenal mucosal architecture, the villus heights were measured using the MetaMorph computer program. At least 5 villi from each mouse were measured.
2.7. Statistical analysis
The data in the tables are presented as the arithmetic mean±standard deviation (SD). Statistical analysis was performed by one-way analysis of variance (ANOVA) using Predictive Analytics Software 18.0. Duncan’s multiple-range test was used, with differences considered to be significant at P<0.05.
3. Results
3.1. Characterization of CM-Pal
CM-Pal was produced by ion exchange processes between copper nitrate and the Pal cation. The copper concentration in the CM-Pal was 51.25 mg/g, as measured by ICP-OES.
3.2. Detection of the antibacterial activity of CM-Pal in vitro
Different masses of CM-Pal were added into 50-ml cultures. The antibacterial rate of CM-Pal is shown in Table 1. The average antibacterial rate of Pal ranged between 26.56% and 56.83%. Pal had antibacterial properties to a certain degree, but CM-Pal had greater ones. As more CM-Pal was added, the antibacterial effect increased. There were no bacteria growing after 1 h (10 and 20 mg CM-Pal) or 2 h (5 mg CM-Pal) of incubation.
Table 1.
Antibacterial rates of CM-Pal and Pal
| Time (h) | Antibacterial rate of CM-Pal (%) |
Antibacterial rate of Pal (%) |
||||
| 0.1 mg/ml | 0.2 mg/ml | 0.4 mg/ml | 0.1 mg/ml | 0.2 mg/ml | 0.4 mg/ml | |
| 0.5 | 72.44±0.34c | 92.06±0.05b | 96.08±0.05a | 26.56±0.94e | 39.31±0.51f | 56.83±0.83d |
| 1 | 95.23±0.13b | 100a | 100a | 28.61±0.95d | 26.41±0.44e | 55.92±0.79c |
| 2 | 100a | 100a | 100a | 27.38±0.85d | 38.18±0.94c | 54.35±0.47b |
| 4 | 100a | 100a | 100a | 29.72±0.77d | 44.74±0.89c | 54.17±0.77b |
| 6 | 100a | 100a | 100a | 28.63±0.66d | 46.76±0.64c | 53.42±0.47b |
Pal, palygorskite; CM-Pal, copper-modified palygorskite. Pal and CM-Pal were added to S. typhimurium cultures. The antibacterial rate was calculated from the difference in the cell numbers at 0 h and other time. The antibacterial rate was the average value±SD. Within rows, different superscripts (a–f) indicate a significant difference (P<0.05)
3.3 Clinical symptoms
Mice were infected with S. typhimurium NJS1, which resulted in diarrhea within 2 h of infection. All of the mice developed frequent watery stools, anorexia, and lethargy. The effects of different treatments on the incidence of diarrhea are shown in Table 2.
Table 2.
Effects of different treatments on the incidence of diarrhea and on fecal scores at 12 h p.i.
| Group | Incidence of diarrhea (%) |
Fecal score (12 h p.i.) | |||
| 12 h | 24 h | 48 h | 96 h | ||
| A | 80 | 0 | 0 | 0 | 1.33±0.49d |
| B | 0 | 0 | 0 | ||
| C | 90 | 30 | 10 | 0 | 1.58±0.50b |
| D | 80 | 30 | 10 | 0 | 1.31±0.47d |
| E | 50 | 0 | 0 | 0 | 1.39±0.50d |
| F | 100 | 90 | 20 | 10 | 1.48±0.63c |
| G | 100 | 60 | 50 | 50 | 1.73±0.64a |
A, CM-Pal prevention group; B, uninfected group; C, Pal group; D, montmorillonite powder group; E, CM-Pal treatment group; F, gentamicin group; G, no treatment group. Within columns, different superscripts (a–d) indicate a significant difference (P<0.05)
At 12 h post infection (p.i.), 8 of the 10 mice in the CM-Pal prevention group (Group A) still showed signs of diarrhea. At 24 h p.i., no mice had diarrhea, and the activity and feed intake of these mice increased. At 48 h p.i., all of the mice had fully recovered from the diarrhea.
None of the mice in the uninfected group (Group B) had diarrhea or clinical symptoms of diarrhea. Nine of the 10 mice in the Pal group (Group C) had diarrhea at 12 h p.i., and 3 mice in this group still had diarrhea at 24 h p.i. At 48 h p.i., one mouse exhibited viscous feces. The activity and feed intake of this group increased until 96 h after inoculation.
The mice in the montmorillonite powder group (Group D) showed a similar curative effect on their diarrhea as the Pal group.
At 12 h p.i., 5 of the 10 mice in the CM-Pal treatment group (Group E) still had diarrhea, and certain mice began eating and drinking. At 24 p.i., no mice had diarrhea, and the activity and feed intake of the mice increased. At 48 h p.i., all of the mice had fully recovered from the diarrhea.
Among the mice in the gentamicin group (Group F), 10, 9, 2, and 1 showed signs of diarrhea at 12, 24, 48, and 96 h p.i., respectively. In the no-treatment group (Group G) 5 mice still showed signs of diarrhea at 96 h p.i.
3.4. Effects of different treatments on the diarrhea index
The diarrhea index (the incidence of diarrhea combined with the fecal score) at 12 h p.i. is shown in Table 2. The CM-Pal prevention group, Pal group, montmorillonite powder group, and CM-Pal treatment group experienced varying degrees of improvement in their diarrhea at 12 h p.i. Half of the mice in the CM-Pal treatment group had no diarrhea. However, all of the mice in the gentamicin group and no-treatment group still had diarrhea. The fecal scores of the CM-Pal prevention group, montmorillonite powder group, and CM-Pal treatment group were lower than the scores of the other groups (P<0.05).
3.5. Effects of different treatments on growth performance
The average weight of each group was measured at 1, 4, 7, 9, and 12 d. There was no significant difference in body weight among Groups C, D, E, F, and G (Table 3). However, the average weight of Group A was higher than that of the other groups. CM-Pal had a good effect on weight gain.
Table 3.
Effects of different treatments on the body weight of mice
| Group | Body weight (g) |
||||
| 1 d | 4 d | 7 d | 9 d | 12 d | |
| A | 20.39±1.22a | 23.61±0.51a | 26.85±1.47a | 24.00±0.41a | 26.47±0.44a |
| B | 20.20±0.68a | 21.59±0.23ab | 23.26±0.49b | 25.53±0.64a | 28.05±1.12a |
| C | 19.25±0.88a | 20.27±0.47b | 22.04±0.54b | 21.10±0.78b | 22.26±0.43b |
| D | 20.55±0.25a | 21.18±1.41b | 23.87±0.26b | 21.39±0.55b | 22.75±0.57b |
| E | 19.94±0.32a | 20.56±0.63b | 22.23±0.40b | 21.83±0.29b | 22.49±0.45b |
| F | 20.06±0.33a | 21.10±0.32b | 23.02±0.31b | 22.18±0.64b | 23.00±0.68b |
| G | 20.74±0.28a | 21.04±1.17b | 23.90±0.56b | 21.04±0.51b | 22.53±0.27b |
A, CM-Pal prevention group; B, uninfected group; C, Pal group; D, montmorillonite powder group; E, CM-Pal treatment group; F, gentamicin group; G, no treatment group. Within columns, different superscripts (a–b) indicate a significant difference (P<0.05)
3.6. Histopathological examination
Villi were damaged by the infection with S. typhimurium. Many disrupted villi were found in the intestinal lumen (Fig. 1). The values for villus height are shown in Table 4. Villus height decreased at 1 d p.i. The villus height level in the Group E recovered on 3 d p.i., whereas the recovery time of the other groups was longer.
Fig. 1.
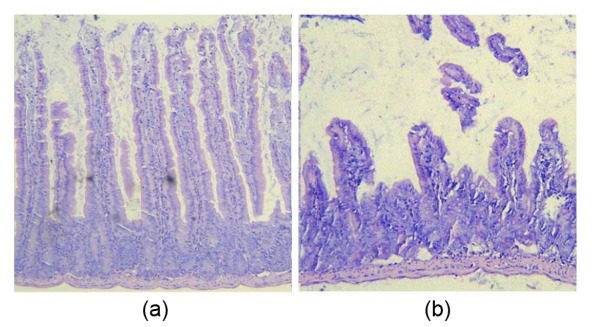
Duodenal villus morphology uninfected (a) and infected (b)
Table 4.
Effects of different treatments on villus height
| Group | Villus height (μm) |
|||
| 3 d | 8 d | 10 d | 12 d | |
| A | 898.00±80.07a | 309.33±49.10cde | 593.00±78.00b | 672.33±36.02c |
| B | 774.67±14.01b | 788.61±11.09a | 790.32±22.10a | 789.18±12.07b |
| C | 375.33±59.23cd | 419.00±23.90c | 659.67±52.62c | |
| D | 421.33±42.57c | 393.33±44.84c | 554.67±30.86d | |
| E | 548.33±53.89b | 871.00±51.88a | 879.33±28.75a | |
| F | 303.33±25.93de | 428.67±9.29c | 528.00±16.82d | |
| G | 206.00±16.09e | 331.00±29.60c | 533.33±33.13d | |
A, CM-Pal prevention group; B, uninfected group; C, Pal group; D, montmorillonite powder group; E, CM-Pal treatment group; F, gentamicin group; G, no treatment group. Within columns, different superscripts (a–e) indicate a significant difference (P<0.05)
4. Discussion
In this study, CM-Pal was produced by ion exchange processes between copper nitrate and the Pal cation. The efficacy of the CM-Pal in preventing and treating animal diarrhea caused by Salmonella was then examined in vitro and in vivo.
This research suggested that the CM-Pal had greater antibacterial activity than Pal in a dose-and time-dependent manner. Living organisms need Cu2+ at low concentrations as cofactors for metalloproteins and enzymes; however, at high concentrations, Cu2+ induces an inhibition of growth in bacteria (Gordon et al., 1994). Many researchers have suggested that Cu2+ has antibacterial activity against harmful bacteria (Faundez et al., 2004; Hu and Xia, 2006; Kanhed et al., 2014). The exchange of montmorillonite with Cu2+ enhances the antibacterial activity and leads to improved growth performance and a decreased incidence of diarrhea in pigs (Hu and Xia, 2006). In the research presented here, CM-Pal showed greater antibacterial activity than Pal. The density of Cu2+ on the Pal surface was much higher than that in solution. Therefore, Cu2+ bridging between the Pal particle and the bacteria plays an important role in the antibacterial activity of CM-Pal (Hu and Xia, 2006). The in vitro antibacterial test results for CM-Pal have shown its capacity to be used successfully as an inorganic antibacterial material. Inorganic antibacterial materials are more favorable than organic antibacterial materials in terms of stability, thermal resistance, safety for the user, and long-lasting effects (Karel et al., 2015).
Bacterial diarrhea is not easy to treat with traditional antibiotics due to antibiotic resistance. In the present study, there was one diarrheic mouse at 96 h p.i. in the antibiotic-treated group (gentamicin group), whereas all of the mice had fully recovered from the diarrhea at 48 h p.i. in the CM-Pal group. The incidence of diarrhea in the antibiotic-treated group (gentamicin group) was 100% at 12 h p.i., whereas the incidence in the CM-Pal group was only half of that. Judging from clinical symptoms and the diarrhea index (the incidence of diarrhea combined with the fecal score), CM-Pal was an effective antibiotic substitute for the treatment of bacterial diarrhea. Pal and montmorillonite powder were somewhat effective in treating diarrhea but less effective than CM-Pal. The clinical symptoms of diarrhea include anorexia, and none of the mice wanted to eat or drink after infection. The mice in the CM-Pal treatment group were the first to eat and drink, and the recovery time was shorter in this group than in the other groups. All of these results showed that the effects of CM-Pal on the diarrhea were remarkable.
In addition to its therapeutic effect, CM-Pal had a prophylactic effect on diarrhea. Although all of the mice in the prevention group developed frequent watery stools, these mice had fully recovered from the diarrhea without any treatment at 48 h p.i.
Previous work on Pal has shown that dietary supplementation with Pal improved growth performance, ameliorated liver damage, and increased dry matter, energy and crude protein utilization (Lv et al., 2015). In the present study, the average weight of the prevention group, which was given CM-Pal before infection, was higher than that of the other groups. This result suggested that CM-Pal could improve growth performance.
Villi are important structures in the small intestine, which is involved mainly in nutrient absorption (Yang et al., 2016). The gastrointestinal tissues are the tissues that are primarily affected in animals infected with Salmonella. The pathogens persist and proliferate in the gastrointestinal tract and invade the sub-epithelial tissues, and mainly the ileum, leading to marked disruption of the small intestinal villus epithelial cells (Naughton et al., 1995). The research presented here showed a large number of desquamated and shortened villi after infection. However, the villus height in the CM-Pal group decreased at 1 d p.i. It is possible that CM-Pal could protect the villi from damage by Salmonella. On the one hand, the Salmonella is killed by the Cu2+ in the CM-Pal. On the other hand, the large specific surface area of Pal allows it to become distributed over the surface of the gut mucosa to form a barrier immediately after ingestion (Slamova et al., 2011; Lv et al., 2015). Pal can protect the intestinal mucosa by interacting with digestive mucus (Guarino et al., 2009; Zhang et al., 2013).
Footnotes
Project supported by the Foundation of Key Laboratory for Palygorskite Science and Applied Technology of Jiangsu Province (No. HPK201506), the Natural Science Foundation of Jiangsu Province (No. BK20130686), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), China
Compliance with ethics guidelines: Da-wei YAO, Ze-zhong YU, Na LI, Yu-nong HOU, Jia-rong XU, and De-ji YANG declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Faundez G, Troncoso M, Navarrete P, et al. Antimicrobial activity of copper surfaces against suspensions of Salmonella enterica and Campylobacter jejuni . BMC Microbiol. 2004;4:19. doi: 10.1186/1471-2180-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gordon AS, Howell LD, Harwood V. Responses of diverse heterotrophic bacteria to elevated copper concentrations. Can J Microbiol. 1994;40(5):408–411. doi: 10.1139/m94-067. [DOI] [PubMed] [Google Scholar]
- 3.Guarino A, Lo Vecchio A, Pirozzi MR. Clinical role of diosmectite in the management of diarrhea. Expert Opin Drug Metab Toxicol. 2009;5(4):433–440. doi: 10.1517/17425250902865594. [DOI] [PubMed] [Google Scholar]
- 4.Hu CH, Xia MS. Adsorption and antibacterial effect of copper-exchanged montmorillonite on Escherichia coli K-88. Appl Clay Sci. 2006;31(3-4):180–184. doi: 10.1016/j.clay.2005.10.010. [DOI] [Google Scholar]
- 5.Isabel Carretero M, Pozo M. Clay and non-clay minerals in the pharmaceutical industry Part I. Excipients and medical applications. Appl Clay Sci. 2009;46(1):73–80. doi: 10.1016/j.clay.2009.07.017. [DOI] [Google Scholar]
- 6.Isabel Carretero M, Pozo M. Clay and non-clay minerals in the pharmaceutical and cosmetic industries Part II. Active ingredients. Appl Clay Sci. 2010;47(3-4):171–181. doi: 10.1016/j.clay.2009.10.016. [DOI] [Google Scholar]
- 7.Kanhed P, Birla S, Gaikwad S, et al. In vitro antifungal efficacy of copper nanoparticles against selected crop pathogenic fungi. Mater Lett. 2014;115:13–17. doi: 10.1016/j.matlet.2013.10.011. [DOI] [Google Scholar]
- 8.Karel FB, Koparal AS, Kaynak E. Development of silver ion doped antibacterial clays and investigation of their antibacterial activity. Adv Mater Sci Eng. 2015;2015:1–6. doi: 10.1155/2015/409078. [DOI] [Google Scholar]
- 9.Liu Z, Li P, Ning Z, et al. A modified attapulgite clay for controlling infiltration of reclaimed water riverbed. Environ Earth Sci. 2015;73(7):3887–3900. doi: 10.1007/s12665-014-3673-z. [DOI] [Google Scholar]
- 10.Lv Y, Tang C, Wang X, et al. Effects of dietary supplementation with palygorskite on nutrient utilization in weaned piglets. Livest Sci. 2015;174:82–86. doi: 10.1016/j.livsci.2015.02.004. [DOI] [Google Scholar]
- 11.Murray HH. Traditional and new applications for kaolin, smectite, and palygorskite: a general overview. Appl Clay Sci. 2000;17(5-6):207–221. doi: 10.1016/S0169-1317(00)00016-8. [DOI] [Google Scholar]
- 12.Naughton PJ, Grant G, Ewen SWB, et al. Salmonella typhimurium and Salmonella enteritidis induce gut growth and increase the polyamine content of the rat small intestine in vivo. FEMS Immunol Med Microbiol. 1995;12(3-4):251–257. doi: 10.1016/0928-8244(95)00081-2. [DOI] [PubMed] [Google Scholar]
- 13.Slamova R, Trckova M, Vondruskova H, et al. Clay minerals in animal nutrition. Appl Clay Sci. 2011;51(4):395–398. doi: 10.1016/j.clay.2011.01.005. [DOI] [Google Scholar]
- 14.Stahl CH, Callaway TR, Lincoln LM, et al. Inhibitory activities of colicins against Escherichia coli strains responsible for postweaning diarrhea and edema disease in swine. Antimicrob Agents Chemother. 2004;48(8):3119–3121. doi: 10.1128/AAC.48.8.3119-3121.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia MS, Hu CH, Xu ZR, et al. Effects of copper-bearing montmorillonite (Cu-MMT) on Escherichia coli and diarrhea on weanling pigs. Asian Austral J Anim Sci. 2004;17(12):1712–1716. doi: 10.5713/ajas.2004.1712. [DOI] [Google Scholar]
- 16.Yang HS, Wu F, Long LN, et al. Effects of yeast products on the intestinal morphology, barrier function, cytokine expression, and antioxidant system of weaned piglets. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2016;17(10):752–762. doi: 10.1631/jzus.B1500192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J, Lv Y, Tang C, et al. Effects of dietary supplementation with palygorskite on intestinal integrity in weaned piglets. Appl Clay Sci. 2013;86:185–189. doi: 10.1016/j.clay.2013.10.009. [DOI] [Google Scholar]
- 18.Zhou G, Hu Z, Wang Y, et al. An inquiry into preparing diarrhea model of mice and application of diarrhea index. Chin Tradit Herb Drugs. 1994;25:195–199. (in Chinese) [Google Scholar]
- 19.Zhou YH, Xia MS, Ye Y, et al. Antimicrobial ability of Cu2+-montmorillonite. Appl Clay Sci. 2004;27(3-4):215–218. doi: 10.1016/j.clay.2004.06.002. [DOI] [Google Scholar]


