Abstract
Objective: Bovine endometritis is one of the most common reproductive disorders in cattle. The aim of this study was to investigate the anti-inflammation potential of punicalagin in lipopolysaccharide (LPS)-induced bovine endometrial epithelial cells (bEECs) and to uncover the underlying mechanisms. Methods: bEECs were stimulated with different concentrations (1, 10, 30, 50, and 100 μg/ml) of LPS for 3, 6, 9, 12, and 18 h. MTT assay was used to assess cell viability and to identify the conditions for inflammatory injury and effective concentrations of punicalagin. Quantitative real-time polymerase chain reaction (qRT-PCR) was used to assess gene expression of pro-inflammatory cytokines. Western blotting was used to assess levels of inflammation-related proteins. Results: Treatment of bEECs with 30 µg/ml LPS for 12 h induced cell injury and reduced cell viability. Punicalagin (5, 10, or 20 µg/ml) pretreatment significantly decreased LPS-induced productions of interleukin (IL)-1β, IL-6, IL-8, and tumor necrosis factor-α (TNF-α) in bEECs. Molecular research showed that punicalagin inhibited the activation of the upstream mediator nuclear factor-κB (NF-κB) by suppressing the production of inhibitor κBα (IκBα) and phosphorylation of p65. Results also indicated that punicalagin can suppress the phosphorylation of mitogen-activated protein kinases (MAPKs) including p38, c-Jun N-terminal kinase (JNK), and extracellular signal-regulated kinase (ERK). Conclusions: Punicalagin may attenuate LPS-induced inflammatory injury and provide a potential option for the treatment of dairy cows with Escherichia coli endometritis.
Keywords: Bovine endometrial epithelial cell, Cytokine, Inflammatory injury, Punicalagin
1. Introduction
Endometritis is one of the most common reproductive disorders caused by bacteria in cattle throughout the world. It damages the endometrium, delaying the onset of the ovarian cycle activity after calving, extending luteal phases, reducing fertility, and causing significant economic losses (Sheldon et al., 2006; Azawi, 2008; Kasimanickam et al., 2013). Therefore, methods are needed to eradicate the causative bacteria earlier and control inflammation. Although antibiotics have been effective, increasing drug resistance and concerns about food safety limit their use (Malinowski et al., 2011; Zhao et al., 2014; Mackeen et al., 2015; Ward and Duff, 2016).
Escherichia coli and other Gram-negative bacteria are the major pathogens associated with endometritis (Janowski et al., 2013; Sens and Heuwieser, 2013; Brodzki et al., 2014; Wagener et al., 2014). Lipopolysaccharides (LPSs) are the major structural components of the cell wall of Gram-negative bacteria. LPS can be transferred to cluster of differentiation 14 (CD14) by LPS-binding protein (LBP) and is recognized by Toll-like receptor 4 (TLR4) on the cell surface (Shimada et al., 2005; Khan et al., 2009; Wu et al., 2016). The interaction between LPS and TLR4 results in the activation of intracellular signaling through myeloid differentiation factor 88 (MyD88) and TIR-domain-containing adapter-inducing interferon-β (TRIF) pathways, leading to the activation of major mitogen-activated protein kinases (MAPKs) and translocation of nuclear transcription factor-κB (NF-κB) (Sheldon and Roberts, 2010; Kawai and Akira, 2011; Huang et al., 2016). NF-κB regulates the expression of cytokines, including interleukin (IL)-1β, IL-6, IL-8, and tumor necrosis factor-α (TNF-α), which are essential mediators of the inflammation response (Wang et al., 2012; Liu et al., 2014; Huang et al., 2016).
The endometrium is the first line of defense against microbial invasion, and endometrial epithelial cells are thought to play a key role in local innate immunity (Herath et al., 2006; Soboll et al., 2006; Turner et al., 2014). Endometrial epithelial cells line the uterine mucosal surface, forming a physical barrier to protect the host from pathogen invasion (Wira et al., 2005). Endometrial epithelial cells in the bovine uterus were found to recognize and respond to pathogens via surface receptors such as TLRs (Chapwanya et al., 2013; Turner et al., 2014).
Punicalagin, a hydrolysable tannin of pomegranate juice, is a powerful nutrient that promotes overall health (Yaidikar et al., 2014). Punicalagin exhibits multiple biological effects, including antioxidant, antiproliferative, antiviral, and antimicrobial activities (Taguri et al., 2004; Aqil et al., 2012; Yang et al., 2012; Xu et al., 2016). Moreover, it has shown anti-inflammatory properties both in vitro and in vivo (Jean-Gilles et al., 2013; Olajide et al., 2014; Xu et al., 2014). However, the effects of punicalagin on endometritis have not been investigated. This study was therefore designed to investigate whether punicalagin could protect against LPS-induced inflammatory injury in bovine endometrial epithelial cells (bEECs) and to clarify its possible mechanisms of action.
2. Materials and methods
2.1. Reagents
Punicalagin (>98% high-performance liquid chromatography (HPLC) purity) was purchased from Tauto Biotech (Shanghai, China). LPS (E. coli O55:B5), 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), trypsin, collagenase II, bovine serum albumin (BSA), and DNase I were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium/Ham’s F-12 nutrient mixture (DMEM/F12), fetal bovine serum (FBS), antibiotic, and TRIzol reagent were purchased from Gibco (Grand Island, NY, USA). A bicinchoninic acid (BCA) protein assay kit was purchased from Pierce (Rockford, IL, USA). Antibodies against β-actin, inhibitor κBα (IκBα), phosphorylated p65 (p-p65), phosphorylated p38 (p-p38), phosphorylated c-Jun N-terminal kinase (p-JNK), and phosphorylated extracellular signal-regulated kinase (p-ERK) were purchased from Cell Signaling Technology (Danvers, MA, USA). Goat anti-mouse antibody was purchased from Li-CDR Odyssey (Lincoln, NE, USA).
2.2. Cell culture and treatment
Uteruses from five non-pregnant Holstein cows were obtained from a local abattoir immediately after slaughter and kept on ice until further processing in the laboratory. The animals had no evidence of genital disease based on visual inspection and the uteruses were obtained in accordance with protocols approved by the local Institutional Animal Care and Use Committee. Primary bEECs were isolated from the endometrium as described previously (Chapwanya et al., 2013). In brief, the endometrium was sliced into small pieces measuring about 3–5 mm3 and digested with sterile filtered digestive solution (50 mg trypsin, 50 mg collagenase II, 100 mg BSA, and 10 mg DNase I) for 1 h in a gently agitating water bath at 37 °C. Cells were resuspended in DMEM/F12 containing 10% FBS and antibiotics (100 U/ml penicillin and 100 U/ml streptomycin) at 37 °C in a humidified incubator with 5% CO2.
2.3. Cell viability assay
The bEECs were seeded in 96-well plates at 1×104 cells per well and allowed to grow to 90% confluence in complete medium. After 24 h, cells were washed twice with phosphate-buffered saline (PBS) and incubated with serum-free medium for 1 h at 37 °C in 5% CO2. Cells were then treated with different concentrations of LPS (0, 1, 10, 30, 50, and 100 µg/ml) for 3, 6, 9, 12, or 18 h in the incubator. Cell viability was measured by the MTT assay. To examine the cytotoxicity of punicalagin in bEECs, cells were treated with different concentrations of punicalagin (5, 10, 20, 25, 30, and 50 µg/ml) for 24 h. The medium was then removed and DMEM/F12 containing 10% MTT was added to each well. Formazan complex was dissolved in 100 µl dimethyl sulfoxide (DMSO) after 4 h of incubation. The optical density was measured using a microplate reader (Bio-Rad, USA) at 490 nm.
2.4. Quantitative real-time PCR analysis
To detect the effects of punicalagin on gene expression in LPS-stimulated cells, bEECs were pre-incubated in six-well plates (1×106 cells/well) and pretreated with punicalagin (5, 10, and 20 µg/ml) for 2 h prior to LPS (30 µg/ml) treatment in an incubator at 37 °C and 5% CO2 for 3, 6, 9, or 12 h. Total RNA was isolated using a phenol and guanidine isothiocyanate-based TRIzol reagent according to the manufacturer’s instructions. The concentration and integrity of total RNA were measured at a 260/280 nm ratio. Quantitative polymerase chain reaction (PCR) analysis was performed using the DNA Engine Mx3000P® (Agilent, Santa Clara, CA, USA) fluorescence detection system against a double-stranded DNA-specific fluorescent dye (Stratagene, La Jolla, CA, USA) according to optimized PCR protocols. β-Actin was amplified in parallel with the target genes and used as a normalization control. The cycling conditions were as follows: 95 °C for 10 min, followed by 40 cycles of 95 °C for 30 s, 60 °C for 60 s, and 72 °C for 60 s. Expression levels were determined using the relative threshold cycle (Ct) method as described by the manufacturer (Stratagene). The PCR reaction system (25 µl in total) contained 12.5 µl of SYBR Green PCR mix, 0.375 µl of reference dye, 1 µl of each primer (both, 10 μmol/L), 1 µl of complementary DNA (cDNA) template, and 9.125 µl of diethyl phosphorocyanidate (DEPC)-treated water. Table 1 lists the gene-specific oligonucleotide primers used for real-time PCR (RT-PCR).
Table 1.
Primers for RT-PCR
| Gene | Accession number1 | Primer sequence (5'→3') | Product (bp) |
| β-actin | NM_173979.3 | F: CAGAAGGACTCGTACGTGGG R: TTGGCCTTGGGGTTCAGGG | 199 |
| TNF-α | NM_173966.3 | F: CTTCATTGCCAGGTTTCTG R: CAGGTGTTGGATGCAGCTCT | 141 |
| IL-1β | NM_174093.1 | F: ATGACTTCTGCTTTCCCTACCC R: GCTGCTTTCACACTCATCATTC | 179 |
| IL-6 | NM_173923.2 | F: GCAFFTATTTGTGAAGAGAGCTG R: CACAGAACATGAGGCACTGAA | 148 |
| IL-8 | NM_173925.2 | F: ACGGGCTTTACCTCATCTACTC R: GCTCTTGATGGCAGACAGG | 140 |
Primers were designed from the sequences published in the GenBank database under the indicated accession numbers. F: forward primer; R: reverse primer
2.5. Western blot analysis
The bEECs cultured in tissue culture flasks for 24 h were pretreated with punicalagin for 2 h prior to treatment with LPS (30 µg/ml) for 15, 45, or 90 min in an incubator at 37 °C and 5% CO2. Cells were then harvested on ice, washed twice using ice-cold PBS, and suspended in 500 µl lysis buffer supplemented with protease inhibitor. After incubating on ice for 30 min, cell extracts were subjected to centrifugation (12 000g) at 4 °C for 15 min and proteins were quantified using a BCA protein assay kit. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electro-transferred to nitrocellulose membranes (Pierce Biotechnology Inc.), which were then hybridized with the specific antibodies. β-Actin was used to correct for protein loading. Densitometric values of immunoblot signals were obtained from three separate experiments using Image J software (National Institutes of Health, USA).
2.6. Statistical analysis
The results are expressed as mean±the standard error of the mean (SEM) and differences between mean values of normally distributed data were assessed by one-way analysis of variance (ANOVA) multiple comparisons. A P-value of 0.05 or less was regarded as significant.
3. Results
3.1. Effect of punicalagin on cell viability
We first examined the cytotoxicity of different concentrations of punicalagin (5, 10, 20, 25, 30, and 50 µg/ml) on bEECs by evaluating cell viability using the MTT assay. The results showed that punicalagin in concentrations from 0 to 30 µg/ml had no cytotoxic effect on bEECs (Fig. 1), suggesting that the inhibitory effect of punicalagin on LPS-induced inflammation was not a result of cytotoxicity caused by a reduction in cell viability.
Fig. 1.
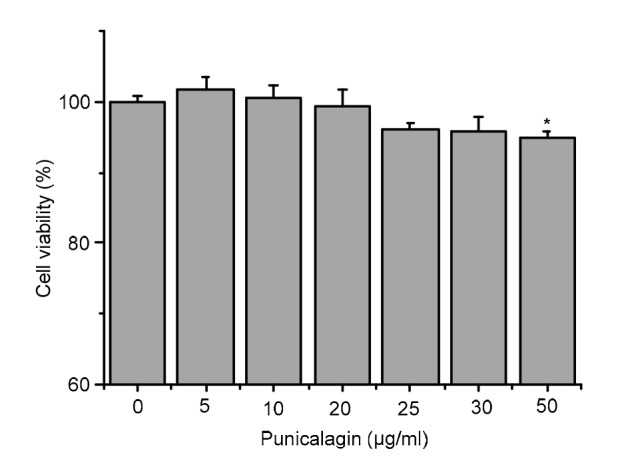
Punicalagin cytotoxicity in bEECs
Cells were treated with different concentrations (5, 10, 20, 25, 30, and 50 µg/ml) of punicalagin for 24 h. Cell viability was evaluated by MTT assay. Data represent the mean±SEM of three independent experiments. * P<0.05 vs. the control group
3.2. Inhibition of LPS-induced pro-inflammatory cytokine production by punicalagin
To determine the appropriate LPS concentration and time for stimulation, cell viability was tested after LPS treatment with different concentrations (0, 1, 10, 30, 50, and 100 µg/ml) for 3, 6, 9, 12, and 18 h. Cell viability was not altered by the treatment with 1 and 10 µg/ml of LPS before 12 h (Fig. 2). However, stimulation of cells with 30 µg/ml LPS for 12 h significantly reduced cell vitality (P<0.05), and compared with the control group (Figs. 3a–3c), the treated cells showed obvious floating, rounding, and nuclear pycnosis (Figs. 3d–3f). The effects of 50 and 100 µg/ml LPS on cell viability after 12 h were more pronounced (P<0.01 each). Therefore, we selected cell stimulation with 30 µg/ml LPS for 12 h for our experimental analyses.
Fig. 2.
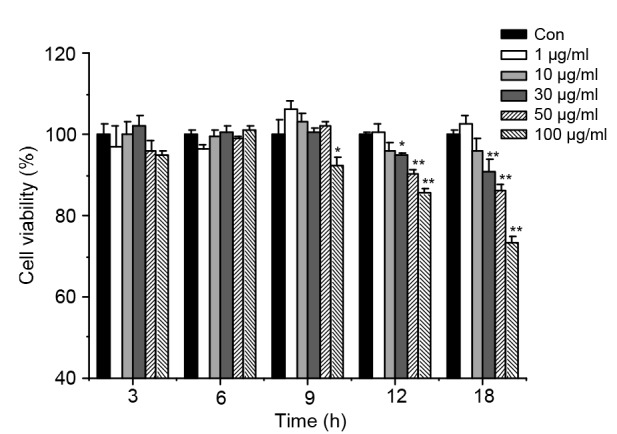
Cell viability of bEECs induced by LPS
Cells were incubated with different concentrations (1, 10, 30, 50, and 100 µg/ml) of LPS for 3, 6, 9, 12, and 18 h. Cell viability was significantly reduced by 30 µg/ml of LPS at 12 h (* P<0.05). Data represent the mean±SEM of three independent experiments. * P<0.05, ** P<0.01 vs. the control (Con) group
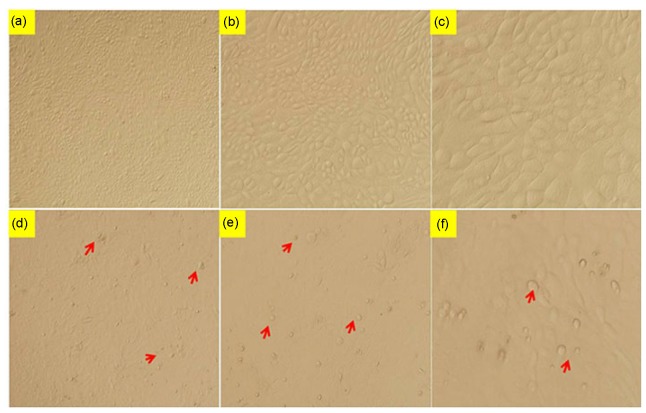
Fig. 3.
Morphology of bEECs induced by LPS
Cells were incubated with or without 30 µg/ml LPS for 12 h and cell morphology was observed using an inverted microscope. (a–c) Control group: the distribution of bEECs was compact and regular. (d–f) Model group: bEECs exposed to 30 µg/ml LPS for 12 h became enlarged, lost their cuboidal shape, and showed disrupted cell-cell contacts (arrowheads). (a, d) 4× microscope; (b, e) 10× microscope; (c, f) 20× microscope
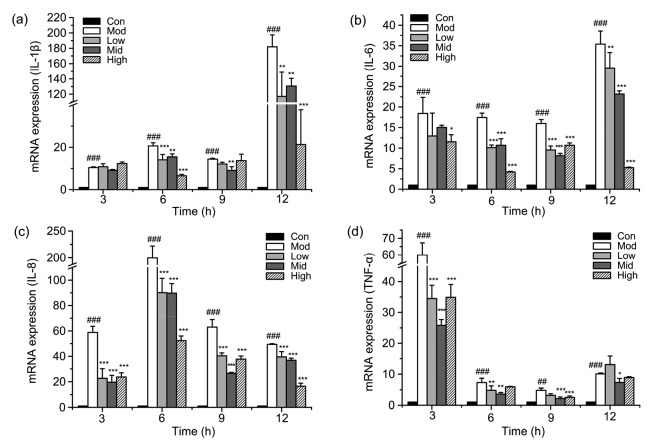
To determine the effect of punicalagin on LPS-induced pro-inflammatory cytokine expression, total RNA was extracted and examined by RT-PCR. The expression levels of IL-1β, IL-6, IL-8, and TNF-α induced by LPS were significantly (P<0.001) upregulated at indicated time points (Fig. 4). This effect was markedly inhibited by punicalagin in a dose-dependent manner, confirming that punicalagin had anti-inflammatory effects.
Fig. 4.
Effect of punicalagin on LPS-induced pro-inflammatory cytokine mRNA expression
Cells were pretreated with punicalagin (5, 10, and 20 µg/ml) for 2 h and exposed to 30 µg/ml LPS for 3, 6, 9, and 12 h. The levels of IL-1β (a), IL-6 (b), IL-8 (c), and TNF-α (d) mRNAs were quantified using RT-PCR analysis. Data represent the mean±SEM of three independent experiments. ## P<0.01, ### P<0.001 vs. the control (Con) group. * P<0.05, ** P<0.01, *** P<0.001 vs. the model (Mod) group. Con: control; Mod: treated with LPS (30 µg/ml) only; Low: punicalagin (5 µg/ml)+LPS (30 µg/ml); Mid: punicalagin (10 µg/ml)+LPS (30 µg/ml); High: punicalagin (20 µg/ml)+LPS (30 µg/ml)
3.3. Inhibition of LPS-induced NF-κB activation by punicalagin
NF-κB is an important transcription factor that induces cytokine mRNA after stimulation by LPS. Therefore, critical proteins involved in NF-κB signaling pathway were examined by Western blotting. The results showed an almost complete degradation of IκBα and a significant increase in phosphorylation of p65 after cells were treated with LPS for 15 min (Figs. 5a and 5b), indicating an increase in NF-κB activity. The degradation of IκBα and phosphorylation of p65 induced by LPS were partially inhibited by punicalagin, which indicated a weakening activity of NF-κB. After 45 min, the degradation of IκBα and phosphorylation of p65 had decreased in all groups. These results show that NF-κB activity in bEECs induced by LPS was significantly inhibited by punicalagin.
Fig. 5.
Effect of punicalagin on LPS-induced NF-κB activation
Cells were pretreated with punicalagin (5, 10, and 20 µg/ml) for 2 h, exposed to 30 µg/ml LPS for 15, 45, and 90 min, and analyzed by Western blotting. IκBα (a) and phosphorylated p65 (b) were analyzed using anti-IκBα and phosphor-specific anti-p65 antibodies. Data represent the mean±SEM of three independent experiments. # P<0.05, ### P<0.001 vs. the control (Con) group. * P<0.05, ** P<0.01, *** P<0.001 vs. the model (Mod) group. Con: control; Mod: treated with LPS (30 µg/ml) only; Low: punicalagin (5 µg/ml)+LPS (30 µg/ml); Mid: punicalagin (10 µg/ml)+LPS (30 µg/ml); High: punicalagin (20 µg/ml)+LPS (30 µg/ml)
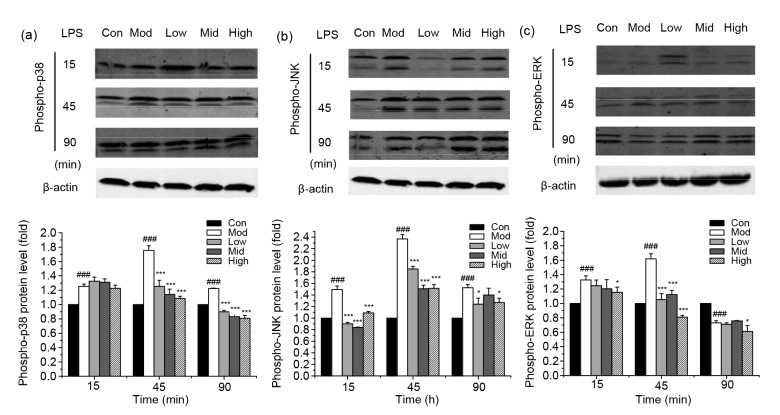
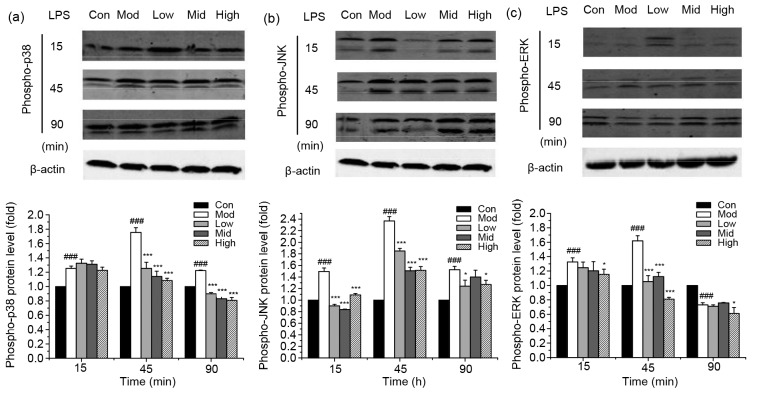
3.4. Inhibition of LPS-induced MAPK activation by punicalagin
To further expound the mechanism of the inhibitory effect on LPS-induced pro-inflammatory cytokine expression by punicalagin, we then investigated the effect of punicalagin on LPS-induced activation via the MAPK signaling pathway by assessing the phosphorylation of p38, JNK, and ERK (Fig. 6). Treatment of bEECs with LPS significantly (P<0.001) increased the activation of MAPKs by strengthening the phosphorylation of p38, JNK, and ERK. However, the phosphorylation levels were attenuated to some degree in punicalagin-pretreated cells compared with LPS-treated cells.
Fig. 6.
Effect of punicalagin on LPS-induced MAPK activation
Cells were pretreated with punicalagin (5, 10, and 20 µg/ml) for 2 h, exposed to 30 µg/ml LPS for 15, 45, and 90 min, and analyzed by Western blotting. Phosphorylation levels of p38 (a), JNK (b), and ERK (c) were analyzed using phospho-specific anti-p38, phospho-specific anti-JNK, and phospho-specific anti-ERK antibodies. Data represent the mean±SEM of three independent experiments. ### P<0.001 vs. the control (Con) group. * P<0.05, *** P<0.001 vs. the model (Mod) group. Con: control; Mod: treated with LPS (30 µg/ml) only; Low: punicalagin (5 µg/ml)+LPS (30 µg/ml); Mid: punicalagin (10 µg/ml)+LPS (30 µg/ml); High: punicalagin (20 µg/ml)+LPS (30 µg/ml)
4 Discussion
LPS, a cell wall component of Gram-negative bacteria, is a well-known inducer of inflammation, and exposure of cells to LPS has been shown to promote the release of pro-inflammatory cytokines (Lim et al., 2012; Fu et al., 2013; Wang et al., 2013), making LPS a good stimulant of inflammation. In many previous reports, the concentrations of LPS used in vitro were less than 1 µg/ml (Choi et al., 2007; Wang et al., 2012; Qi et al., 2016). However, in our study, the treatment with LPS up to 30 µg/ml for 3, 6, or 9 h did not inhibit cell viability. This may have been because of the high tolerance of primary cells to LPS and species specificity. However, incubation of cells with 30 µg/ml LPS for 12 h significantly reduced cell vitality and induced modality change, and therefore we selected this concentration and time as the experimental conditions in this study.
Clinical treatment of inflammation caused by bacterial infection and its associated symptoms involves the use of antibiotic drugs (Ugurlu et al., 2010; Pandrea et al., 2016). However, the use of these drugs is associated with severe side effects (Kumar et al., 2016; Yoon et al., 2016), indicating the need for natural methods of inflammation control. Punicalagin, a large natural polyphenolic compound found in pomegranates, is a traditional medicine reported to exhibit anti-inflammatory activity (Jean-Gilles et al., 2013; Olajide et al., 2014; Peng et al., 2015; Yaidikar and Thakur, 2015). Jean-Gilles et al. (2013) showed that punicalagin may be toxic to cattle and rats in vivo. In this study, punicalagin did not have toxic effects on bEECs at concentrations of 0–30 µg/ml. These results are consistent with those of Kulkarni et al. (2007) showing that punicalagin is toxic only at higher concentrations in cells of Vero (a normal African green monkey kidney cell line), Hep-2 (a human larynx epithelial cancer cell line), and A-549 (a human small cell lung carcinoma cell line). This indicates that the inhibition of LPS-induced inflammatory injury in bEECs was not a result of toxicity.
E. coli induces a rapid inflammation response in endometrial epithelial cells, which release a large number of pro-inflammatory cytokines, including IL-1β, IL-6, IL-8, and TNF-α (Chapwanya et al., 2013; Huang et al., 2016). These cytokines attract immune effector cells to fight infection; however, excessive expression of these cytokines injures the cells. In this study, the potential effect of punicalagin on the expression of pro-inflammatory cytokines (IL-1β, IL-6, IL-8, and TNF-α) was analyzed by RT-PCR. As expected, the expression of IL-1β, IL-6, IL-8, and TNF-α was upregulated at all time points after LPS stimulation. Punicalagin inhibited the expression of IL-1β, IL-6, IL-8, and TNF-α in a dose-dependent manner, which protected bEECs from LPS.
NF-κB plays a central role in regulating immune and inflammatory processes, and thus is a target in developing novel treatments for inflammatory diseases (Checker et al., 2012; Ling et al., 2012). Before activation, NF-κB is bound in the cytoplasm by IκBα, an inhibitory protein that keeps NF-κB in an inactive state. When activated by various stimulants such as LPS, IκBα is phosphorylated by IκB kinase (IKK) and is separated from NF-κB. Free NF-κB then translocates from the cytoplasm into the nucleus, where it binds specifically to certain DNA sequences, promoting the expression of specific target genes (Corbetta et al., 2005; Gu et al., 2012). Previous studies reported that treatments of BV2 and THP1 cells with LPS greatly upregulated the phosphorylation level of NF-κB p65, indicating activation of the NF-κB signal pathway (Lim et al., 2012; Wang et al., 2013). To confirm the specific effect of punicalagin on NF-κB activity in bEECs, Western blotting was performed to detect levels of IκBα and phosphorylated p65. Punicalagin significantly inhibited LPS-induced NF-κB signaling in bEECs by suppressing the degradation of IκBα and the phosphorylation of p65 within 15, 45, and 90 min of LPS stimulation. We therefore propose that punicalagin prevented inflammation in the LPS-activated bEECs through the NF-κB pathway.
The TLR-4-mediated NF-κB pathway is well established as a signaling pathway responsible for inflammatory responses. In addition to NF-κB activation, TLR-4 can initiate MAPK signaling (Risco et al., 2012; Hines et al., 2013). Phosphorylation of MAPK family members including ERK, p38, and JNK activates a series of transcription factors, such as activator protein 1 (AP-1), cAMP-response element binding protein (CREB), and signal transducers and activators of transcription (STAT), and subsequently promotes transcription of cytokines (Morimoto et al., 2009; Ruimi et al., 2010). Punicalagin has been shown to inhibit the expression of pro-inflammatory genes by regulating the phosphorylation of proteins of the MAPK pathway (Olajide et al., 2014; Xu et al., 2014). However, whether the MAPK signaling pathway undergoes any changes in bEECs had not been determined. In the current study, LPS enhanced the phosphorylation levels of p38, JNK, and ERK in bEECs, but these effects were markedly downregulated by punicalagin in a dose-and time-dependent manner. These results indicate that attenuation of p38, JNK, and ERK activation may be involved in the reduction of cytokine production by punicalagin.
5 Conclusions
This study demonstrated the protective effect of punicalagin on LPS-induced inflammatory injury in bEECs. This effect was at least partly achieved by the decreased production of pro-inflammatory cytokines mediated by reducing the degradation of IκBα and phosphorylation of p65, p38, JNK, and ERK in the NF-κB and MAPK pathways. However, other possible pathways and targets related to the anti-inflammatory effect of punicalagin in bEECs need to be researched in the future.
Acknowledgments
We are thankful for help from the members of China Agricultural University-Beijing University of Agriculture Traditional Chinese Veterinary Medicine (CAU-BUA TCVM) teaching and research team.
Footnotes
Project supported by the National Key Technology R & D Program of China (No. 2013BAD10B04) and the Importation and Development of High-Caliber Talents Project of Beijing Municipal Institutions (No. CIT&TCD20130324), China
Compliance with ethics guidelines: An LYU, Jia-jia CHEN, Hui-chuan WANG, Xiao-hong YU, Zhi-cong ZHANG, Ping GONG, Lin-shu JIANG, and Feng-hua LIU declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Aqil F, Munagala R, Vadhanam MV, et al. Anti-proliferative activity and protection against oxidative DNA damage by punicalagin isolated from pomegranate husk. Food Res Int. 2012;49(1):345–353. doi: 10.1016/j.foodres.2012.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azawi OI. Postpartum uterine infection in cattle. Anim Reprod Sci. 2008;105(3-4):187–208. doi: 10.1016/j.anireprosci.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Brodzki P, Bochniarz M, Brodzki A, et al. Trueperella pyogenes and Escherichia coli as an etiological factor of endometritis in cows and the susceptibility of these bacteria to selected antibiotics. Pol J Vet Sci. 2014;17(4):657–664. doi: 10.2478/pjvs-2014-0096. [DOI] [PubMed] [Google Scholar]
- 4.Chapwanya A, Meade KG, Doherty ML, et al. Endometrial epithelial cells are potent producers of tracheal antimicrobial peptide and serum amyloid A3 gene expression in response to E. coli stimulation. Vet Immunol Immunopathol. 2013;151(1-2):157–162. doi: 10.1016/j.vetimm.2012.09.042. [DOI] [PubMed] [Google Scholar]
- 5.Checker R, Patwardhan RS, Sharma D, et al. Schisandrin B exhibits anti-inflammatory activity through modulation of the redox-sensitive transcription factors Nrf2 and NF-κB. Free Radic Biol Med. 2012;53(7):1421–1430. doi: 10.1016/j.freeradbiomed.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Choi HJ, Kang OH, Park PS, et al. Mume fructus water extract inhibits pro-inflammatory mediators in lipopolysaccharide-stimulated macrophages. J Med Food. 2007;10(3):460–466. doi: 10.1089/jmf.2006.198. [DOI] [PubMed] [Google Scholar]
- 7.Corbetta S, Vicentini L, Ferrero S, et al. Activity and function of the nuclear factor kappaB pathway in human parathyroid tumors. Endocr Relat Cancer. 2005;12(4):929–937. doi: 10.1677/erc.1.00970. [DOI] [PubMed] [Google Scholar]
- 8.Fu Y, Liu B, Feng X, et al. Lipopolysaccharide increases Toll-like receptor 4 and downstream Toll-like receptor signaling molecules expression in bovine endometrial epithelial cells. Vet Immunol Immunopathol. 2013;151(1-2):20–27. doi: 10.1016/j.vetimm.2012.09.039. [DOI] [PubMed] [Google Scholar]
- 9.Gu JH, Ge JB, Li M, et al. Inhibition of NF-κB activation is associated with anti-inflammatory and anti-apoptotic effects of Ginkgolide B in a mouse model of cerebral ischemia/reperfusion injury. Eur J Pharm Sci. 2012;47(4):652–660. doi: 10.1016/j.ejps.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 10.Herath S, Fischer DP, Werling D, et al. Expression and function of Toll-like receptor 4 in the endometrial cells of the uterus. Endocrinology. 2006;147(1):562–570. doi: 10.1210/en.2005-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hines DJ, Choi HB, Hines RM, et al. Prevention of LPS-induced microglia activation, cytokine production and sickness behavior with TLR4 receptor interfering peptides. PLoS ONE. 2013;8(3):e60388. doi: 10.1371/journal.pone.0060388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang B, Xiao D, Tan B, et al. Chitosan oligosaccharide reduces intestinal inflammation that involves calcium-sensing receptor (CaSR) activation in lipopolysaccharide (LPS)-challenged piglets. J Agric Food Chem. 2016;64(1):245–252. doi: 10.1021/acs.jafc.5b05195. [DOI] [PubMed] [Google Scholar]
- 13.Janowski T, Barański W, Łukasik K, et al. Prevalence of subclinical endometritis in repeat breeding cows and mRNA expression of tumor necrosis factor α and inducible nitric oxide synthase in the endometrium of repeat breeding cows with and without subclinical endometritis. Pol J Vet Sci. 2013;16(4):693–699. doi: 10.2478/pjvs-2013-0098. [DOI] [PubMed] [Google Scholar]
- 14.Jean-Gilles D, Li L, Vaidyanathan VG, et al. Inhibitory effects of polyphenol punicalagin on type-II collagen degradation in vitro and inflammation in vivo. Chem Biol Interact. 2013;205(2):90–99. doi: 10.1016/j.cbi.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 15.Kasimanickam RK, Kasimanickam VR, Olsen JR, et al. Associations among serum pro- and anti-inflammatory cytokines, metabolic mediators, body condition, and uterine disease in postpartum dairy cows. Reprod Biol Endocrinol. 2013;11(1):103. doi: 10.1186/1477-7827-11-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34(5):637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Khan KN, Kitajima M, Hiraki K, et al. Toll-like receptors in innate immunity: role of bacterial endotoxin and Toll-like receptor 4 in endometrium and endometriosis. Gynecol Obstet Invest. 2009;68(1):40–52. doi: 10.1159/000212061. [DOI] [PubMed] [Google Scholar]
- 18.Kulkarni AP, Mahal HS, Kapoor S, et al. In vitro studies on the binding, antioxidant, and cytotoxic actions of punicalagin. J Agric Food Chem. 2007;55(4):1491–1500. doi: 10.1021/jf0626720. [DOI] [PubMed] [Google Scholar]
- 19.Kumar H, Kaur A, Kishor N, et al. Prevalence of multiple antibiotic resistant nasal carriage MRSA among healthy population of border villages in Amritsar Region, Punjab, India. J Clin Diagn Res. 2016;10(5):DL01–DL02. doi: 10.7860/JCDR/2016/16131.7781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim JY, Hwang BY, Hwang KW, et al. Methylalpinumisoflavone inhibits lipopolysaccharide-induced inflammation in microglial cells by the NF-kappaB and MAPK signaling pathway. Phytother Res. 2012;26(12):1948–1956. doi: 10.1002/ptr.4810. [DOI] [PubMed] [Google Scholar]
- 21.Ling M, Li Y, Xu Y, et al. Regulation of miRNA-21 by reactive oxygen species-activated ERK/NF-κB in arsenite-induced cell transformation. Free Radic Biol Med. 2012;52(9):1508–1518. doi: 10.1016/j.freeradbiomed.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 22.Liu M, Song S, Li H, et al. The protective effect of caffeic acid against inflammation injury of primary bovine mammary epithelial cells induced by lipopolysaccharide. J Dairy Sci. 2014;97(5):2856–2865. doi: 10.3168/jds.2013-7600. [DOI] [PubMed] [Google Scholar]
- 23.Mackeen AD, Packard RE, Ota E, et al. Antibiotic regimens for postpartum endometritis. Cochrane Database Syst Rev. 2015;(2):CD001067. doi: 10.1002/14651858.CD001067.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malinowski E, Lassa H, Markiewicz H, et al. Sensitivity to antibiotics of Arcanobacterium pyogenes and Escherichia coli from the uteri of cows with metritis/endometritis. Vet J. 2011;187(2):234–238. doi: 10.1016/j.tvjl.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Morimoto Y, Kikuchi K, Ito T, et al. MK615 attenuates Porphyromonas gingivalis lipopolysaccharide-induced pro-inflammatory cytokine release via MAPK inactivation in murine macrophage-like RAW264.7 cells. Biochem Biophys Res Commun. 2009;389(1):90–94. doi: 10.1016/j.bbrc.2009.08.103. [DOI] [PubMed] [Google Scholar]
- 26.Olajide OA, Kumar A, Velagapudi R, et al. Punicalagin inhibits neuroinflammation in LPS-activated rat primary microglia. Mol Nutr Food Res. 2014;58(9):1843–1851. doi: 10.1002/mnfr.201400163. [DOI] [PubMed] [Google Scholar]
- 27.Pandrea I, Xu C, Stock JL, et al. Antibiotic and antiinflammatory therapy transiently reduces inflammation and hypercoagulation in acutely SIV-infected pigtailed macaques. PLoS Pathog. 2016;12(1):e1005384. doi: 10.1371/journal.ppat.1005384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng J, Wei D, Fu Z, et al. Punicalagin ameliorates lipopolysaccharide-induced acute respiratory distress syndrome in mice. Inflammation. 2015;38(2):493–499. doi: 10.1007/s10753-014-9955-5. [DOI] [PubMed] [Google Scholar]
- 29.Qi Z, Qi S, Ling L, et al. Salidroside attenuates inflammatory response via suppressing JAK2-STAT3 pathway activation and preventing STAT3 transfer into nucleus. Int Immunopharmacol. 2016;35:265–271. doi: 10.1016/j.intimp.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Risco A, del Fresno C, Mambol A, et al. p38γ and p38δ kinases regulate the Toll-like receptor 4 (TLR4)-induced cytokine production by controlling ERK1/2 protein kinase pathway activation. Proc Natl Acad Sci USA. 2012;109(28):11200–11205. doi: 10.1073/pnas.1207290109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruimi N, Rwashdeh H, Wasser S, et al. Daedalea gibbosa substances inhibit LPS-induced expression of iNOS by suppression of NF-κB and MAPK activities in RAW 264.7 macrophage cells. Int J Mol Med. 2010;25(3):421–432. doi: 10.3892/ijmm_00000361. [DOI] [PubMed] [Google Scholar]
- 32.Sens A, Heuwieser W. Presence of Escherichia coli, Trueperella pyogenes, α-hemolytic streptococci, and coagulase-negative staphylococci and prevalence of subclinical endometritis. J Dairy Sci. 2013;96(10):6347–6354. doi: 10.3168/jds.2013-6646. [DOI] [PubMed] [Google Scholar]
- 33.Sheldon IM, Roberts MH. Toll-like receptor 4 mediates the response of epithelial and stromal cells to lipopolysaccharide in the endometrium. PLoS ONE. 2010;5(9):e12906. doi: 10.1371/journal.pone.0012906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheldon IM, Lewis GS, LeBlanc S, et al. Defining postpartum uterine disease in cattle. Theriogenology. 2006;65(8):1516–1530. doi: 10.1016/j.theriogenology.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 35.Shimada K, Daida H, Ma-Krupa W, et al. Lipopolysaccharide, CD14 and Toll-like receptors: an emerging link between innate immunity and atherosclerotic disease. Future Cardiol. 2005;1(5):657–674. doi: 10.2217/14796678.1.5.657. [DOI] [PubMed] [Google Scholar]
- 36.Soboll G, Schaefer TM, Wira CR. Effect of Toll-like receptor (TLR) agonists on TLR and microbicide expression in uterine and vaginal tissues of the mouse. Am J Reprod Immunol. 2006;55(6):434–446. doi: 10.1111/j.1600-0897.2006.00381.x. [DOI] [PubMed] [Google Scholar]
- 37.Taguri T, Tanaka T, Kouno I. Antimicrobial activity of 10 different plant polyphenols against bacteria causing food-borne disease. Biol Pharm Bull. 2004;27(12):1965–1969. doi: 10.1248/bpb.27.1965. [DOI] [PubMed] [Google Scholar]
- 38.Turner ML, Cronin JG, Healey GD, et al. Epithelial and stromal cells of bovine endometrium have roles in innate immunity and initiate inflammatory responses to bacterial lipopeptides in vitro via Toll-like receptors TLR2, TLR1, and TLR6. Endocrinology. 2014;155(4):1453–1465. doi: 10.1210/en.2013-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ugurlu O, Yaris M, Oztekin CV, et al. Impacts of antibiotic and anti-inflammatory therapies on serum prostate-specific antigen levels in the presence of prostatic inflammation: a prospective randomized controlled trial. Urol Int. 2010;84(2):185–190. doi: 10.1159/000277596. [DOI] [PubMed] [Google Scholar]
- 40.Wagener K, Grunert T, Prunner I, et al. Dynamics of uterine infections with Escherichia coli, Streptococcus uberis and Trueperella pyogenes in post-partum dairy cows and their association with clinical endometritis. Vet J. 2014;202(3):527–532. doi: 10.1016/j.tvjl.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 41.Wang HW, Wu T, Qi JY, et al. Salidroside attenuates LPS-stimulated activation of THP-1 cell-derived macrophages through down-regulation of MAPK/NF-κB signaling pathways. J Huazhong Univ Sci Technol Med Sci. 2013;33(4):463–469. doi: 10.1007/s11596-013-1143-6. [DOI] [PubMed] [Google Scholar]
- 42.Wang Z, Jiang W, Zhang Z, et al. Nitidine chloride inhibits LPS-induced inflammatory cytokines production via MAPK and NF-kappaB pathway in RAW 264.7 cells. J Ethnopharmacol. 2012;144(1):145–150. doi: 10.1016/j.jep.2012.08.041. [DOI] [PubMed] [Google Scholar]
- 43.Ward E, Duff P. A comparison of 3 antibiotic regimens for prevention of postcesarean endometritis: an historical cohort study. Am J Obstet Gynecol. 2016;214(6):751.e1–751e4. doi: 10.1016/j.ajog.2016.02.037. [DOI] [PubMed] [Google Scholar]
- 44.Wira CR, Grant-Tschudy KS, Crane-Godreau MA. Epithelial cells in the female reproductive tract: a central role as sentinels of immune protection. Am J Reprod Immunol. 2005;53(2):65–76. doi: 10.1111/j.1600-0897.2004.00248.x. [DOI] [PubMed] [Google Scholar]
- 45.Wu H, Zhao G, Jiang K, et al. Engeletin alleviates lipopolysaccharide-induced endometritis in mice by inhibiting TLR4-mediated NF-κB activation. J Agric Food Chem. 2016;64(31):6171–6178. doi: 10.1021/acs.jafc.6b02304. [DOI] [PubMed] [Google Scholar]
- 46.Xu L, He S, Yin P, et al. Punicalagin induces Nrf2 translocation and HO-1 expression via PI3K/Akt, protecting rat intestinal epithelial cells from oxidative stress. Int J Hyperthermia. 2016;32(5):465–473. doi: 10.3109/02656736.2016.1155762. [DOI] [PubMed] [Google Scholar]
- 47.Xu X, Yin P, Wan C, et al. Punicalagin inhibits inflammation in LPS-induced RAW264.7 macrophages via the suppression of TLR4-mediated MAPKs and NF-κB activation. Inflammation. 2014;37(3):956–965. doi: 10.1007/s10753-014-9816-2. [DOI] [PubMed] [Google Scholar]
- 48.Yaidikar L, Thakur S. Punicalagin attenuated cerebral ischemia-reperfusion insult via inhibition of proinflammatory cytokines, up-regulation of Bcl-2, down-regulation of Bax, and caspase-3. Mol Cell Biochem. 2015;402(1-2):141–148. doi: 10.1007/s11010-014-2321-y. [DOI] [PubMed] [Google Scholar]
- 49.Yaidikar L, Byna B, Thakur SR. Neuroprotective effect of punicalagin against cerebral ischemia reperfusion-induced oxidative brain injury in rats. J Stroke Cerebrovasc Dis. 2014;23(10):2869–2878. doi: 10.1016/j.jstrokecerebrovasdis.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 50.Yang Y, Xiu J, Zhang L, et al. Antiviral activity of punicalagin toward human enterovirus 71 in vitro and in vivo. Phytomedicine. 2012;20(1):67–70. doi: 10.1016/j.phymed.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 51.Yoon YK, Park DW, Sohn JW, et al. Effects of inappropriate empirical antibiotic therapy on mortality in patients with healthcare-associated methicillin-resistant Staphylococcus aureus bacteremia: a propensity-matched analysis. BMC Infect Dis. 2016;16(1):331. doi: 10.1186/s12879-016-1650-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao HX, Zhao JL, Shen JZ, et al. Prevalence and molecular characterization of fluoroquinolone resistance in Escherichia coli isolates from dairy cattle with endometritis in China. Microb Drug Resist. 2014;20(2):162–169. doi: 10.1089/mdr.2013.0073. [DOI] [PubMed] [Google Scholar]