Abstract
The aim of this study was to evaluate the possible therapeutic or protective effects of Helichrysum plicatum DC. subsp. plicatum ethanol extract (HPE) against gentamicin-induced nephrotoxicity. Thirty-six Sprague Dawley male rats weighing between 200 and 250 g were used as live material. They were formed into six groups containing 6 rats each and were allowed to adapt to laboratory conditions for 7 d. Group I: control, 5% DMSO intraperitoneal (i.p.); Group II: HPE 100 mg/(kg·d) i.p.; Group III: HPE 200 mg/(kg·d) i.p.; Group IV: gentamicin as 80 mg/(kg·d) i.p.; Group V: gentamicin as 80 mg/(kg·d) i.p.+HPE 100 mg/(kg·d) i.p.; and Group VI: gentamicin as 80 mg/(kg·d) i.p.+HPE 200 mg/(kg·d) i.p. for 8 d. Following treatment, serum, liver, and kidney tissues were used to assess blood urea nitrogen (BUN), creatinine, enzymatic and non-enzymatic antioxidants, and lipid peroxidation. Gentamicin significantly increased serum BUN, creatinin, and liver and kidney levels of malondialdehyde (MDA). It also decreased the activity of catalase (CAT), glutathione peroxidase (GPx), and superoxide dismutase (SOD). Treatment with the HPE 100 mg/kg reversed gentamicin-induced alterations as evidenced by decreased serum BUN and creatinin, liver and kidney oxidant marker, and tubular necrosis as well as by an increase in antioxidant enzymes. It was found that HPE 200 mg/kg significantly increased liver and kidney tissue MDA levels in nephrotoxicity in rats. As a result, these findings support the proposition that HPE in 100 mg/kg dose demonstrates in the kidney and liver as free radicals and scavenger to prevent the toxic effects of gentamicin in both the biochemical and histopathology parameters.
Keywords: Antioxidants, Extract, Gentamicin, Helichrysum plicatum DC. subsp. Plicatum, Nephrotoxicity, Oxidative stress
1. Introduction
Aminoglycoside antibiotic gentamicin sulphate is frequently used for the treatment of Gram-negative bacteria such as Pseudomonas, Proteus, and Serratia (Corona et al., 2014; Shrestha and Haylor, 2014). It has been a most powerful therapeutic drug against bacterial strains that are resistant to other antibiotics in many conditions; however, its use is limited due to the side effects of nephrotoxicity and hepatotoxicity (Martínez-Salgado et al., 2007; Nayma et al., 2012; Mahmood et al., 2014). When the effects of gentamicin-induced nephrotoxicity are not completely known, suggested pathological effects include induction of apoptosis, necrosis, oxidative stress, an increase in monocyte/macrophages infiltration, and elevation of endothelin I (Sumbul et al., 2003; Süzgec et al., 2005). Gentamicin-induced nephrotoxicity is characterized physiologically by increases in the levels of serum creatinine, blood urea nitrogen (BUN), tubular necrosis and glomerular congestion, and decreases in the glomerular filtration rate (Balakumar et al., 2010; Nasri, 2012). It is morphologically characterized by epithelial edema, proximal tubule epithelial desquamation, tubular necrosis, and glomerular hypertrophy (Stafford et al., 2005). Gentamicin increases the production of reactive oxygen species (ROS), for example, super oxide anions, hydrogen peroxide, hydroxyl radicals, and reactive nitrogen species in the kidney (Tavafi and Ahmadvand, 2011; Tavafi, 2013; Moreira et al., 2014). Gentamicin reduces the activity of renal antioxidant enzymes such as catalase (CAT), glutathione peroxidase (GPx), superoxide dismutase (SOD), and glutathione (GSH) (Ojano-Dirain et al., 2014; Kandemir et al., 2015).
Helichrysum species belonging to the family Asteraceae include approximately 185 genera and more than 1240 species, which are widespread throughout the world (Europe, Asia, Africa, and Madagascar) (Bayer et al., 2007). Helichrysum genus is represented by 24 species and 30 taxa, of which 17 are endemic and are widely found in the flora of Turkey (Guner, 2012). The biological activity of many Helichrysum species has been researched in different countries, but there is little information about those belonging to Turkish flora (Aslan et al., 2007).
Helichrysum species are largely known as “ölmez çiçek, altınotu, or mantuvar” and are commonly used for the treatment of clearing kidney stones, stomach ulcers, otitis, wounds, burns, and nephritis in Turkey (Sezik et al., 2001). Aerial parts of the plants have been used as an herbal tea. These effects of Helichrysum species are due to the flavonoids contained within them (Morone-Fortunato et al., 2010). They are used for wound wrappings and stomach ache relaxation; anti-inflammatory (Sala et al., 2003), antioxidant (Albayrak et al., 2009), and antimicrobial activity (Sagdic et al., 2003); anti-infective, antibacterial (Smirnov et al., 1982), detoxifying, hepatoprotective, cholagogic, and choleretic effects; to stimulate the secretion of gastric juices; and treatment of coughs, erythema, diabetes mellitus, and renal failure. In Turkish folk medicine, H. plicatum DC. subsp. plicatum has been used as a diuretic, lithagogue, and for stomach ache (Aslan, 2000).
The chemical composition of the H. plicatum subsp. plicatum belonging to Turkish flora has been researched. The major components of the capitula of H. plicatum subsp. plicatum are several flavonoids such as helichrysin A and B, apigenin, naringenin, isoastragalin, and isosalipurposide (Aslan et al., 2007).
This study was to evaluate both the anti-nephrotoxicity effect of H. plicatum DC. subsp. plicatum ethanol extract (HPE) on gentamicin-induced rats and the antioxidant activity by measuring the levels of malondialdehyde (MDA), CAT, GPx, and SOD in the liver and kidney tissues and serum BUN and creatinine of the nephrotoxicity in the rats.
2. Materials and methods
2.1. Animals
The experiments were administered according to the ethical conditions confirmed by the Ethic Committee of Experimental Animal Teaching and Researcher Center, Ataturk University, Erzurum, Turkey (No. 05.11.2013 36643897-932-ATA-140). Rats were obtained from the Medical and Experimental Application and Research Center (ATADEM), Erzurum, Turkey. Sprague-Dawley male rats weighing between 200 and 250 g were housed under standard conditions at (25±2) °C (constant temperature), a relative humidity of (60±5)%, a 12-h light-dark rhythm, and had free access to a standard diet of food pellets and tap water ad libitum during the study period.
2.2. Drugs and chemicals
Gentamicin sulphate was bought from Eczacibasi (Gentasol Flacon, Istanbul, Turkey). All other chemicals used were of analytical level and were bought from the Sigma Chemical Co. (St. Louis, MO, USA).
2.3. Plant materials
Helichrysum plicatum DC. subsp. plicatum (abbreviated as HP) aerial part was collected in July 2013 from Kop Mountain 2100 m (Bayburt, Turkey) and identified by Meryem SENGUL (Department of Botanic, Faculty of Agriculture, Ataturk University, Erzurum, Turkey). A voucher specimen has been deposited in the Herbarium of Ataturk University (voucher No. ATA9562), Erzurum, Turkey.
2.4. Preparation of the test samples
The dried plant samples were powdered in a crusher and then 100 g of plant sample was extracted individually with 500 ml ethanol for 48 h at room temperature. The extract was filtered using Whatman filter paper (No. 1), evaporated to dryness in a vacuum under reduced pressure at 40 °C with a rotary evaporator (RV 05 Basic 1B, IKA Group, Wilmington, NC, USA). The extract was dissolved in 5% dimethyl sulfoxide (DMSO) for further study. The dried extracts were stored at 4 °C until used.
2.5. Experimental protocol
A total of 36 Spraque-Dawley male rats were used in the present study. Thirty-six rats were randomly assigned into 6 experimental groups (6 rats per group). They were allowed to adapt to laboratory conditions for 7 d. The study was conducted for 8 d. Rats were divided into the following groups: Group I: control group, received only vehicle (5% DMSO) intraperitoneal (i.p.); Group II: HPE1-administered group, HPE 100 mg/(kg·d) i.p., 8 d; Group III: HPE2-administered group, HPE 200 mg/(kg·d) i.p., 8 d; Group IV: GM (gentamicin)-administered group, gentamicin 80 mg/(kg·d) i.p., 8 d; Group V: GM-plus-HPE1-treated group, gentamicin 80 mg/(kg·d) i.p.+HPE 100 mg/(kg·d) i.p., 8 d; Group VI: GM-plus-HPE2-treated group, gentamicin 80 mg/(kg·d) i.p.+HPE 200 mg/(kg·d) i.p., 8 d.
At the end of these processes, rats were anaesthetized with pentobarbital sodium (60 mg/kg i.p.). The blood was withdrawn and then centrifuged at 4000 r/min to separate the serum and stored at −20 °C for estimating BUN and serum creatinine levels and dissected liver and kidney tissues. Meanwhile, both the kidneys and liver were harvested; one of the kidneys and a part of the liver were immediately kept in 10% neutral buffered formalin, embedded in paraffin, and used for histopathological assay. For biochemical estimation, other tissues were snap frozen in liquid nitrogen and stored at −80 °C until analysis.
2.6. Assessment of renal function test
Creatinine and BUN levels were analyzed in the serum using commercially available kits (Diasis Diagnostic Systems, Istanbul, Turkey).
2.7. Assessments of biochemical parameters
The kidney and liver tissues were homogenized in a Teflon-glass homogenizer using a 0.1 mol/L phosphate buffer (pH 7.4) to obtain a 1:10 (w/v) homogenate. Homogenates MDA content as a lipid peroxidation marker was measured using the thiobarbituric acid reaction according to the method of Placer et al. (1966). CAT activity was measured as the difference in H2O2 extinction per unit time as described previously for assessed CAT enzyme activity (Góth, 1991). The protein concentration was also measured in the supernatant according to the method of Mert (1996). The GPx activity was determined according to the method of Matkovics et al. (1988). The generation of superoxide radicals produced by xanthine and xanthine oxidase, following the reaction of nitro blue tetrazolium and the formation of formazan dye, was used to measure SOD activity (Sun et al., 1988).
2.8. Histological examination
Other tissues were fixed in 10% neutral buffered formalin. After dehydration in a graded ethanol series and clearing with xylene, the sample material was embedded in paraffin and 4-μm-thick tissue sections were cut using microtome (LEICA, RM2255). Sections were stained with haematoxylin and eosin (H & E) and periodic acid Schiff (PAS) for observation under the light microscope (OLYMPUS, BX51).
The tissue section was evaluated by high power light microscopic examination. For each specimen, the histopathological stain was examined in 10 randomly selected areas of approximately ×40 objective. The scores were derived semi-quantitatively using light microscopy on the preparations from each animal and were reported as follows: none, 0; mild, 1; moderate, 2; severe, 3; and very strong, 4.
2.9. Statistical analysis
Statistical analysis was done by one-way analysis of variance (ANOVA) using the SPSS software package, Version 20.00. Data between groups were tested by ANOVA and post-hoc Tukey’s test was used to compare the studied parameters between the groups. P values of <0.05 were considered significantly different for all parameters. The results are expressed as mean±standard error means (SEM) for 6 rats in each group.
3. Results
3.1. Effect of H. plicatum extract on renal function in gentamicin-induced nephrotoxicity
The levels of serum BUN and creatinine are shown in Table 1. To evaluate whether H. plicatum extract protected renal function, serum BUN and creatinine levels were measured in all groups. The levels of serum BUN and creatinine in the gentamicin-administered rats (Group IV) were significantly increased in comparison to those of the control, which reflects injury to kidney and the HPE-administered rats (P<0.001) and the levels of serum BUN and creatinine in the GM-plus-HPE1-treated rats were significantly decreased in comparison to those of the gentamicin-administered rats (Group IV) (P<0.001) but were still significantly higher than those of the control group (P<0.001) (Table 1). HPE2 improved the renal function, but significant normalization of serum creatinine (P<0.001) and BUN levels (P<0.001) was observed only with the lower dose 100 mg/kg of HPE. This indicates that kidney injury was decreased with HPE 100 mg/kg (Group V) treatment.
Table 1.
Effects of HPE on serum BUN and creatinine in gentamicin-induced nephrotoxicity in rats
| Group | BUN (mg/dl) | Creatinine (mg/dl) |
| I | 34.68±0.18f | 0.59±0.01d |
| II | 31.32±0.41e | 0.64±0.02d |
| III | 43.22±0.53d | 0.72±0.01d |
| IV | 79.88±0.33a | 4.81±0.03a |
| V | 48.03±1.66c | 1.72±0.05c |
| VI | 73.02±0.56b | 3.43±0.18b |
|
| ||
| P | *** | *** |
Group I: control group; Group II: HPE1-administered group; Group III: HPE2-administered group; Group IV: gentamicin-administered group; Group V: gentamicin-plus-HPE1-treated group; Group VI: gentamicin-plus-HPE2-treated group. Data are expressed as mean±SEM (n=6). Values with different superscripts within one column differ significantly.
P<0.001
3.2 Effect of H. plicatum extract on oxidative stress in gentamicin-induced nephrotoxicity
Results of biochemical tests of MDA, CAT, GPx, and SOD are shown in Table 2. In the gentamicin-administered group rats, there was significant (P<0.001) depletion of antioxidants CAT, GPx, and SOD along with increase in lipid peroxidation as compared with the control group. GM-plus-HPE1 decreased MDA level and significantly restored the anti-oxidant status as demonstrated by increase in CAT content, GPx and SOD activity in comparison to the gentamicin-administered and control groups.
Table 2.
Effects of H. plicatum extract on biochemical parameters of liver and kidney tissues
| Group | MDA (nmol/g) | CAT (kU/g) | GPx (U/g) | SOD (EU/mg) |
| Liver | ||||
| I | 47.72±1.69b | 257.56±24.16ab | 10.26±0.88bc | 11.57±0.68a |
| II | 45.94±1.36b | 262.63±23.07a | 16.98±1.10a | 11.87±0.73a |
| III | 51.27±2.00b | 186.79±32.62bc | 11.27±0.55bc | 11.64±0.57a |
| IV | 64.24±6.64a | 91.48±4.80d | 9.36±0.13bc | 5.51±0.44c |
| V | 49.68±2.06b | 232.30±31.57abc | 12.48±0.78b | 8.08±0.43b |
| VI | 51.99±0.73b | 177.43±12.74c | 9.62±0.54bc | 5.91±0.36c |
|
| ||||
| P | ** | *** | *** | *** |
|
| ||||
| Kidney | ||||
| I | 55.46±0.62c | 255.29±50.96b | 16.62±2.73a | 12.28±0.88a |
| II | 52.70±3.00c | 379.22±30.31a | 19.83±1.73a | 14.76±1.53a |
| III | 61.90±3.91bc | 357.33±41.98a | 17.61±2.49a | 14.54±0.95a |
| IV | 108.43±5.88a | 64.59±13.31e | 6.54±0.11c | 7.46±0.56b |
| V | 55.67±3.09c | 223.08±11.35b | 7.42±0.25c | 12.61±0.29a |
| VI | 73.30±5.77b | 127.56±20.56cde | 7.05±0.08c | 7.77±0.64b |
|
| ||||
| P | *** | *** | *** | *** |
Group I: control group; Group II: HPE1-administered group; Group III: HPE2-administered group; Group IV: gentamicin-administered group; Group V: gentamicin-plus-HPE1-treated group; Group VI: gentamicin-plus-HPE2-treated group. EU: enzyme unit. Data are expressed as mean±SEM (n=6). Values with different superscripts within one column differ significantly.
P<0.01,
P<0.001
3.3. Effect of H. plicatum extract on histopathological changes in gentamicin-induced nephrotoxicity
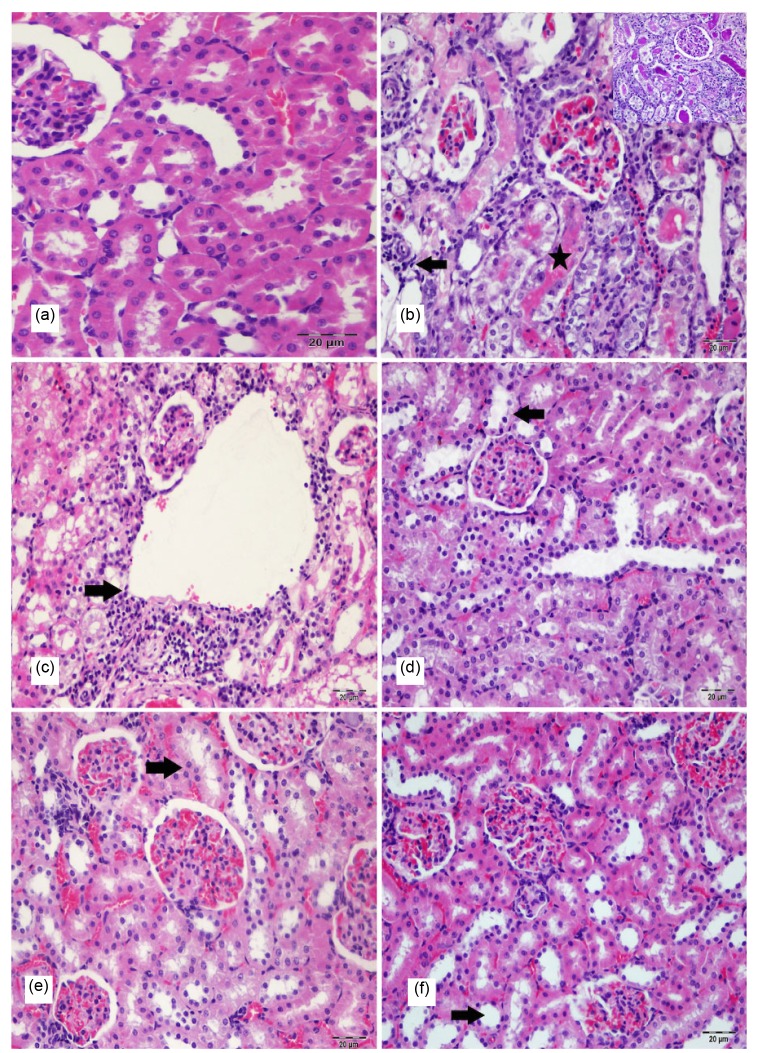
Figs. 1 and 2 show histopathological images of the kidney and liver tissues of all groups. No histopathological finding was observed in the kidney in the control group (Fig. 1a). Gentamicin-administered group (Group IV) showed congestion and dilatation in the renal blood vessels and severe degeneration (vacuolar or hydrophic degeneration) and necrosis in the epithelial cells of the proximal tubules. The lumens of these tubules were filled with degenerate and desquamated epithelial cells. At the same time hyaline casts were seen in some dilated renal tubules. In addition to these changes, mononuclear cells had infiltrated in intertubular areas (Fig. 1b). Lesions similar to those observed in Group IV were also observed in the kidney of the rats included in Group VI (Fig. 1c), Group V (Fig. 1d), Group III (Fig. 1e), and Group II (Fig. 1f). These degenerative and inflammatory changes generally were reduced in Group V when compared with Groups IV and VI.
Fig. 1.
Histopathological appearance in H & E-PAS-stained rat kidney sections of all groups
(a) Normal kidney tissue (control group); (b) Severe tubulo epithelial degeneration, necrosis (star) and interstitial mononuclear cells infiltration (arrow) in kidney (Group IV); (c) Severe tubulo epithelial degeneration, necrosis and interstitial mononuclear cells infiltration (arrow) in kidney (Group VI); (d) Moderate tubulo epithelial degeneration (arrow) in kidney (Group V); (e) Moderate tubulo epithelial degeneration (arrow) in kidney (Group III); (f) Mild tubulo epithelial degeneration (arrow) in kidney (Group II). Bar: 20 μm
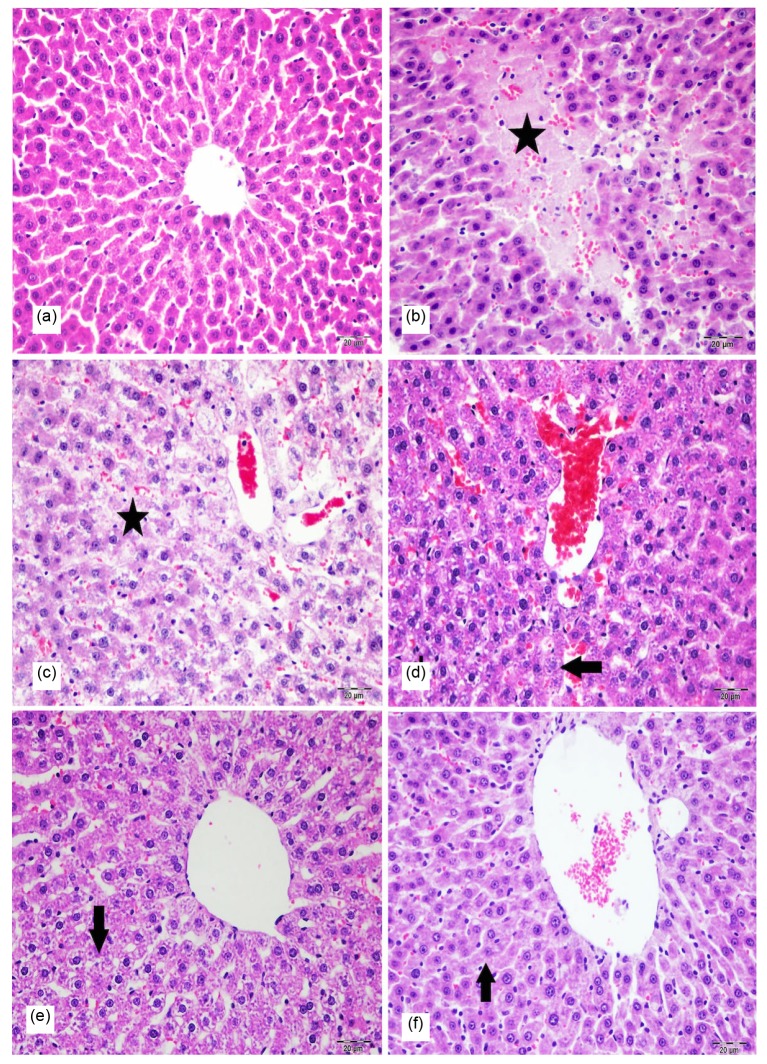
Fig. 2.
Histopathological appearance in H & E-stained rat liver sections of all groups
(a) Normal hepatic tissue (control group); (b) Severe degeneration and necrosis (star) in hepatocytes of liver (Group IV); (c) Severe degeneration and necrosis (star) in hepatocytes of liver (Group VI); (d) Moderate degeneration (arrow) in hepatocytes of liver (Group V); (e) Moderate degeneration (arrow) in hepatocytes of liver (Group III); (f) Mild degeneration (arrow) in hepatocytes of liver (Group II). Bar: 20 μm
No histopathological finding was observed in the liver in the control group (Fig. 2a). Gentamicin-administered group (Fig. 2b) showed remarkable degenerative changes in the hepatocytes. At the same time there was necrosis in some hepatocytes. In addition to these changes, mononuclear cells had infiltrated within portal areas. Furthermore, proliferation of Kupffer cells and hyperplasia of bile ducts were observed. Lesions similar to those observed in Group IV were also observed in the liver of the rats included in Group VI (Fig. 2c), Group V (Fig. 2d), Group III (Fig. 2e), and Group II (Fig. 2f). These degenerative and inflammatory changes generally were reduced in Group V when compared with Groups IV and VI.
Table 3 shows the scores derived semi-quantitatively using light microscopy on the preparations from each animal.
Table 3.
Assessments of degeneration, necrotic changes graded as none, mild, moderate, severe, and extremely severe by light microscopy of kidney sections at 40× magnification in 10 randomly selected areas
| Group | Degeneration | Necrosis | Inflammatory cell | Biliary hiperplasia |
| Liver | ||||
| I | 0.00±0.00b | 0.00±0.00b | 0.00±0.00c | 0.00±0.00c |
| II | 0.83±0.31b | 0.00±0.00b | 0.00±0.00c | 0.00±0.00c |
| III | 2.33±0.21c | 1.17±0.31c | 1.17±0.31c | 1.50±0.43b |
| IV | 3.67±0.21a | 2.50±0.22a | 2.50±0.22a | 2.67±0.21a |
| V | 1.83±0.17bc | 0.83±0.17bc | 0.33±0.21b | 1.17±0.31b |
| VI | 3.00±0.26ab | 1.83±0.17ab | 1.83±0.17ab | 1.83±0.31ab |
|
| ||||
| P | *** | *** | *** | *** |
|
| ||||
| Kidney | ||||
| I | 0.00±0.00c | 0.00±0.00c | 0.00±0.00c | |
| II | 0.50±0.22c | 0.00±0.00c | 0.00±0.00c | |
| III | 2.50±0.22b | 1.00±0.37b | 0.83±0.31bc | |
| IV | 4.00±0.00a | 3.17±0.17a | 2.00±0.26a | |
| V | 2.00±0.00b | 1.17±0.17b | 0.50±0.22c | |
| VI | 3.33±0.21a | 2.33±0.21a | 1.67±0.21ab | |
|
| ||||
| P | *** | *** | *** | |
Group I: control group; Group II: HPE1-administered group; Group III: HPE2-administered group; Group IV: gentamicin-administered group; Group V: gentamicin-plus-HPE1-treated group; Group VI: gentamicin-plus-HPE2-treated group. Data are expressed as mean±SEM (n=6). Values with different superscripts within one column differ significantly.
P<0.001
4. Discussion
Gentamicin is a well-known potent, broad spectrum aminoglycoside antibiotic with a low cost and high effectiveness when used against Gram-negative infections (Banday et al., 2008; Salem et al., 2010). However, gentamicin use has been restricted and gentamicin is used carefully owing to its effect of causing nephrotoxicity and hepatotoxicity (Stojiljkovic and Stoiljkovic, 2006; Raju et al., 2011; Nayma et al., 2012; Masakazu et al., 2014). The present study has shown the effects of HPE and how it develops gentamicin-induced nephrotoxicity in rats.
Gentamicin-induced renal toxicity is clinically characterized and is linked with a marked increase in lipid peroxidation, nitrotyrosine formation, and protein oxidation in the renal cortex by an increase in nitrogenous waste products in the blood (BUN and serum creatinine),$?@%">nitrogenous waste products in the blood (BUN and serum creatinine),and decreased excretion in urine and glomerular filtration rate (Balakumar et al., 2010). In this study, the level of both the serum BUN and creatinine significantly decreased in the gentamicin-induced nephrotoxicity groups treated with HPE1 and HPE2 when compared to the gentamicin-induced nephrotoxicity group not treated with the plant extract. Those levels of the control that reflected injury to the kidneys as biomarkers in the HPE-treated rats (P<0.001) and the levels of serum BUN and creatinine in the GM-plus-HPE1-treated rats were significantly decreased in comparison to those of the gentamicin rats. While HPE2 improved the renal function, significant normalization of serum creatinine and BUN levels were measured only with the lower dose 100 mg/kg of HPE. This indicates that kidney injuries decreased with HPE 100 mg/kg treatment. Researchers have reported similar findings of increase in serum BUN and creatinine levels in the model of nephrotoxicity (Ali, 2003; Singh et al., 2012).
Nephrotoxicity and hepatotoxicity, major side effects of the use of gentamicin sulphate, have resulted in ROS production. Antioxidant compounds have played effective roles in the decline of kidney and liver injuries induced by the use of gentamicin. Oxidative stress is one of the agents responsible for renal and liver injury (Wang et al., 2004; Tavafi, 2013; Mahmood et al., 2014). Gentamicin has caused severe renal injuries in rats, which were revealed by a marked elevation in both the plasma BUN and creatinine and histopathological changes in the proximal tubule cells. Gentamicin increases the generation of ROS, like reactive nitrogen species, hydroxyl radicals, hydrogen peroxide, and super oxide anions in the kidney and is implicated in the pathophysiology of gentamicin-induced nephrotoxicity. ROS cause cellular injury, degeneration, and necrosis by such mechanisms as peroxidation of membrane lipids, protein denaturation, and DNA damage (Ouédraogo et al., 2013).
Antioxidants have an important role in the prevention and treatment of diseases (Peng et al., 2000). Gentamicin reduced the activity of important endogenous antioxidants such as CAT, GPx, and SOD (Moreira et al., 2014). Gentamicin-induced acute renal damage increased the level of lipid peroxidation in both kidney and liver tissues. This increase in lipid peroxidation was significantly reduced by HPE in a dose-dependent manner, which confirmed that the plant extract is capable of attenuating oxidative stress.
In the GM-plus-HPE1-treated group, it was shown that the activity of liver and kidney CAT, GPx, and SOD increased and MDA levels decreased. HPE of 100 mg/kg dose demonstrated a positive effect on the nephrotoxicity of rat livers and renal tissues by partially supporting an antioxidant defense in nephrotoxicity in rats and it had therapeutic effects on liver and renal pathology and biochemical parameters. It was found that HPE 200 mg/kg significantly decreased SOD, GPx, and CAT activity and increased MDA level in nephrotoxicity in rats.
Some reported research has reinforced our results since they found that a gentamicin-administrated group caused a highly significant increase in kidney and liver MDA levels. They also showed a highly significant decrease in hepatic and renal CAT and SOD, and reduced GSH activity as compared to the control group (Yaman and Balikci, 2010; Kamel et al., 2015). These investigations indicated that gentamicin-induced nephrotoxicity results are in line with those of other studies (Tavafi and Ahmadvand, 2011; Al-Kenanny et al., 2012; Ademiluyi et al., 2013; El-Kashef and El-Kenawi, 2015) and these findings related well to the kidney and liver histological results.
Evidence has indicated that the renal toxicity of gentamicin is due to its selective accumulation in the renal proximal convoluted tubules and its long-term stay, which subsequently leads to the loss of brush border integrity, severe degeneration (vacuolar or hydrophic degeneration) and necrosis in epithelial cells of the proximal tubules, and mononuclear cells have infiltrated in intertubular areas (Raju et al., 2011; Liu et al., 2016; Veljković et al., 2016). In this study, gentamicin-administered group (Group IV) showed congestion and dilatation in the renal blood vessels and severe degeneration (vacuolar or hydrophic degeneration) and necrosis in epithelial cells of the proximal tubules.
Evidence has indicated that the hepatotoxicity of gentamicin subsequently leads to necrosis and degenerative changes in the hepatocytes (Stojiljkovic and Stoiljkovic, 2006; Nayma et al., 2012; Mahmood et al., 2014; Masakazu et al., 2014). In this study, gentamicin-administered groups (Group IV) showed remarkable degenerative changes and necrosis in the hepatocytes; mononuclear cells had infiltrated within portal areas and proliferation of Kupffer cells.
The biochemical analysis of our study was confirmed by the histopathological analysis, where the GM group showed necrosis of the proximal tubules, vacuolization of the cytoplasm, and massive mononuclear inflammatory infiltrates in the interstitium. Both biochemical results and histopathological evidence showed that administration of a 100 mg/kg HPE dose reduced the gentamicin-induced nephrotoxicity. Co-administration of HPE with gentamicin had a renoprotective and hepatoprotective effect and showed that only mild infiltrations had normal glomeruli and alleviated tubular degeneration.
5. Conclusions
The present study showed that a 100 mg/kg HPE dose has beneficial effects in decreasing the elevated serum BUN and creatinine and liver and kidney levels of MDA, and increased CAT, GPx, and SOD in nephrotoxic rats. Histological analyses of the liver and kidney indicated that the extract reduced the damage as compared to the gentamicin group. The protective effects of HPE on histopathological and biochemical parameters of liver and kidney tissues in lesions of gentamicin-induced nephrotoxicity rats had not previously been reported. This study showed that HPE might prevent gentamicin-induced hepatorenal toxicity and the related oxidative stress by inhibiting free radical generation and by restoration of the antioxidant systems.
Acknowledgments
The authors would like to thank Unsal Sami AKTAS (Ani-Med Pharmacy, Turkey) for the financial support and Meryem SENGUL (Department of Botanic, Faculty of Agriculture, Ataturk University, Erzurum, Turkey) for identifying Helichrysum plicatum DC. subsp. plicatum plant.
Footnotes
Compliance with ethics guidelines: Betul APAYDIN YILDIRIM, Saban KORDALI, Kubra Asena TERIM KAPAKIN, Fatih YILDIRIM, Esra AKTAS SENOCAK, and Serdar ALTUN declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Ademiluyi AO, Oboh G, Owoloye TR, et al. Modulatory effect of dietary inclusion of garlic (Allium Sativum) on gentamicin-induced hepatotoxicity and oxidative stress in rats. Asian Pac J Trop Biomed. 2013;3(6):470–475. doi: 10.1016/S2221-1691(13)60098-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albayrak S, Aksoy A, Sagdic O, et al. Composition, antioxidant and antimicrobial activities of Helicrysum (Asteraceae) species collected from Turkey. Food Chem. 2009;119(1):114–122. doi: 10.1016/j.foodchem.2009.06.003. [DOI] [Google Scholar]
- 3.Ali BH. Agents ameliorating or augmenting experimental gentamicin nephrotoxicity: some recent research. Food Chem Toxicol. 2003;41(11):1447–1452. doi: 10.1016/S0278-6915(03)00186-8. [DOI] [PubMed] [Google Scholar]
- 4.Al-Kenanny ER, Al-Hayaly LK, Al-Badrany AG. Protective effect of Arabic gum on liver injury experimentally induced by gentamicin in mice. Kufa J Vet Med Sci. 2012;3:174–189. [Google Scholar]
- 5.Aslan M. Seker hastalıgına karsı halkilacı olarak kullanılan bitkiler üzerinde Farmakognozik Arastırmalar. Doktora tezi, Gazi Üniversitesi Saglık Bilimler Enstitüsü farmakognozik Anabilimdalı, Ankara; 2000. p. 216. (in Turkish) [Google Scholar]
- 6.Aslan M, Orhan DD, Orhan N, et al. In vivo antidiabetic and antioxidant potential of Helichrysum plicatum ssp. plicatum capitulums in streptozotocin-induced-diabetic rats. J Ethnopharmacol. 2007;109(1):54–59. doi: 10.1016/j.jep.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Balakumar P, Rohilla A, Thangathirupathi A. Gentamicin-induced nephrotoxicity: do we have a promising therapeutic approach to blunt it? Pharmacol Res. 2010;62(3):179–186. doi: 10.1016/j.phrs.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Banday AA, Farooq N, Priyamvada S, et al. Time dependent effects of gentamicin on the enzymes of carbohydrate metabolism, brush border membrane and oxidative stress in rat kidney tissues. Life Sci. 2008;82(9-10):450–459. doi: 10.1016/j.lfs.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 9.Bayer RJ, Breitwieaser I, Ward J, et al. Tribe Gnaphalieae (Cass.) Lecoq & Juillet (1831) In: Kadereit JW, Jeffrey C, editors. The Families and Genera of Vascular Plants, Asterales. Springer, Berlin; 2007. pp. 246–284. [Google Scholar]
- 10.Corona PS, Espinal L, Rodriguez-Pardo D, et al. Antibiotic susceptibility in Gram-positive chronic joint arthroplasty infections: increased aminoglycoside resistance rate in patients with prior aminoglycoside-impregnated cement spacer use. J Arthropl. 2014;29(8):1617–1621. doi: 10.1016/j.arth.2014.03.029. [DOI] [PubMed] [Google Scholar]
- 11.El-Kashef DH, El-Kenawi AE, et al. Protective effect of allicin against gentamicin-induced nephrotoxicity in rats. Int Immunopharmacol. 2015;29(2):679–686. doi: 10.1016/j.intimp.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Góth LA. Simple method for determination of serum catalase activity and revision of serum catalase activity and revision of reference range. Clin Chim Acta. 1991;196(2-3):143–152. doi: 10.1016/0009-8981(91)90067-M. [DOI] [PubMed] [Google Scholar]
- 13.Guner A. Turkiye Bitkileri Listesi (The List of Turkish Plants), Namas Matbaacilik San. Tic. A.Ş., Istanbul; 2012. pp. 163–165. (in Turkish) [Google Scholar]
- 14.Kamel MA, Hosny Abdel Fadil I, Noha MA. Prevention of Hepato-renal toxicity with vitamin E, vitamin C and their combination in gentamicin treated rats. Int J Pharma Sci. 2015;5(6):1289–1296. [Google Scholar]
- 15.Kandemir FM, Özkaraca M, Apaydin Yildirim B, et al. Rutin attenuates gentamicin-induced renal damage by reducing the oxidative stress, inflammation, apoptosis and autophagy in rats. Ren Fail. 2015;23:1–8. doi: 10.3109/0886022X.2015. [DOI] [PubMed] [Google Scholar]
- 16.Liu P, Feng Y, Dong D, et al. Enhanced renoprotective effect of IGF-1 modified human umbilical cord-derived mesenchymal stem cells on gentamicin-induced acute kidney injury. Sci Rep-UK. 2016;6:20287. doi: 10.1038/srep20287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahmood N, Haleh M, Mohammad P, et al. Pathological changes of gentamicin in liver tissue and antioxidant property of Cinnamon extract on Wistar rats. Biomed Pharmacol J. 2014;7(1):341–347. doi: 10.13005/bpj/496. [DOI] [Google Scholar]
- 18.Masakazu K, Yoshiko E, Masashi E. Acquired resistance of Listeria monocytogenes in and escaped from liver parenchymal cells to gentamicin is caused by being coated with their plasma membrane. Microb Infect. 2014;16(3):237–243. doi: 10.1016/j.micinf.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Martínez-Salgado C, López-Hernández FJ, López-Novoa JM. Glomerular nephrotoxicity of amino nucleosides. Toxicol Appl Pharmacol. 2007;223(1):86–98. doi: 10.1016/j.taap.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Matkovics B, Szabo L, Varga IS. Determination of enzyme activities in lipid peroxidation and glutathione pathways. Laboratoriumi Diagnosztika. 1988;15:248–249. (in Hungarian) [Google Scholar]
- 21.Mert N. Veteriner Klinik Biyokimya. Uludag Universitesi Veteriner Fakultesi Guclendirme Vakfı Yayın 12, Bursa; 1996. pp. 151–153. (in Turkish) [Google Scholar]
- 22.Moreira MA, Nascimento MA, Bozzo TA, et al. Ascorbic acid reduces gentamicin induced nephrotoxicity in rats through the control of reactive oxygen species. Clin Nutr. 2014;33(2):296–301. doi: 10.1016/j.clnu.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Morone-Fortunato I, Montemurro C, Ruta C, et al. Essential oils, genetic relationships and in vitro establishment of Helichrysum italicum (Roth) G. Don subsp. italicum from wild Mediterranean germplasm. Ind Crops Prod. 2010;32(3):639–649. doi: 10.1016/j.indcrop.2010.07.023. [DOI] [Google Scholar]
- 24.Nasri H. Acute kidney injury and beyond. J Ren Inj Prev. 2012;1(1):1–2. doi: 10.12861/jrip.2012.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nayma S, Sadia CS, M Tanveer HP, et al. Effects of Ashwagandha (Withania somnifera) root extract on some serum liver marker enzymes (AST, ALT) in gentamicin intoxicated rats. J Bangladesh Soc Physiol. 2012;7(1):1–7. doi: 10.3329/jbsp.v7i1.11152. [DOI] [Google Scholar]
- 26.Ojano-Dirain CP, Antonelli PJ, le Prell CG. Mitochondria-targeted antioxidant MitoQ reduces gentamicin-induced ototoxicity. Otol Neurotol. 2014;35(3):533–539. doi: 10.1097/MAO.0000000000000192. [DOI] [PubMed] [Google Scholar]
- 27.Ouédraogo M, Lamien-Sanoub A, Ramdeb N, et al. Protective effect of Moringa oleifera leaves against gentamicin-induced nephrotoxicity in rabbits. Exp Toxicol Pathol. 2013;65(3):335–339. doi: 10.1016/j.etp.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Peng J, Gones GL, Watson K. Stress protein as biomarkers of oxidative stress: effects of antioxidant supplement. Free Radic Biol Med. 2000;28(11):1598–1606. doi: 10.1016/S0891-5849(00)00276-8. [DOI] [PubMed] [Google Scholar]
- 29.Placer ZA, Cushmanni LL, Johnson BC. Estimation of products of lipid peroxidation (as malondialdehyde) in biochemical systems. Anal Biochem. 1966;16(2):359–364. doi: 10.1016/0003-2697(66)90167-9. [DOI] [PubMed] [Google Scholar]
- 30.Raju S, Kavimani S, Maheshwara rao VU, et al. Floral extract of Tecoma stans: a potent inhibitor of gentamicin-induced nephrotoxicity in vivo . Asian Pac J Trop Med. 2011;4(9):680–685. doi: 10.1016/S1995-7645(11)60173-9. [DOI] [PubMed] [Google Scholar]
- 31.Sagdic O, Karahan AG, Ozcan M, et al. Effect of some spice extracts on bacterial inhibition. Food Sci Technol Int. 2003;9(5):353–356. doi: 10.1177/1082013203038976. [DOI] [Google Scholar]
- 32.Sala A, Recio MC, Schinella GR, et al. Assessment of the anti-inflammatory activity and free radical scavenger activity of tiliroside. Eur J Pharmacol. 2003;461(1):53–61. doi: 10.1016/S0014-2999(02)02953-9. [DOI] [PubMed] [Google Scholar]
- 33.Salem EA, Salem NA, Kamel M, et al. Amelioration of gentamicin nephrotoxicity by green tea extract in uninephrectomized rats as a model of progressive renal failure. Ren Fail. 2010;32(10):1210–1215. doi: 10.3109/0886022X.2010.517350. [DOI] [PubMed] [Google Scholar]
- 34.Sezik E, Yesilada E, Honda G, et al. Traditional medicine in Turkey. X. Folk medicine in Central Anatolia. J Ethnopharmacol. 2001;75(2-3):95–115. doi: 10.1016/S0378-8741(00)00399-8. [DOI] [PubMed] [Google Scholar]
- 35.Shrestha B, Haylor J. Experimental rat models of chronic allograft nephropathy: a review. Int J Nephrol Renovasc Dis. 2014;23(7):315–322. doi: 10.2147/IJNRD.S65604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh AP, Junemann A, Muthuraman A, et al. Animal models of acute renal failure. Pharmacol Rep. 2012;64(1):31–44. doi: 10.1016/S1734-1140(12)70728-4. [DOI] [PubMed] [Google Scholar]
- 37.Smirnov V, Preobrazheskaia N, Kalashnikov I. Antibacterial properties of Helichrysum plicatum . Mikrobiol Z. 1982;44:71–72. [PubMed] [Google Scholar]
- 38.Stafford GI, Jäger AK, van Staden J. Effect of storage on the chemical composition and biological activity of several popular South African medicinal plants. J Ethnopharmacol. 2005;97(1):107–115. doi: 10.1016/j.jep.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 39.Stojiljkovic N, Stoiljkovic M. Micromorphological and histochemical characteristics of a rat’s liver treated with gentamicin. Acta Medica Medianae. 2006;45(3):24–28. [Google Scholar]
- 40.Sumbul H, Gokturk RS, Dussen OD. A new endemic species of Helichrysum Gaertn. (Asteraceae-Inuleae) from south Anatolia. Bot J Linn Soc. 2003;141(2):251–254. doi: 10.1046/j.1095-8339.2003.00109.x. [DOI] [Google Scholar]
- 41.Sun Y, Oberley LW, Li YA. Simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34(3):497–500. [PubMed] [Google Scholar]
- 42.Süzgec S, Mericli AH, Houghton PJ, et al. Flavonoids of Helichrysum compactum and their antioxidant and antibacterial activity. Fitoterapia. 2005;76(2):269–272. doi: 10.1016/j.fitote.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 43.Tavafi M. Protection of renal tubules against gentamicin induced nephrotoxicity. J Ren Inj Prev. 2013;2(1):5–6. doi: 10.12861/jrip.2013.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tavafi M, Ahmadvand H. Effect of rosmarinic acid on inhibition of gentamicin induced nephrotoxicity in rats. Tissue Cell. 2011;43(6):392–397. doi: 10.1016/j.tice.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 45.Veljković M, Pavlović DR, Stojiljković N, et al. Morphological and morphometric study of protective effect of green tea in gentamicin-induced nephrotoxicity in rats. Life Sci. 2016;147:85–91. doi: 10.1016/j.lfs.2016.01.035. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y, Iwatani H, Ito T, et al. Fetal cells in mother rats contribute to the remodeling of liver and kidney after injury. Biochem Bioph Res Commun. 2004;325(3):961–967. doi: 10.1016/j.bbrc.2004.10.105. [DOI] [PubMed] [Google Scholar]
- 47.Yaman I, Balikci S. Protective effect of Nigella sativa against gentamicin-induced nephrotoxicity in rats. Exp Toxicol Path. 2010;62(2):183–190. doi: 10.1016/j.etp.2009.03.006. [DOI] [PubMed] [Google Scholar]